Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability
Abstract
1. Introduction
2. Materials and Methods
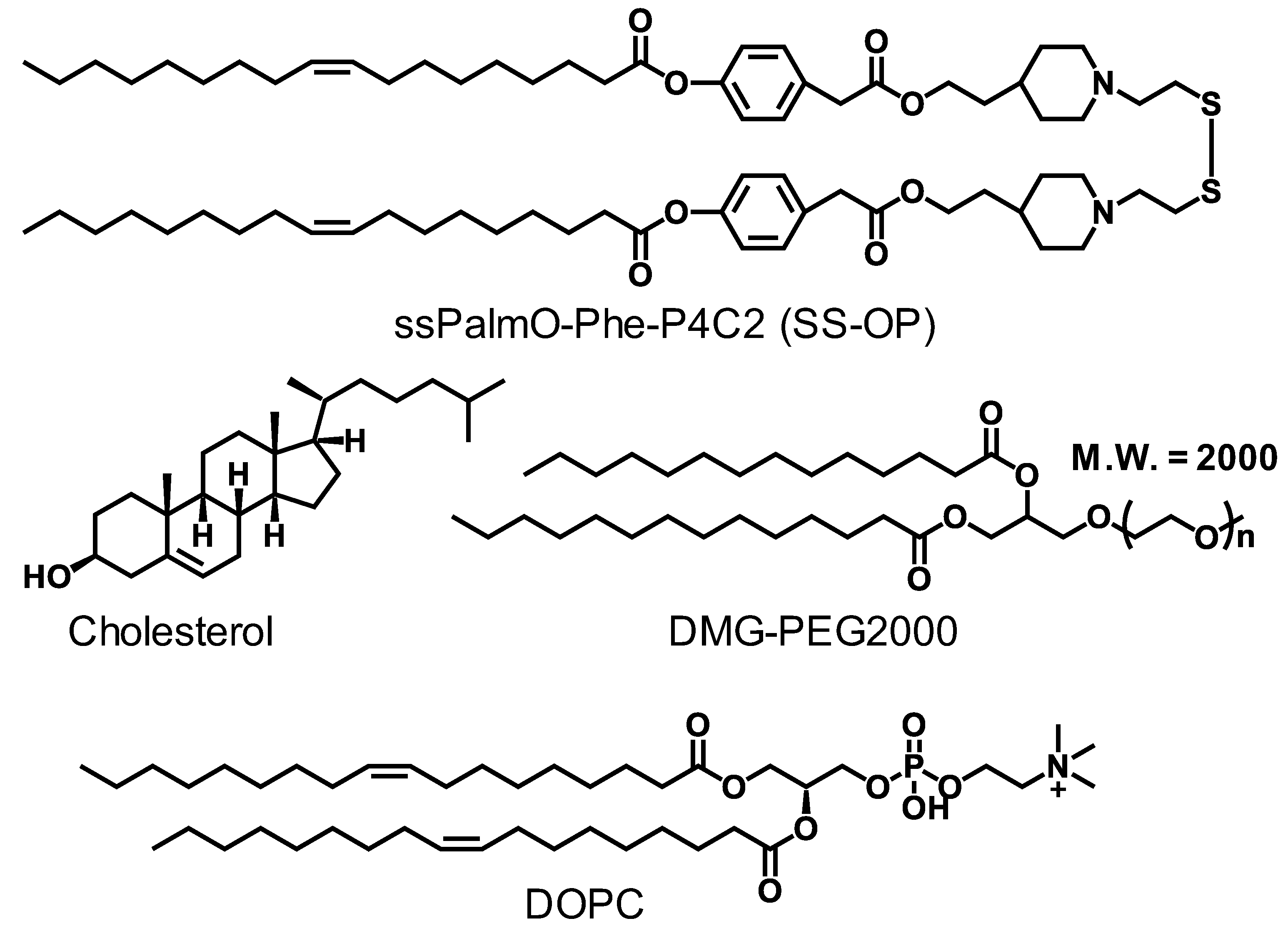
2.1. Materials
2.2. Animals
2.3. In Vitro Transcription to Prepare the IVT-mRNA
2.3.1. pDNA Linearization
2.3.2. In Vitro Transcription
2.3.3. Cellulose Column Chromatography
2.3.4. Capping
2.3.5. Poly(A) Tailing
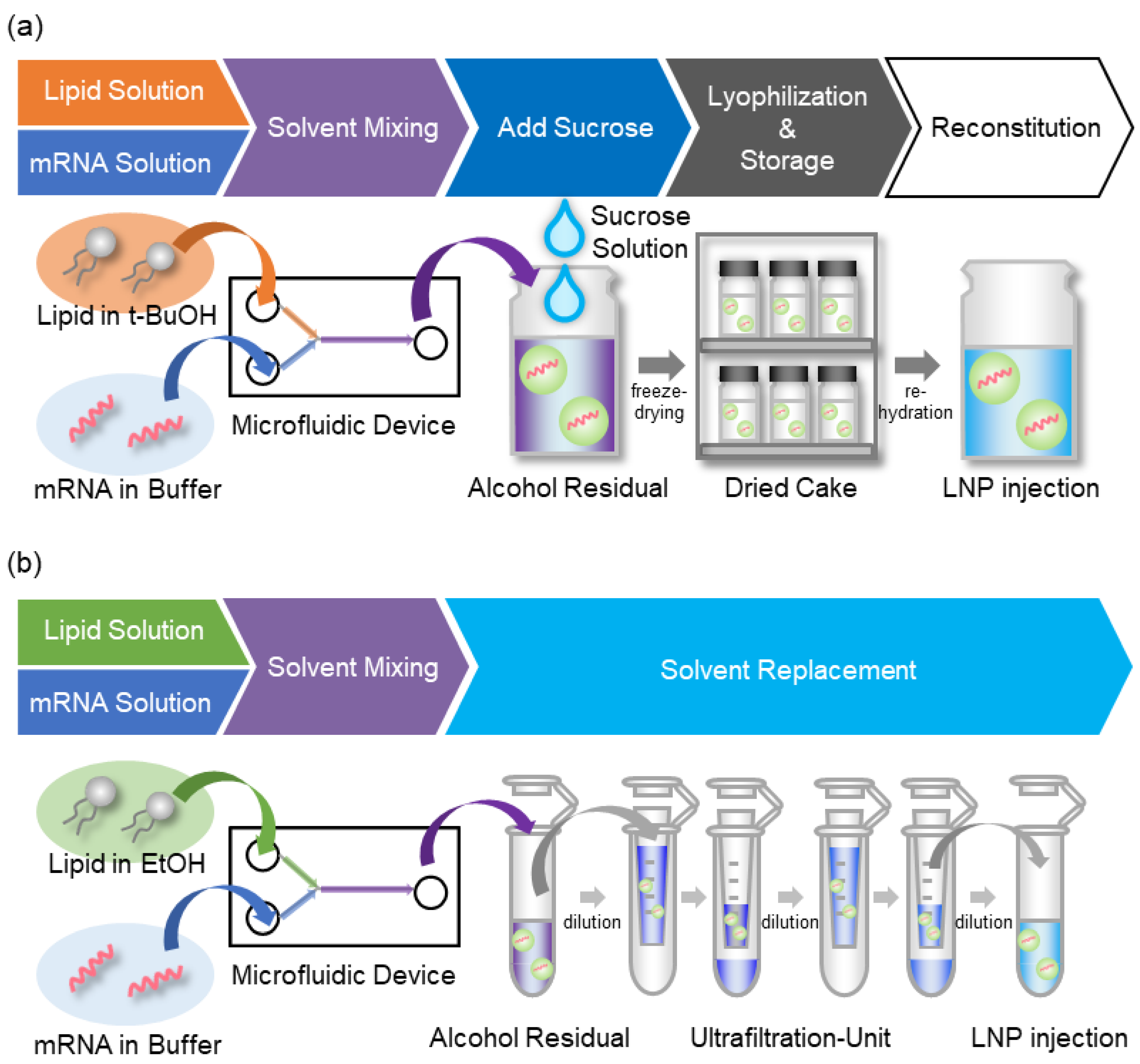
2.4. The Conventional Alcohol Dilution Method for mRNA-LNP (mRNA-LNPc)
2.5. Alcohol Dilution–Lyophilization Method for mRNA-LNP (mRNA-LNPad)
2.6. Characterization of the Particles
2.7. Evaluation of Hepatic Gene Expression Efficiency (In Vivo Luciferase Assay)
2.8. Evaluation of Hepatic Gene Expression Efficiency (IVIS Imaging Assay)
2.9. Human Erythropoietin ELISA Assay
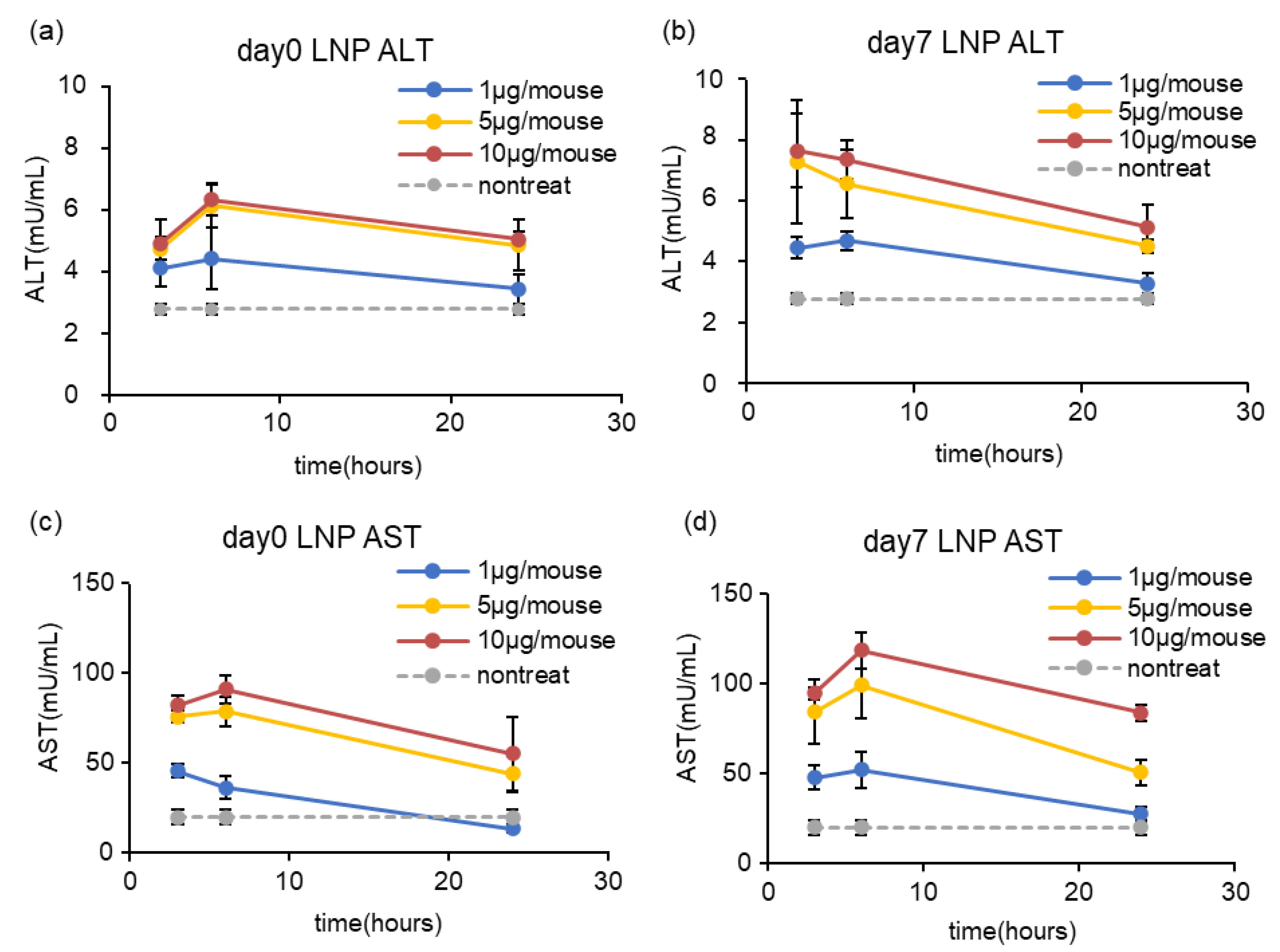
2.10. Evaluation of Hepatotoxicity of LNP (AST/ALT ELISA Assay)
3. Results
3.1. Strategy for the Alcohol Dilution–Lyophilization Method
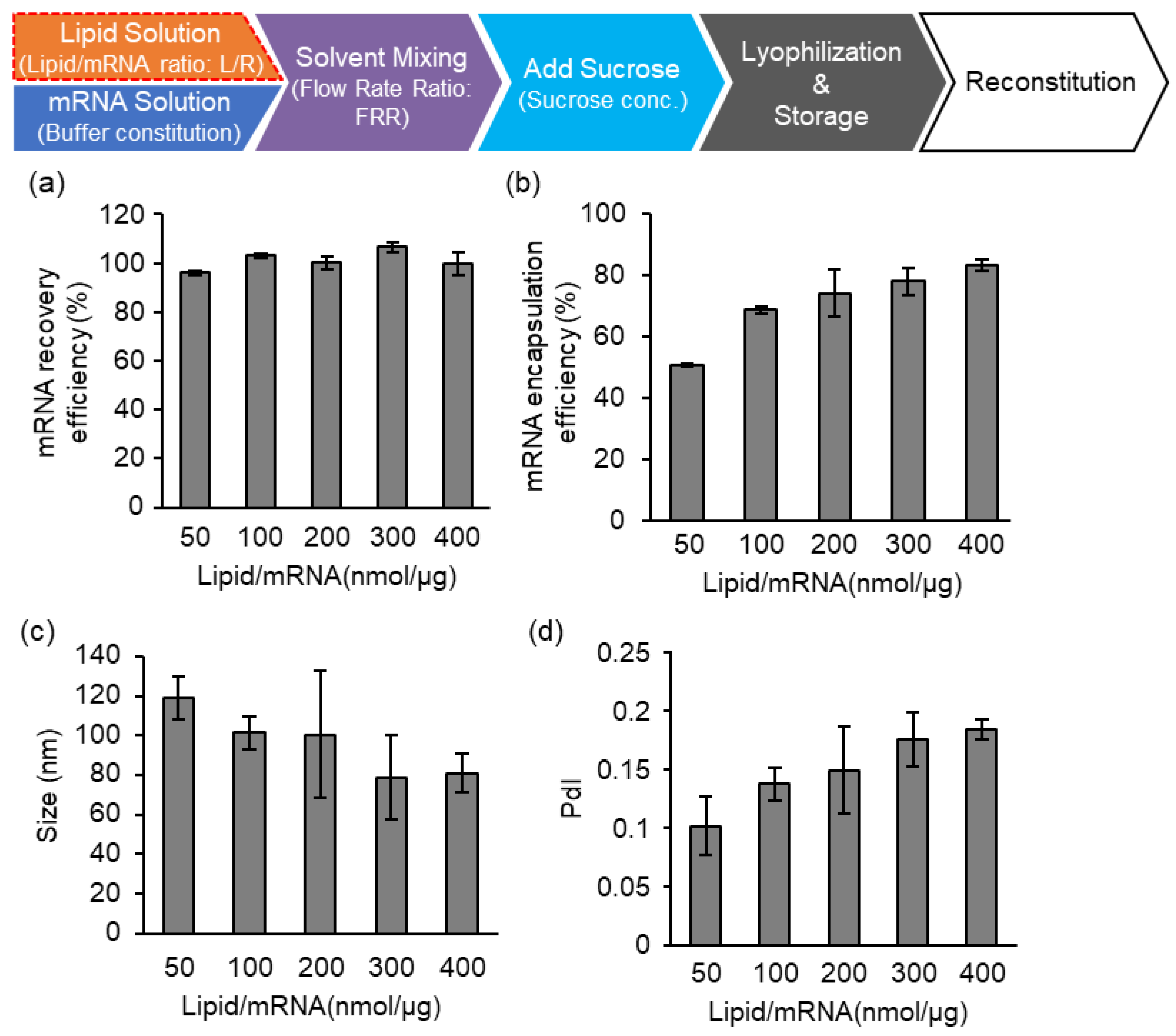
3.2. Effects of Flow Rate Ratio (FRR) of the Lipid/mRNA Solution and Sucrose Concentration
3.3. The Effects of the Buffers and Lipid/mRNA Ratio
