Practical Considerations for Next-Generation Adjuvant Development and Translation
Abstract
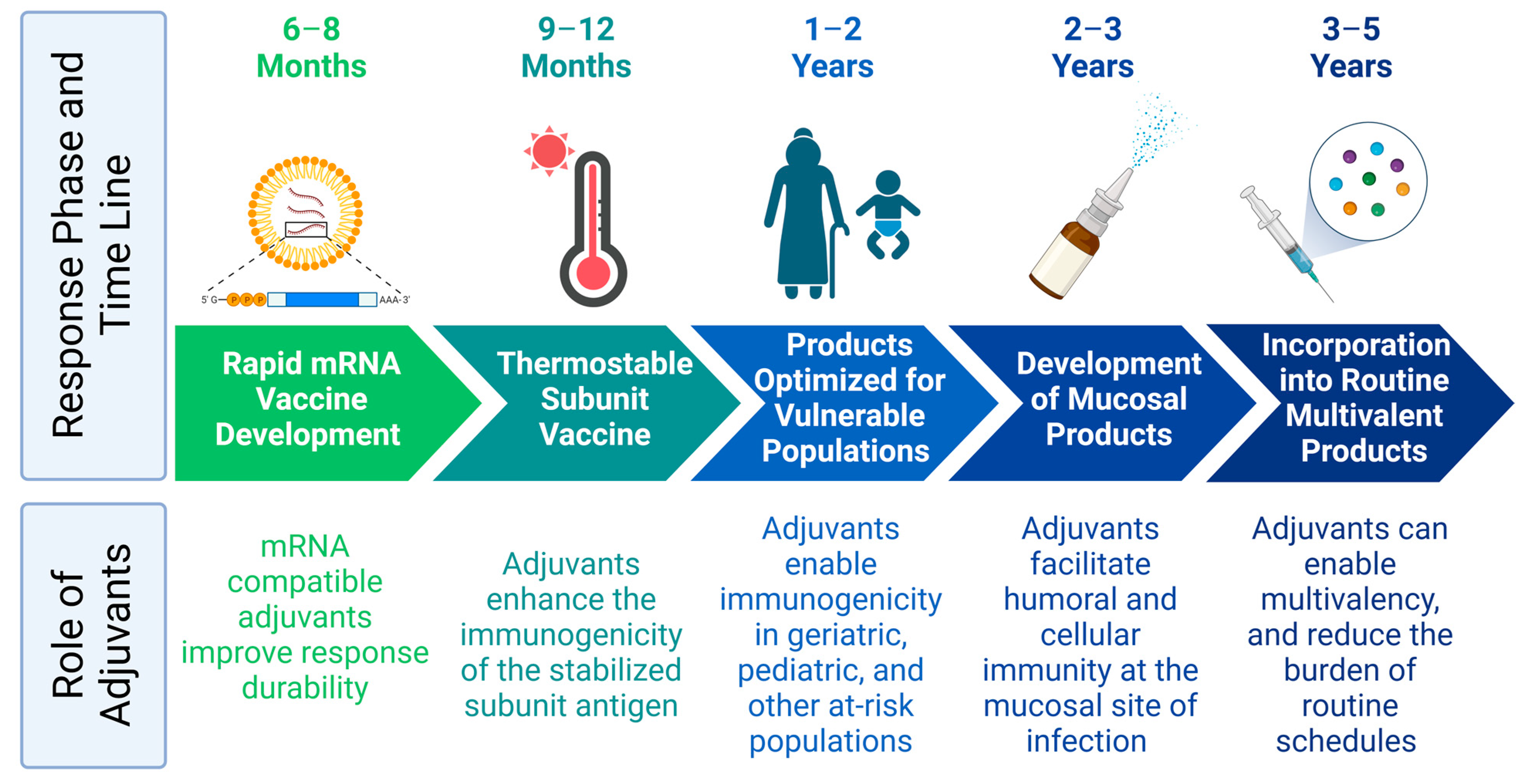
1. Introduction
2. Sustainable Raw Materials
3. Thermostability
4. Alternative Routes of Delivery
5. Control of Exposure Duration and Kinetics
6. Combining Multiple Adjuvants and Vaccine Platforms
7. Optimization via Aggregate Metrics
8. Conclusions
- Derisk the environmental and supply chain sustainability of materials. Consider alternate compounds or production methods to reduce dependency on animal or unsustainable sources.
- Incorporate thermostability approaches early in development.
- Define the need that a new adjuvant would address (e.g., mucosal immunity and durability of immunity) and tailor subsequent activities to this need.
