Protein Adsorption, Calcium-Binding Ability, and Biocompatibility of Silver Nanoparticle-Loaded Polyvinyl Alcohol (PVA) Hydrogels Using Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
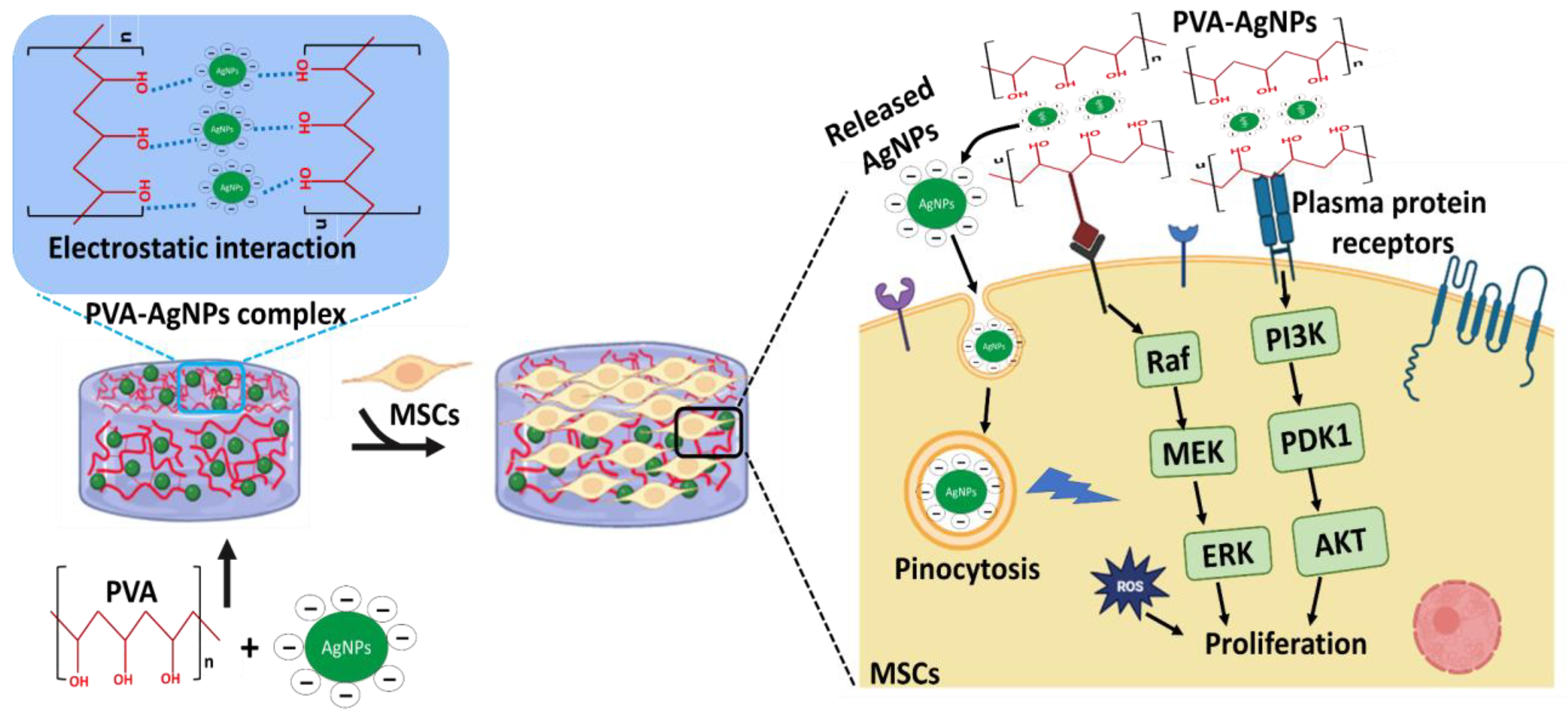
1. Introduction
2. Materials and Methods
2.1. Materials
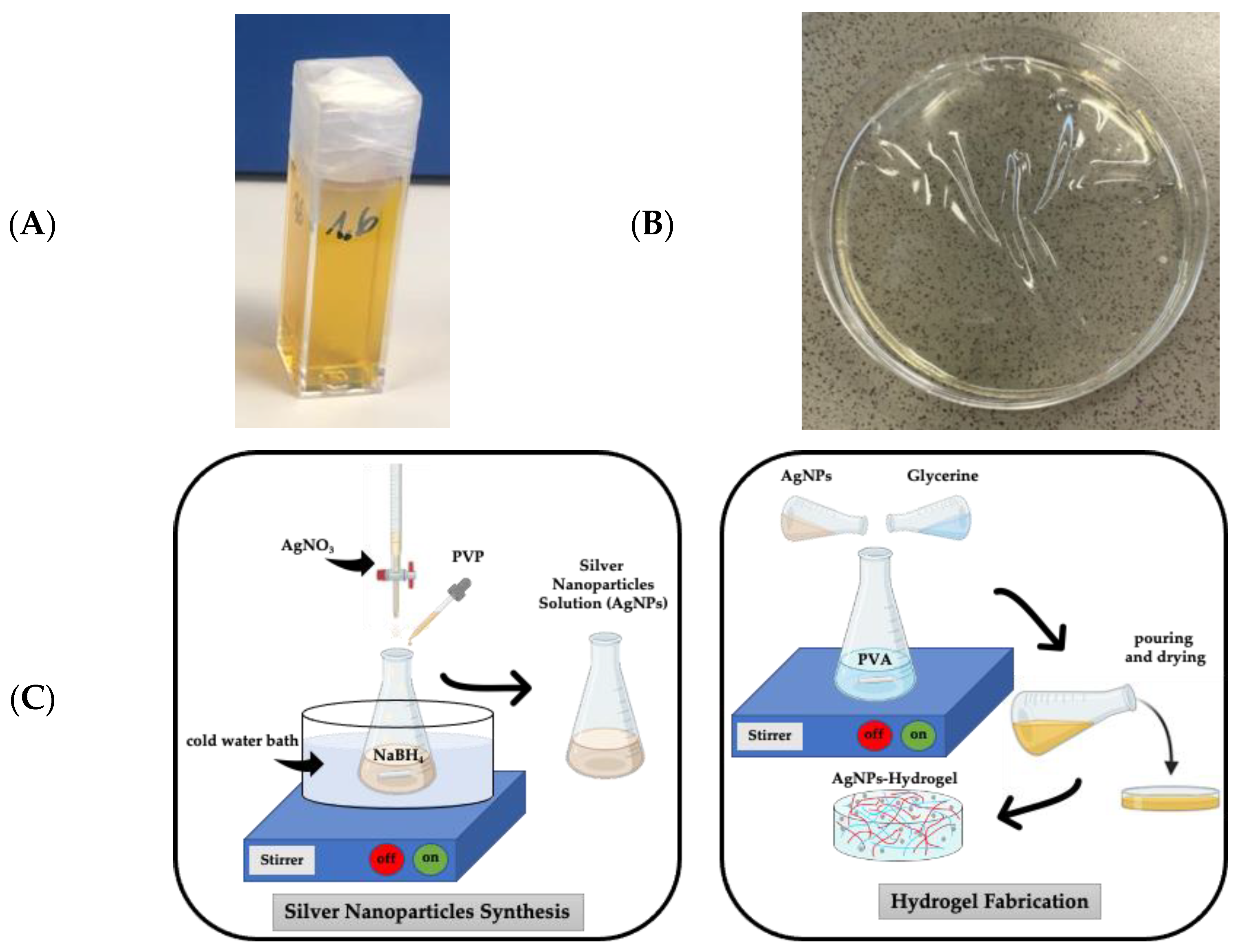
2.2. Fabrication of PVA-AgNP Hydrogels
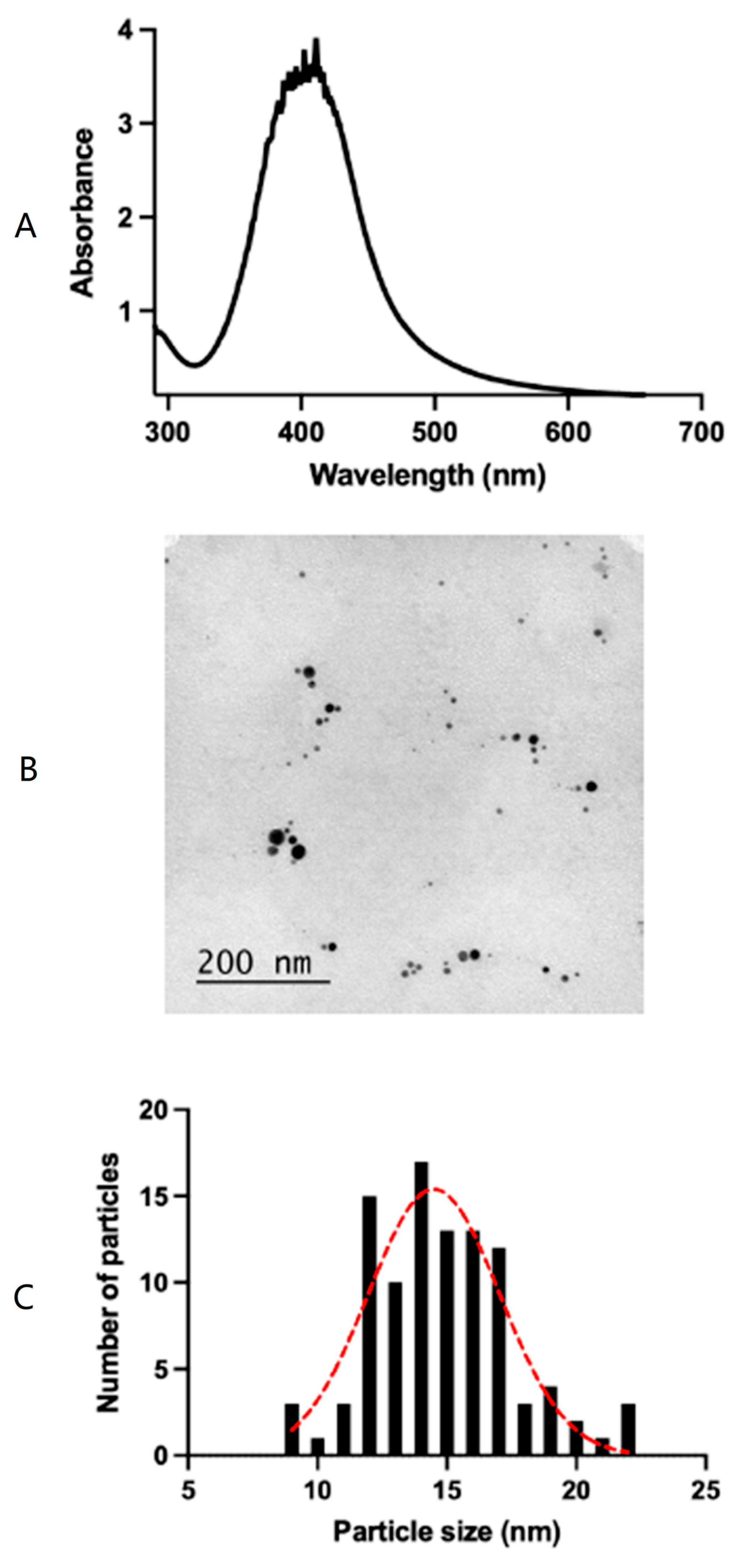
2.3. Silver Nanoparticles Characterization
2.4. Morphology of Silver Nanoparticles
2.5. Water Holding Capacity
2.6. Swelling Ratio
2.7. Calcium-Binding Ability
2.8. Protein-Binding Ability
2.9. Cell Culture
2.10. Cell Proliferation
2.11. Cytotoxicity
2.12. Histological Staining
2.13. Fluorescence Microscopy
2.14. Statistical Analysis
3. Results
3.1. Silver Nanoparticles Characterization
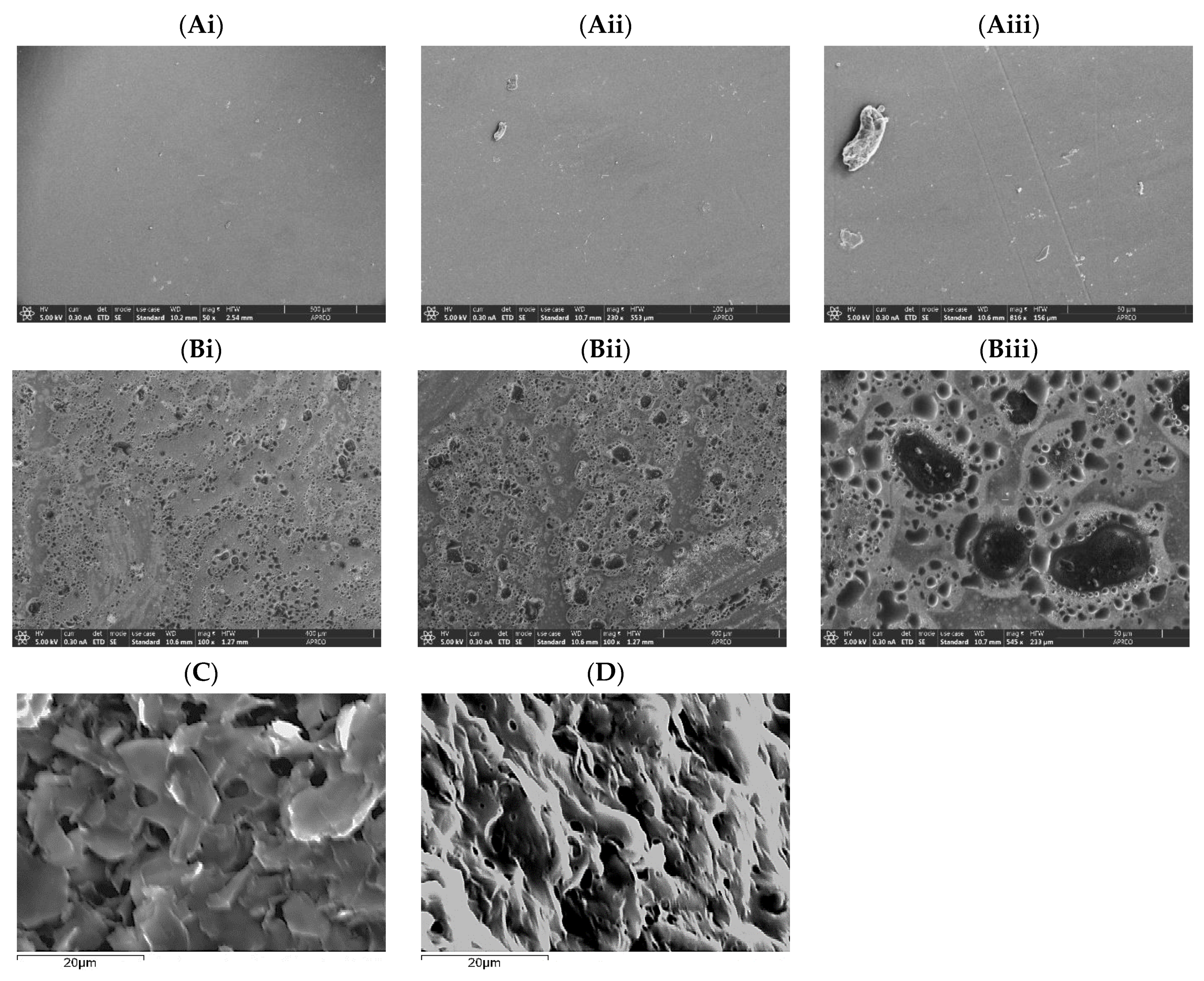
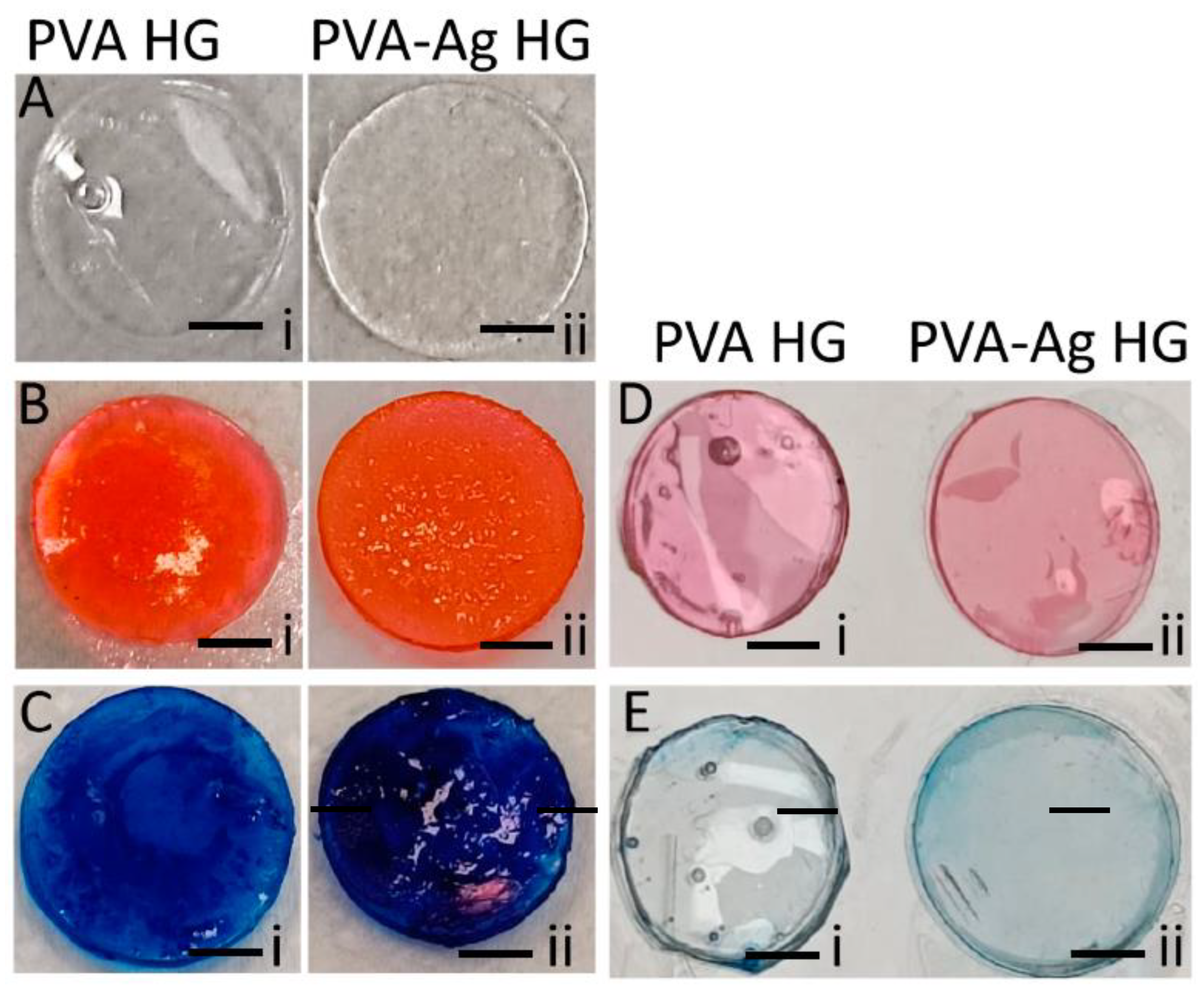
3.2. PVA and PVA-AgNPs Hydrogel’s Microstructure
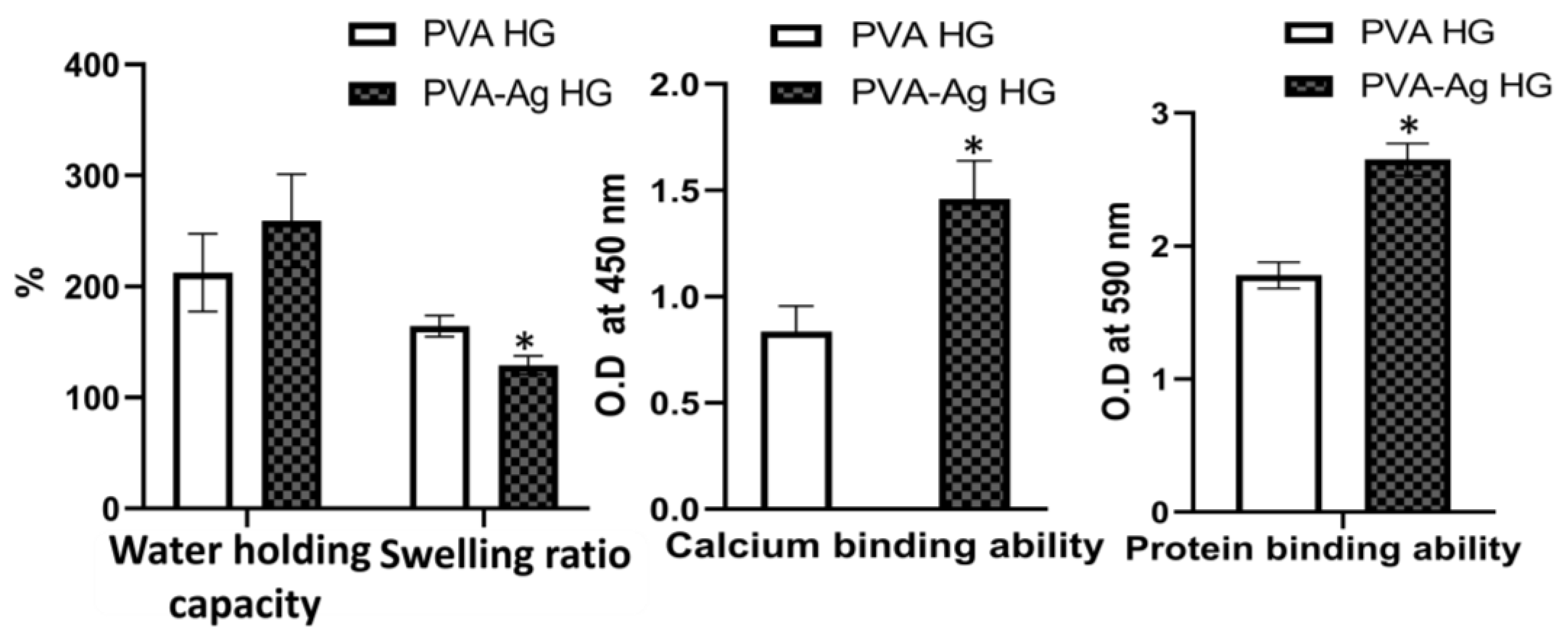
3.3. Water-Holding Capacity
3.4. Swelling Ratio
3.5. Calcium-Binding Ability
3.6. Protein-Binding Ability
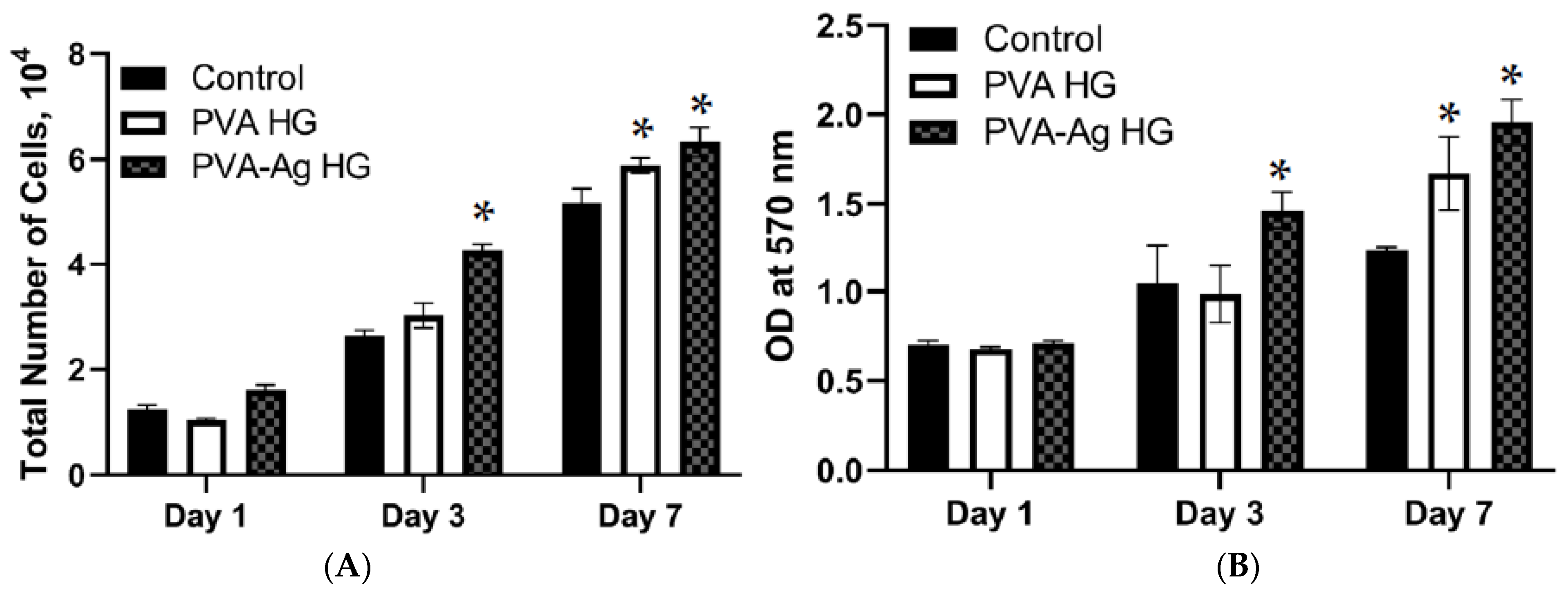
3.7. Cell Proliferation
3.8. Cytotoxicity
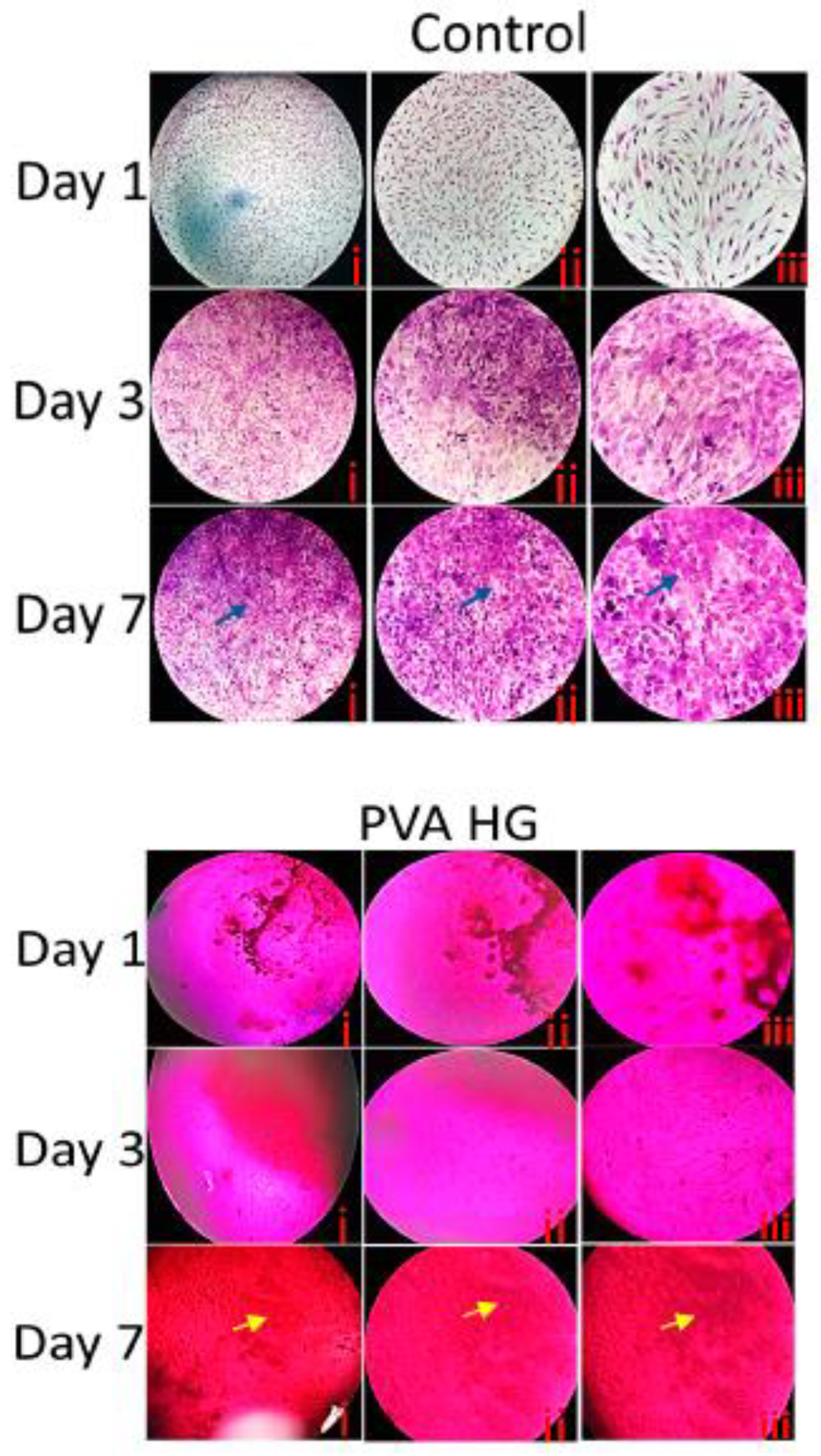
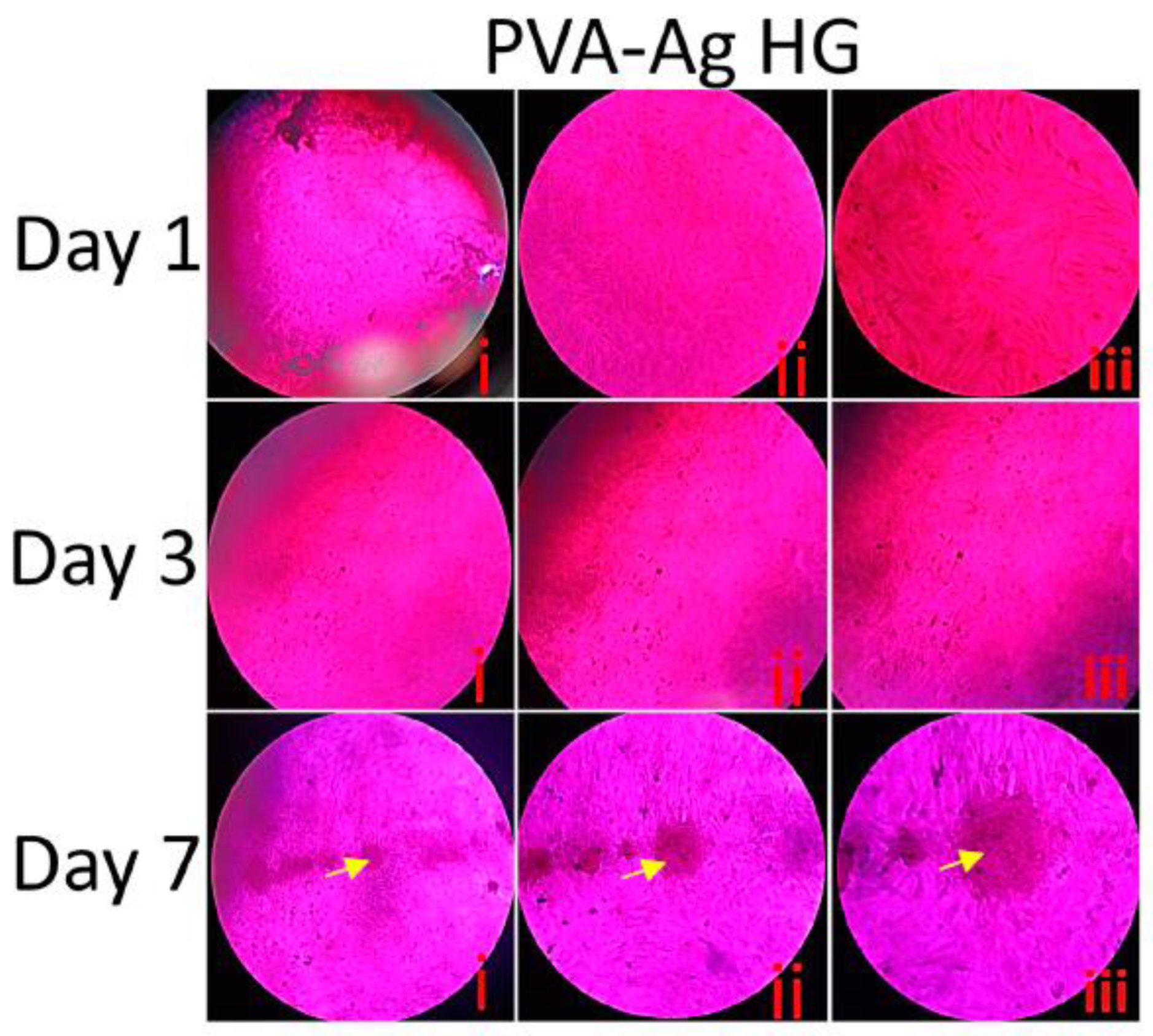
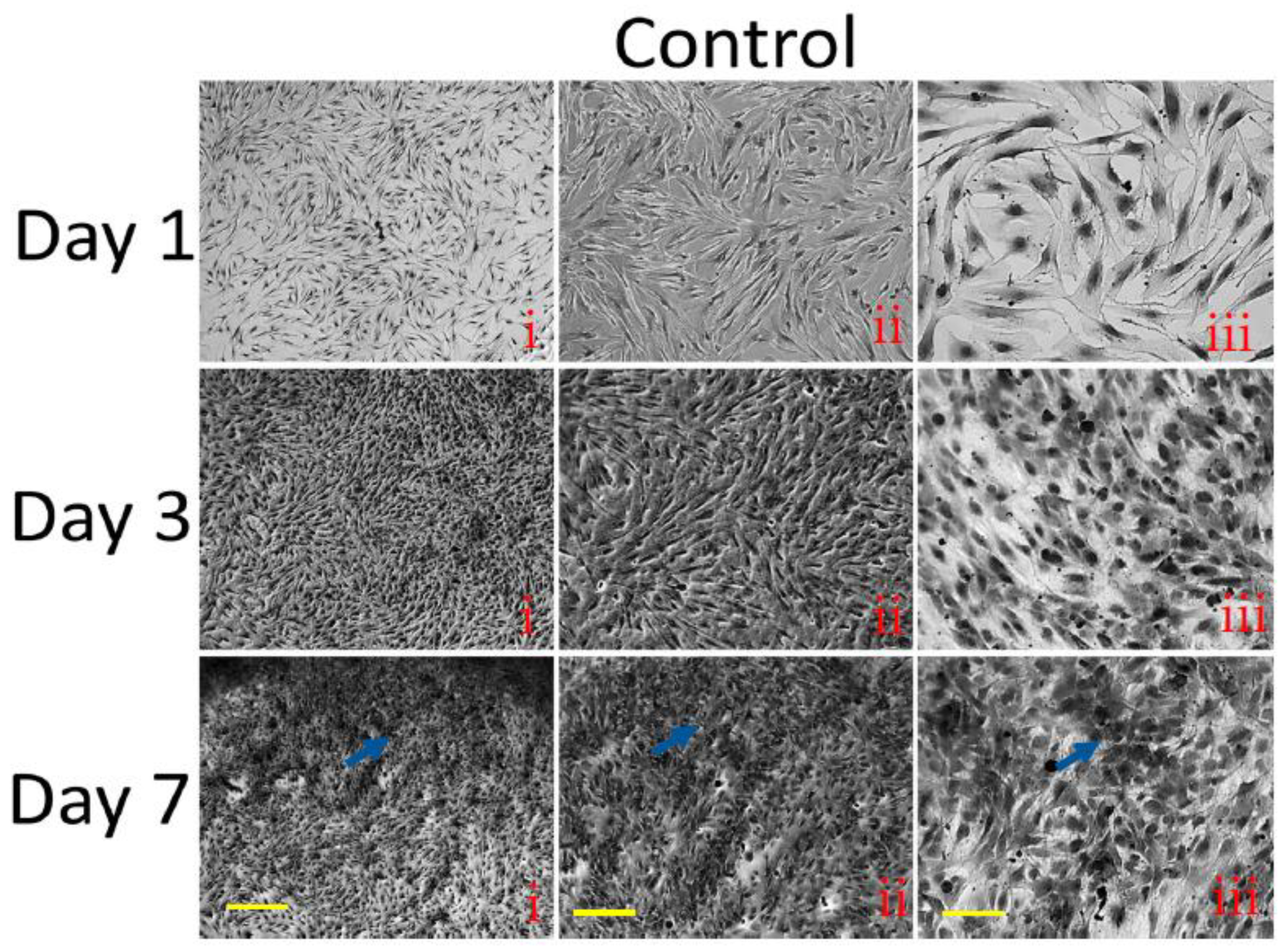
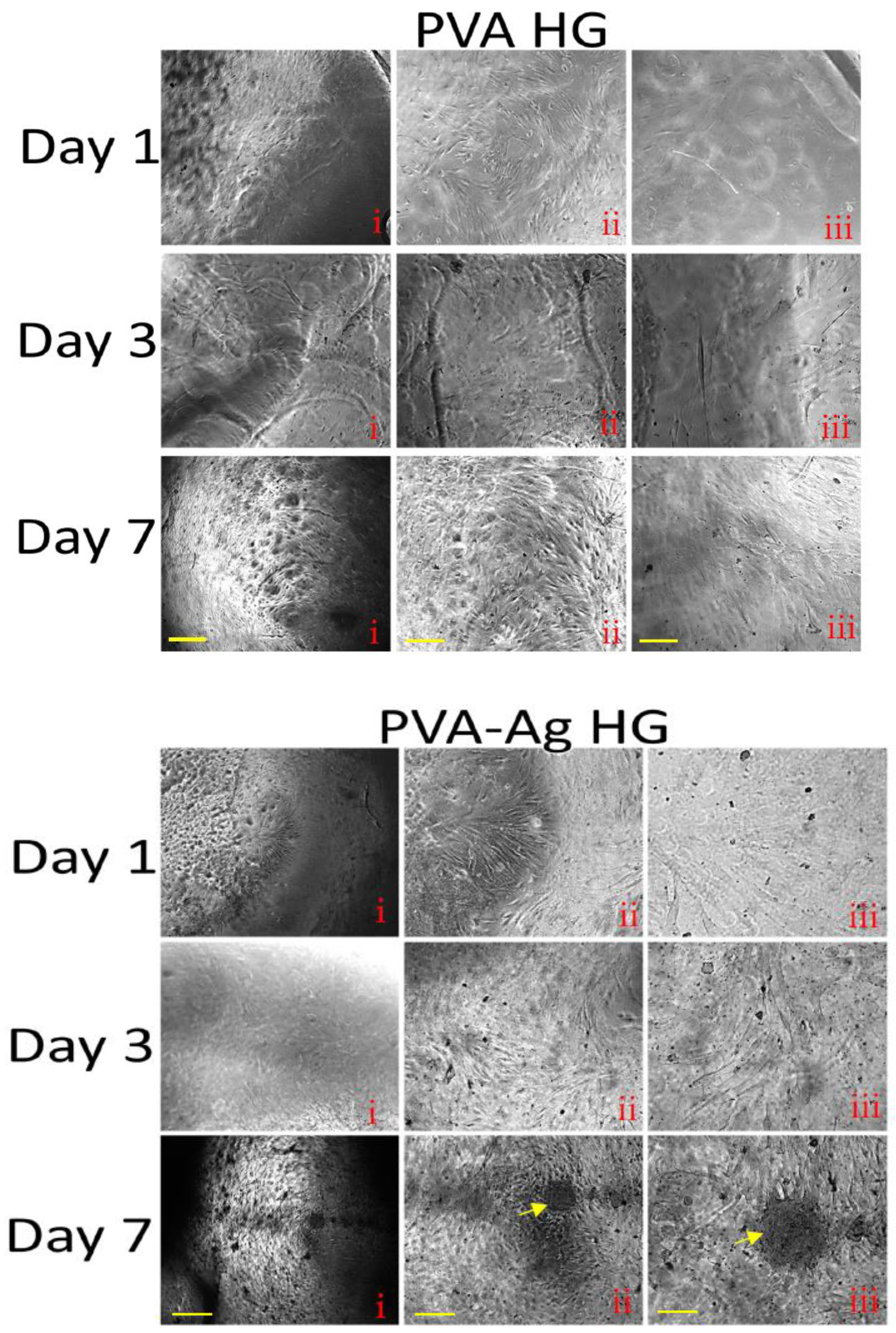
3.9. Histological Staining
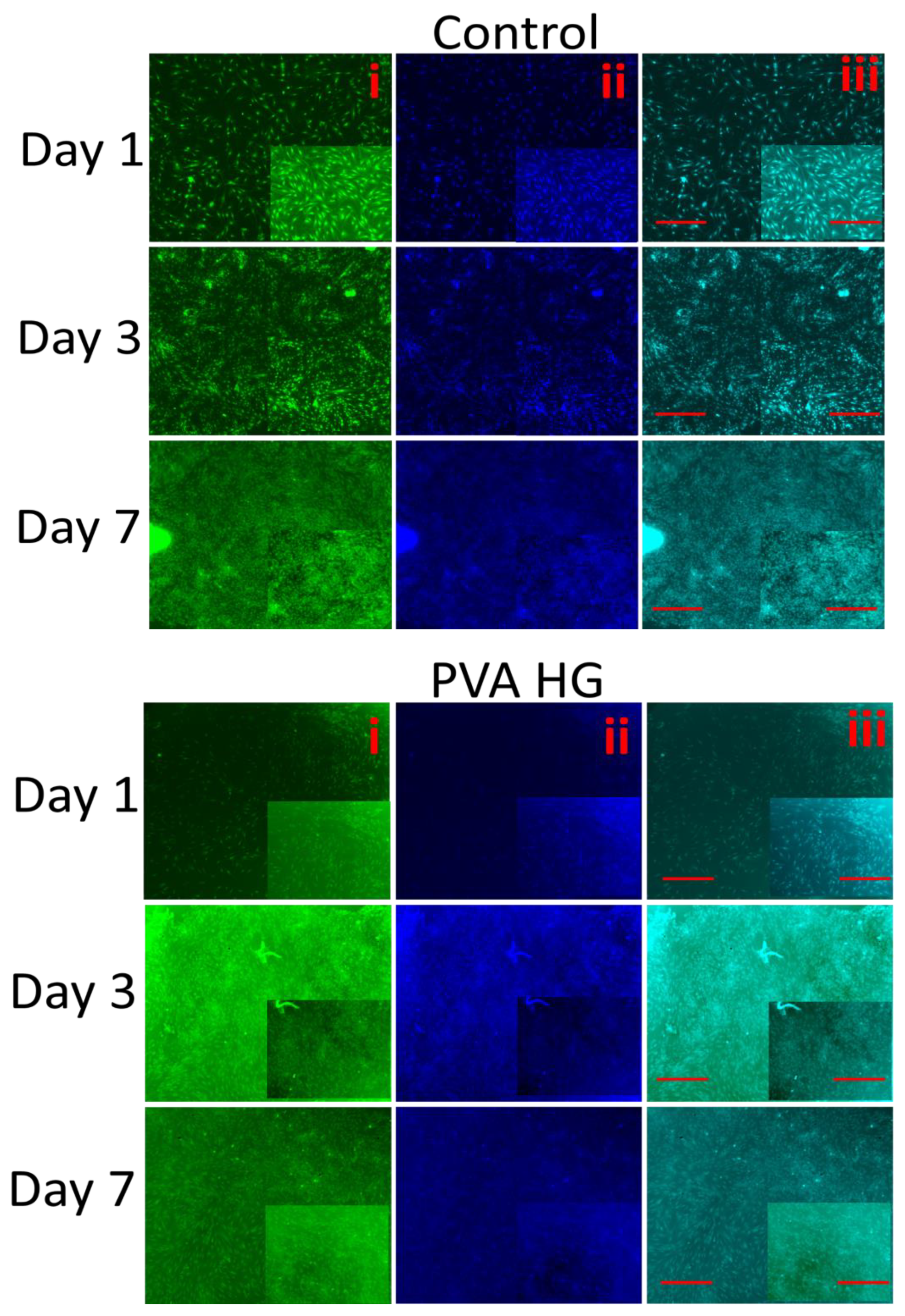
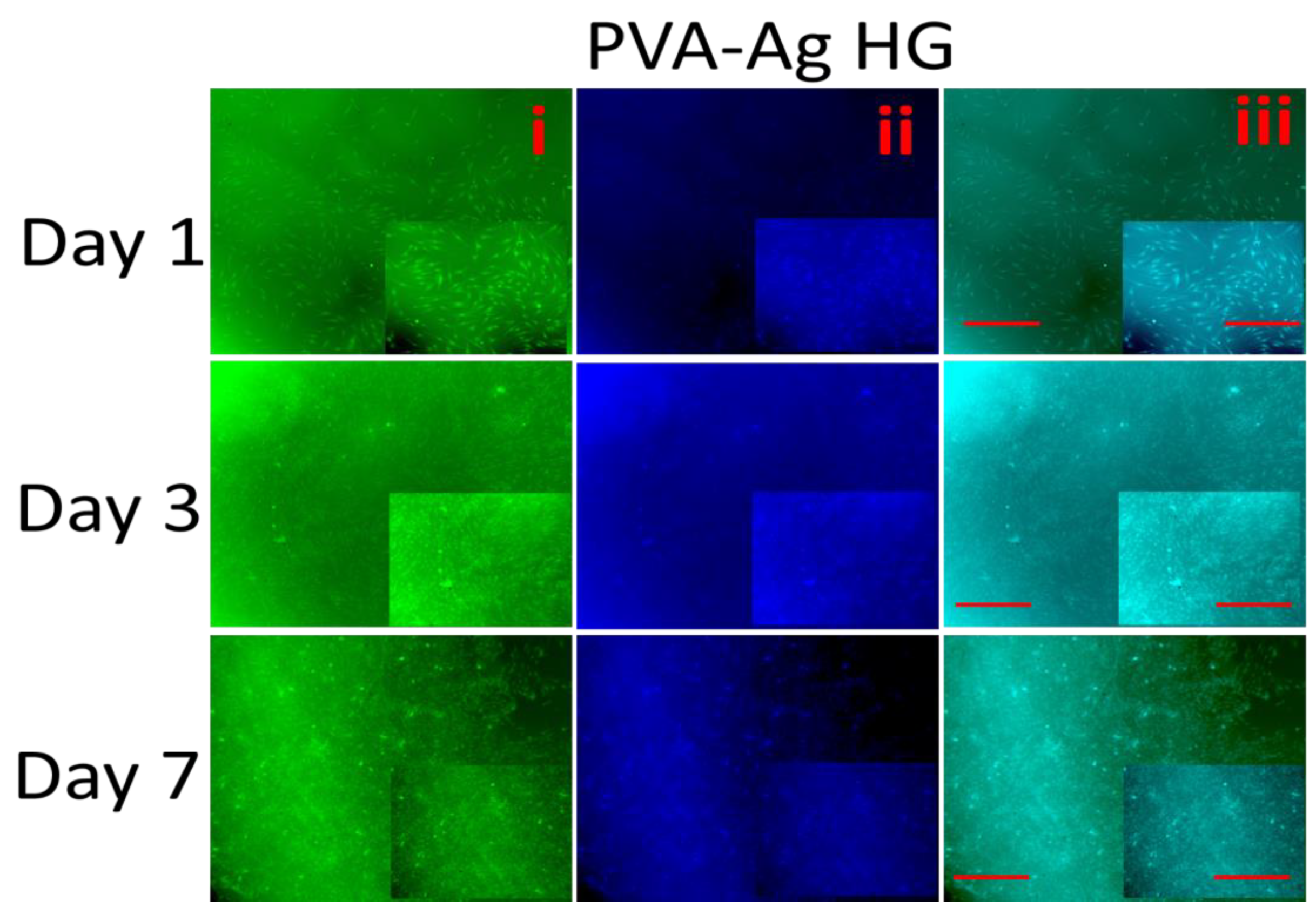
3.10. Fluorescence Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of poly (vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.Y. Cartilage regeneration using biodegradable oxidized alginate/hyaluronate hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 4519–4525. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yang, H.; Xu, Z.L. Influence of post-treatments on the properties of porous poly (vinyl alcohol) membranes. J. Appl. Polym. Sci. 2008, 107, 1423–1429. [Google Scholar] [CrossRef]
