Indole Antitumor Agents in Nanotechnology Formulations: An Overview
Abstract
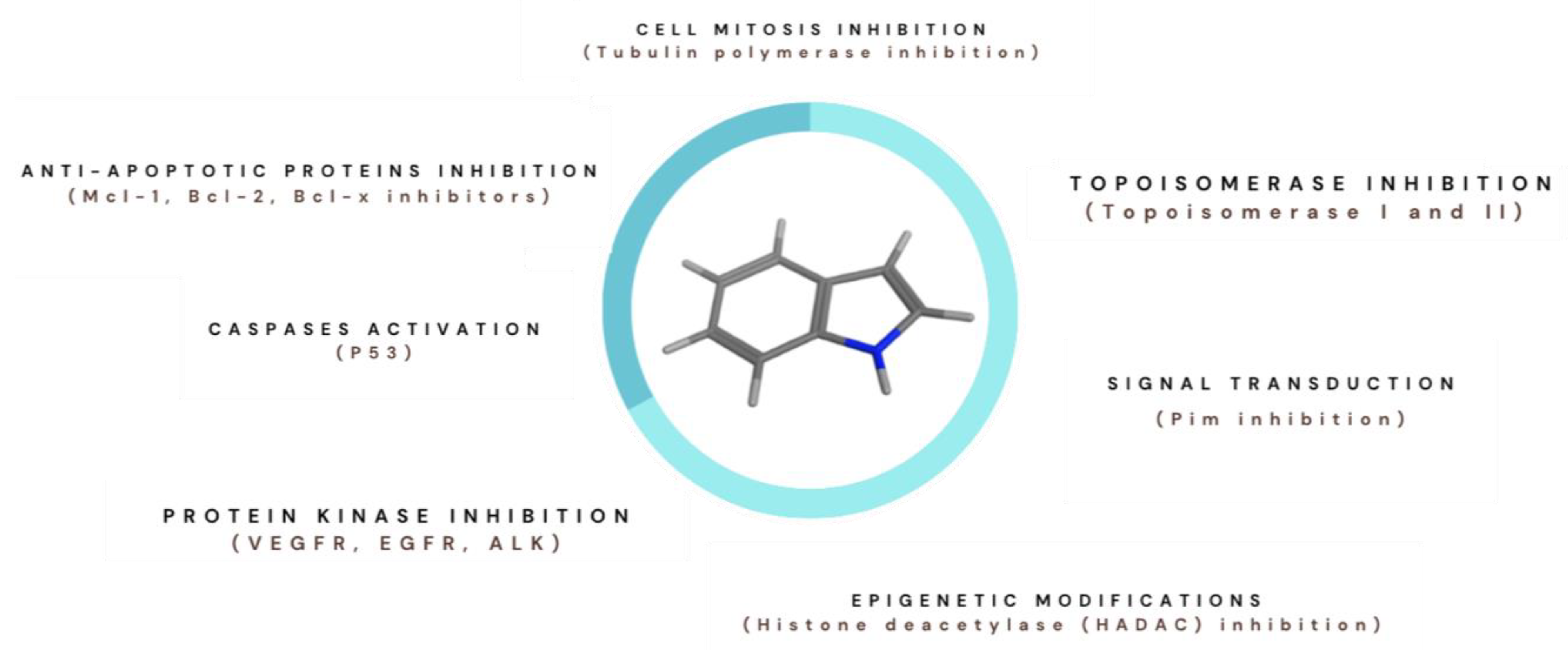
1. Introduction
1.1. Indole Derivatives and Cancer
1.2. Nanoparticles for Cancer Treatment
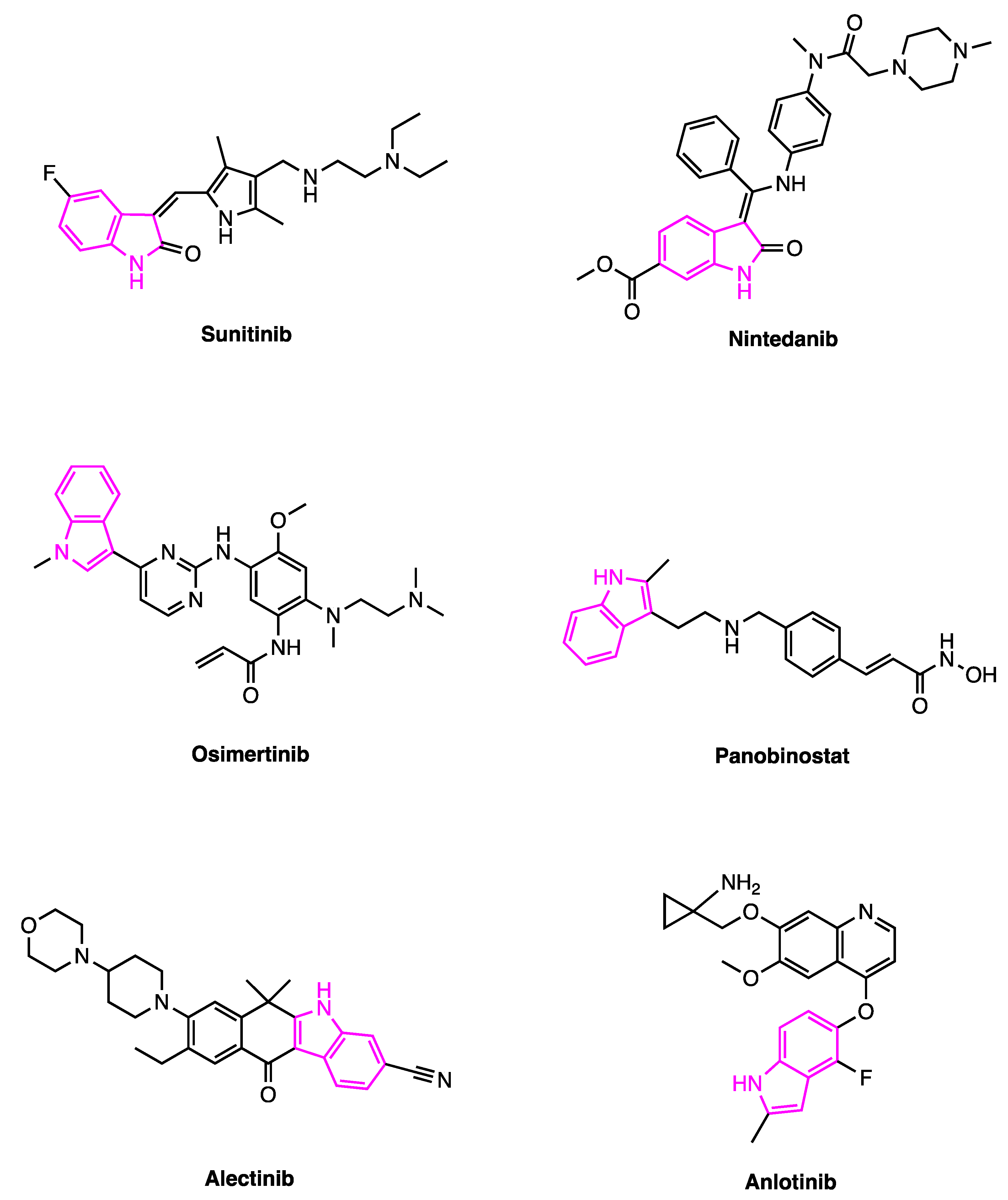
2. Sunitinib
2.1. Liposome–Sunitinib
2.2. Chitosan Nanoparticles—Sunitinib
2.3. Magnetic Nanoparticles–Sunitinib
2.4. Solid Lipid Nanoparticles–Sunitinib
2.5. Micellar Nanocomplex–Sunitinib
2.6. Miscellaneous Nanoformulations-Sunitinib
3. Nintedanib
4. Osimertinib
5. Panobinostat
6. Alectinib and Anlotinib
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALCL | Anaplastic large cell lymphoma |
| ALE | Alectinib |
| ALK | Anaplastic lymphoma kinase |
| ANLO | Anlotinib |
| APTES | 3-aminopropyltriethoxysilane |
| BBB | Blood–brain barrier |
| BSA | Bovine serum albumin |
| CDN | Cyclodextrin network |
| CHI | Chitosan |
| Chol | Cholesterol |
| CMC | Carboxymethyl cellulose |
| CNS | Central nervous system |
| CNT | Carbon nanotube |
| COS | Chitooligosaccharides |
| Cur | Curcumin |
| DL | Drug loading |
| DLS | Dynamic light scattering |
| dPGS-SS-PCL | Dendritic (poly glycerol sulfate)-SS-poly(ε-caprolactone) |
| dPGS-SS-PLA | Dendritic (poly glycerol sulfate)-SS-poly(lactide) |
| dPGS-SS-PLGA | Dendritic (poly glycerol sulfate)-SS-poly(lactide-co-glycolide) |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| EE | Encapsulation efficiency |
| EGFR | Epidermal growth factor receptor |
| EMA | European Medicines Agency |
| EPOPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine |
| EXO | Exosome |
| FA | Folic acid |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth factor receptor |
| FLT3 | Fms-like tyrosine kinase 3 |
| FTIR | Fourier transform infrared spectroscopy |
| HA | Hyaluronic acid |
| HADAC | Histone deacetylase |
| HAS | Human serum albumin |
| HAT | Histone acetylase |
| HCC | Hepatocellular carcinoma |
| HPMC | Hydroxypropyl methylcellulose |
| i.v. | Intravenous |
| ILD | Interstitial lung disease |
| IPF | Idiopathic pulmonary fibrosis |
| LIFU | Low-intensity focused ultrasound |
| LPHNP | Lipid polymer hybrid nanoparticle |
| MMSNP | Magnetic mesoporous silica nanoparticles |
| MNC | Micellar nanocomplex |
| MNP | Magnetic nanoparticle |
| mPEG-PCL | Methoxy poly-(ethylene glycol)-poly(ε-caprolactone) |
| NDDS | Nano drug delivery systems |
| NINTE | Nintedanib |
| NIR | Near infrared light |
| NLC | Nanostructured lipid carrier |
| NMPA | China National Medical Products Administration |
| NSCLC | Non-small-cell lung cancer |
| NsqNSCLC | Non-squamous non-small-cell lung cancer |
| OSI | Osimertinib |
| PANO | Panobinostat |
| PAsp(DBP) | Poly(aspartic acid-dibutyl-1,3-propanediamine) |
| PBS | Phosphate-buffered saline |
| PCL | Polycaprolactone |
| Pd-Exo | Palladium modified exosomes |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDGFR | Platelet-derived growth factor receptor |
| PDI | Polydispersity index |
| PEG | Polyethylene glycol |
| PEGEGCG | Poly(ethylene glycol)-conjugated epigallocatechin-3-O-gallate |
| PFCE | Perfluoro-15-crown-5-ether |
| PK | Pharmacokinetics |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| RCC | Renal cell carcinoma |
| RTK | Receptor tyrosine kinases |
| SEM | Scanning electron microscopy |
| SLN | Solid lipid nanocarriers |
| SMA | Styrene-co-maleic anhydride |
| SPIO | Supermagnetic iron oxide nanoparticles |
| SSc-ILD | Systemic sclerosis or scleroderma |
| SNEDDS | Self-nanoemulsifying drug delivery system |
| SUNI | Sunitinib |
| TEM | Transmission electron microscopes |
| TK | Tyrosine kinase |
| TKI | Tyrosine kinase inhibitor |
