Optimization and Characterization of Novel ALCAM-Targeting Antibody Fragments for Transepithelial Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant ALCAM Protein
2.2. Cloning, Expression, Purification, and Characterization of Proteins
2.3. Antibody Format Design
2.4. Sequence Optimization Generating OPT
2.5. Construction of the Affinity Maturation Library
2.6. Selection of Affinity-Matured Anti-ALCAM Antibodies by Phage Display
2.7. Bacterial Expression of Phagemid scFv
2.8. ELISA Screening of Bacterial Supernatant Expressing scFv
2.9. SPR-Based Screening of scFv-Expressing Bacterial Supernatants
2.10. CD6 Competition ELISA
2.11. ELISA to Determine Species Cross-Reactivity
2.12. Affinity Measurements of Purified Antibodies by SPR
2.13. Chemical and Thermal Stability
2.14. Stability Studies
2.15. Solubility
2.16. Culture of Immortalized Murine Lymphatic Endothelial Cells
2.17. Generation of Bone Marrow-Derived DCs
2.18. In Vitro DC Transmigration Assay
2.19. Flow Cytometry
2.20. Flow Cytometry Detection of ALCAM Expression by Human Corneal Epithelial Cells
2.21. Corneal Penetration Model
2.22. AlphaLISA
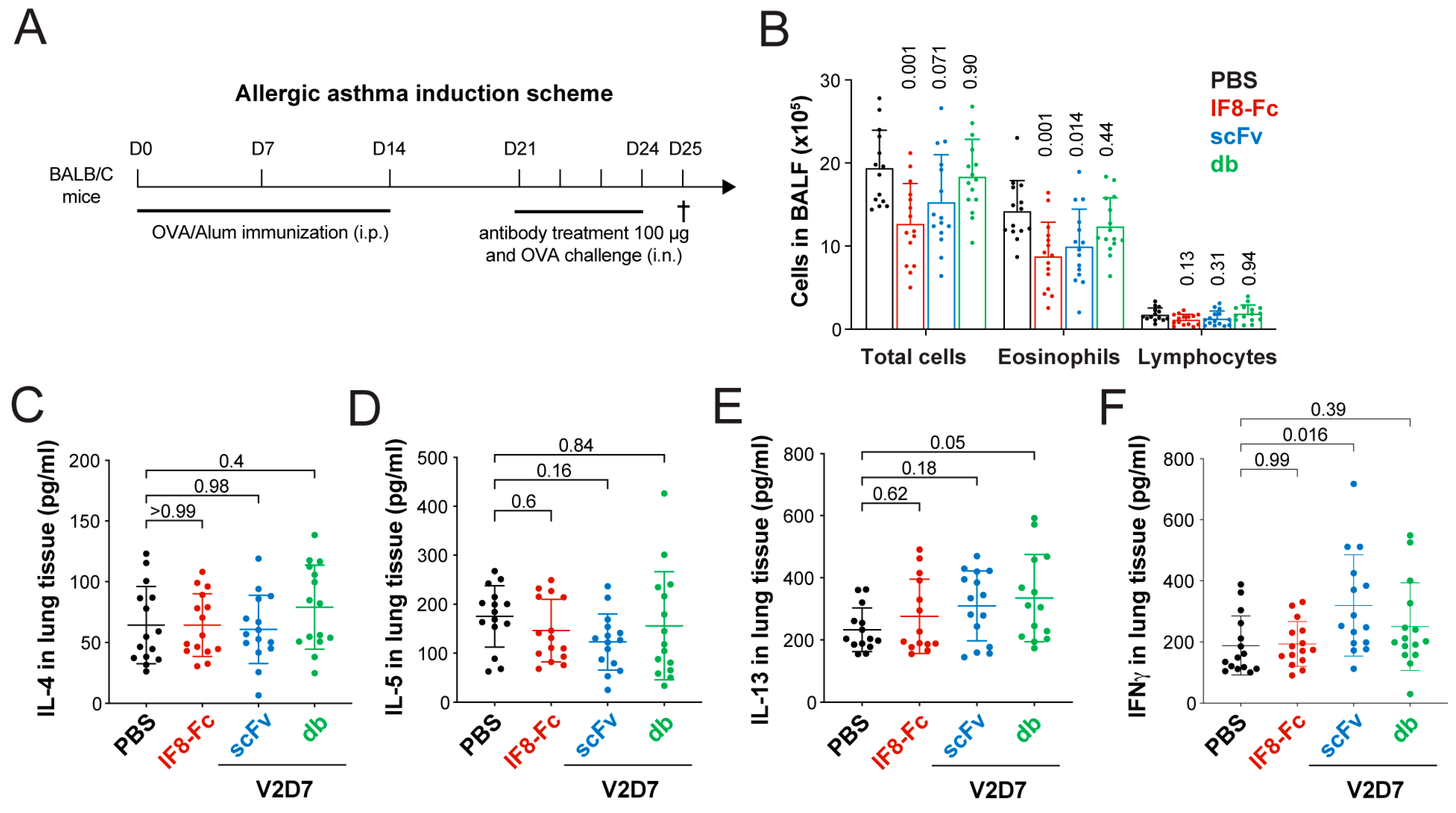
2.23. Allergic Asthma Study
2.24. Statistical Analysis
3. Results
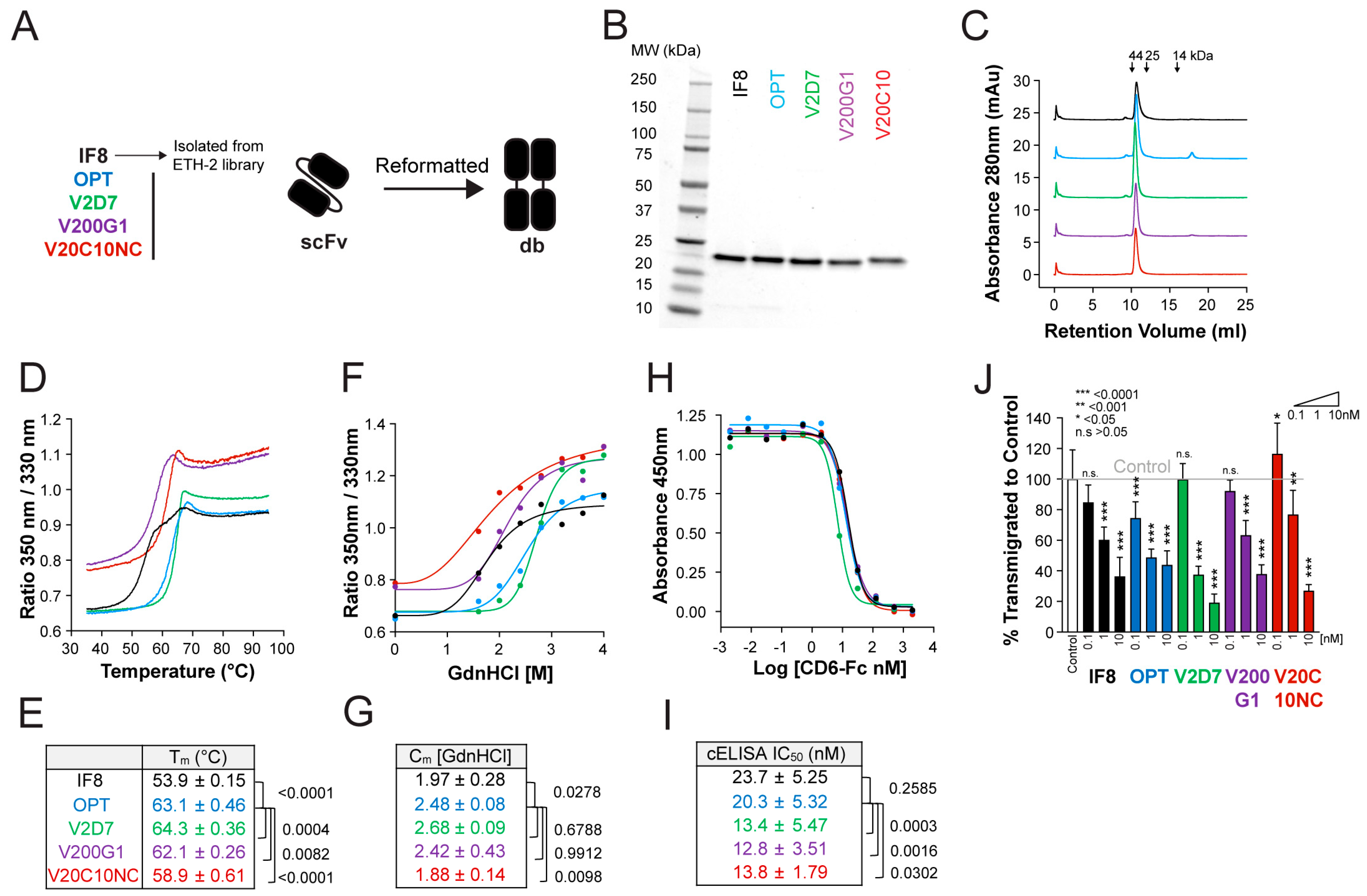
3.1. Stability Improvement of the ALCAM-Targeting Single-Chain Variable Fragment IF8
3.2. Affinity Maturation of the ALCAM-Targeting scFv OPT
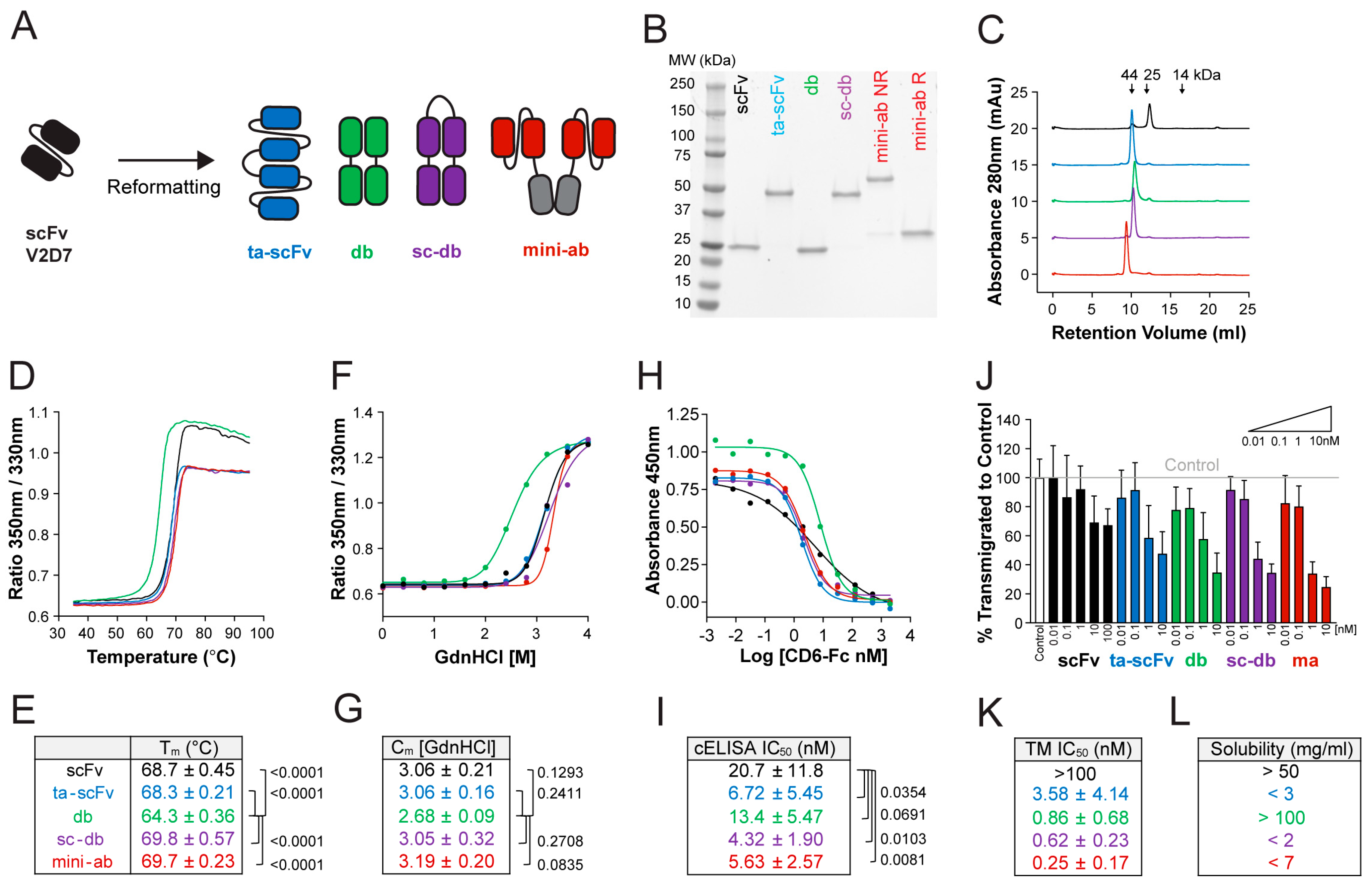
3.3. Generation of Stable, High-Affinity ALCAM-Targeting Fragments of Various Formats
3.4. Improvements in Stability and Affinity Are Similar between Antibody Clones in scFv and db Formats
3.5. Smaller Antibodies Penetrate Better through In Vitro 3D Human Corneal Epithelium
3.6. Topical Treatment with IF8-Fc and scFv V2D7 Reduces Immune Cell Infiltration in a Mouse Model of Allergic Asthma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Kempen, L.C.; Nelissen, J.M.; Degen, W.G.; Torensma, R.; Weidle, U.H.; Bloemers, H.P.; Figdor, C.G.; Swart, G.W. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J. Biol. Chem. 2001, 276, 25783–25790. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Quertermous, T. Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J. Biol. Chem. 2004, 279, 55315–55323. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.W.; Joosten, B.; Torensma, R.; Parnes, J.R.; van Leeuwen, F.N.; Figdor, C.G. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 2006, 107, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Iolyeva, M.; Karaman, S.; Willrodt, A.H.; Weingartner, S.; Vigl, B.; Halin, C. Novel role for ALCAM in lymphatic network formation and function. FASEB J. 2013, 27, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, A.H.; Beffinger, M.; Vranova, M.; Protsyuk, D.; Schuler, K.; Jadhav, M.; Heikenwalder, M.; van den Broek, M.; Borsig, L.; Halin, C. Stromal Expression of Activated Leukocyte Cell Adhesion Molecule Promotes Lung Tumor Growth and Metastasis. Am. J. Pathol. 2017, 187, 2558–2569. [Google Scholar] [CrossRef]