- Kiene, K.; Porta, F.; Topacogullari, B.; Detampel, P.; Huwyler, J. Self-assembling chitosan hydrogel: A drug-delivery device enabling the sustained release of proteins. J. Appl. Polym. Sci. 2018, 135, 45638. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar]
- Tian, Z.; Liu, W.; Li, G. The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 2016, 130, 264–270. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly (vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 15623. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Chircov, C.; Niculescu, A.G.; Hildegard, H.; Baltă, C.; Roșu, M.; Mladin, B.; Gherasim, O.; Mihaiescu, D.E.; Vasile, B.Ș. H2O2-PLA-(Alg)2Ca Hydrogel Enriched in Matrigel® Promotes Diabetic Wound Healing. Pharmaceutics 2023, 15, 857. [Google Scholar] [CrossRef]
- Elango, J.; Lijnev, A.; Zamora-Ledezma, C.; Alexis, F.; Wu, W.; Marín, J.M.G.; de Val, J.E.M.S. The Relationship of Rheological Properties and the Performance of Silk Fibroin Hydrogels in Tissue Engineering Application. Process Biochem. 2022, 125, 198–211. [Google Scholar] [CrossRef]
- Guamba, E.; Vispo, N.S.; Whitehead, D.C.; Singh, A.K.; Santos-Oliveira, R.; Niebieskikwiat, D.; Zamora-Ledezma, C.; Alexis, F. Cellulose-based hydrogels towards an antibacterial wound dressing. Biomater. Sci. 2023, 11, 3461–3468. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, C.; Peng, X.; Liu, T.; Shi, Y.; Liang, M.; Wang, H. Biomimetic anisotropic poly (vinyl alcohol) hydrogels with significantly enhanced mechanical properties by freezing–thawing under drawing. J. Mater. Chem. B 2019, 7, 3243–3249. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- González, A.; Noguez, C.; Beránek, J.; Barnard, A. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Mavani, K.; Shah, M. Synthesis of silver nanoparticles by using sodium borohydride as a reducing agent. Int. J. Eng. Res. Technol. 2013, 2, 1–5. [Google Scholar]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef]
- Jeevithan, E.; Shakila, R.J.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Physico-functional and mechanical properties of chitosan and calcium salts incorporated fish gelatin scaffolds. Int. J. Biol. Macromol. 2013, 60, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Lijnev, A.; Elango, J.; Gómez-López, V.M.; Pérez-Albacete Martínez, C.; Granero Marín, J.M.; Maté Sánchez De Val, J.E. Antibacterial and Proliferative Effects of NaOH-Coated Titanium, Zirconia, and Ceramic-Reinforced PEEK Dental Composites on Bone Marrow Mesenchymal Stem Cells. Pharmaceutics 2022, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Dominiei, M.; Le Blanc, K.; Mueller, I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zhu, H.-W.; Zhang, J.-N.; Su, P.; Liu, T.; He, C.; Feng, D.; Wang, H. Strong adhesion of poly (vinyl alcohol)–glycerol hydrogels onto metal substrates for marine antifouling applications. Soft Matter 2020, 16, 709–717. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the size of silver nanoparticles from their optical properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- Dong, Q.; Zu, D.; Kong, L.; Chen, S.; Yao, J.; Lin, J.; Lu, L.; Wu, B.; Fang, B. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater. Res. 2022, 26, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wu, K.