| TPGS | D-alpha-tocopheryl polyethylene glycol 1000 succinate |
| US | Ultrasound |
| Vd | Distribution volume |
| VEGFR | Vascular endothelial growth factor receptors |
| XRD | X-ray diffraction |
References
- Kumari, A.; Singh, R.K. Medicinal Chemistry of Indole Derivatives: Current to Future Therapeutic Prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Dhuguru, J.; Skouta, R. Role of Indole Scaffolds as Pharmacophores in the Development of Anti-Lung Cancer Agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology of Tyrosine Kinase Inhibitors in Cancer Therapy: A Perspective. Int. J. Mol. Sci. 2021, 22, 6538. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087. [Google Scholar] [CrossRef]
- Sundberg, R.J. Electrophilic Substitution Reactions of Indoles. In Heterocyclic Scaffolds II: Reactions and Applications of Indoles; Springer: Heidelberg, Germany, 2010; Volume 26, pp. 47–115. [Google Scholar]
- Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A.R.; Mayr, H. Nucleophilic Reactivities of Indoles. J. Org. Chem. 2006, 71, 9088–9095. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 4th ed.; W.H. Freeman Publishing: New York, NY, USA, 2005. [Google Scholar]
- Little, R.; Wittes, R.E.; Longo, D.L.; Wilson, W.H. Vinblastine for Recurrent Hodgkin’s Disease Following Autologous Bone Marrow Transplant. J. Clin. Oncol. 1998, 16, 584–588. [Google Scholar] [CrossRef]
- Mintzer, D.M.; Real, F.X.; Jovino, L.; Krown, S.E.; York, N. Treatment of Kaposi’s Sarcoma and Thrombocytopenia with Vincristine in Patients with the Acquired Immunodeficiency Syndrome. Ann. Intern. Med. 1985, 102, 200–202. [Google Scholar] [CrossRef]
- Jackson, D.V.; Paschold, E.H.; Spurr, C.L.; Muss, H.B.; Richards, F.; Cooper, M.R.; White, D.R.; Stuart, J.J.; Hopkins, J.O.; Rich, R.B.S., Jr.; et al. Treatment of Advanced Non-Hodgkin’s Lymphoma with Vincristine Infusion. Cancer 1984, 53, 2601–2606. [Google Scholar] [CrossRef]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole Alkaloids from Catharanthus roseus: Bioproduction and Their Effect on Human Health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef]
- Tu, Y.; Cheng, S.; Zhang, S.; Sun, H.; Xu, Z. Vincristine Induces Cell Cycle Arrest and Apoptosis in SH-SY5Y Human Neuroblastoma Cells. Int. J. Mol. Med. 2013, 31, 113–119. [Google Scholar] [CrossRef]
- Ahmad, A.; Sakr, W.A.; Wahidur Rahman, K. Anticancer Properties of Indole Compounds: Mechanism of Apoptosis Induction and Role in Chemotherapy. Curr. Drug Targets 2010, 11, 652–656. [Google Scholar] [CrossRef]
- Brancale, A.; Silvestri, R. Indole, a Core Nucleus for Potent Inhibitors of Tubulin Polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef]
- Mondal, D.; Amin, S.A.; Moinul, M.; Das, K.; Jha, T.; Gayen, S. How the Structural Properties of the Indole Derivatives Are Important in Kinase Targeted Drug Design? A Case Study on Tyrosine Kinase Inhibitors. Bioorg. Med. Chem. 2022, 53, 116534. [Google Scholar] [CrossRef]
- Jiang, B.E.; Hu, J.; Liu, H.; Liu, Z.; Wen, Y.; Liu, M.; Zhang, H.K.; Pang, X.; Yu, L.F. Design, Synthesis, and Biological Evaluation of Indole-Based Hydroxamic Acid Derivatives as Histone Deacetylase Inhibitors. Eur. J. Med. Chem. 2022, 227, 113893. [Google Scholar] [CrossRef]
- Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 11 April 2023).
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 20 March 2023).