- Ohneda, O.; Ohneda, K.; Arai, F.; Lee, J.; Miyamoto, T.; Fukushima, Y.; Dowbenko, D.; Lasky, L.A.; Suda, T. ALCAM (CD166): Its role in hematopoietic and endothelial development. Blood 2001, 98, 2134–2142. [Google Scholar] [CrossRef]
- Willrodt, A.H.; Salabarria, A.C.; Schineis, P.; Ignatova, D.; Hunter, M.C.; Vranova, M.; Golding-Ochsenbein, A.M.; Sigmund, E.; Romagna, A.; Strassberger, V.; et al. ALCAM Mediates DC Migration through Afferent Lymphatics and Promotes Allospecific Immune Reactions. Front. Immunol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Masedunskas, A.; King, J.A.; Tan, F.; Cochran, R.; Stevens, T.; Sviridov, D.; Ofori-Acquah, S.F. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 2006, 580, 2637–2645. [Google Scholar] [CrossRef]
- Lyck, R.; Lecuyer, M.A.; Abadier, M.; Wyss, C.B.; Matti, C.; Rosito, M.; Enzmann, G.; Zeis, T.; Michel, L.; Garcia Martin, A.B.; et al. ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2017, 37, 2894–2909. [Google Scholar] [CrossRef]
- Kim, M.N.; Hong, J.Y.; Shim, D.H.; Sol, I.S.; Kim, Y.S.; Lee, J.H.; Kim, K.W.; Lee, J.M.; Sohn, M.H. Activated Leukocyte Cell Adhesion Molecule Stimulates the T-Cell Response in Allergic Asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 994–1008. [Google Scholar] [CrossRef]
- Li, Y.; Singer, N.G.; Whitbred, J.; Bowen, M.A.; Fox, D.A.; Lin, F. CD6 as a potential target for treating multiple sclerosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Consuegra-Fernandez, M.; Julia, M.; Martinez-Florensa, M.; Aranda, F.; Catala, C.; Armiger-Borras, N.; Arias, M.T.; Santiago, F.; Guilabert, A.; Esteve, A.; et al. Genetic and experimental evidence for the involvement of the CD6 lymphocyte receptor in psoriasis. Cell. Mol. Immunol. 2018, 15, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.A.; Ayilam Ramachandran, R.; Garcia, S.J.; Der, E.; Herlitz, L.; Ampudia, J.; Chu, D.; Jordan, N.; Zhang, T.; Parodis, I.; et al. The CD6/ALCAM pathway promotes lupus nephritis via T cell-mediated responses. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Hong, J.Y.; Kim, M.N.; Kwak, E.J.; Kim, S.Y.; Kim, E.G.; Lee, K.E.; Kim, Y.S.; Jee, H.M.; Kim, S.H.; et al. Activated Leukocyte Cell Adhesion Molecule Modulates Th2 Immune Response in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2019, 11, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.N.; Lee, K.E.; Hong, J.Y.; Oh, M.S.; Kim, S.Y.; Kim, K.W.; Sohn, M.H. Activated leucocyte cell adhesion molecule (ALCAM/CD166) regulates T cell responses in a murine model of food allergy. Clin. Exp. Immunol. 2018, 192, 151–164. [Google Scholar] [CrossRef]