- Keep compositions as simple as possible but consider the added value of combination approaches when necessary.
- Employ experimental design methods and systems immunology approaches to comprehensively characterize the immune profile of the new adjuvant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The Continued Advance of Vaccine Adjuvants—‘We can work it out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Hogenesch, H.; Orr, M.T.; Fox, C.B. Vaccine Adjuvants: Mechanisms of Action. In Vaccine Development: From Concept to Clinic; Prasad, A.K., Ed.; Drug Discovery Series; Royal Society of Chemistry: London, UK, 2023; pp. 214–236. [Google Scholar]
- Burny, W.; Callegaro, A.; Bechtold, V.; Clement, F.; Delhaye, S.; Fissette, L.; Janssens, M.; Leroux-Roels, G.; Marchant, A.; van den Berg, R.A.; et al. Different Adjuvants Induce Common Innate Pathways That Are Associated with Enhanced Adaptive Responses against a Model Antigen in Humans. Front. Immunol. 2017, 8, 943. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of Adaptive Immunity by the Human Vaccine Adjuvant AS01 Depends on Activated Dendritic Cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant Protein Vaccines, a Proven Approach against Coronavirus Pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Ghimire, T.R.; Benson, R.A.; Garside, P.; Brewer, J.M. Alum Increases Antigen Uptake, Reduces Antigen Degradation and Sustains Antigen Presentation by DCs in Vitro. Immunol. Lett. 2012, 147, 55–62. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Fox, C.B.; Haensler, J. An Update on Safety and Immunogenicity of Vaccines Containing Emulsion-Based Adjuvants. Expert Rev. Vaccines 2013, 12, 747–758. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The Mechanism of Action of MF59—An Innately Attractive Adjuvant Formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Wenbin Tuo, D.Z. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2016, 3, e113. [Google Scholar] [CrossRef]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; de Oliveira, M.H.S.; Catahay, J.A.; Khan, S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the Significant Decline in Humoral Immune Response Six Months Post-SARS-CoV-2 MRNA Vaccination: A Systematic Review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Milligan, E.C.; Olstad, K.; Williams, C.A.; Mallory, M.; Cano, P.; Cross, K.A.; Munt, J.E.; Garrido, C.; Lindesmith, L.; Watanabe, J.; et al. Infant Rhesus Macaques Immunized against SARS-CoV-2 Are Protected against Heterologous Virus Challenge 1 Year Later. Sci. Transl. Med. 2023, 15, eadd6383. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Braga, F.C.; Buenz, E.J.; Campana, P.R.V.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.; Niwasabutra, K.; Acevedo, R.; Igoli, J.; Altwaijry, N.A.; Tusiimire, J.; Gray, A.I.; Watson, D.G.; Ferro, V.A. Natural Vaccine Adjuvants and Immunopotentiators Derived From Plants, Fungi, Marine Organisms, and Insects. In Immunopotentiators in Modern Vaccines; Elsevier: London, UK, 2017; pp. 211–229. ISBN 978-0-12-804019-5. [Google Scholar]
- Fernández-Tejada, A.; Tan, D.S.; Gin, D.Y. Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis. Acc. Chem. Res. 2016, 49, 1741–1756. [Google Scholar] [CrossRef]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Fox, C.B. A Two-Step Orthogonal Chromatographic Process for Purifying the Molecular Adjuvant QS-21 with High Purity and Yield. J. Chromatogr. A 2021, 1635, 461705. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Orme, A.; El-Demerdash, A.; Owen, C.; Martin, L.B.B.; Misra, R.C.; Kikuchi, S.; Rejzek, M.; Martin, A.C.; Harkess, A.; et al. Elucidation of the Pathway for Biosynthesis of Saponin Adjuvants from the Soapbark Tree. Science 2023, 379, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tejada, A.; Walkowicz, W.E.; Tan, D.S.; Gin, D.Y. Semisynthesis of Analogues of the Saponin Immunoadjuvant QS-21. In Vaccine Adjuvants: Methods and Protocols; Fox, C.B., Ed.; Springer New York: New York, NY, USA, 2017; pp. 45–71. ISBN 978-1-4939-6445-1. [Google Scholar]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Baudner, B.; Gallorini, S.; Santos, G.; O’Hagan, D.T.; Singh, M. An Alternative Renewable Source of Squalene for Use in Emulsion Adjuvants. Vaccine 2011, 29, 6262–6268. [Google Scholar] [CrossRef]