- Ropero, S.; Esteller, M. The Role of Histone Deacetylases (HDACs) in Human Cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Villar-Garea, A.; Boix-Chornet, M.; Espada, J.; Schotta, G.; Bonaldi, T.; Haydon, C.; Ropero, S.; Petrie, K.; et al. Loss of Acetylation at Lys16 and Trimethylation at Lys20 of Histone H4 Is a Common Hallmark of Human Cancer. Nat. Genet. 2005, 37, 391–400. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Kalff, A.; Chow, A.; Khong, T.; Spencer, A. Dysregulated Class I Histone Deacetylases Are Indicators of Poor Prognosis in Multiple Myeloma. Epigenetics 2014, 9, 1511–1520. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Panobinostat: First Global Approval. Drugs 2015, 75, 695–704. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-22-2660 (accessed on 30 March 2023).
- Suzuki, K.; Sunami, K.; Matsumoto, M.; Maki, A.; Shimada, F.; Suzuki, K.; Shimizu, K. Phase II, Multicenter, Single-Srm, Open-Label Study to Evaluate the Efficacy and Safety of Panobinostat in Combination with Bortezomib and Dexamethasone in Japanese Patients with Relapsed or Relapsed-and-Refractory Multiple Myeloma. Acta Haematol. 2021, 144, 264–274. [Google Scholar] [CrossRef]
- Shi, W.; Palmer, J.D.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Bar-Ad, V.; Judy, K.; Farrell, C.; et al. Phase I Trial of Panobinostat and Fractionated Stereotactic Re-Irradiation Therapy for Recurrent High Grade Gliomas. J. Neurooncol. 2016, 127, 535–539. [Google Scholar] [CrossRef]
- Robertson, S.C.; Tynan, J. RTK Mutations and Human Syndromes: When Good Receptors Turn Bad. Trends Genet. 2000, 16, 265–368. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of Tyrosine Kinase Inhibitors in Cancer Therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef]
- Rosenzweig, S.A. Acquired Resistance to Drugs Targeting Tyrosine Kinases. Adv. Cancer Res. 2018, 138, 71–98. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y. shan Advances in Studies of Tyrosine Kinase Inhibitors and Their Acquired Resistance. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular Basis for Sunitinib Efficacy and Future Clinical Development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef]
- Hao, Z.; Sadek, I. Sunitinib: The Antiangiogenic Effects and Beyond. OncoTargets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Rini, B.I.; Atkins, M.B. Resistance to Targeted Therapy in Renal-Cell Carcinoma. Lancet Oncol. 2009, 10, 992–1000. [Google Scholar] [CrossRef]
- DuBois, S.G.; Shusterman, S.; Reid, J.M.; Ingle, A.M.; Ahern, C.H.; Baruchel, S.; Glade-Bender, J.; Ivy, P.; Adamson, P.C.; Blaney, S.M. Tolerability and Pharmacokinetic Profile of a Sunitinib Powder Formulation in Pediatric Patients with Refractory Solid Tumors: A Children’s Oncology Group Study. Cancer Chemother. Pharmacol. 2012, 69, 1021–1027. [Google Scholar] [CrossRef]
- Verschuur, A.C.; Bajčiová, V.; Mascarenhas, L.; Khosravan, R.; Lin, X.; Ingrosso, A.; Janeway, K.A. Sunitinib in Pediatric Patients with Advanced Gastrointestinal Stromal Tumor: Results from a Phase I/II Trial. Cancer Chemother. Pharmacol. 2019, 84, 41–50. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, H.V.; Molyneaux, P.L.; Maher, T.M. Reducing Lung Function Decline in Patients with Idiopathic Pulmonary Fibrosis: Potential of Nintedanib. Drug Des. Dev. Ther. 2013, 7, 503–510. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=205832 (accessed on 11 April 2023).
- Tan, C.S.; Gilligan, D.; Pacey, S. Treatment Approaches for EGFR-Inhibitor-Resistant Patients with Non-Small-Cell Lung Cancer. Lancet Oncol. 2015, 16, e447–e459. [Google Scholar] [CrossRef]
- Liao, B.C.; Lin, C.C.; Yang, J.C.H. Second and Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Advanced Nonsmall Cell Lung Cancer. Curr. Opin. Oncol. 2015, 27, 94–101. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208065 (accessed on 11 April 2023).