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Synthesis of cellulose-based double-network hydrogels demonstrating high strength, self-healing, and antibacterial properties. Carbohydr. Polym. 2017, 168, 112–120. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Compos. Part A Appl. Sci. Manuf. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf. B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly (vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 53. [Google Scholar] [CrossRef]
- Nadtoka, O.; Virych, P.; Bezugla, T.; Doroschuk, V.; Lelyushok, S.; Pavlenko, V.; Yeshchenko, O.; Kutsevol, N. Antibacterial hybrid hydrogels loaded with nano silver. Appl. Nanosci. 2021, 12, 629–636. [Google Scholar] [CrossRef]
- Lee, W.F.; Tsao, K.T. Preparation and properties of nanocomposite hydrogels containing silver nanoparticles by ex situ polymerization. J. Appl. Polym. Sci. 2006, 100, 3653–3661. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Tian, Y.; Ren, L. Hydroxyapatite gradient on poly (vinyl alcohol) hydrogels surface to mimic calcified cartilage zone for cartilage repair. J. Biomater. Appl. 2022, 36, 1579–1587. [Google Scholar] [CrossRef]
- Ye, M.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C 2014, 44, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cheng, J.; Xu, S.; Cheng, X.; Zhao, J.; Low, Z.W.K.; Chee, P.L.; Lu, Z.; Zheng, L.; Kai, D. PVA/pectin composite hydrogels inducing osteogenesis for bone regeneration. Mater. Today Bio 2022, 16, 100431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef]