- Fox, C.B.; Anderson, R.C.; Dutill, T.S.; Goto, Y.; Reed, S.G.; Vedvick, T.S. Monitoring the Effects of Component Structure and Source on Formulation Stability and Adjuvant Activity of Oil-in-Water Emulsions. Colloids Surf. B Biointerfaces 2008, 65, 98–105. [Google Scholar] [CrossRef]
- Verma, K.C. Assessment of Squalene Variability and Its Enhancement in Amaranthus Populations: With Application to Vaccine Development. Biotechnol. Appl. Biochem. 2022, 69, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.J.; Kinsey, R.; Mohamath, R.; Phan, T.; Liang, H.; Orr, M.T.; Lykins, W.R.; Guderian, J.A.; Bakken, J.; Argilla, D.; et al. Semi-Synthetic Terpenoids with Differential Adjuvant Properties as Sustainable Replacements for Shark Squalene in Vaccine Emulsions. Npj Vaccines 2023, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Van Hoeven, N.; Granger, B.; Lin, S.; Guderian, J.A.; Hartwig, A.; Marlenee, N.; Bowen, R.A.; Soultanov, V.; Carter, D. Vaccine Adjuvant Activity of Emulsified Oils from Species of the Pinaceae Family. Phytomedicine 2019, 64, 152927. [Google Scholar] [CrossRef] [PubMed]
- Tateno, M.; Stone, B.J.; Srodulski, S.J.; Reedy, S.; Gawriluk, T.R.; Chambers, T.M.; Woodward, J.; Chappell, J.; Kempinski, C.F. Synthetic Biology-Derived Triterpenes as Efficacious Immunomodulating Adjuvants. Sci. Rep. 2020, 10, 17090. [Google Scholar] [CrossRef] [PubMed]
- Adlington, K.; El Harfi, J.; Li, J.; Carmichael, K.; Guderian, J.A.; Fox, C.B.; Irvine, D.J. Molecular Design of Squalene/Squalane Countertypes via the Controlled Oligomerization of Isoprene and Evaluation of Vaccine Adjuvant Applications. Biomacromolecules 2016, 17, 165–172. [Google Scholar] [CrossRef]
- Coler, R.N.; Bertholet, S.; Moutaftsi, M.; Guderian, J.A.; Windish, H.P.; Baldwin, S.L.; Laughlin, E.M.; Duthie, M.S.; Fox, C.B.; Carter, D.; et al. Development and Characterization of Synthetic Glucopyranosyl Lipid Adjuvant System as a Vaccine Adjuvant. PLoS ONE 2011, 6, e16333. [Google Scholar] [CrossRef]
- Fox, C.B.; Carter, D.; Kramer, R.M.; Beckmann, A.M.; Reed, S.G. Current Status of Toll-Like Receptor 4 Ligand Vaccine Adjuvants. In Immunopotentiators in Modern Vaccines; Schijns, V.E.J.C., O’Hagan, D.T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 105–127. ISBN 978-0-12-804019-5. [Google Scholar]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and Immunogenicity of the Chlamydia Vaccine Candidate CTH522 Adjuvanted with CAF01 Liposomes or Aluminium Hydroxide: A First-in-Human, Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- Chen, D.; Zehrung, D. Desirable Attributes of Vaccines for Deployment in Low-Resource Settings. J. Pharm. Sci. 2013, 102, 29–33. [Google Scholar] [CrossRef]
- Lydon, P.; Zipursky, S.; Tevi-Benissan, C.; Djingarey, M.H.; Gbedonou, P.; Youssouf, B.O.; Zaffran, M. Economic Benefits of Keeping Vaccines at Ambient Temperature during Mass Vaccination: The Case of Meningitis A Vaccine in Chad. Bull. World Health Organ. 2014, 92, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Review of COVID-19 MRNA Vaccines: BNT162b2 and MRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of MRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Thermostable Vaccines: An Innovative Concept in Vaccine Development. Expert Rev. Vaccines 2022, 21, 811–824. [Google Scholar] [CrossRef]
- Qi, Y.; Fox, C.B. Development of Thermostable Vaccine Adjuvants. Expert Rev. Vaccines 2021, 20, 497–517. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef]
- Emami, F.; Keihan Shokooh, M.; Mostafavi Yazdi, S.J. Recent Progress in Drying Technologies for Improving the Stability and Delivery Efficiency of Biopharmaceuticals. J. Pharm. Investig. 2023, 53, 35–57. [Google Scholar] [CrossRef]