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR—Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Zwierenga, F.; van Veggel, B.; Hendriks, L.E.L.; Hiltermann, T.J.N.; Hiddinga, B.I.; Hijmering Kappelle, L.B.M.; ter Elst, A.; Hashemi, S.M.S.; Dingemans, A.M.C.; van der Leest, C.; et al. High Dose Osimertinib in Patients with Advanced Stage EGFR Exon 20 Mutation-Positive NSCLC: Results from the Phase 2 Multicenter POSITION20 Trial. Lung Cancer 2022, 170, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, M.; Zhang, A. Alectinib: A Novel Second Generation Anaplastic Lymphoma Kinase (ALK) Inhibitor for Overcoming Clinically-Acquired Resistance. Acta Pharm. Sin. B 2015, 5, 34–37. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a Kinase Gene, ALK, to a Nucleolar Protein Gene, NPM, in Non-Hodgkin’s Lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Kodama, T.; Tsukaguchi, T.; Yoshida, M.; Kondoh, O.; Sakamoto, H. Selective ALK Inhibitor Alectinib with Potent Antitumor Activity in Models of Crizotinib Resistance. Cancer Lett. 2014, 351, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, A.; Yamada, T.; Nanjo, S.; Takeuchi, S.; Ebi, H.; Kita, K.; Matsumoto, K.; Yano, S. Receptor Ligand-Triggered Resistance to Alectinib and Its Circumvention by Hsp90 Inhibition in EML4-ALK Lung Cancer Cells. Oncotarget 2014, 5, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208434 (accessed on 11 April 2023).
- Lin, B.; Song, X.; Yang, D.; Bai, D.; Yao, Y.; Lu, N. Anlotinib Inhibits Angiogenesis via Suppressing the Activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018, 654, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Q.; Zhang, H.; Li, X.; Jiang, Q.; Yao, J. A Small Molecular Multi-Targeting Tyrosine Kinase Inhibitor, Anlotinib, Inhibits Pathological Ocular Neovascularization. Biomed. Pharmacother. 2021, 138, 111493. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, W.; Du, F.; Du, C.; Li, S.; Wang, J.; Li, L.; Wang, F.; Hao, Y.; Li, C.; et al. Safety, Pharmacokinetics, and Antitumor Properties of Anlotinib, an Oral Multi-Target Tyrosine Kinase Inhibitor, in Patients with Advanced Refractory Solid Tumors. J. Hematol. Oncol. 2016, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Anlotinib: First Global Approval. Drugs 2018, 78, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3181972 (accessed on 11 April 2023).
- Li, D.; Chi, Y.; Chen, X.; Ge, M.; Zhang, Y.; Guo, Z.; Wang, J.; Chen, J.; Zhang, J.; Cheng, Y.; et al. Anlotinib in Locally Advanced or Metastatic Medullary Thyroid Carcinoma: A Randomized, Double-Blind Phase IIB Trial. Clin. Cancer Res. 2021, 27, 3567–3575. [Google Scholar] [CrossRef]
- Chi, Y.; Shu, Y.; Ba, Y.; Bai, Y.; Qin, B.; Wang, X.; Xiong, J.; Xu, N.; Zhang, H.; Zhou, J.; et al. Anlotinib Monotherapy for Refractory Metastatic Colorectal Cancer: A Double-Blinded, Placebo-Controlled, Randomized Phase III Trial (ALTER0703). Oncologist 2021, 26, e1693–e1703. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, A.; Zhang, W.; Jiang, Z.; Chen, B.; Zhao, J.; Li, Z.; Wang, L.; Bi, X.; Zhao, H.; et al. Anlotinib in the Treatment of Advanced Hepatocellular Carcinoma: An Open-Label Phase II Study (ALTER-0802 Study). Hepatol. Int. 2021, 15, 621–629. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Svenson, S. What Nanomedicine in the Clinic Right Now Really Forms Nanoparticles? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Multifunctional and Stimuli-Sensitive Pharmaceutical Nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, S. Nanoparticles Escaping RES and Endosome: Challenges for SiRNA Delivery for Cancer Therapy. J. Nanomater. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Bernsen, E.C.; Hogenes, V.J.; Nuijen, B.; Hanff, L.M.; Huitema, A.D.R.; Diekstra, M.H.M. Practical Recommendations for the Manipulation of Kinase Inhibitor Formulations to Age-Appropriate Dosage Forms. Pharmaceutics 2022, 14, 2834. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Christensen, R.; Minkin, P.; Stewart, C.F.; Furman, W.L.; Baker, S. Stability of Sunitinib in Oral Suspension. Ann. Pharmacother. 2008, 42, 962–966. [Google Scholar] [CrossRef]
- Passadouro, M.; Pedroso de Lima, M.C.; Faneca, H. MicroRNA Modulation Combined with Sunitinib as a Novel Therapeutic Strategy for Pancreatic Cancer. Int. J. Nanomed. 2014, 9, 3203–3217. [Google Scholar] [CrossRef]
- Hu, J.; Zong, Y.; Li, J.; Zhou, X.; Zhang, J.; Zhu, T.; Jiao, M.; Su, H.; Bo, B. In Vitro and in Vivo Evaluation of Targeted Sunitinib-Loaded Polymer Microbubbles against Proliferation of Renal Cell Carcinoma. J. Ultrasound Med. 2016, 35, 589–597. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Qian, C.; Guo, Y.; Li, C.; Gao, F.; Yang, Y.; Wang, K.; Oupicky, D.; Sun, M. Near-Infrared Light-Activated IR780-Loaded Liposomes for Anti-Tumor Angiogenesis and Photothermal Therapy. Nanomedicine 2018, 14, 2283–2294. [Google Scholar] [CrossRef]
- Jiao, Y.; Gao, Y.; Wang, J.Y.; An, H.; Li, Y.X.; Zhang, X. Intelligent Porphyrin Nano-Delivery System for Photostimulated and Targeted Inhibition of Angiogenesis. Int. J. Pharm. 2022, 621, 121805. [Google Scholar] [CrossRef]