- Nie, L.; Li, X.; Chang, P.; Liu, S.; Wei, Q.; Guo, Q.; Wu, Q.; Fan, L.; Okoro, O.V.; Shavandi, A. A fast method for in vitro biomineralization of PVA/alginate/biphasic calcium phosphate hydrogel. Mater. Lett. 2022, 308, 131182. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.; Li, P.; Hou, R.; Shavandi, A.; Yang, S. Hydroxyethyl chitosan-reinforced polyvinyl alcohol/biphasic calcium phosphate hydrogels for bone regeneration. ACS Omega 2020, 5, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Ventura, R.; Min, Y.-K.; Lee, B.-T. Genipin cross-linked polyvinyl alcohol-gelatin hydrogel for bone regeneration. J. Biomed. Sci. Eng. 2016, 9, 419–429. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Tai, G.; Yan, F.; Cai, L.; Xin, C.; Al Islam, S. Graphene Oxide-loaded magnetic nanoparticles within 3D hydrogel form High-performance scaffolds for bone regeneration and tumour treatment. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106672. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Liu, Z.; Zeng, Q.; Jiang, G.; Yang, M. Probing the surface structure of hydroxyapatite through its interaction with hydroxyl: A first-principles study. RSC Adv. 2018, 8, 3716–3722. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.-S.; He, J.-Y.; Xu, W.-H.; Huang, X.-J.; Liu, J.-H. Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres. Chem. Eng. J. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.A.; Loutfy, S.A.; El-Fakharany, E.M.; Taha, T.H.; Hussien, Y.; Kamoun, E.A. Influence of chitosan and hydroxyapatite incorporation on properties of electrospun PVA/HA nanofibrous mats for bone tissue regeneration: Nanofibers optimization and in-vitro assessment. J. Drug Deliv. Sci. Technol. 2021, 62, 102417. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. Aaps Pharmscitech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly (vinyl alcohol)-hyaluronic acid membranes for wound dressing applications: Synthesis and in vitro bio-evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A novel high-strength poly (ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Qi, R.; Cao, X.; Shen, M.; Guo, R.; Yu, J.; Shi, X. Biocompatibility of electrospun halloysite nanotube-doped poly (lactic-co-glycolic acid) composite nanofibers. J. Biomater. Sci. Polym. Ed. 2012, 23, 299–313. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Chicaiza-Zambrano, A.; Santiago Vispo, N.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Almeida, C.E.; Alexis, F. Frequency Based Control of Antifouling Properties Using Graphene Nanoplatelet/Poly (Lactic-co-Glycolic Acid) Composite Films. Compos. Interfaces 2021, 28, 1137–1153. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef] [PubMed]
- Baimanov, D.; Cai, R.; Chen, C. Understanding the chemical nature of nanoparticle–protein interactions. Bioconjugate Chem. 2019, 30, 1923–1937. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Samberg, M.E.; Oldenburg, S.J.; Riviere, J.E. Protein binding modulates the cellular uptake of silver nanoparticles into human cells: Implications for in vitro to in vivo extrapolations? Toxicol. Lett. 2013, 220, 286–293. [Google Scholar] [CrossRef]
- Kettler, K.; Giannakou, C.; de Jong, W.H.; Hendriks, A.J.; Krystek, P. Uptake of silver nanoparticles by monocytic THP-1 cells depends on particle size and presence of serum proteins. J. Nanoparticle Res. 2016, 18, 286. [Google Scholar] [CrossRef]
- Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A.A.; Masoud, M.S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021, 16, 035014. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J.L.; Melzig, M.F.; Chen, W.; Haag, R. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 77, 28–37. [Google Scholar] [CrossRef]