- Chan, M.Y.; Dutill, T.S.; Kramer, R.M. Lyophilization of Adjuvanted Vaccines: Methods for Formulation of a Thermostable Freeze-Dried Product. In Vaccine Adjuvants: Methods and Protocols; Fox, C.B., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 215–226. ISBN 978-1-4939-6445-1. [Google Scholar]
- Gomez, M.; Vehring, R. Spray Drying and Particle Engineering in Dosage Form Design for Global Vaccines. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 121–138. [Google Scholar] [CrossRef]
- Preston, K.B.; Randolph, T.W. Stability of Lyophilized and Spray Dried Vaccine Formulations. Adv. Drug Deliv. Rev. 2021, 171, 50–61. [Google Scholar] [CrossRef]
- Sagawa, Z.K.; Goman, C.; Frevol, A.; Blazevic, A.; Tennant, J.; Fisher, B.; Day, T.; Jackson, S.; Lemiale, F.; Toussaint, L.; et al. Safety and Immunogenicity of a Thermostable ID93 + GLA-SE Tuberculosis Vaccine Candidate in Healthy Adults. Nat. Commun. 2023, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization Provides Long-Term Stability for a Lipid Nanoparticle-Formulated, Nucleoside-Modified MRNA Vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chan, A.Y.L.; Chow, M.Y.T.; Lo, F.F.K.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K.W. Spray Freeze Drying of Small Nucleic Acids as Inhaled Powder for Pulmonary Delivery. Asian J. Pharm. Sci. 2018, 13, 163–172. [Google Scholar] [CrossRef]
- Demuth, P.C.; Min, Y.; Irvine, D.J.; Hammond, P.T. Implantable Silk Composite Microneedles for Programmable Vaccine Release Kinetics and Enhanced Immunogenicity in Transcutaneous Immunization. Adv. Healthc. Mater. 2014, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Creighton, R.L.; Woodrow, K.A. Microneedle-Mediated Vaccine Delivery to the Oral Mucosa. Adv. Healthc. Mater. 2018, 8, 1801180. [Google Scholar] [CrossRef]
- Frizzell, H.; Woodrow, K.A. Biomaterial Approaches for Understanding and Overcoming Immunological Barriers to Effective Oral Vaccinations. Adv. Funct. Mater. 2020, 30, 1907170. [Google Scholar] [CrossRef]
- VanBenschoten, H.M.; Woodrow, K.A. Vaginal Delivery of Vaccines. Adv. Drug Deliv. Rev. 2021, 178, 113956. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Sengupta, A.; Azharuddin, M.; Cardona, M.E.; Devito, C.; von Castelmur, E.; Wehlin, A.; Pietras, Z.; Sunnerhagen, M.; Selegård, R.; Aili, D.; et al. Intranasal Coronavirus SARS-CoV-2 Immunization with Lipid Adjuvants Provides Systemic and Mucosal Immune Response against SARS-CoV-2 S1 Spike and Nucleocapsid Protein. Vaccines 2022, 10, 504. [Google Scholar] [CrossRef]
- Kar, S.; Devnath, P.; Emran, T.B.; Tallei, T.E.; Mitra, S.; Dhama, K. Oral and Intranasal Vaccines against SARS-CoV-2: Current Progress, Prospects, Advantages, and Challenges. Immun. Inflamm. Dis. 2022, 10, e604. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F.; Casadevall, A. Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence. Viruses 2022, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Halberg, I.B.; Lyby, K.; Wassermann, K.; Heise, T.; Zijlstra, E.; Plum-Mörschel, L. Efficacy and Safety of Oral Basal Insulin versus Subcutaneous Insulin Glargine in Type 2 Diabetes: A Randomised, Double-Blind, Phase 2 Trial. Lancet Diabetes Endocrinol. 2019, 7, 179–188. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional Oral Delivery Systems for Enhanced Bioavailability of Therapeutic Peptides/Proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Y.; Gungor, S.; Cevher, E. Nasal Delivery of High Molecular Weight Drugs. Molecules 2009, 14, 3754–3779. [Google Scholar] [CrossRef] [PubMed]
- Sodha, S.J.; Patel, M.; Nagarkar, R.; Mohammed, I.A.; Patel, H. Translation of Pulmonary Protein Therapy from Bench to Bedside: Addressing the Bioavailability Challenges. J. Drug Deliv. Sci. Technol. 2021, 64, 102664. [Google Scholar] [CrossRef]
