Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin
Abstract
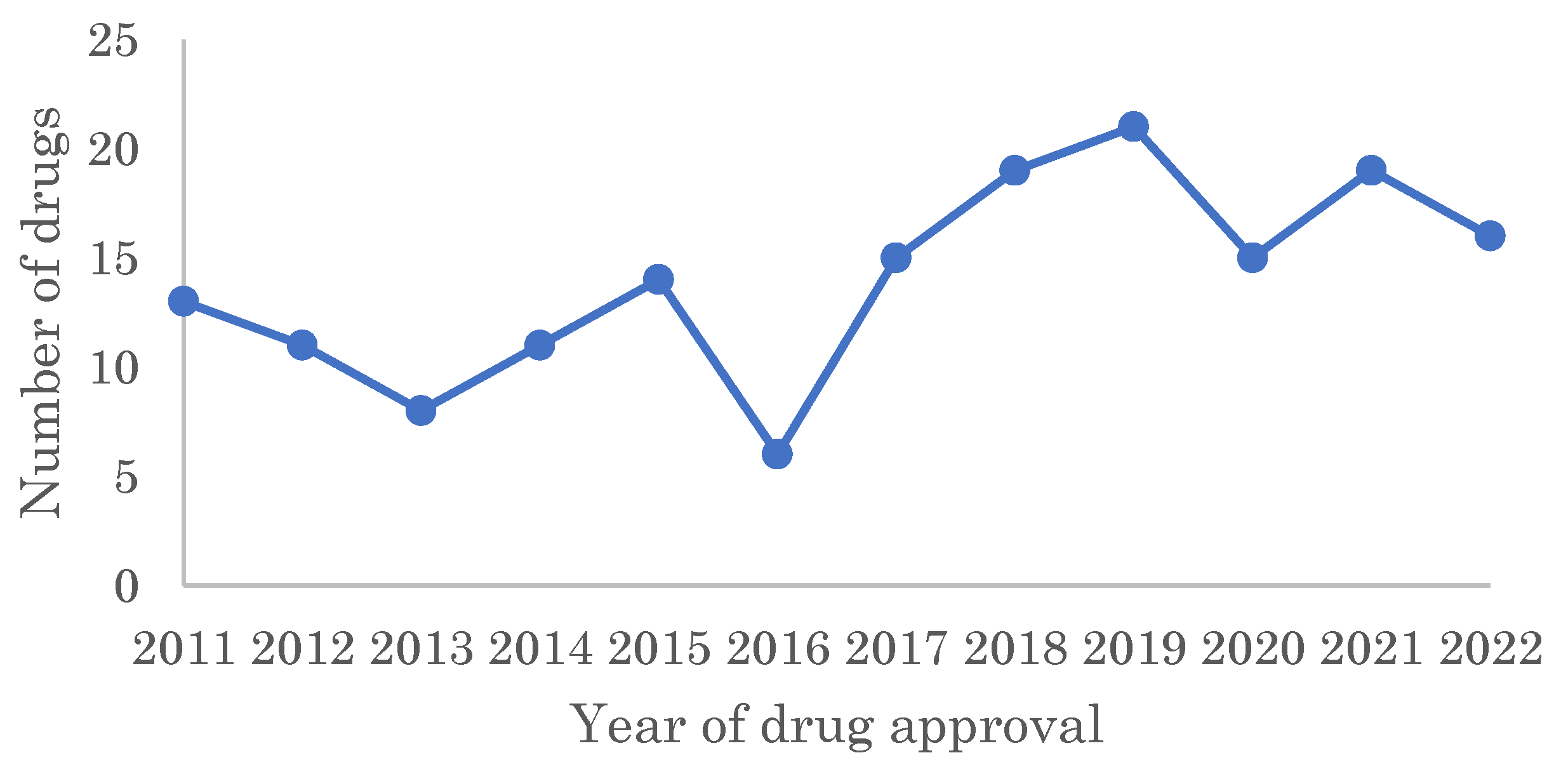
1. Introduction
2. Results
2.1. Target Families of FIC Drugs
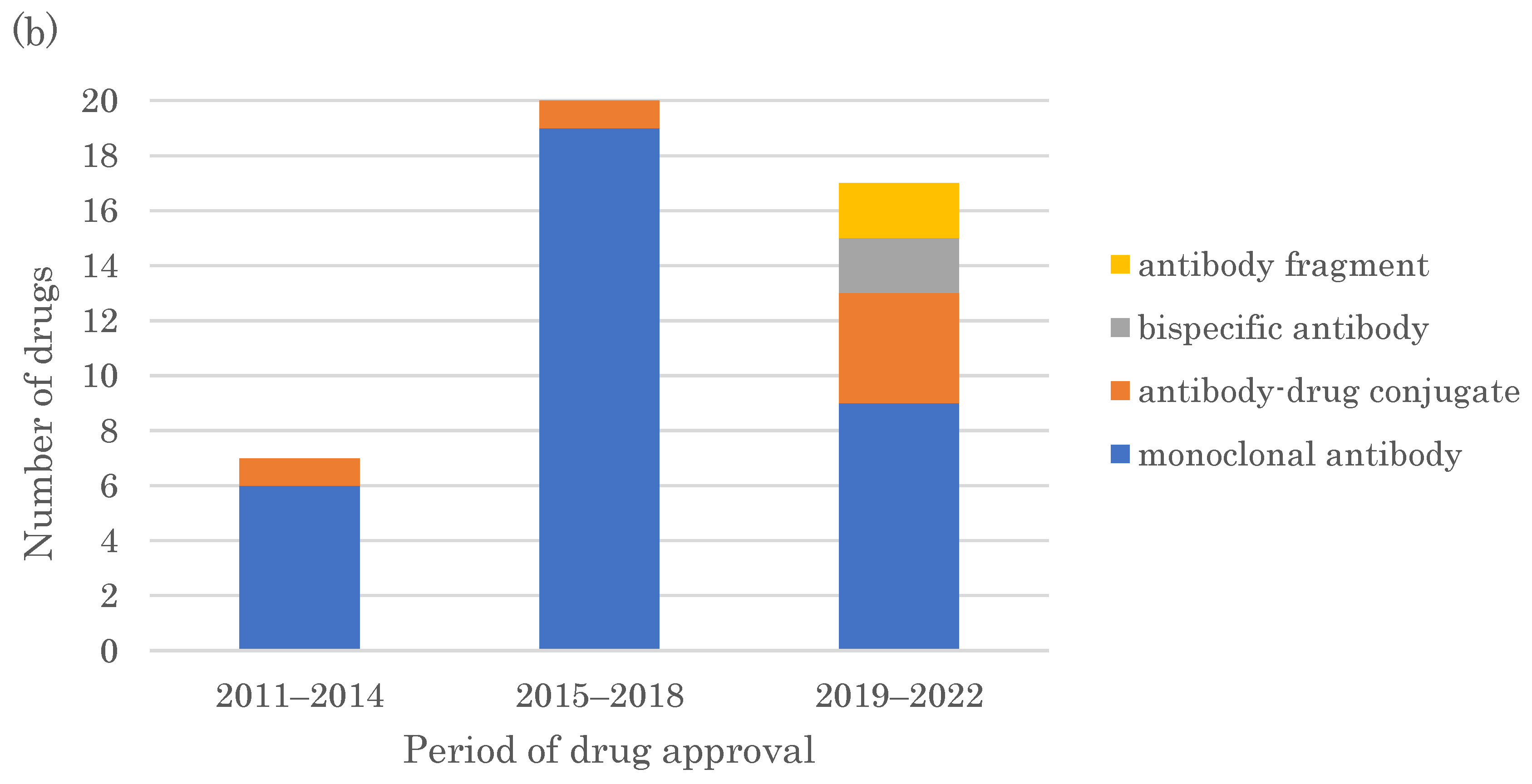
2.2. Modalities of FIC Drugs
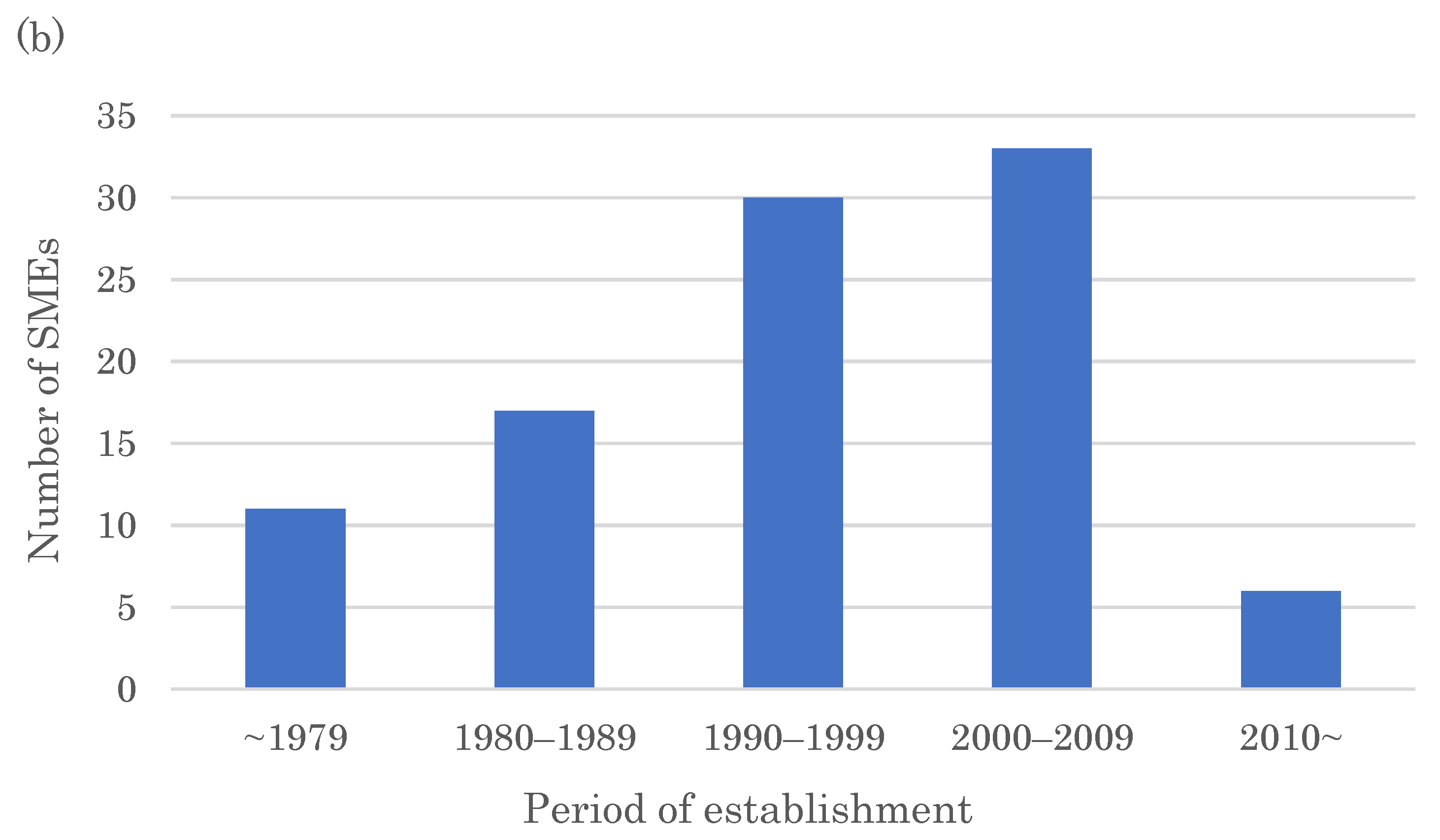
2.3. Originators of FIC Drugs
2.4. SMEs Creating More Than One FIC Drug in 2011–2022 and Their Drugs’ Modalities
3. Discussion
4. Materials and Methods
4.1. Definition of FIC in This Study
4.2. Identification Method of FIC Drugs, Their Target Family, Modality, and Origin
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanthier, M.L.; Kerr, K.W.; Miller, K.L. An Analysis of Follow-On Development in New Drug Classes, January 1986–June 2018. Clin. Pharmacol. Ther. 2019, 106, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Eichler, H.G.; Bloechl-Daum, B.; Abadie, E.; Barnett, D.; Konig, F.; Pearson, S. Relative efficacy of drugs: An emerging issue between regulatory agencies and third-party payers. Nat. Rev. Drug Discov. 