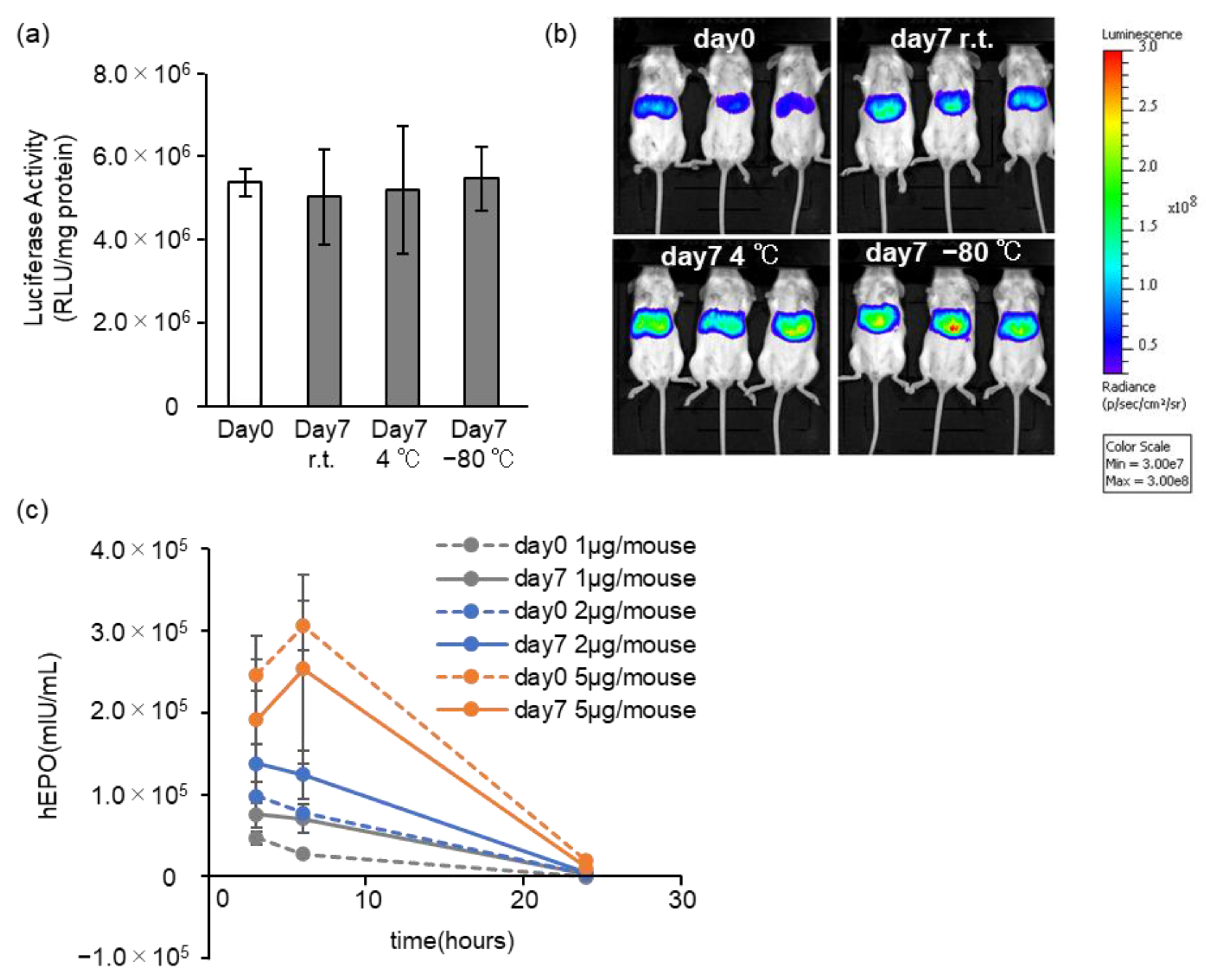
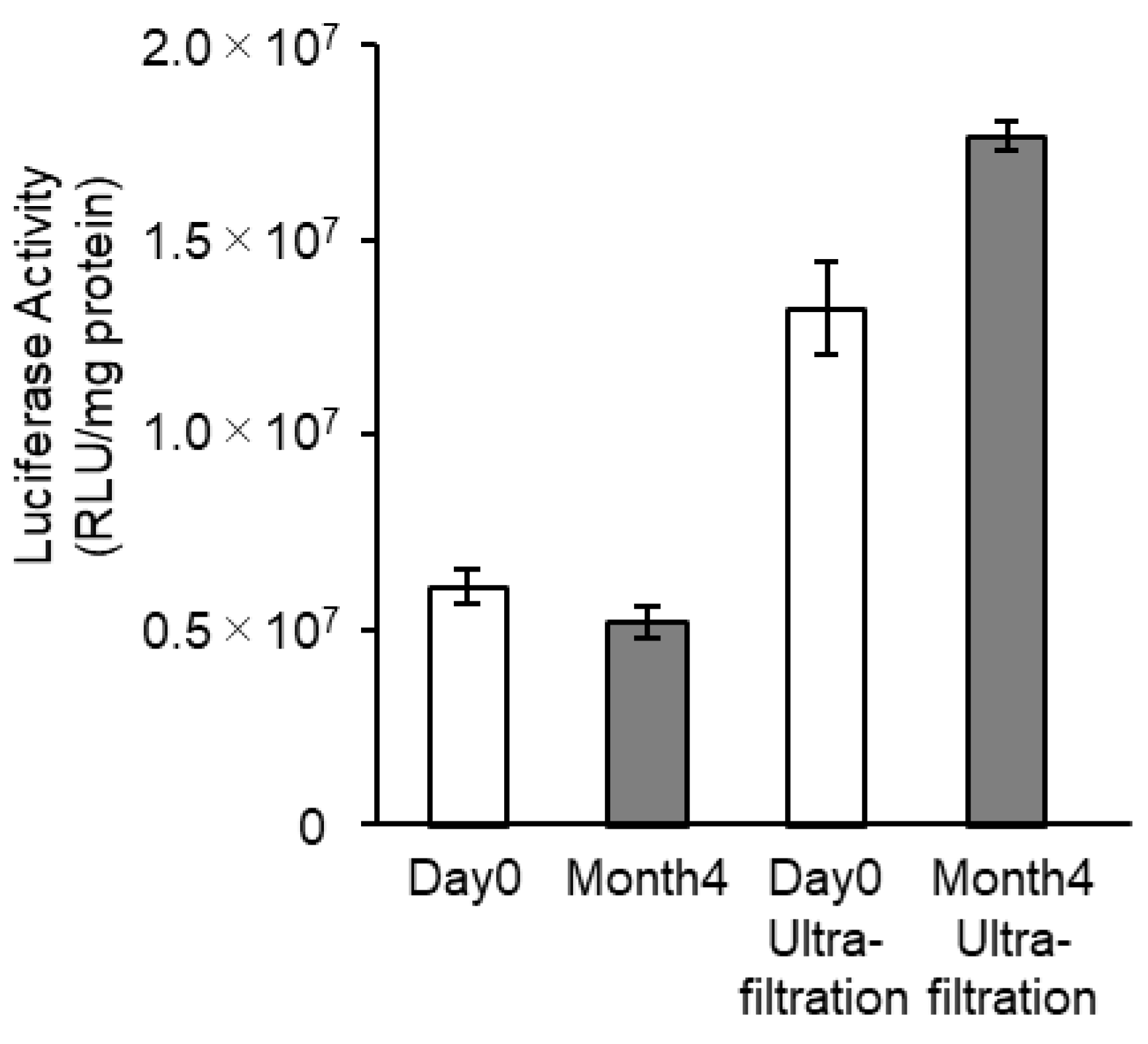
3.4. Effects of Long-Term Storage on In Vivo Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akita, H.; Ishiba, R.; Hatakeyama, H.; Tanaka, H.; Sato, Y.; Tange, K.; Arai, M.; Kubo, K.; Harashima, H. A neutral envelope-type nanoparticle containing pH-responsive and SS-cleavable lipid-like material as a carrier for plasmid DNA. Adv. Healthc. Mater. 2013, 2, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakurai, Y.; Anindita, J.; Akita, H. Development of lipid-like materials for RNA delivery based on intracellular environment-responsive membrane destabilization and spontaneous collapse. Adv. Drug. Deliv. Rev. 2020, 154–155, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Ukawa, M.; Akita, H.; Hayashi, Y.; Ishiba, R.; Tange, K.; Arai, M.; Kubo, K.; Higuchi, Y.; Shimizu, K.; Konishi, S.; et al. Neutralized nanoparticle composed of SS-cleavable and pH-activated lipid-like material as a long-lasting and liver-specific gene delivery system. Adv. Healthc. Mater. 2014, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stoter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development-An introductory review. Br. J. Pharmacol. 