- Gurrea-Rubio, M.; Fox, D.A. The dual role of CD6 as a therapeutic target in cancer and autoimmune disease. Front. Med. 2022, 9, 1026521. [Google Scholar] [CrossRef]
- Bughani, U.; Saha, A.; Kuriakose, A.; Nair, R.; Sadashivarao, R.B.; Venkataraman, R.; Patel, S.; Deshchougule, A.T.; Montero, E.; Pai, H.V.; et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE 2017, 12, e0180088. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Reichert, J.M.; Sleeman, M.; Reckamp, K.L.; Ryffel, B.; Adamczewski, J.P.; Sweeney, T.D.; Vanbever, R.; Diot, P.; Owen, C.A.; et al. Therapeutic monoclonal antibodies for respiratory diseases: Current challenges and perspectives, 31 March–1 April 2016, Tours, France. MAbs 2016, 8, 999–1009. [Google Scholar] [CrossRef]
- Liang, W.; Pan, H.W.; Vllasaliu, D.; Lam, J.K.W. Pulmonary Delivery of Biological Drugs. Pharmaceutics 2020, 12, 1025. [Google Scholar] [CrossRef]
- Loscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Ottiger, M.; Thiel, M.A.; Feige, U.; Lichtlen, P.; Urech, D.M. Efficient intraocular penetration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Investig. Ophthalmol. Vis. Sci. 2009, 50, 779–786. [Google Scholar] [CrossRef]
- Thiel, M.A.; Coster, D.J.; Standfield, S.D.; Brereton, H.M.; Mavrangelos, C.; Zola, H.; Taylor, S.; Yusim, A.; Williams, K.A. Penetration of engineered antibody fragments into the eye. Clin. Exp. Immunol. 2002, 128, 67–74. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Sadrai, Z.; Saban, D.R.; Zhang, Q.; Dana, R. Corneal penetration of topical and subconjunctival bevacizumab. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8718–8723. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Brereton, H.M.; Farrall, A.; Standfield, S.D.; Taylor, S.D.; Kirk, L.A.; Coster, D.J. Topically applied antibody fragments penetrate into the back of the rabbit eye. Eye 2005, 19, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Fishburn, C.S.; Weers, J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug. Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Koussoroplis, S.J.; Paulissen, G.; Tyteca, D.; Goldansaz, H.; Todoroff, J.; Barilly, C.; Uyttenhove, C.; Van Snick, J.; Cataldo, D.; Vanbever, R. PEGylation of antibody fragments greatly increases their local residence time following delivery to the respiratory tract. J. Control. Release 2014, 187, 91–100. [Google Scholar] [CrossRef]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef]