2010, 9, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T. From blockbuster medicine to personalized medicine. Future Med. 2008, 5, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Qattan, M.; Demonacos, C.; Krstic-Demonacos, M. Roadmap to personalized medicine. Croat. Med. J. 2012, 53, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, Q.; Zhang, Q.; You, Q.; Wang, L. A decade of approved first-in-class small molecule orphan drugs: Achievements, challenges and perspectives. Eur. J. Med. Chem. 2022, 243, 114742. [Google Scholar] [CrossRef] [PubMed]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef]
- Phillips, M.I.; Lee, J.R. Recent orphan drugs that are first-in-class. Expert Opin. Orphan Drugs 2014, 2, 759–763. [Google Scholar] [CrossRef]
- Tambuyzer, E.; Vandendriessche, B.; Austin, C.P.; Brooks, P.J.; Larsson, K.; Needleman, K.I.M.; Valentine, J.; Davies, K.; Groft, S.C.; Preti, R.; et al. Therapies for rare diseases: Therapeutic modalities, progress and challenges ahead. Nat. Rev. Drug Discov. 2020, 19, 93–111. [Google Scholar] [CrossRef]
- Eder, J.; Sedrani, R.; Wiesmann, C. The discovery of first-in-class drugs: Origins and evolution. Nat. Rev. Drug Discov. 2014, 13, 577–587. [Google Scholar] [CrossRef]
- Agarwal, P.; Sanseau, P.; Cardon, L.R. Novelty in the target landscape of the pharmaceutical industry. Nat. Rev. Drug Discov. 2013, 12, 575–576. [Google Scholar] [CrossRef]
- Lazo, J.S.; McQueeney, K.E.; Sharlow, E.R. New Approaches to Difficult Drug Targets: The Phosphatase Story. SLAS Discov. 2017, 22, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Fu, H.; Chu, X.; Wen, R.; Zhang, M.; You, T.; Fu, P.; Qin, J.; Cui, T. Research advances in treatment methods and drug development for rare diseases. Front. Pharmacol. 2022, 13, 971541. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ito, Y. Recent chemical approaches for site-specific conjugation of native antibodies: Technologies toward next-generation antibody-drug conjugates. Chembiochem 2019, 20, 2729–2737. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Develop. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef]
- Ikeda, K.; Maezawa, Y.; Yonezawa, T.; Shimizu, Y.; Tashiro, T.; Kanai, S.; Sugaya, N.; Masuda, Y.; Inoue, N.; Niimi, T.; et al. DLiP-PPI library: An integrated chemical database of small-to-medium-sized molecules targeting protein-protein interactions. Front. Chem. 2023, 10, 1090643. [Google Scholar] [CrossRef]
- Moon, S.; Lee, B.H. Chemically Induced Cellular Proteolysis: An Emerging Therapeutic Strategy for Undruggable Targets. Mol. Cells 2018, 41, 933–942. [Google Scholar] [CrossRef]
- Kneller, R. The importance of new companies for drug discovery: Origins of a decade of new drugs. Nat. Rev. Drug Discov. 2010, 9, 867–882. [Google Scholar] [CrossRef]
- Kinch, M.S.; Hoyer, D.; Patridge, E.; Plummer, M. Target selection for FDA-approved medicines. Drug Discov. Today 2015, 20, 784–789. [Google Scholar] [CrossRef]
- Okuyama, R. Nurturing Deep Tech to Solve Social Problems: Learning from COVID-19 mRNA Vaccine Development. Pathogens 2022, 11, 1469. [Google Scholar] [CrossRef]
- Okuyama, R. Strengthening the Competitiveness of Japan’s Pharmaceutical Industry: Analysis of Country Differences I the Origin of New Drugs and Japan’s Highly Productive Firm. Biol. Pharm. Bull. 2023, 46, 718–724. [Google Scholar] [CrossRef]
- Kayki-Mutlu, G.; Aksoyalp, Z.S.; Wojnowski, L.; Michel, M.C. A year in pharmacology: New drugs approved by the US Food and Drug Administration in 2021. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 867–885. [Google Scholar] [CrossRef]
- Graul, A.I.; Sorbera, L.A. The year’s new drugs and biologics 2020. Drugs Today 2021, 57, 101–177. [Google Scholar] [CrossRef] [PubMed]
