Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches
Abstract
1. Introduction
2. Conventional Treatments for Prostate Cancer and Complications
2.1. Surgery
2.2. Radiation Therapy
2.3. Hormone Therapy
2.4. Chemotherapy
2.5. Cellular Immunotherapy
2.6. Other Therapeutic Approaches
3. PDT
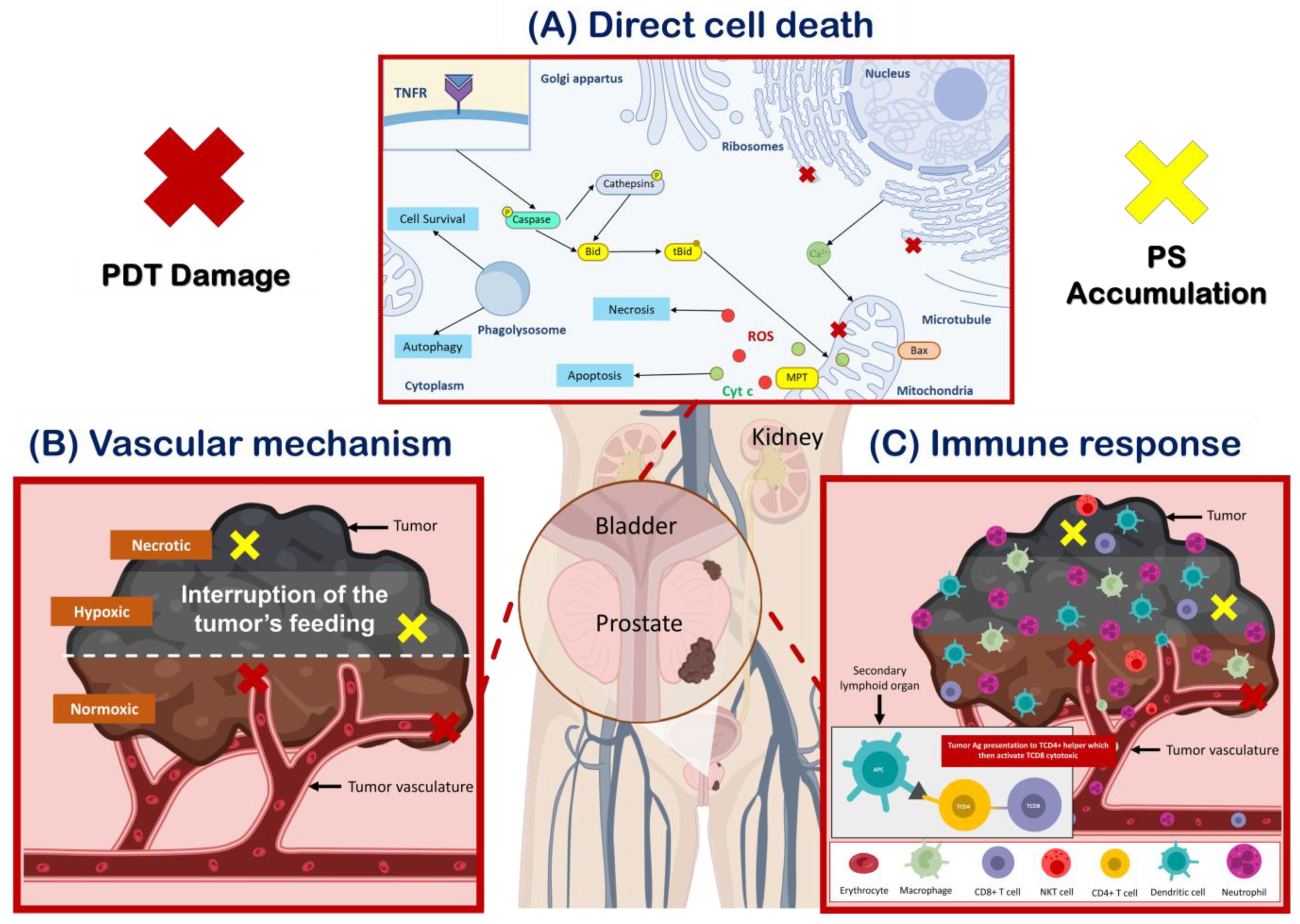
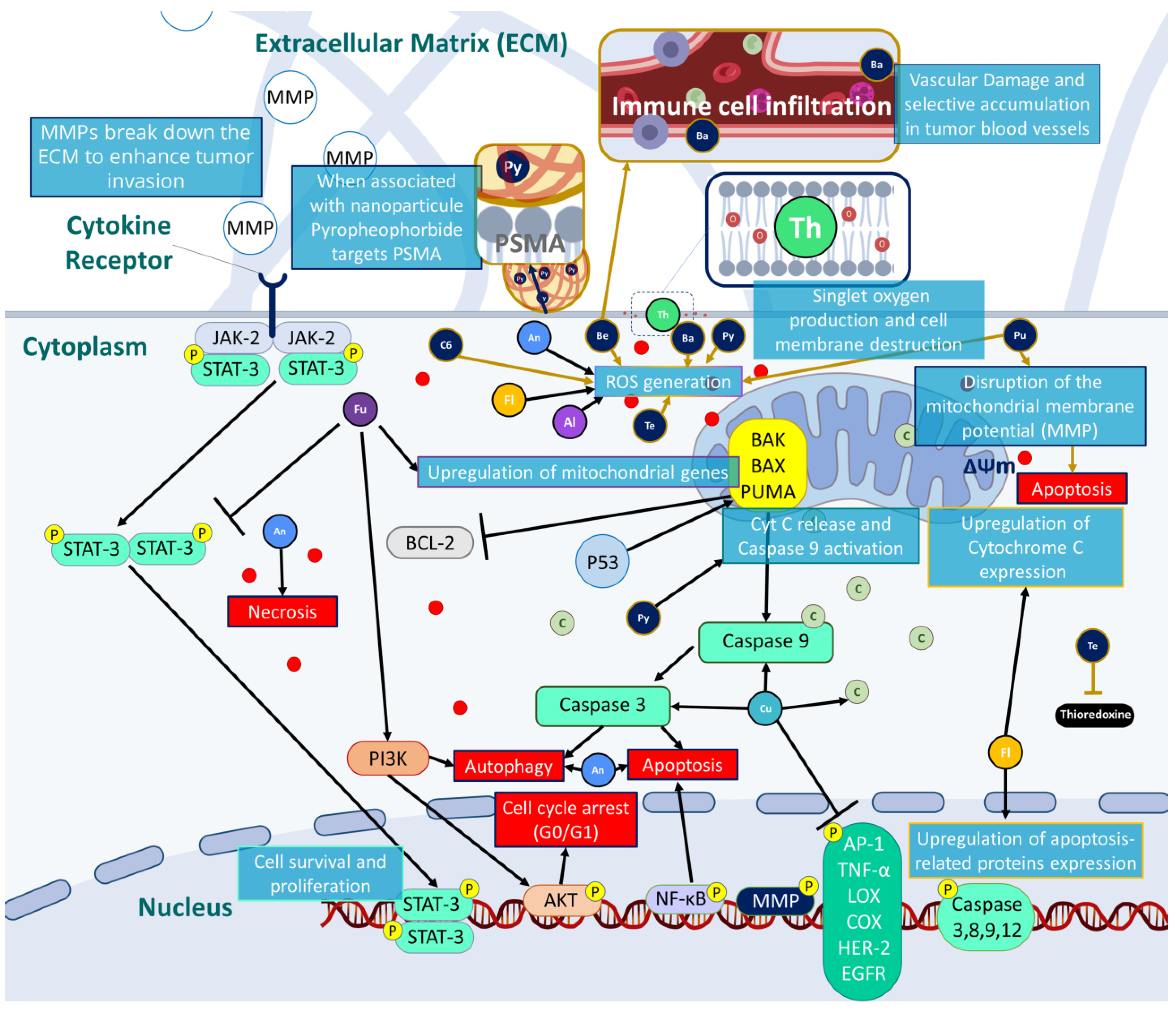
3.1. Mechanism of Action