2022, 1–24. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release Off. J. Control. Release Soc. 2015, 217, 337–344. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Keller, J.M.; Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012, 20, 948–953. [Google Scholar] [CrossRef]
- Fuchs, A.L.; Neu, A.; Sprangers, R. A general method for rapid and cost-efficient large-scale production of 5’ capped RNA. RNA 2016, 22, 1454–1466. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Rodríguez-Abreu, C.; Vila, A. Nano-droplet systems by surfactant self-assembly and applications in the pharmaceutical industry. Curr. Top. Med. Chem. 2014, 14, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Füldner, H.H. Characterization of a third phase transition in multilamellar dipalmitoyllecithin liposomes. Biochemistry 1981, 20, 5707–5710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Mamontov, E. Multiscale lipid membrane dynamics as revealed by neutron spectroscopy. Prog. Lipid Res. 2022, 87, 101179. [Google Scholar] [CrossRef]
- Ter-Minassian-Saraga, L.; Madelmont, G. Subtransition and hydration studies of fully hydrated DPPC gel-phase. J. Colloid. Interface Sci. 1984, 99, 420–426. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Vlatkovic, I. Non-Immunotherapy Application of LNP-mRNA: Maximizing Efficacy and Safety. Biomedicines 2021, 9, 530. [Google Scholar] [CrossRef]
- Shirane, D.; Tanaka, H.; Nakai, Y.; Yoshioka, H.; Akita, H. Development of an Alcohol Dilution-Lyophilization Method for Preparing Lipid Nanoparticles Containing Encapsulated siRNA. Biol. Pharm. Bull. 2018, 41, 1291–1294. [Google Scholar] [CrossRef]
- Tanaka, H.; Takahashi, T.; Konishi, M.; Takata, N.; Gomi, M.; Shirane, D.; Miyama, R.; Hagiwara, S.; Yamasaki, Y.; Sakurai, Y.; et al. Self-Degradable Lipid-Like Materials Based on “Hydrolysis accelerated by the intra-Particle Enrichment of Reactant (HyPER)” for Messenger RNA Delivery. Adv. Funct. Mater. 2020, 30, 1910575. [Google Scholar] [CrossRef]
- Baiersdorfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Kariko, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Akita, H.; Ishiba, R.; Togashi, R.; Tange, K.; Nakai, Y.; Hatakeyama, H.; Harashima, H. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release Off. J. Control. Release Soc. 2015, 200, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, C.; Deng, Y.; Wang, Y.; Wang, W. Freeze-drying of liposomes using tertiary butyl alcohol/water cosolvent systems. Int. J. Pharm. 2006, 312, 131–136. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, T.C.; Li, X.F.; Zhang, N.N.; Li, L.; Zhou, C.; Deng, Y.Q.; Cao, T.S.; Yang, G.; Li, R.T.; et al. Long-term stability and protection efficacy of the RBD-targeting COVID-19 mRNA vaccine in nonhuman primates. Signal. Transduct. Target. Ther. 2021, 6, 438. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyazaki, T.; Muto, H.; Kubara, K.; Mukai, Y.; Watari, R.; Sato, S.; Kondo, K.; Tsukumo, S.I.; Yasutomo, K.; et al. Design and lyophilization of lipid nanoparticles for mRNA vaccine and its robust immune response in mice and nonhuman primates. Mol. Ther. Nucleic Acids 2022, 30, 226–240. [Google Scholar] [CrossRef]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu, P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, L.C.; Trout, B.L.; Pisano, R. From Batch to Continuous: Freeze-Drying of Suspended Vials for Pharmaceuticals in Unit-Doses. Ind. Eng. Chem. Res. 2019, 58, 1635–1649. [Google Scholar] [CrossRef]