- Gomez, M.; Archer, M.; Barona, D.; Wang, H.; Ordoubadi, M.; Bin Karim, S.; Carrigy, N.B.; Wang, Z.; McCollum, J.; Press, C.; et al. Microparticle Encapsulation of a Tuberculosis Subunit Vaccine Candidate Containing a Nanoemulsion Adjuvant via Spray Drying. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2021, 163, 23–37. [Google Scholar] [CrossRef]
- Murphy, B.M.; Chen, J.Z.; Rolo, M.; Eldam, M.; Jordan, L.; Sivananthan, S.J.; Kinsey, R.; Guderian, J.A.; Pedersen, K.; Abhyankar, M.; et al. Intranasal Delivery of a Synthetic Entamoeba Histolytica Vaccine Containing Adjuvant (LecA + GLA-3 M-052 Liposomes): In Vitro Characterization. Int. J. Pharm. 2022, 626, 122141. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Newman, S.P. Drug Delivery to the Lungs: Challenges and Opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Cheng, L.; Wong, H. Food Effects on Oral Drug Absorption: Application of Physiologically-Based Pharmacokinetic Modeling as a Predictive Tool. Pharmaceutics 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.; Ramani, S.; Lopman, B.A.; Church, J.A.; Iturriza-Gómara, M.; Prendergast, A.J.; Grassly, N.C. Causes of Impaired Oral Vaccine Efficacy in Developing Countries. Future Microbiol. 2018, 13, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Picece, V.C.T.M.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing Spatial and Temporal Control of Vaccine Responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling Timing and Location in Vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171. [Google Scholar] [CrossRef]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained Antigen Availability during Germinal Center Initiation Enhances Antibody Responses to Vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef]
- Gale, E.C.; Powell, A.E.; Roth, G.A.; Meany, E.L.; Yan, J.; Ou, B.S.; Grosskopf, A.K.; Adamska, J.; Picece, V.C.T.M.; d’Aquino, A.I.; et al. Hydrogel-Based Slow Release of a Receptor-Binding Domain Subunit Vaccine Elicits Neutralizing Antibody Responses Against SARS-CoV-2. Adv. Mater. 2021, 33, 2104362. [Google Scholar] [CrossRef]
- Shah, N.J.; Najibi, A.J.; Shih, T.Y.; Mao, A.S.; Sharda, A.; Scadden, D.T.; Mooney, D.J. A Biomaterial-Based Vaccine Eliciting Durable Tumour-Specific Responses against Acute Myeloid Leukaemia. Nat. Biomed. Eng. 2020, 4, 40–51. [Google Scholar] [CrossRef]
- Cirelli, K.M.; Crotty, S. Germinal Center Enhancement by Extended Antigen Availability. Curr. Opin. Immunol. 2017, 47, 64–69. [Google Scholar] [CrossRef]
- Bobbala, S.; Hook, S. Vaccine Implants: Current Status and Recent Advancements. Emerg. Top. Life Sci. 2021, 4, 601–612. [Google Scholar] [CrossRef]
- Mudzingwa, E.K.; Parker, I.K. Long-Acting Injections for HIV Prevention among Women in Sub-Saharan Africa. Lancet 2022, 399, 1754–1755. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.T.; Atujuna, M.; Krogstad, E.; Hartmann, M.; Ndwayana, S.; O’Rourke, S.; Bekker, L.-G.; Van Der Straten, A.; Minnis, A.M. The Invisible Product: Preferences for Sustained-Release, Long-Acting Pre-Exposure Prophylaxis to HIV Among South African Youth. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 80, 542–550. [Google Scholar] [CrossRef]
- Saouaf, O.M.; Roth, G.A.; Ou, B.S.; Smith, A.A.A.; Yu, A.C.; Gale, E.C.; Grosskopf, A.K.; Picece, V.C.T.M.; Appel, E.A. Modulation of Injectable Hydrogel Properties for Slow Co-delivery of Influenza Subunit Vaccine Components Enhance the Potency of Humoral Immunity. J. Biomed. Mater. Res. A 2021, 109, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Gale, E.C.; Alcántara-Hernández, M.; Luo, W.; Axpe, E.; Verma, R.; Yin, Q.; Yu, A.C.; Lopez Hernandez, H.; Maikawa, C.L.; et al. Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Cent. Sci. 2020, 6, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.S.; Saouaf, O.M.; Yan, J.; Bruun, T.U.J.; Baillet, J.; Zhou, X.; King, N.P.; Appel, E.A. Broad and Durable Humoral Responses Following Single Hydrogel Immunization of SARS-CoV-2 Subunit Vaccine. Adv. Healthc. Mater. 2023, in press. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next Generation of Immune Checkpoint Inhibitors and Beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Garçon, N.; Di Pasquale, A. From Discovery to Licensure, the Adjuvant System Story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and Molecular Synergy in AS01-Adjuvanted Vaccines Results in an Early IFNγ Response Promoting Vaccine Immunogenicity. Npj Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Weinberger, B. Adjuvant Strategies to Improve Vaccination of the Elderly Population. Curr. Opin. Pharmacol. 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Polhemus, M.E.; Remich, S.A.; Ogutu, B.R.; Waitumbi, J.N.; Otieno, L.; Apollo, S.; Cummings, J.F.; Kester, K.E.; Ockenhouse, C.F.; Stewart, A.; et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in Adults in a High Malaria Transmission Area. PLoS ONE 2009, 4, e6465. [Google Scholar] [CrossRef] [PubMed]
- Dorostkar, F.; Arashkia, A.; Roohvand, F.; Shoja, Z.; Navari, M.; Mashhadi Abolghasem Shirazi, M.; Shahosseini, Z.; Farahmand, M.; Shams Nosrati, M.S.; Jalilvand, S. Co-administration of 2′3′-CGAMP STING Activator and CpG-C Adjuvants with a Mutated Form of HPV 16 E7 Protein Leads to Tumor Growth Inhibition in the Mouse Model. Infect. Agent. Cancer 2021, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, M.M.; Orr, M.T.; Kinsey, R.; Sivananthan, S.; Nafziger, A.J.; Oakland, D.N.; Young, M.K.; Farr, L.; Uddin, M.J.; Leslie, J.L.; et al. Optimizing a Multi-Component Intranasal Entamoeba Histolytica Vaccine Formulation Using a Design of Experiments Strategy. Front. Immunol. 2021, 12, 683157. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous Prime–Boost Strategies for COVID-19 Vaccines. J. Travel Med. 2021, 29, taab191. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, Safety, and Reactogenicity of Heterologous COVID-19 Primary Vaccination Incorporating MRNA, Viral-Vector, and Protein-Adjuvant Vaccines in the UK (Com-COV2): A Single-Blind, Randomised, Phase 2, Non-Inferiority Trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef]
- Saunders, K.O.; Lee, E.; Parks, R.; Martinez, D.R.; Li, D.; Chen, H.; Edwards, R.J.; Gobeil, S.; Barr, M.; Mansouri, K.; et al. Neutralizing Antibody Vaccine for Pandemic and Pre-Emergent Coronaviruses. Nature 2021, 594, 553–559. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a Subunit COVID-19 Vaccine to Induce Protective Immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef]
- Rice, A.; Verma, M.; Voigt, E.; Battisti, P.; Beaver, S.; Reed, S.; Dinkins, K.; Mody, S.; Zakin, L.; Tanaka, S.; et al. Heterologous SaRNA Prime, DNA Dual-Antigen Boost SARS-CoV-2 Vaccination Elicits Robust Cellular Immunogenicity and Cross-Variant Neutralizing Antibodies. Front. Immunol. 2022, 13, 910136. [Google Scholar] [CrossRef]
- Jalah, R.; Kulkarni, V.; Patel, V.; Rosati, M.; Alicea, C.; Bear, J.; Yu, L.; Guan, Y.; Shen, X.; Tomaras, G.D.; et al. DNA and Protein Co-Immunization Improves the Magnitude and Longevity of Humoral Immune Responses in Macaques. PLoS ONE 2014, 9, e91550. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine into MRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G.; et al. Type I IFN Counteracts the Induction of Antigen-Specific Immune Responses by Lipid-Based Delivery of MRNA Vaccines. Mol. Ther. 2013, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Roose, K.; Ballegeer, M.; Zhong, Z.; Sanders, N.N.; De Koker, S.; Saelens, X.; Van Lint, S. The Opposing Effect of Type I IFN on the T Cell Response by Non-Modified MRNA-Lipoplex Vaccines Is Determined by the Route of Administration. Mol. Ther.-Nucleic Acids 2020, 22, 373–381. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. A Vaccine Strategy That Protects against Genital Herpes by Establishing Local Memory T Cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Awasthi, S.; Lu, P.; Pullum, D.A.; Dixon, D.A.; Iwasaki, A.; Friedman, H.M. Successful Application of Prime and Pull Strategy for a Therapeutic HSV Vaccine. Npj Vaccines 2019, 4, 33. [Google Scholar] [CrossRef]