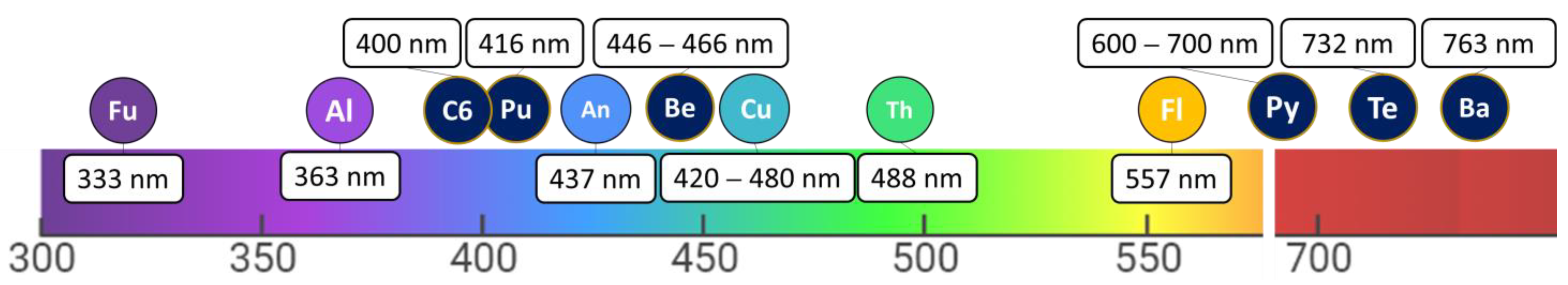
3.2. Photosensitizers (PSs)
3.3. Natural and Synthetic PS
3.4. Ongoing Clinical Studies in Prostate Cancer
3.5. Multimodal Synergistic Therapies to Overcome PDT Limitations
3.5.1. PDT/Surgery
3.5.2. PDT/Sonodynamic Therapy
3.5.3. PDT/Photoimmunotherapy
3.5.4. PDT/Chemotherapy
3.5.5. PDT/Photothermal Therapy

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Windahl, T.; Andersson, S.O.; Lofgren, L. Photodynamic therapy of localised prostatic cancer. Lancet 1990, 336, 1139. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef]
- PDQ® Adult Treatment Editorial Board. Prostate Cancer Treatment: Health Professional Version. Updated: 13 February 2023. Available online: https://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq (accessed on 14 May 2023).