- Cristina Mendonça Nogueira, T.; Vinicius Nora de Souza, M. New FDA oncology small molecule drugs approvals in 2020: Mechanism of action and clinical applications. Bioorg. Med. Chem. 2021, 46, 116340. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Morris, J. Antibody-drug Conjugate Targets, Drugs, and Linkers. Curr. Cancer Drug Targets 2022, 22, 463–529. [Google Scholar] [CrossRef]
- Talap, J.; Zhao, J.; Shen, M.; Song, Z.; Zhou, H.; Kang, Y.; Sun, L.; Yu, L.; Zeng, S.; Cai, S. Recent advances in therapeutic nucleic acids and their analytical methods. J. Pharm. Biomed. Anal. 2021, 206, 114368. [Google Scholar] [CrossRef]
- Kennedy, K.H.; Gomez, K.; Thovmasian, N.J.; Chang, D.C. Small biotechs versus large pharma: Who drives first-in-class innovation in oncology? Drug Discov. Today 2023, 28, 103456. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Green, A.R. Me-too pharmaceutical products: History, definitions, examples, and relevance to drug shortages and essential medicines lists. Br. J. Clin. Pharmacol. 2020, 86, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
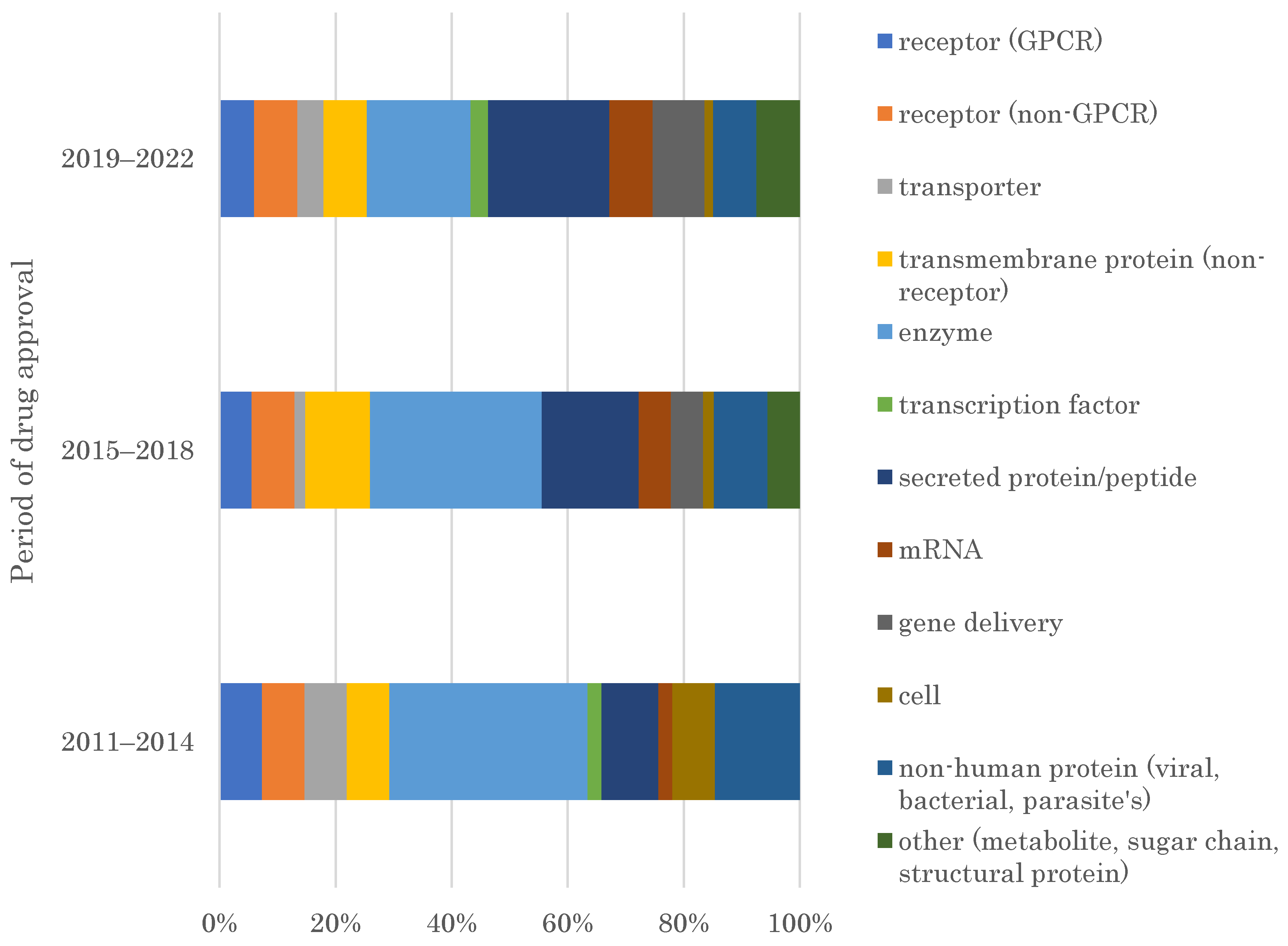
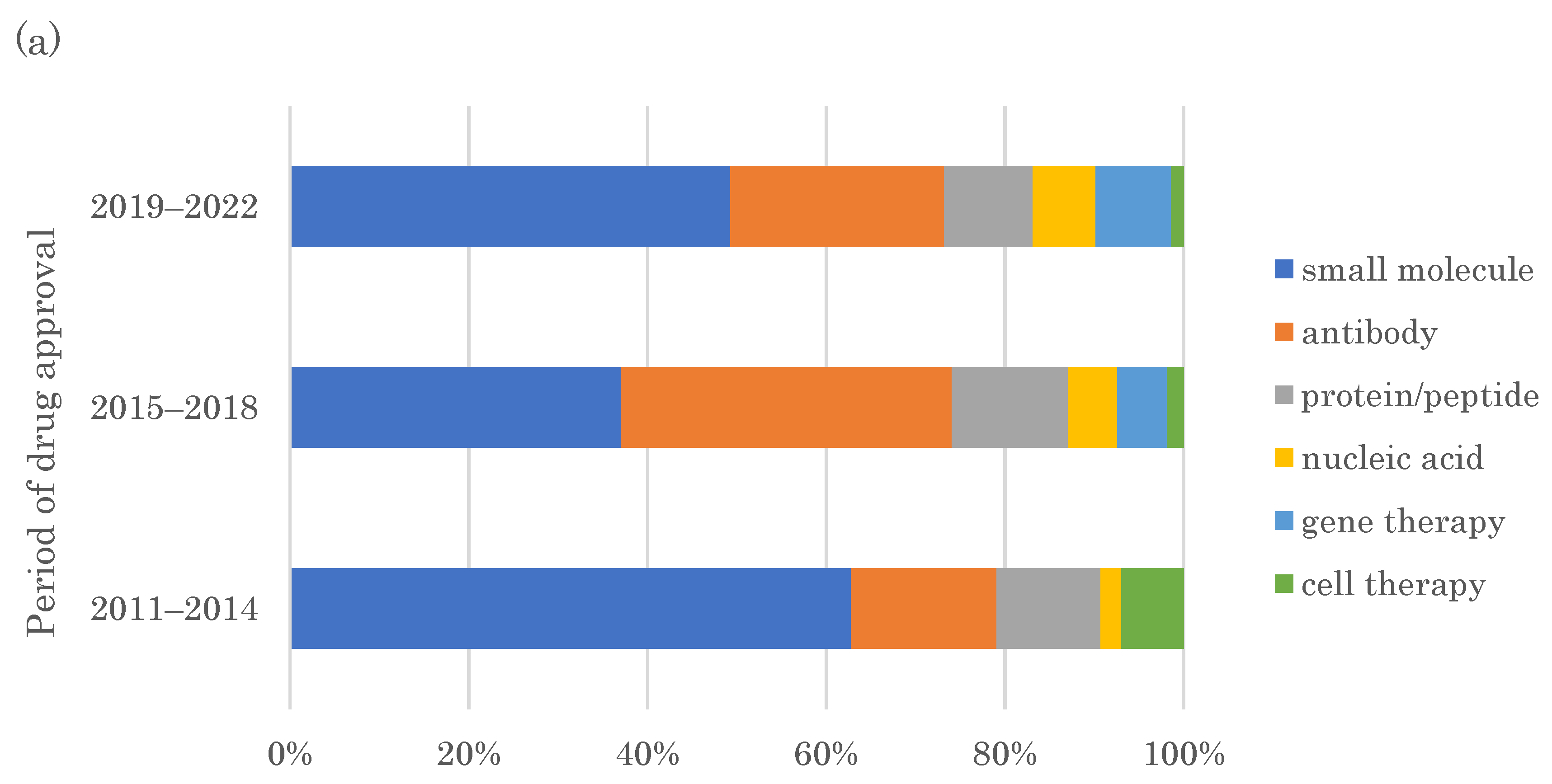
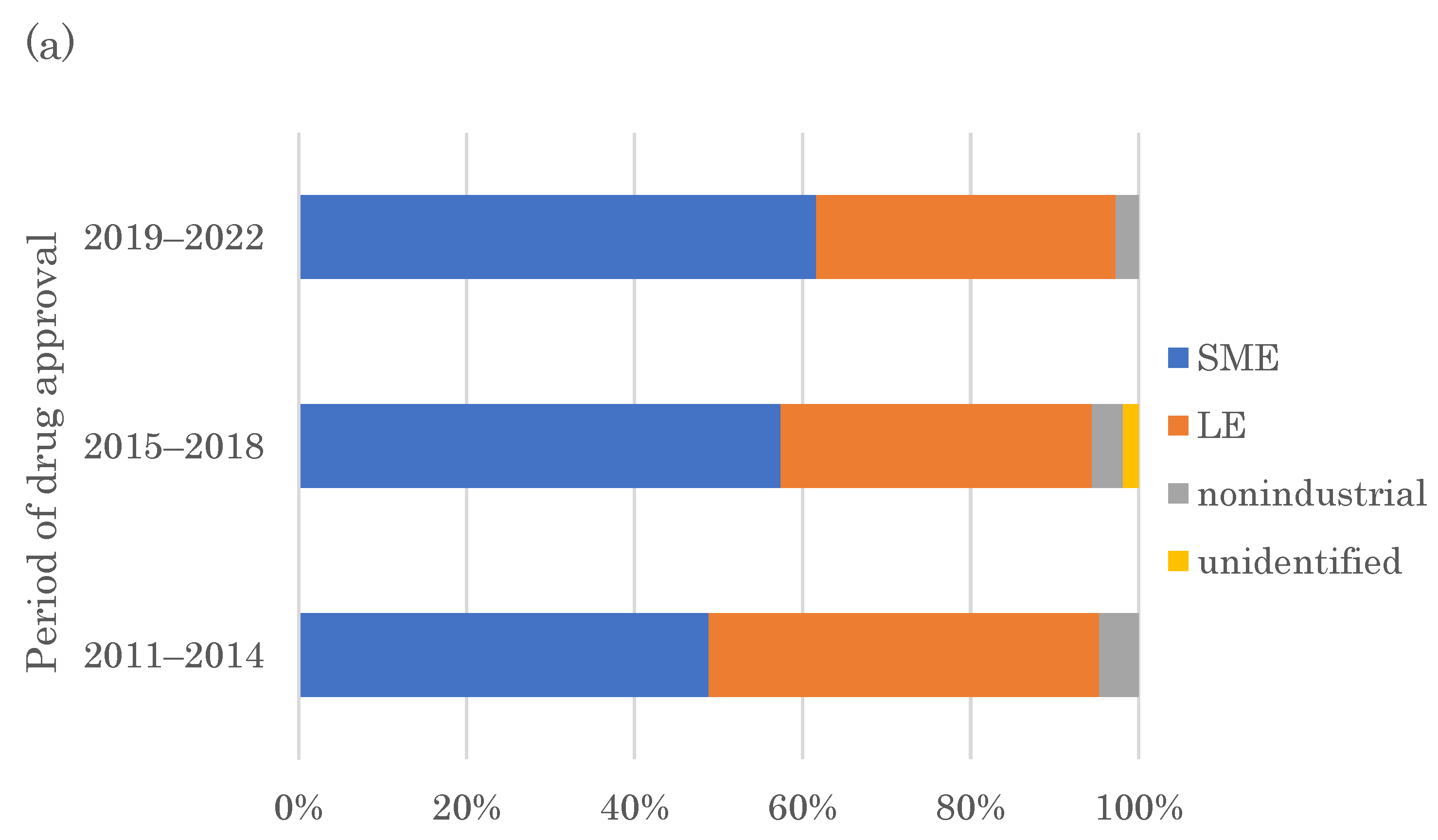
| Company | Drug Name | Modality |
|---|---|---|
| Agios Pharmaceuticals | mitapivat | small molecule |
| ivosidenib | small molecule | |
| enasidenib | small molecule | |
| Alexion Pharmaceuticals | sebelipase alfa | recombinant protein |
| asfotase alfa | recombinant protein | |
| Alnylam Pharmaceuticals | lumasiran | siRNA |
| givosiran | siRNA | |
| patisiran | siRNA | |
| BioMarin Pharmaceutical | vosoritide | peptide |
| cerliponase alfa | recombinant protein | |
| elosulfase alfa | recombinant protein | |
| Bluebird Bio, Inc. | elivaldogene autotemcel | gene therapy |
| betibeglogene autotemcel | gene therapy | |
| idecabtagene vicleucel | gene therapy | |
| Genmab | tisotumab vedotin | antibody–drug conjugate |
| teprotumumab | monoclonal antibody | |
| daratumumab | monoclonal antibody | |
| Ionis Pharmaceuticals | nusinersen | antisense oligonucleotide |
| mipomersen | antisense oligonucleotide | |
| PDL BioPharma | elotuzumab | monoclonal antibody |
| mepolizumab | monoclonal antibody | |
| Sarepta Therapeutics | casimersen | antisense oligonucleotide |
| golodirsen | antisense oligonucleotide | |
| eteplirsen | antisense oligonucleotide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, R. Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin. Pharmaceutics 2023, 15, 1794. https://doi.org/10.3390/pharmaceutics15071794
Okuyama R. Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin. Pharmaceutics. 2023; 15(7):1794. https://doi.org/10.3390/pharmaceutics15071794
Chicago/Turabian StyleOkuyama, Ryo. 2023. "Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin" Pharmaceutics 15, no. 7: 1794. https://doi.org/10.3390/pharmaceutics15071794
APA StyleOkuyama, R. (2023). Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin. Pharmaceutics, 15(7), 1794. https://doi.org/10.3390/pharmaceutics15071794