- Mayor, A.; Thibert, B.; Huille, S.; Respaud, R.; Audat, H.; Heuze-Vourc’h, N. Inhaled antibodies: Formulations require specific development to overcome instability due to nebulization. Drug. Deliv. Transl. Res. 2021, 11, 1625–1633. [Google Scholar] [CrossRef]
- Piazza, T.; Cha, E.; Bongarzone, I.; Canevari, S.; Bolognesi, A.; Polito, L.; Bargellesi, A.; Sassi, F.; Ferrini, S.; Fabbi, M. Internalization and recycling of ALCAM/CD166 detected by a fully human single-chain recombinant antibody. J. Cell Sci. 2005, 118, 1515–1525. [Google Scholar] [CrossRef]
- Strassberger, V.; Gutbrodt, K.L.; Krall, N.; Roesli, C.; Takizawa, H.; Manz, M.G.; Fugmann, T.; Neri, D. A comprehensive surface proteome analysis of myeloid leukemia cell lines for therapeutic antibody development. J. Proteom. 2014, 99, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Fairhead, M.; Howarth, M. Site-specific biotinylation of purified proteins using BirA. Methods Mol. Biol. 2015, 1266, 171–184. [Google Scholar] [CrossRef]
- Cousin, N.; Bartel, S.; Scholl, J.; Tacconi, C.; Egger, A.; Thorhallsdottir, G.; Neri, D.; Dieterich, L.C.; Detmar, M. Antibody-Mediated Delivery of VEGF-C Promotes Long-Lasting Lymphatic Expansion That Reduces Recurrent Inflammation. Cells 2022, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, E.K.; Klemm, J.D.; Kim, P.S.; Alber, T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 1991, 254, 539–544. [Google Scholar] [CrossRef]
- Durr, E.; Jelesarov, I.; Bosshard, H.R. Extremely fast folding of a very stable leucine zipper with a strengthened hydrophobic core and lacking electrostatic interactions between helices. Biochemistry 1999, 38, 870–880. [Google Scholar] [CrossRef]
- Tissot, K.; Ewert, S.; Auf Der Maur, A.; Barberis, A.; Escher, D. Immunoglobulin Frameworks which Demonstrate Enhanced Stability in the Intracellular Environment and Methods of Identifying Same. Internation Patent Number WO/2003/097697, 2003. [Google Scholar]
- Ewert, S.; Honegger, A.; Pluckthun, A. Stability improvement of antibodies for extracellular and intracellular applications: CDR grafting to stable frameworks and structure-based framework engineering. Methods 2004, 34, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, M.P. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2001, 29, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Raybould, M.I.J.; Marks, C.; Krawczyk, K.; Taddese, B.; Nowak, J.; Lewis, A.P.; Bujotzek, A.; Shi, J.; Deane, C.M. Five computational developability guidelines for therapeutic antibody profiling. Proc. Natl. Acad. Sci. USA 2019, 116, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Trachsel, E.; Kaspar, M.; Schliemann, C.; Sommavilla, R.; Rybak, J.N.; Rosli, C.; Borsi, L.; Neri, D. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int. J. Cancer 2008, 122, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.P.; Tomlinson, I.M.; Winter, G. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur. J. Immunol. 1994, 24, 827–836. [Google Scholar] [CrossRef]