- Ramanathan, R.; Park, J.; Hughes, S.M.; Lykins, W.R.; Bennett, H.R.; Hladik, F.; Woodrow, K.A. Effect of Mucosal Cytokine Administration on Selective Expansion of Vaginal Dendritic Cells to Support Nanoparticle Transport. Am. J. Reprod. Immunol. 2015, 74, 333–344. [Google Scholar] [CrossRef]
- Khan, A.A.; Srivastava, R.; Vahed, H.; Roy, S.; Walia, S.S.; Kim, G.J.; Fouladi, M.A.; Yamada, T.; Ly, V.T.; Lam, C.; et al. Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/ Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107+ CD8+ T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge. J. Virol. 2018, 92, e00535-18. [Google Scholar] [CrossRef]
- Shamseldin, M.M.; Kenney, A.; Zani, A.; Evans, J.P.; Zeng, C.; Read, K.A.; Hall, J.M.; Chaiwatpongsakorn, S.; Mahesh, K.C.; Lu, M.; et al. Prime-Pull Immunization of Mice with a BcfA-Adjuvanted Vaccine Elicits Sustained Mucosal Immunity That Prevents SARS-CoV-2 Infection and Pathology. J. Immunol. 2023, 210, 1257–1271. [Google Scholar] [CrossRef]
- Chung, H.; Kim, E.-A.; Chang, J. A “Prime and Deploy” Strategy for Universal Influenza Vaccine Targeting Nucleoprotein Induces Lung-Resident Memory CD8 T Cells. Immune Netw. 2021, 21, e28. [Google Scholar] [CrossRef]
- Melero, I.; Berman, D.M.; Aznar, M.A.; Korman, A.J.; Gracia, J.L.P.; Haanen, J. Evolving Synergistic Combinations of Targeted Immunotherapies to Combat Cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Lell, B.; Agnandji, S.; von Glasenapp, I.; Haertle, S.; Oyakhiromen, S.; Issifou, S.; Vekemans, J.; Leach, A.; Lievens, M.; Dubois, M.C.; et al. A Randomized Trial Assessing the Safety and Immunogenicity of AS01 and AS02 Adjuvanted RTS,S Malaria Vaccine Candidates in Children in Gabon. PLoS ONE 2009, 4, e7611. [Google Scholar] [CrossRef] [PubMed]
- Spring, M.D.; Cummings, J.F.; Ockenhouse, C.F.; Dutta, S.; Reidler, R.; Angov, E.; Bergmann-Leitner, E.; Stewart, V.A.; Bittner, S.; Juompan, L.; et al. Phase 1/2a Study of the Malaria Vaccine Candidate Apical Membrane Antigen-1 (AMA-1) Administered in Adjuvant System AS01B or AS02A. PLoS ONE 2009, 4, e5254. [Google Scholar] [CrossRef] [PubMed]
- Rangel, C.; Angus, J.; Ghahramani, Z.; Lioumi, M.; Sotheran, E.; Gaiba, A.; Wild, D.L.; Falciani, F. Modeling T-Cell Activation Using Gene Expression Profiling and State-Space Models. Bioinformatics 2004, 20, 1361–1372. [Google Scholar] [CrossRef]
- Eftimie, R.; Gillard, J.J.; Cantrell, D.A. Mathematical Models for Immunology: Current State of the Art and Future Research Directions. Bull. Math. Biol. 2016, 78, 2091–2134. [Google Scholar] [CrossRef] [PubMed]
- Andrew, S.M.; Baker, C.T.H.; Bocharov, G.A. Rival Approaches to Mathematical Modelling in Immunology. J. Comput. Appl. Math. 2007, 205, 669–686. [Google Scholar] [CrossRef]
- Grebennikov, D.S.; Donets, D.O.; Orlova, O.G.; Argilaguet, J.; Meyerhans, A.; Bocharov, G.A. Mathematical Modeling of the Intracellular Regulation of Immune Processes. Mol. Biol. 2019, 53, 718–731. [Google Scholar] [CrossRef]
- Baguelin, M.; LeFèvre, J.; Richard, J.-P. How to Deal with Potentially Huge Dimensional State Space: The Meta-Dynamics Approach—Application to a Model of the Co-Evolution of Bacterio-Phage Populations. J. Comput. Appl. Math. 2007, 205, 687–695. [Google Scholar] [CrossRef]
- Lienert, F.; Lohmueller, J.J.; Garg, A.; Silver, P.A. Synthetic Biology in Mammalian Cells: Next Generation Research Tools and Therapeutics. Nat. Rev. Mol. Cell Biol. 2014, 15, 95–107. [Google Scholar] [CrossRef]
- Davis, M.M.; Tato, C.M.; Furman, D. Systems Immunology: Just Getting Started. Nat. Immunol. 2017, 18, 725–732. [Google Scholar] [CrossRef]