- Júnior, D.M.; Hausen, M.A.; Asami, J.; Higa, A.M.; Leite, F.L.; Mambrini, G.P.; Rossi, A.L.; Komatsu, D.; Duek, E.A.d.R. A new dermal substitute containing polyvinyl alcohol with silver nanoparticles and collagen with hyaluronic acid: In vitro and In vivo approaches. Antibiotics 2021, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Gebauer, J.; Diendorf, J.; Treuel, L.; Ruiz, L.; Gonzalez-Calbet, J.; Vallet-Regi, M.; Zellner, R.; Köller, M. The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. J. Mater. Chem. 2010, 20, 512–518. [Google Scholar] [CrossRef]
- Nersisyan, H.; Lee, J.; Son, H.; Won, C.; Maeng, D. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion. Mater. Res. Bull. 2003, 38, 949–956. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef]
- Emam, H.E.; El-Bisi, M. Merely Ag nanoparticles using different cellulose fibers as removable reductant. Cellulose 2014, 21, 4219–4230. [Google Scholar] [CrossRef]
- Emam, H.E.; Saleh, N.; Nagy, K.S.; Zahran, M. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int. J. Biol. Macromol. 2015, 78, 249–256. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van De Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef]
- Kettler, K.; Krystek, P.; Giannakou, C.; Hendriks, A.J.; de Jong, W.H. Exploring the effect of silver nanoparticle size and medium composition on uptake into pulmonary epithelial 16HBE14o-cells. J. Nanoparticle Res. 2016, 18, 182. [Google Scholar] [CrossRef]
- Bhol, K.; Schechter, P. Topical nanocrystalline silver cream suppresses inflammatory cytokines and induces apoptosis of inflammatory cells in a murine model of allergic contact dermatitis. Br. J. Dermatol. 2005, 152, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Li, C.-W.; Wang, Q.; Shi, S.