- Charkhat Gorgich, E.A.; Kasbiyan, H.; Shabani, R.; Mehdizadeh, M.; Hajiahmadi, F.; Ajdary, M.; Barati, M.; Moradi, F.; Ahmadvand, D. Smart Chlorotoxin-Functionalized Liposomes for Sunitinib Targeted Delivery into Glioblastoma Cells. J. Drug Deliv. Sci. Technol. 2022, 77, 103908. [Google Scholar] [CrossRef]
- Lai, X.; Liu, X.L.; Pan, H.; Zhu, M.H.; Long, M.; Yuan, Y.; Zhang, Z.; Dong, X.; Lu, Q.; Sun, P.; et al. Light-Triggered Efficient Sequential Drug Delivery of Biomimetic Nanosystem for Multimodal Chemo-, Antiangiogenic, and Anti-MDSC Therapy in Melanoma. Adv. Mater. 2022, 34, 2106682. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based Nanomaterials: A State-of-the-Art Review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Sangeetha, D.; Gomathi, T. Sunitinib Loaded Chitosan Nanoparticles Formulation and Its Evaluation. Int. J. Biol. Macromol. 2016, 82, 952–958. [Google Scholar] [CrossRef]
- Saber, M.M.; Bahrainian, S.; Dinarvand, R.; Atyabi, F. Targeted Drug Delivery of Sunitinib Malate to Tumor Blood Vessels by CRGD-Chiotosan-Gold Nanoparticles. Int. J. Pharm. 2017, 517, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Mahdavinia, G.R.; Kazemi, B.; Ehrlich, H.; Joseph, Y.; Rahimi-Nasrabadi, M. Highly Efficient Sunitinib Release from PH-Responsive MHPMC@Chitosan Core-Shell Nanoparticles. Carbohydr. Polym. 2021, 258, 117719. [Google Scholar] [CrossRef]
- Alinavaz, S.; Jabbari, P.; Mahdavinia, G.R.; Jafari, H.; Sharifi, S.; Lighvan, Z.M.; Akbari, A. Novel Magnetic Carboxymethylcellulose/Chitosan Bio-Nanocomposites for Smart Co-Delivery of Sunitinib Malate Anticancer Compound and Saffron Extract. Polym. Int. 2022, 71, 1243–1251. [Google Scholar] [CrossRef]
- Karimi, M.H.; Mahdavinia, G.R.; Massoumi, B. PH-Controlled Sunitinib Anticancer Release from Magnetic Chitosan Nanoparticles Crosslinked with κ-Carrageenan. Mater. Sci. Eng. C 2018, 91, 705–714. [Google Scholar] [CrossRef]
- Rajan, A.; Sahu, N.K. Review on Magnetic Nanoparticle-Mediated Hyperthermia for Cancer Therapy. J. Nanopart. Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Chen, S.; Liang, Q.; Liu, E.; Yu, Z.; Sun, L.; Ye, J.; Shin, M.C.; Wang, J.; He, H. Curcumin/Sunitinib Co-Loaded BSA-Stabilized SPIOs for Synergistic Combination Therapy for Breast Cancer. J. Mater. Chem. B 2017, 5, 4060–4072. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Akakuru, O.; Xu, W.; Wu, A.; Zhang, Y. ICG and Sunitinib-Loaded NH2-MOFs for Folate-Mediated Hepatocellular Carcinoma Dual-Modal Therapy. Chem. Res. Chin. Univ. 2021, 37, 967–974. [Google Scholar] [CrossRef]
- Torabi, M.; Aghanejad, A.; Savadi, P.; Barzegari, A.; Omidi, Y.; Barar, J. Targeted Delivery of Sunitinib by MUC-1 Aptamer-Capped Magnetic Mesoporous Silica Nanoparticles. Molecules 2023, 28, 411. [Google Scholar] [CrossRef]
- Taymouri, S.; Alem, M.; Varshosaz, J.; Rostami, M.; Akbari, V.; Firoozpour, L. Biotin Decorated Sunitinib Loaded Nanostructured Lipid Carriers for Tumor Targeted Chemotherapy of Lung Cancer. J. Drug Deliv. Sci. Technol. 2019, 50, 237–247. [Google Scholar] [CrossRef]
- Khaledian, S.; Kahrizi, D.; Moradi, S.; Martinez, F. An Experimental and Computational Study to Evaluation of Chitosan/Gum Tragacanth Coated-Natural Lipid-Based Nanocarriers for Sunitinib Delivery. J. Mol. Liq. 2021, 334, 116075. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Castro, G.; Liu, C.H.; Lau, P. Advanced Nanotechnology: An Arsenal to Enhance Immunotherapy in Fighting Cancer. Clin. Chim. Acta 2019, 492, 12–19. [Google Scholar] [CrossRef]
- Yongvongsoontorn, N.; Chung, J.E.; Gao, S.J.; Bae, K.H.; Yamashita, A.; Tan, M.H.; Ying, J.Y.; Kurisawa, M. Carrier-Enhanced Anticancer Efficacy of Sunitinib-Loaded Green Tea-Based Micellar Nanocomplex beyond Tumor-Targeted Delivery. ACS Nano 2019, 13, 7591–7602. [Google Scholar] [CrossRef]
- Zeng, X.; Teng, Y.; Zhu, C.; Li, Z.; Liu, T.; Sun, Y.; Han, S. Combined Ibuprofen-Nanoconjugate Micelles with E-Selectin for Effective Sunitinib Anticancer Therapy. Int. J. Nanomed. 2022, 17, 6031–6046. [Google Scholar] [CrossRef]
- He, J.; Xiao, H.; Li, B.; Peng, Y.; Li, X.; Wang, Y.; Adamus, G.; Kowalczuk, M.; Shuai, X. The Programmed Site-Specific Delivery of the Angiostatin Sunitinib and Chemotherapeutic Paclitaxel for Highly Efficient Tumor Treatment. J. Mater. Chem. B 2019, 7, 4953–4962. [Google Scholar] [CrossRef]
- Qin, T.; Xu, X.; Zhang, Z.; Li, J.; You, X.; Guo, H.; Sun, H.; Liu, M.; Dai, Z.; Zhu, H. Paclitaxel/Sunitinib-Loaded Micelles Promote an Antitumor Response in Vitro through Synergistic Immunogenic Cell Death for Triple-Negative Breast Cancer. Nanotechnology 2020, 31, 365101. [Google Scholar] [CrossRef]
- Shih, Y.H.; Peng, C.L.; Chiang, P.F.; Shieh, M.J. Dual-Functional Polymeric Micelles Co-Loaded with Antineoplastic Drugs and Tyrosine Kinase Inhibitor for Combination Therapy in Colorectal Cancer. Pharmaceutics 2022, 14, 768. [Google Scholar] [CrossRef]
- Braatz, D.; Dimde, M.; Ma, G.; Zhong, Y.; Tully, M.; Grötzinger, C.; Zhang, Y.; Mavroskoufis, A.; Schirner, M.; Zhong, Z.; et al. Toolbox of Biodegradable Dendritic (Poly Glycerol Sulfate)-SS-Poly(Ester) Micelles for Cancer Treatment: Stability, Drug Release, and Tumor Targeting. Biomacromolecules 2021, 22, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Sangeetha, D.; Shivashankar, M. In Vitro Release and Cytotoxic Studies of Novel Alginate Nanocarrier for the Antitumor Drug: Sunitinib. Regen. Eng. Transl. Med. 2019, 5, 220–227. [Google Scholar] [CrossRef]