- Mohan, R.; Schellhammer, P.F. Treatment options for localized prostate cancer. Am. Fam. Physician 2011, 84, 413–420. [Google Scholar]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Wallis, C.J.; Herschorn, S.; Saskin, R.; Su, J.; Klotz, L.H.; Chang, M.; Kulkarni, G.S.; Lee, Y.; Kodama, R.T.; Narod, S.A.; et al. Complications after radical prostatectomy or radiotherapy for prostate cancer: Results of a population-based, propensity score-matched analysis. Urology 2015, 85, 621–627. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Cotter, S.E.; Gargollo, P.C.; Zietman, A.L.; Dahl, D.M.; Smith, M.R. Management of Complications of Prostate Cancer Treatment. CA Cancer J. Clin. 2008, 58, 196–213. [Google Scholar] [CrossRef]
- Garcia, J.A. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: An insight for oncologists. Ther. Adv. Med. Oncol. 2011, 3, 101–108. [Google Scholar] [CrossRef]
- Fitzgerald, F. Photodynamic Therapy (PDT); Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2017. [Google Scholar]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; de Sousa Junior, W.T.; Mundim, T.; Vale, C.L.C.; de Oliveira, J.V.; Ganassin, R.; Pacheco, T.J.A.; Vasconcelos Morais, J.A.; Longo, J.P.F.; Azevedo, R.B.; et al. Induction of Immunogenic Cell Death by Photodynamic Therapy Mediated by Aluminum-Phthalocyanine in Nanoemulsion. Pharmaceutics 2022, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.G.B. Development of a Novel Photosensitizer for Photodynamic Therapy of Cancer. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2016. [Google Scholar]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Tomasz, K.; Beata, C.-G.; Katarzyna, K.; Magdalena, S.; Daria, W.; Sebastian, L.; Tomasz, K.; Tomasz, G. Nurses and Pharmacists in Interdisciplinary Team of Health Care Providers in Photodynamic Therapy. In Photomedicine; Yohei, T., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 7. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Nayak, C.S. Photodynamic therapy in dermatology. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Zou, M.Z.; Zhang, M.; Wang, X.S.; Zeng, X.; Cong, H.; Zhang, X.Z. π-Extended Benzoporphyrin-Based Metal—Organic Framework for Inhibition of Tumor Metastasis. ACS Nano 2018, 12, 4630–4640. [Google Scholar] [CrossRef]
- Dai, L.; Shen, G.; Wang, Y.; Yang, P.; Wang, H.; Liu, Z. PSMA-targeted melanin-like nanoparticles as a multifunctional nanoplatform for prostate cancer theranostics. J. Mater. Chem. B 2021, 9, 1151–1161. [Google Scholar] [CrossRef]
- Stallivieri, A.; Colombeau, L.; Jetpisbayeva, G.; Moussaron, A.; Myrzakhmetov, B.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorganic Med. Chem. 2017, 25, 1–10. [Google Scholar] [CrossRef]
- Stallivieri, A.; Baros, F.; Jetpisbayeva, G.; Myrzakhmetov, B.; Frochot, C. The Interest of Folic Acid in Targeted Photodynamic Therapy. Curr. Med. Chem. 2015, 22, 3185–3207. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef]
- Laville, I.; Figueiredo, T.; Loock, B.; Pigaglio, S.; Maillard, P.; Grierson, D.S.; Carrez, D.; Croisy, A.; Blais, J. Synthesis, cellular internalization and photodynamic activity of glucoconjugated derivatives of tri and tetra(meta-hydroxyphenyl)chlorins. Bioorganic Med. Chem. 2003, 11, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, E.E.; Gazzali, A.M.; Acherar, S.; Frochot, C.; Barberi-Heyob, M.; Boura, C.; Chaimbault, P.; Sibille, E.; Wahab, H.A.; Vanderesse, R. New Peptide-Conjugated Chlorin-Type Photosensitizer Targeting Neuropilin-1 for Anti-Vascular Targeted Photodynamic Therapy. Int. J. Mol. Sci. 2015, 16, 24059–24080. [Google Scholar] [CrossRef]