- Tomlinson, I.M.; Walter, G.; Marks, J.D.; Llewelyn, M.B.; Winter, G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J. Mol. Biol. 1992, 227, 776–798. [Google Scholar] [CrossRef] [PubMed]
- Viti, F.; Nilsson, F.; Demartis, S.; Huber, A.; Neri, D. Design and use of phage display libraries for the selection of antibodies and enzymes. Methods Enzymol. 2000, 326, 480–505. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Gabra, H.; Hill, F.; Evan, G.; Sikora, K. A novel tumour marker related to the c-myc oncogene product. Mol. Cell. Probes 1987, 1, 73–82. [Google Scholar] [CrossRef]
- Vigl, B.; Aebischer, D.; Nitschke, M.; Iolyeva, M.; Rothlin, T.; Antsiferova, O.; Halin, C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011, 118, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, R.L.; Shakhar, G.; Dudziak, D.; Wardemann, H.; Eisenreich, T.; Dustin, M.L.; Nussenzweig, M.C. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004, 5, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rossner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zal, T.; Volkmann, A.; Stockinger, B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 1994, 180, 2089–2099. [Google Scholar] [CrossRef]
- Pini, A.; Viti, F.; Santucci, A.; Carnemolla, B.; Zardi, L.; Neri, P.; Neri, D. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J. Biol. Chem. 1998, 273, 21769–21776. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Kinuthia, M.W.; Truong, T.; Lapointe, A.M.; Hayden, P.; Klausner, M. New Human Organotypic Corneal Tissue Model for Ophthalmic Drug Delivery Studies. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2880–2898. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Kinuthia, M.W.; Lapointe, A.M.; Truong, T.; Klausner, M.; Hayden, P. Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Exp. Eye Res. 2020, 190, 107867. [Google Scholar] [CrossRef]
- Smith, N.R.; Davies, P.S.; Levin, T.G.; Gallagher, A.C.; Keene, D.R.; Sengupta, S.K.; Wieghard, N.; El Rassi, E.; Wong, M.H. Cell Adhesion Molecule CD166/ALCAM Functions Within the Crypt to Orchestrate Murine Intestinal Stem Cell Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 389–409. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tatsumi, K.; Kosaka, K.; Sato, Y.; Higuchi, T.; Yoshioka, S.; Maeda, M.; Ueda, M.; Fujii, S. Human blastocysts and endometrial epithelial cells express activated leukocyte cell adhesion molecule (ALCAM/CD166). J. Clin. Endocrinol. Metab. 