- Bannigan, P.; Bao, Z.; Hickman, R.J.; Aldeghi, M.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine Learning Models to Accelerate the Design of Polymeric Long-Acting Injectables. Nat. Commun. 2023, 14, 35. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems Biology Approach Predicts Immunogenicity of the Yellow Fever Vaccine in Humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 MRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H.I.; Clutterbuck, E.; Kazmin, D.; Wang, L.; Cortese, M.; Bosinger, S.E.; Patel, N.B.; Zak, D.E.; Aderem, A.; Dong, T.; et al. Systems Biology of Immunity to MF59-Adjuvanted versus Nonadjuvanted Trivalent Seasonal Influenza Vaccines in Early Childhood. Proc. Natl. Acad. Sci. USA 2016, 113, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Li, S.; Nakaya, H.I. Systems Vaccinology. Immunity 2010, 33, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Rubinstein, L.; Kinders, R.; Parchment, R.E.; Gutierrez, M.E.; Murgo, A.J.; Ji, J.; Mroczkowski, B.; Pickeral, O.K.; Simpson, M.; et al. Phase 0 Clinical Trials: Conceptions and Misconceptions. Cancer J. 2008, 14, 133–137. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of Experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Durakovic, B. Design of Experiments Application, Concepts, Examples: State of the Art. Period. Eng. Nat. Sci. 2017, 5, 421–439. [Google Scholar] [CrossRef]
- Poncet, D.; Hessler, C.; Liang, H.; Gautheron, S.; Sergent, M.; Rintala, N.D.; Seydoux, E.; Huang, P.-W.D.; Argilla, D.; Ruiz, S.; et al. Preclinical Optimization of an Enterotoxigenic Escherichia Coli Adjuvanted Subunit Vaccine Using Response Surface Design of Experiments. NPJ Vaccines 2020, 5, 83. [Google Scholar] [CrossRef]
- Festing, M.F. Randomized Block Experimental Designs Can Increase the Power and Reproducibility of Laboratory Animal Experiments. ILAR J. 2014, 55, 472–476. [Google Scholar] [CrossRef]
- Ly, H.H.; Daniel, S.; Soriano, S.K.V.; Kis, Z.; Blakney, A.K. Optimization of Lipid Nanoparticles for SaRNA Expression and Cellular Activation Using a Design-of-Experiment Approach. Mol. Pharm. 2022, 19, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Wilkhu, J.S.; McNeil, S.E.; Anderson, D.E.; Perrie, Y. Characterization and Optimization of Bilosomes for Oral Vaccine Delivery. J. Drug Target. 2013, 21, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kutle, L.; Pavlović, N.; Dorotić, M.; Zadro, I.; Kapustić, M.; Halassy, B. Robustness Testing of Live Attenuated Rubella Vaccine Potency Assay Using Fractional Factorial Design of Experiments. Vaccine 2010, 28, 5497–5502. [Google Scholar] [CrossRef] [PubMed]
- Block, S.L.; Szenborn, L.; Daly, W.; Jackowska, T.; D’Agostino, D.; Han, L.; Dull, P.M.; Smolenov, I. A Comparative Evaluation of Two Investigational Meningococcal ABCWY Vaccine Formulations: Results of a Phase 2 Randomized, Controlled Trial. Vaccine 2015, 33, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.M.; Archer, M.C.; Orr, M.T.; Dubois Cauwelaert, N.; Beebe, E.A.; Huang, P.-W.D.; Dowling, Q.M.; Schwartz, A.M.; Fedor, D.M.; Vedvick, T.S.; et al. Development of a Thermostable Nanoemulsion Adjuvanted Vaccine against Tuberculosis Using a Design-of-Experiments Approach. Int. J. Nanomed. 2018, 13, 3689–3711. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an Evidence Based Approach for the Development of Adjuvanted Vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ng’uni, T.; Chasara, C.; Ndhlovu, Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020, 11, 590780. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykins, W.R.; Fox, C.B. Practical Considerations for Next-Generation Adjuvant Development and Translation. Pharmaceutics 2023, 15, 1850. https://doi.org/10.3390/pharmaceutics15071850
Lykins WR, Fox CB. Practical Considerations for Next-Generation Adjuvant Development and Translation. Pharmaceutics. 2023; 15(7):1850. https://doi.org/10.3390/pharmaceutics15071850
Chicago/Turabian StyleLykins, William R., and Christopher B. Fox. 2023. "Practical Considerations for Next-Generation Adjuvant Development and Translation" Pharmaceutics 15, no. 7: 1850. https://doi.org/10.3390/pharmaceutics15071850
APA StyleLykins, W. R., & Fox, C. B. (2023). Practical Considerations for Next-Generation Adjuvant Development and Translation. Pharmaceutics, 15(7), 1850. https://doi.org/10.3390/pharmaceutics15071850