- Chaleix, V.; Sol, V.; Huang, Y.-M.; Guilloton, M.; Granet, R.; Blais, J.C.; Krausz, P. RGD-Porphyrin Conjugates: Synthesis and Potential Application in Photodynamic Therapy. Eur. J. Org. Chem. 2003, 2003, 1486–1493. [Google Scholar] [CrossRef]
- Sol, V.; Lamarche, F.; Enache, M.; Garcia, G.; Granet, R.; Guilloton, M.; Blais, J.C.; Krausz, P. Polyamine conjugates of meso-tritolylporphyrin and protoporphyrin IX: Potential agents for photodynamic therapy of cancers. Bioorganic Med. Chem. 2006, 14, 1364–1377. [Google Scholar] [CrossRef]
- Garcia, G.; Sarrazy, V.; Sol, V.; Morvan, C.L.; Granet, R.; Alves, S.; Krausz, P. DNA photocleavage by porphyrin–polyamine conjugates. Bioorganic Med. Chem. 2009, 17, 767–776. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Foglietta, F.; Pinnelli, V.; Giuntini, F.; Barbero, N.; Panzanelli, P.; Durando, G.; Terreno, E.; Serpe, L.; Canaparo, R. Sonodynamic Treatment Induces Selective Killing of Cancer Cells in an In Vitro Co-Culture Model. Cancers 2021, 13, 3852. [Google Scholar] [CrossRef]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A.; Pandey, R.K. Sonodynamic therapy in combination with photodynamic therapy shows enhanced long-term cure of brain tumor. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Shi, H.; Chen, H.; Tao, J.; Shen, R.; Wang, T. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance. Cancer Sci. 2018, 109, 94–102. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagnosis Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Samat, N.; Tan, P.J.; Shaari, K.; Abas, F.; Lee, H.B. Prioritization of natural extracts by LC-MS-PCA for the identification of new photosensitizers for photodynamic therapy. Anal. Chem. 2014, 86, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Arambula, J.F.; Preihs, C.; Borthwick, D.; Magda, D.; Sessler, J.L. Texaphyrins: Tumor localizing redox active expanded porphyrins. Anticancer Agents Med. Chem. 2011, 11, 222–232. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Rizza, P.; Pellegrino, M.; Zito, D.; Giordano, C.; Mauro, L.; Catalano, S.; Aquila, S.; Sisci, D.; et al. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res. Treat. 2012, 136, 443–455. [Google Scholar] [CrossRef]
- Van Aelst, B.; Devloo, R.; Zachee, P.; t’Kindt, R.; Sandra, K.; Vandekerckhove, P.; Compernolle, V.; Feys, H.B. Psoralen and Ultraviolet A Light Treatment Directly Affects Phosphatidylinositol 3-Kinase Signal Transduction by Altering Plasma Membrane Packing. J. Biol. Chem. 2016, 291, 24364–24376. [Google Scholar] [CrossRef]
- Mastrangelopoulou, M.; Grigalavicius, M.; Berg, K.; Menard, M.; Theodossiou, T.A. Cytotoxic and Photocytotoxic Effects of Cercosporin on Human Tumor Cell Lines. Photochem. Photobiol. 2019, 95, 387–396. [Google Scholar] [CrossRef]
- Ge, Z.-C.; Qu, X.; Yu, H.-F.; Zhang, H.-M.; Wang, Z.-H.; Zhang, Z.-T. Antitumor and apoptotic effects of bergaptol are mediated via mitochondrial death pathway and cell cycle arrest in human breast carcinoma cells. Bangladesh J. Pharmacol. 2016, 11, 489–494. [Google Scholar] [CrossRef]
- Liszewski, W.; Naym, D.G.; Biskup, E.; Gniadecki, R. Psoralen with ultraviolet A-induced apoptosis of cutaneous lymphoma cell lines is augmented by type I interferons via the JAK1-STAT1 pathway. Photodermatol. Photoimmunol. Photomed. 2017, 33, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.; Amos, S. Other plant metabolites. In Pharmacognosy; Elsevier: Cham, Switzerland, 2017; pp. 267–280. [Google Scholar]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.-H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol. Rep. 2007, 17, 537–540. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Sun, D.; Hao, J.-B.; Wei, Y.-F.; Yin, L.-F.; Liu, X. The effect of dietary soyabean isoflavones on photodynamic therapy in K562 leukemia cells. J. Photochem. Photobiol. B Biol. 2012, 110, 28–33. [Google Scholar] [CrossRef] [PubMed]