2003, 88, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Wee, S.F.; Whichard, L.P.; Bowen, M.A.; Pesando, J.M.; Aruffo, A.; Haynes, B.F. Identification and characterization of a 100-kD ligand for CD6 on human thymic epithelial cells. J. Exp. Med. 1995, 181, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.J.; Jiang, D.G.; Jiang, W.G.; Harding, K.G.; Patel, G.K. Activated leukocyte cell adhesion molecule impacts on clinical wound healing and inhibits HaCaT migration. Int. Wound J. 2011, 8, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Seong, B.L. Solubility, Stability, and Avidity of Recombinant Antibody Fragments Expressed in Microorganisms. Front. Microbiol. 2020, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A. Engineering antibodies for stability and efficient folding. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 47–68. [Google Scholar] [CrossRef]
- Ma, H.; O’Fagain, C.; O’Kennedy, R. Antibody stability: A key to performance—Analysis, influences and improvement. Biochimie 2020, 177, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Long, N.E.; Sullivan, B.J.; Ding, H.; Doll, S.; Ryan, M.A.; Hitchcock, C.L.; Martin, E.W., Jr.; Kumar, K.; Tweedle, M.F.; Magliery, T.J. Linker engineering in anti-TAG-72 antibody fragments optimizes biophysical properties, serum half-life, and high-specificity tumor imaging. J. Biol. Chem. 2018, 293, 9030–9040. [Google Scholar] [CrossRef]
- Whitlow, M.; Bell, B.A.; Feng, S.L.; Filpula, D.; Hardman, K.D.; Hubert, S.L.; Rollence, M.L.; Wood, J.F.; Schott, M.E.; Milenic, D.E.; et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993, 6, 989–995. [Google Scholar] [CrossRef]
- Molgaard, K.; Compte, M.; Nunez-Prado, N.; Harwood, S.L.; Sanz, L.; Alvarez-Vallina, L. Balanced secretion of anti-CEA x anti-CD3 diabody chains using the 2A self-cleaving peptide maximizes diabody assembly and tumor-specific cytotoxicity. Gene Ther. 2017, 24, 208–214. [Google Scholar] [CrossRef]
- Compte, M.; Alvarez-Cienfuegos, A.; Nunez-Prado, N.; Sainz-Pastor, N.; Blanco-Toribio, A.; Pescador, N.; Sanz, L.; Alvarez-Vallina, L. Functional comparison of single-chain and two-chain anti-CD3-based bispecific antibodies in gene immunotherapy applications. Oncoimmunology 2014, 3, e28810. [Google Scholar] [CrossRef]
- Maltby, S.; Tay, H.L.; Yang, M.; Foster, P.S. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology 2017, 22, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.; Caiazzo, E.; McSharry, C.; Guzik, T.J.; Maffia, P. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020, 160, 105189. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, W. Molecular mechanisms of glucocorticoid resistance. Eur. J. Clin. Investig. 2023, 53, e13901. [Google Scholar] [CrossRef] [PubMed]