-J.; Hu, M.; Zhang, Q.; Cui, H.-H.; Sun, J.-B.; Zhou, M.; Wu, G.-L. The cellular and molecular mechanisms underlying silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofiber-mediated wound healing. J. Biomed. Nanotechnol. 2017, 13, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.H. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, J.; Zamora-Ledezma, C.; Alexis, F.; Wu, W.; Maté-Sánchez de Val, J.E. Protein Adsorption, Calcium-Binding Ability, and Biocompatibility of Silver Nanoparticle-Loaded Polyvinyl Alcohol (PVA) Hydrogels Using Bone Marrow-Derived Mesenchymal Stem Cells. Pharmaceutics 2023, 15, 1843. https://doi.org/10.3390/pharmaceutics15071843
Elango J, Zamora-Ledezma C, Alexis F, Wu W, Maté-Sánchez de Val JE. Protein Adsorption, Calcium-Binding Ability, and Biocompatibility of Silver Nanoparticle-Loaded Polyvinyl Alcohol (PVA) Hydrogels Using Bone Marrow-Derived Mesenchymal Stem Cells. Pharmaceutics. 2023; 15(7):1843. https://doi.org/10.3390/pharmaceutics15071843
Chicago/Turabian StyleElango, Jeevithan, Camilo Zamora-Ledezma, Frank Alexis, Wenhui Wu, and José Eduardo Maté-Sánchez de Val. 2023. "Protein Adsorption, Calcium-Binding Ability, and Biocompatibility of Silver Nanoparticle-Loaded Polyvinyl Alcohol (PVA) Hydrogels Using Bone Marrow-Derived Mesenchymal Stem Cells" Pharmaceutics 15, no. 7: 1843. https://doi.org/10.3390/pharmaceutics15071843
APA StyleElango, J., Zamora-Ledezma, C., Alexis, F., Wu, W., & Maté-Sánchez de Val, J. E. (2023). Protein Adsorption, Calcium-Binding Ability, and Biocompatibility of Silver Nanoparticle-Loaded Polyvinyl Alcohol (PVA) Hydrogels Using Bone Marrow-Derived Mesenchymal Stem Cells. Pharmaceutics, 15(7), 1843. https://doi.org/10.3390/pharmaceutics15071843