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and Characterization of Ascorbic Acid Based Therapeutic Deep Eutectic Solvent as a New Ion-Gel for Delivery of Sunitinib Malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512. [Google Scholar] [CrossRef]
- Scrivano, L.; Parisi, O.I.; Iacopetta, D.; Ruffo, M.; Ceramella, J.; Sinicropi, M.S.; Puoci, F. Molecularly Imprinted Hydrogels for Sustained Release of Sunitinib in Breast Cancer Therapy. Polym. Adv. Technol. 2019, 30, 743–748. [Google Scholar] [CrossRef]
- Keutgen, X.M.; Ornell, K.J.; Vogle, A.; Lakiza, O.; Williams, J.; Miller, P.; Mistretta, K.S.; Setia, N.; Weichselbaum, R.R.; Coburn, J.M. Sunitinib-Loaded Chondroitin Sulfate Hydrogels as a Novel Drug-Delivery Mechanism for the Treatment of Pancreatic Neuroendocrine Tumors. Ann. Surg. Oncol. 2021, 28, 8532–8543. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Scrivano, L.; Sinicropi, M.S.; Cesario, M.G.; Candamano, S.; Puoci, F.; Sisci, D. Controlled Release of Sunitinib in Targeted Cancer Therapy: Smart Magnetically Responsive Hydrogels as Restricted Access Materials. RSC Adv. 2015, 5, 65308–65315. [Google Scholar] [CrossRef]
- Alshetaili, A.S.; Anwer, M.K.; Alshahrani, S.M.; Alalaiwe, A.; Alsulays, B.B.; Ansari, M.J.; Imam, F.; Alshehri, S. Characteristics and Anticancer Properties of Sunitinib Malate-Loaded Poly-Lactic-Co-Glycolic Acid Nanoparticles against Human Colon Cancer HT-29 Cells Lines. Trop. J. Pharm. Res. 2018, 17, 1263–1269. [Google Scholar] [CrossRef]
- Dehneshin, N.; Raissi, H.; Hasanzade, Z.; Farzad, F. Using Molecular Dynamics Simulation to Explore the Binding of the Three Potent Anticancer Drugs Sorafenib, Streptozotocin, and Sunitinib to Functionalized Carbon Nanotubes. J. Mol. Model. 2019, 25, 159. [Google Scholar] [CrossRef]
- Domvri, K.; Petanidis, S.; Anestakis, D.; Porpodis, K.; Bai, C.; Zarogoulidis, P.; Freitag, L.; Hohenforst-Schmidt, W.; Katopodi, T. Dual Photothermal MDSCs-Targeted Immunotherapy Inhibits Lung Immunosuppressive Metastasis by Enhancing T-Cell Recruitment. Nanoscale 2020, 12, 7051–7062. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, Q.; Lu, S.; Chen, X.; Xu, W.; Shi, F. Co-Delivery of Bufalin and Nintedanib via Albumin Sub-Microspheres for Synergistic Cancer Therapy. J. Control. Release 2021, 338, 705–718. [Google Scholar] [CrossRef]
- Zha, Q.; Zhang, L.; Guo, Y.; Bao, R.; Shi, F.; Shi, Y. Preparation and Study of Folate Modified Albumin Targeting Microspheres. J. Oncol. 2022, 2022, 3968403. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; He, T.; Zhang, Y.; Wang, M.; Yuan, H.; Yang, M. Biguanides Decorated Albumin Nanoparticles Loading Nintedanib for Synergic Enhanced Hepatocellular Carcinoma Therapy. Colloids Surf. B Biointerfaces 2021, 207, 112020. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.G.; Chinni, S. Bioavailability Enhancement of Vitamin E TPGS Liposomes of Nintedanib Esylate: Formulation Optimization, Cytotoxicity and Pharmacokinetic Studies. Drug Deliv. Transl. Res. 2022, 12, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Kallus, S.; Englinger, B.; Senkiv, J.; Laemmerer, A.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of Anticancer FGFR Inhibitors with Improved Therapeutic Index. Nanomedicine 2018, 14, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, M. Enhanced Oral Bioavailability of Nintedanib Esylate with Nanostructured Lipid Carriers by Lymphatic Targeting: In Vitro, Cell Line and in Vivo Evaluation. Eur. J. Pharm. Sci. 2021, 159, 105715. [Google Scholar] [CrossRef] [PubMed]
- Dhavale, R.P.; Dhavale, R.P.; Bhatia, M.S.; Jadhav, S.U.; Dhanavade, M.J.; Barale, S.S.; Pathak, S.; Parale, V.G.; Sonawane, K.D. Exploring Anticancer Potential of Nintedanib Conjugated Magnetic Nanoparticles: In-Vitro and in-Silico Studies. J. Drug Deliv. Sci. Technol. 2023, 81, 104213. [Google Scholar] [CrossRef]
- Karade, V.C.; Sharma, A.; Dhavale, R.P.; Dhavale, R.P.; Shingte, S.R.; Patil, P.S.; Kim, J.H.; Zahn, D.R.T.; Chougale, A.D.; Salvan, G.; et al. APTES Monolayer Coverage on Self-Assembled Magnetic Nanospheres for Controlled Release of Anticancer Drug Nintedanib. Sci. Rep. 2021, 11, 5674. [Google Scholar] [CrossRef]
- Shukla, K.S.; Nguyen, V.; Goyal, M.; Gupta, V. Cationically Modified Inhalable Nintedanib Niosomes: Enhancing Therapeutic Activity against Non-Small-Cell Lung Cancer. Nanomedicine 2022, 17, 935–958. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 632–644. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Skupin-Mrugalska, P.; Minko, T. Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells. Pharmaceutics 2020, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Patil, S.M.; Shukla, S.K.; Kulkarni, N.S.; Gupta, V.; Kunda, N.K. Pulmonary Delivery of Osimertinib Liposomes for Non-Small Cell Lung Cancer Treatment: Formulation Development and in Vitro Evaluation. Drug Deliv. Transl. Res. 2021, 12, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Bin-Jumah, M.N.; Imam, S.S.; Zafar, A.; Yasir, M.; Alshehri, S.; Ghuneim, M.M. Formulation of Osimertinib Nano Lipid Carriers: Optimization, Characterization and Cytotoxicity Assessment. J. Clust. Sci. 2022, 34, 1051–1063. [Google Scholar] [CrossRef]