- Adatia, A.; Vliagoftis, H. Challenges in severe asthma: Do we need new drugs or new biomarkers? Front. Med. 2022, 9, 921967. [Google Scholar] [CrossRef]
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020, 55, 1900588. [Google Scholar] [CrossRef]
- Corren, J. New Targeted Therapies for Uncontrolled Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1394–1403. [Google Scholar] [CrossRef]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Pistocchini, E.; Ritondo, B.L.; Rogliani, P.; Chetta, A. Investigational Treatments in Phase I and II Clinical Trials: A Systematic Review in Asthma. Biomedicines 2022, 10, 2330. [Google Scholar] [CrossRef]
- Fieux, M.; Le Quellec, S.; Bartier, S.; Coste, A.; Louis, B.; Giroudon, C.; Nourredine, M.; Bequignon, E. FcRn as a Transporter for Nasal Delivery of Biologics: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6475. [Google Scholar] [CrossRef]
- Guilleminault, L.; Azzopardi, N.; Arnoult, C.; Sobilo, J.; Herve, V.; Montharu, J.; Guillon, A.; Andres, C.; Herault, O.; Le Pape, A.; et al. Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J. Control. Release 2014, 196, 344–354. [Google Scholar] [CrossRef]
- Czechtizky, W.; Su, W.; Ripa, L.; Schiesser, S.; Hoijer, A.; Cox, R.J. Advances in the design of new types of inhaled medicines. Prog. Med. Chem. 2022, 61, 93–162. [Google Scholar] [CrossRef]


| scFv | VH—GGGGSGGGGS *—VL |
| db | VH—GGSGG—VL |
| sc-db | VH—GGSGG—VL—GGGGSGGGGSGGGGS—VH—GGSGG—VL |
| ta-scFv | VH—GGGGSGGGGSGGGG—VL—GGGGSGGGGS—VH—GGGGSGGGGSGGGG—VL |
| mini-ab | VH—GGGGSGGGGSGGGG—VL—TPLGDTTHTSG—RMKQLEDKVEELLSKNYHLENEVARLKKLVGERGGCGG |
| CDR1 affinity-maturation library primers | ||
| a | LMB3long | 5′—CAG GAA ACA GCT ATG ACC ATG ATT AC—3′ |
| b | DP47CDR1rev | 5′—AGC CTG GCG GAC CCA GCT CAT MNN MNN MNN GCT AAA GGT GAA TCC AGA GGC TGC—3′ |
| c | DP47CDR1for | 5′—GAG CTG GGT CCG CCA GGC TCC—3′ |
| d | DPL16CDR1rev | 5′—TCC TGG CTT CTG CTG GTA CCA GCT TGC MNN MNN MNN TCT GAG GCT GTC TCC TTG—3′ |
| e | DPL16CDR1for | 5′—TGG TAC CAG CAG AAG CCA GGA—3′ |
| f | Fdseqlong | 5′—GAC GTT AGT AAA TGA ATT TTC TGT ATG AGG– 3′ |
| CDR2 affinity-maturation library primers | ||
| a | LMB3long | 5′—CAG GAA ACA GCT ATG ACC ATG ATT AC—3′ |
| b | DP47CDR2rev | 5′—GCC CTT CAC GGA GTC TGC GTA GTA TGT MNN ACC ACC MNN MNN MNN AAT AGC TGA GAC CCA CTC C— 3′ |
| c | DP47CDR2for | 5′—ACA TAC TAC GCA GAC TCC GTG AAG GGC—3′ |
| d | DPL16CDR2revOpt | 5′—TTC TGG GAT CCC TGA GGG CCG MNN MNN TTT MNN ATA CAC GAC AAG TAC AGG GGC C—3′ |
| e | DPL16CDR2forOpt | 5′—CGG CCC TCA GGG ATC CCA GAA—3′ |
| f | Fdseqlong | 5′—GAC GTT AGT AAA TGA ATT TTC TGT ATG AGG—3′ |