- Lamoot, A.; Lammens, J.; De Lombaerde, E.; Zhong, Z.; Gontsarik, M.; Chen, Y.; De Beer, T.R.M.; De Geest, B.G. Successful batch and continuous lyophilization of mRNA LNP formulations depend on cryoprotectants and ionizable lipids. Biomater. Sci. 2023, 11, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Meulewaeter, S.; Nuytten, G.; Cheng, M.H.Y.; De Smedt, S.C.; Cullis, P.R.; De Beer, T.; Lentacker, I.; Verbeke, R. Continuous freeze-drying of messenger RNA lipid nanoparticles enables storage at higher temperatures. J. Control. Release Off. J. Control. Release Soc. 2023, 357, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J.; White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 6777. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, E.; Pinchuk, I.; Lichtenberg, D. Peroxidation of liposomal lipids. Eur. Biophys. J. 2007, 36, 499–515. [Google Scholar] [CrossRef]
- Araseki, M.; Yamamoto, K.; Miyashita, K. Oxidative stability of polyunsaturated fatty acid in phosphatidylcholine liposomes. Biosci. Biotechnol. Biochem. 2002, 66, 2573–2577. [Google Scholar] [CrossRef]



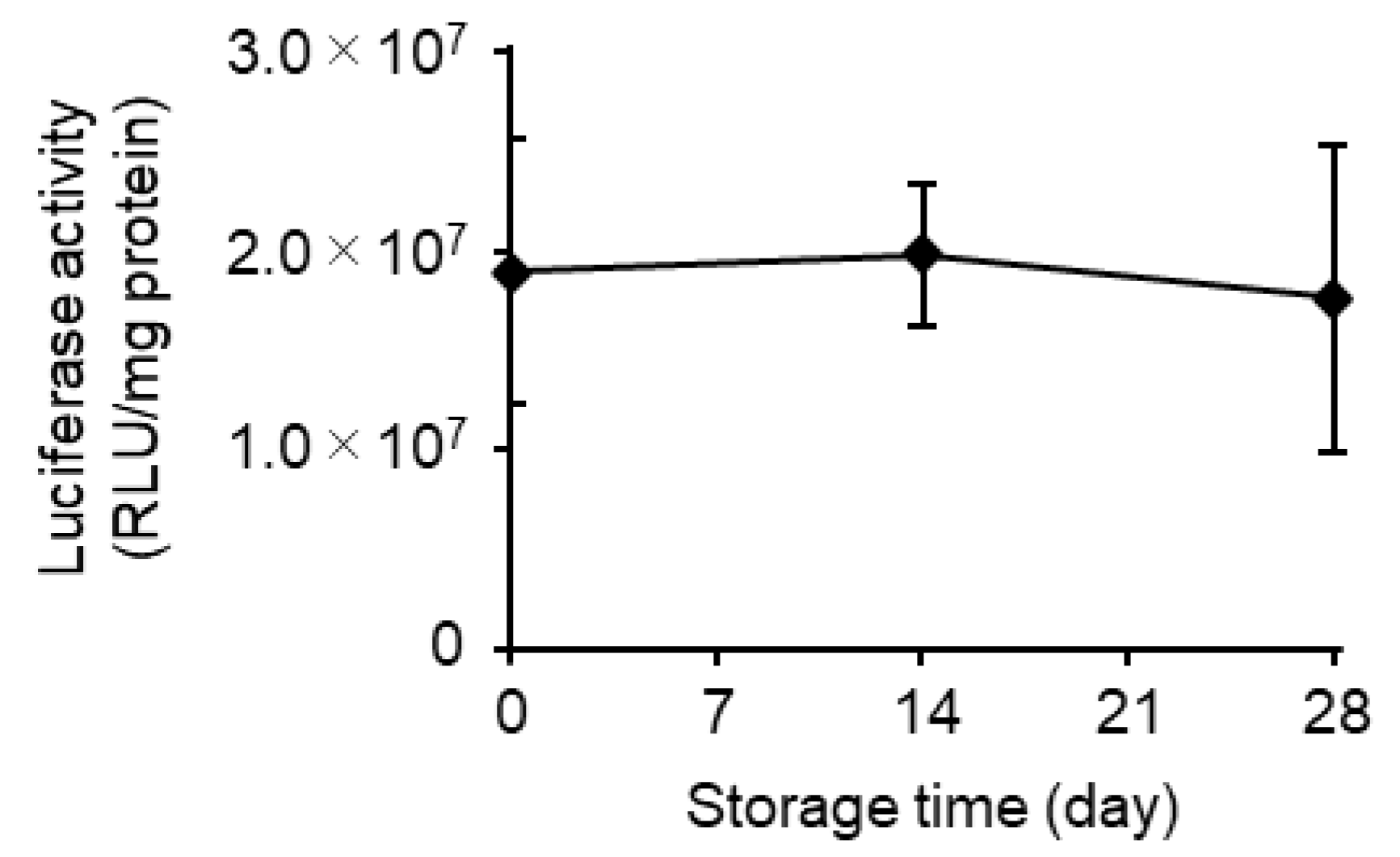
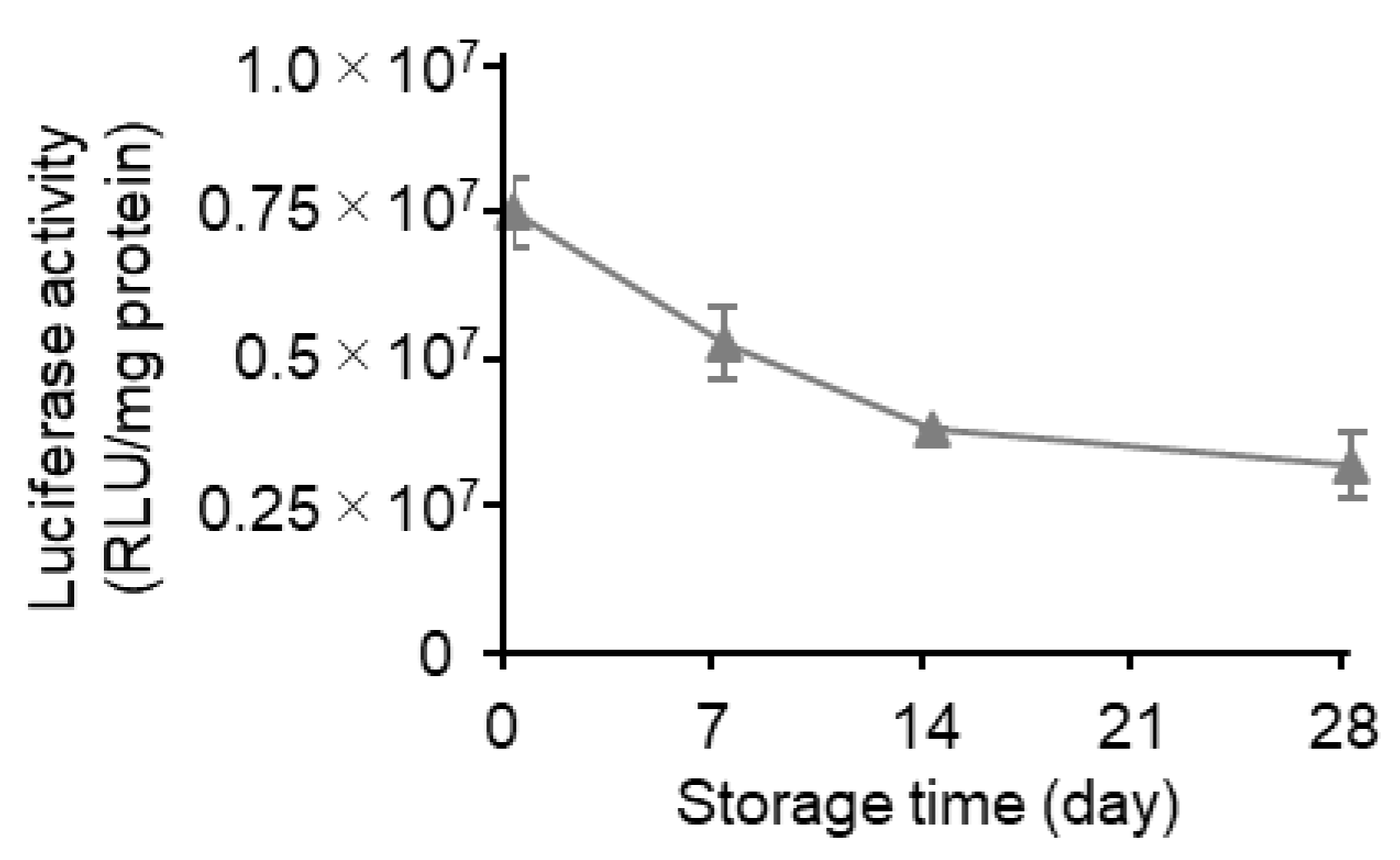

| Storage (Days) | mRNA Recovery Efficiency (%) (a) | mRNA Encapsulation Efficiency (%) (a) | Size (nm) (b) | PdI (b) | Zeta Potential (mV) (b) |
|---|---|---|---|---|---|
| 0 | 96.5 | 88.9 | 102.2 | 0.066 | −1.28 |
| 7 | 98.6 | 90.6 | 106.2 | 0.056 | −2.34 |
| 14 | 90.2 | 86.9 | 100.2 | 0.079 | −2.83 |
| 28 | 91.1 | 89.4 | 108.1 | 0.046 | −3.45 |
| Storage (Days) | mRNA Recovery Efficiency (%) (a) | mRNA Encapsulation Efficiency (%) (a) | Size (nm) (b) | PdI (b) | Zeta Potential (mV) (b) |
|---|---|---|---|---|---|
| 0 | 103.9 | 77.2 | 78.5 | 0.202 | 2.03 |
| 7 | 105.5 | 76.3 | 73.5 | 0.198 | 1.59 |
| 14 | 104.3 | 80.9 | 70.0 | 0.183 | −0.74 |
| 28 | 103.3 | 82.2 | 71.3 | 0.202 | 1.07 |
| Samples | Storage Time (Months) | mRNA Encapsulation Efficiency (%) (a) | Size (nm) (b) | PdI (b) | Z-Potential (mV) (b) |
|---|---|---|---|---|---|
| After Reconstitution | 0 | 60.1 | 75.1 | 0.177 | −3.78 |
| 4 | 68.1 | 106.8 | 0.192 | −2.60 | |
| Reconstitution and Ultrafiltration | 0 | 67.2 | 124.6 | 0.154 | −3.46 |
| 4 | 67.4 | 111.9 | 0.190 | −2.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirane, D.; Tanaka, H.; Sakurai, Y.; Taneichi, S.; Nakai, Y.; Tange, K.; Ishii, I.; Akita, H. Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability. Pharmaceutics 2023, 15, 1819. https://doi.org/10.3390/pharmaceutics15071819
Shirane D, Tanaka H, Sakurai Y, Taneichi S, Nakai Y, Tange K, Ishii I, Akita H. Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability. Pharmaceutics. 2023; 15(7):1819. https://doi.org/10.3390/pharmaceutics15071819
Chicago/Turabian StyleShirane, Daiki, Hiroki Tanaka, Yu Sakurai, Sakura Taneichi, Yuta Nakai, Kota Tange, Itsuko Ishii, and Hidetaka Akita. 2023. "Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability" Pharmaceutics 15, no. 7: 1819. https://doi.org/10.3390/pharmaceutics15071819
APA StyleShirane, D., Tanaka, H., Sakurai, Y., Taneichi, S., Nakai, Y., Tange, K., Ishii, I., & Akita, H. (2023). Development of an Alcohol Dilution–Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability. Pharmaceutics, 15(7), 1819. https://doi.org/10.3390/pharmaceutics15071819