- Chen, W.; Yu, D.; Sun, S.Y.; Li, F. Nanoparticles for Co-Delivery of Osimertinib and Selumetinib to Overcome Osimertinib-Acquired Resistance in Non-Small Cell Lung Cancer. Acta Biomater. 2021, 129, 258–268. [Google Scholar] [CrossRef]
- Wang, X.; Mao, W.; Wang, Z.; Li, X.; Xiong, Y.; Lu, H.; Wang, X.; Yin, H.; Cao, X.; Xin, H. Enhanced Anti-Brain Metastasis from Non-Small Cell Lung Cancer of Osimertinib and Doxorubicin Co-Delivery Targeted Nanocarrier. Int. J. Nanomed. 2020, 15, 5491–5501. [Google Scholar] [CrossRef]
- Chen, R.; Zhai, R.; Wang, C.; Liang, S.; Wang, J.; Liu, Z.; Li, W. Compound Capecitabine Colon-Targeted Microparticle Prepared by Coaxial Electrospray for Treatment of Colon Tumors. Molecules 2022, 27, 590. [Google Scholar] [CrossRef]
- Hu, X.; Chen, S.; Yin, H.; Wang, Q.; Duan, Y.; Jiang, L.; Zhao, L. Chitooligosaccharides-Modified PLGA Nanoparticles Enhance the Antitumor Efficacy of AZD9291 (Osimertinib) by Promoting Apoptosis. Int. J. Biol. Macromol. 2020, 162, 262–272. [Google Scholar] [CrossRef]
- Kumar, S.K.; Choppala, A.D. Development and Optimization of Osimertinib-Loaded Biodegradable Polymeric Nanoparticles Enhance In-Vitro Cytotoxicity in Mutant EGFR NSCLC Cell Models and In-Vivo Tumor Reduction in H1975 Xenograft Mice Models. AAPS PharmSciTech 2022, 23, 159. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Sun, J.; Zou, H.; Sun, Y.; Luo, J.; Xie, Q.; Rong, A.; Wang, H.; Li, X.; et al. An Osimertinib-Perfluorocarbon Nanoemulsion with Excellent Targeted Therapeutic Efficacy in Non-Small Cell Lung Cancer: Achieving Intratracheal and Intravenous Administration. ACS Nano 2022, 16, 12590–12605. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ettari, R.; Giofrè, S.; Eid, A.H.; Bitto, A. Recent Advances in Nanotherapeutics for Multiple Myeloma. Cancers 2020, 12, 3144. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.; Lu, Y.J.; Hung, J.T.; Yu, A.L.; Chen, J.P. Co-Delivery of CPT-11 and Panobinostat with Anti-GD2 Antibody Conjugated Immunoliposomes for Targeted Combination Chemotherapy. Cancers 2020, 12, 3211. [Google Scholar] [CrossRef]
- He, Y.; Fang, Y.; Zhang, M.; Zhao, Y.; Tu, B.; Shi, M.; Muhitdinov, B.; Asrorov, A.; Xu, Q.; Huang, Y. Remodeling “Cold” Tumor Immune Microenvironment via Epigenetic-Based Therapy Using Targeted Liposomes with in Situ Formed Albumin Corona. Acta Pharm. Sin. B 2022, 12, 2057–2073. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, S.I.; Dias, J.N.R.; André, A.S.; Silva, M.L.; Martins, D.; Carrapiço, B.; Castanho, M.; Carriço, J.; Cavaco, M.; Gaspar, M.M.; et al. Highly Specific Blood-Brain Barrier Transmigrating Single-Domain Antibodies Selected by an in Vivo Phage Display Screening. Pharmaceutics 2021, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-SiRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. 2022, 9, 2200353. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Rubio-Ruiz, B.; Pérez-López, A.M.; Sebastián, V.; Martín-Duque, P.; Arruebo, M.; Santamaría, J.; Unciti-Broceta, A. Cancer-Derived Exosomes Loaded with Ultrathin Palladium Nanosheets for Targeted Bioorthogonal Catalysis. Nat. Catal. 2019, 2, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.G.; Collins, A.M.; Bienemann, A.S.; Killick-Cole, C.L.; Haynes, H.R.; Asby, D.J.; Butts, C.P.; Wyatt, M.J.; Barua, N.U.; Gill, S.S. Convection Enhanced Delivery of Panobinostat (LBH589)-Loaded Pluronic Nano-Micelles Prolongs Survival in the F98 Rat Glioma Model. Int. J. Nanomed. 2017, 12, 1385–1399. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Fowler, M.J.; Baker, C.; Stopka, S.A.; Regan, M.S.; Sablatura, L.; Broughton, C.W.; Knight, B.E.; Stabenfeldt, S.E.; Agar, N.Y.R.; et al. β-Cyclodextrin-Poly (β-Amino Ester) Nanoparticles Are a Generalizable Strategy for High Loading and Sustained Release of HDAC Inhibitors. ACS Appl. Mater. Interfaces 2021, 13, 20960–20973. [Google Scholar] [CrossRef]
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; de Marinis, F.; et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non–Small Cell Lung Cancer in the Global Phase III ALEX Study. J. Thorac. Oncol. 2019, 14, 1233–1243. [Google Scholar] [CrossRef]
- Wang, M.; Gong, Y.; Cheng, Y.; Yang, L.; Wang, W.; Lei, X. Synchronal Pulmonary Sarcomatoid Carcinoma and Lung Adenocarcinoma EML4-ALK Fusion: A Case Report. Oncol. Lett. 2022, 24, 343. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, S.A.; Min, K.A.; Jee, J.P.; Jin, S.G.; Cho, K.H. Development of Alectinib-Suspended SNEDDS for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, M.; Shahab, S.; Alnajjar, R.; Ahmadianarog, M. Adsorption Properties of the New Anti-Cancer Drug Alectinib on CNT(6,6-6) Nanotube: Geometry Optimization, Molecular Structure, Spectroscopic (NMR, UV/Vis, Excited State), FMO, MEP and HOMO–LUMO Investigations. J. Clust. Sci. 2019, 30, 83–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Feng, N.; Xin, X.; Xu, Y.; Huo, P.; Wang, X.; Zhang, N. Construction and Antitumor Effects of Antitumor Micelles with Cyclic RGD-Modified Anlotinib. Nanomedicine 2020, 28, 102224. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tang, S.; Chen, H.; Chen, H.; Li, X.J.; Jiang, Y.Q.; Fu, S.Z.; Lin, S. Intratumoral Injection of Anlotinib Hydrogel Enhances Antitumor Effects and Reduces Toxicity in Mouse Model of Lung Cancer. Drug Deliv. 2020, 27, 1524–1534. [Google Scholar] [CrossRef] [PubMed]