| Affinity maturation of CDR1 of the OPT scFv clone | |||
| Biotinylated hALCAM V1 | Competition | Comment | |
| Round 1 | 500 nM | n.a. | n.a. |
| Round 2 | 50 nM | 200 nM Opt scFv | Led to V2D7 clone |
| Affinity maturation of CDR2 of the V2D7 scFv clone | |||
| Round 1 | 5 nM | n.a. | n.a. |
| Round 2a | 5 nM | 20 nM V2D7 scFv | Led to V20C10NC clone |
| Round 2b | 5 nM | 200 nM V2D7 scFv | Led to V200G1 clone |
| Clone | CDR1 | CDR2 | CDR3 | |
|---|---|---|---|---|
| Heavy chain (DP47) | IF8 | SYAMS | AISGSGGSTYYADSVKG | GYVAFDY |
| Opt | SYAMS | AISGSGGSTYYADSVKG | GYVAFDY | |
| V2D7 | STGAMS | AISGSGGSTYYADSVKG | GYVAFDY | |
| V20C10NC | STGAMS | AISG*TGGTTYYADSVKG | GYVAFDY | |
| V200G1 | STGAMS | AISGSGGSTYYADSVKG | GYVAFDY | |
| Light chain (DPL16) | IF8 | QGDSLRSYYAS | GKNNRPS | NSSPPFSAEVV |
| Opt | QGDSLRSYYAS | GKNNRPS | NSSPPFSAEVV | |
| V2D7 | QGDSLRSGYAS | GKNNRPS | NSSPPFSAEVV | |
| V20C10NC | QGDSLRSGYAS | PKM * SRPS | NSSPPFSAEVV | |
| V200G1 | QGDSLRSGYAS | GKTGRPS | NSSPPFSAEVV |
| Biophysical | Functional | ||||
|---|---|---|---|---|---|
| MW (kDa) | Thermal Stability Tm (°C) | Chemical Stability Cm (M GdnHCl) | cELISA IC50 (nM) | DC TM IC50 (nM) | |
| IF8-Fc | 100 | 64 ± 0.66 | 3.06 ± 0.13 | 7.33 ± 3.91 | 0.44 ± 0.39 |
| scFv V2D7 | 24 | 68.7 ± 0.45 | 3.06 ± 0.21 | 20.7 ± 11.8 | >100 |
| db V2D7 | 48 | 64.3 ± 0.36 | 2.68 ± 0.09 | 13.4 ± 5.47 | 0.86 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, A.; Klassa, S.; Herbst, A.; Maccioni, C.; Abhamon, W.; Segueni, N.; Kaluzhny, Y.; Hunter, M.C.; Halin, C. Optimization and Characterization of Novel ALCAM-Targeting Antibody Fragments for Transepithelial Delivery. Pharmaceutics 2023, 15, 1841. https://doi.org/10.3390/pharmaceutics15071841
Bauer A, Klassa S, Herbst A, Maccioni C, Abhamon W, Segueni N, Kaluzhny Y, Hunter MC, Halin C. Optimization and Characterization of Novel ALCAM-Targeting Antibody Fragments for Transepithelial Delivery. Pharmaceutics. 2023; 15(7):1841. https://doi.org/10.3390/pharmaceutics15071841
Chicago/Turabian StyleBauer, Aline, Sven Klassa, Anja Herbst, Cristina Maccioni, William Abhamon, Noria Segueni, Yulia Kaluzhny, Morgan Campbell Hunter, and Cornelia Halin. 2023. "Optimization and Characterization of Novel ALCAM-Targeting Antibody Fragments for Transepithelial Delivery" Pharmaceutics 15, no. 7: 1841. https://doi.org/10.3390/pharmaceutics15071841
APA StyleBauer, A., Klassa, S., Herbst, A., Maccioni, C., Abhamon, W., Segueni, N., Kaluzhny, Y., Hunter, M. C., & Halin, C. (2023). Optimization and Characterization of Novel ALCAM-Targeting Antibody Fragments for Transepithelial Delivery. Pharmaceutics, 15(7), 1841. https://doi.org/10.3390/pharmaceutics15071841