| Compound | Number of Completed 1 Clinical Trials | Approval Date | Competent Authority | Indication/Disease |
|---|---|---|---|---|
| Sunitinib | 309 | 2006 | FDA | Gastrointestinal stromal tumors and advanced renal cell carcinoma (RCC) |
| 2011 | Rare type of pancreatic cancer | |||
| 2017 | Adjuvant treatment of adult patients at high risk of recurrent RCC | |||
| Nintedanib | 87 | 2014 | FDA | Interstitial lung disease (ILD) associated with systemic sclerosis or scleroderma (SSc-ILD) |
| 2019 | Idiopathic pulmonary fibrosis (IPF) | |||
| 2020 | First treatment for chronic fibrosing ILDs with a progressive phenotype | |||
| Osimertinib | 35 | 2015 | FDA | EGFR T790M mutation-positive non-small-cell lung cancer (NSCLC) |
| 2017 | EGFR T790M mutation-positive NSCLC, full approval | |||
| 2018 | First-line treatment for EGFR-mutated NSCLC | |||
| 2020 | Adjuvant treatment of patients with early stage EGFR-mutated NSCLC | |||
| Panobinostat | 75 | 2015 | FDA | Multiple myeloma |
| Alectinib | 10 | 2015 | FDA | ALK-positive NSCLC |
| 2017 | First-line treatment for ALK-positive metastatic NSCLC | |||
| Anlotinib | 29 | 2018 | NMPA | Locally advanced or metastatic n NSCLC patients who have undergone progression or recurrence after ≥2 lines of systemic chemotherapy |
| Nano Delivery System | Authors | Year | Ref |
|---|---|---|---|
| Liposome nanoparticles | Passadouro, M. et al. | 2014 | [65] |
| Hu, J. et al. | 2016 | [66] | |
| Yang, X. et al. | 2018 | [67] | |
| Jiao, Y. et al. | 2022 | [68] | |
| Charkhat Gorgich, E.A et al. | 2022 | [69] | |
| Lai, X. et al. | 2022 | [70] | |
| Chitosan nanoparticles | Joseph, J.J. et al. | 2016 | [72] |
| Saber, M.M. et al. | 2017 | [73] | |
| Jafari, H. et al. | 2021 | [74] | |
| Alinavaz, S. et al. | 2022 | [75] | |
| Karimi, M.H. et al. | 2022 | [76] | |
| Magnetic nanoparticles | Chen, S. et al. | 2017 | [78] |
| Zhang, Z. et al. | 2021 | [79] | |
| Torabi, M. et al. | 2023 | [80] | |
| Solid lipid nanoparticles | Taymouri, S. et al. | 2019 | [81] |
| Khaledian, S. et al. | 2021 | [82] | |
| Ahmed, M.M. et al. | 2022 | [83] | |
| Micellar nanocomplex | Yongvongsoontorn, N. et al. | 2019 | [85] |
| Zeng, X. et al. | 2022 | [86] | |
| He, J. et al. | 2019 | [87] | |
| Qin, T. et al. | 2020 | [88] | |
| Shih, Y.H. et al. | 2022 | [89] | |
| Braatz, D. et al. | 2021 | [90] |
| Drug | Nano Delivery System | Authors | Year | Ref |
|---|---|---|---|---|
| Nintedanib (NINTE) | Albumin nanoparticles | Xu, Y et al. | 2021 | [99] |
| Zha, Q. et al. | 2022 | [100] | ||
| Xu, Y. et al. | 2021 | [101] | ||
| Liposome nanoparticles | Kala, S.G et al. | 2022 | [102] | |
| Kallus, S. et al. | 2018 | [103] | ||
| Nanostructured lipid carriers (NLCs) | Patel, P. et al. | 2021 | [104] | |
| Magnetic nanoparticles | Dhavale, R.P. et al. | 2023 | [105] | |
| Karade, V.C. et al. | 2021 | [106] | ||
| Niosomes | K Shukla, S et al. | 2022 | [107] | |
| Osimertinib (OSI) | Liposome nanoparticles | Skupin-Mrugalska, P. et al. | 2020 | [111] |
| Sawant, S.S. et al. | 2021 | [112] | ||
| Nanostructured lipid carriers (NLCs) | Gilani, S.J. et al. | 2022 | [113] | |
| Conjugates | Chen, W. et al. | 2021 | [114] | |
| Wang, X. et al. | 2020 | [115] | ||
| Chen, R. et al. | 2022 | [116] | ||
| Polymeric nanoparticles | Hu, X. et al. | 2020 | [117] | |
| Kumar, S.K. et al. | 2022 | [118] | ||
| Yang, J. et al. | 2022 | [119] | ||
| Panobinostat (PANO) | Liposome nanoparticles | Jose, G. et al. | 2020 | [121] |
| He, Y. et al. | 2022 | [122] | ||
| Aguiar, S.I. et al. | 2021 | [123] | ||
| Exosomes | Shan, S. et al. | 2022 | [124] | |
| Sancho-Albero, M. et al. | 2019 | [125] | ||
| Nanomicelles | Singleton, W.G. et al. | 2017 | [126] | |
| b-cyclodextrin networks | Chaudhuri, S. et al. | 2021 | [127] | |
| Alectinib (ALE) | Self-nanoemulsifyng drug delivery system (SNEDDS) | Park, E.J. et al. | 2022 | [130] |
| Carbon nanotubes (CNTs) | Sheikhi, M. et al. | 2019 | [131] | |
| Anlotinib (ANLO) | Nanomicelles | Zhang, Y. et al. | 2020 | [132] |
| Hydrogel | Gao, Q. et al. | 2020 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Grondona, C.; Brullo, C.; Spallarossa, A.; Villa, C.; Tasso, B. Indole Antitumor Agents in Nanotechnology Formulations: An Overview. Pharmaceutics 2023, 15, 1815. https://doi.org/10.3390/pharmaceutics15071815
Russo E, Grondona C, Brullo C, Spallarossa A, Villa C, Tasso B. Indole Antitumor Agents in Nanotechnology Formulations: An Overview. Pharmaceutics. 2023; 15(7):1815. https://doi.org/10.3390/pharmaceutics15071815
Chicago/Turabian StyleRusso, Eleonora, Carola Grondona, Chiara Brullo, Andrea Spallarossa, Carla Villa, and Bruno Tasso. 2023. "Indole Antitumor Agents in Nanotechnology Formulations: An Overview" Pharmaceutics 15, no. 7: 1815. https://doi.org/10.3390/pharmaceutics15071815
APA StyleRusso, E., Grondona, C., Brullo, C., Spallarossa, A., Villa, C., & Tasso, B. (2023). Indole Antitumor Agents in Nanotechnology Formulations: An Overview. Pharmaceutics, 15(7), 1815. https://doi.org/10.3390/pharmaceutics15071815