- de Paula Rodrigues, R.; Tini, I.R.; Soares, C.P.; da Silva, N.S. Effect of photodynamic therapy supplemented with quercetin in HEp-2 cells. Cell Biol. Int. 2014, 38, 716–722. [Google Scholar] [CrossRef]
- Núñez Montoya, S.C.; Comini, L.R.; Rumie Vittar, B.; Fernández, I.M.; Rivarola, V.A.; Cabrera, J.L. Phototoxic effects of Heterophyllaea pustulata (Rubiaceae). Toxicon 2008, 51, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Vittar, N.B.R.; Awruch, J.; Azizuddin, K.; Rivarola, V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int. J. Biochem. Cell Biol. 2010, 42, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Perez Martin, J.M.; Labrador, V.; Fernandez Freire, P.; Molero, M.L.; Hazen, M.J. Ultrastructural changes induced in HeLa cells after phototoxic treatment with harmine. J. Appl. Toxicol. 2004, 24, 197–201. [Google Scholar] [CrossRef]
- Burgeiro, A.; Gajate, C.; Dakir, E.L.H.; Villa-Pulgarín, J.A.; Oliveira, P.J.; Mollinedo, F. Involvement of mitochondrial and B-RAF/ERK signaling pathways in berberine-induced apoptosis in human melanoma cells. Anti-Cancer Drugs 2011, 22, 507–518. [Google Scholar] [CrossRef]
- Youn, M.-J.; So, H.-S.; Cho, H.-J.; Kim, H.-J.; Kim, Y.; Lee, J.-H.; Sohn, J.S.; Kim, Y.K.; Chung, S.-Y.; Park, R. Berberine, a Natural Product, Combined with Cisplatin Enhanced Apoptosis through a Mitochondria/Caspase-Mediated Pathway in HeLa Cells. Biol. Pharm. Bull. 2008, 31, 789–795. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef]
- Wondrak, G.T. Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef]
- Pavlickova, V.; Rimpelova, S.; Jurasek, M.; Zaruba, K.; Fahnrich, J.; Krizova, I.; Bejcek, J.; Rottnerova, Z.; Spiwok, V.; Drasar, P.; et al. PEGylated Purpurin 18 with Improved Solubility: Potent Compounds for Photodynamic Therapy of Cancer. Molecules 2019, 24, 4477. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, B.; Yuan, Q.; Zhang, X.; Leung, W.; Xu, C. Photodynamic treatment with purpurin 18 effectively inhibits triple negative breast cancer by inducing cell apoptosis. Lasers Med. Sci. 2021, 36, 339–347. [Google Scholar] [CrossRef]
- Yu, T.T.; Hu, J.; Li, Q.R.; Peng, X.C.; Xu, H.Z.; Han, N.; Li, L.G.; Yang, X.X.; Xu, X.; Yang, Z.Y.; et al. Chlorin e6-induced photodynamic effect facilitates immunogenic cell death of lung cancer as a result of oxidative endoplasmic reticulum stress and DNA damage. Int. Immunopharmacol. 2023, 115, 109661. [Google Scholar] [CrossRef]
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef]
- Harmatys, K.M.; Overchuk, M.; Chen, J.; Ding, L.; Chen, Y.; Pomper, M.G.; Zheng, G. Tuning Pharmacokinetics to Improve Tumor Accumulation of a Prostate-Specific Membrane Antigen-Targeted Phototheranostic Agent. Bioconjugate Chem. 2018, 29, 3746–3756. [Google Scholar] [CrossRef]
- Matroule, J.-Y.; Carthy, C.M.; Granville, D.J.; Jolois, O.; Hunt, D.W.C.; Piette, J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene 2001, 20, 4070–4084. [Google Scholar] [CrossRef]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2004, 73, 1–28. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Pendse, D.; Emberton, M. Photodynamic therapy for prostate cancer—A review of current status and future promise. Nat. Clin. Pract. Urol. 2009, 6, 18–30. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ (accessed on 14 May 2023).
- Taneja, S.S.; Bennett, J.; Coleman, J.; Grubb, R.; Andriole, G.; Reiter, R.E.; Marks, L.; Azzouzi, A.R.; Emberton, M. Final Results of a Phase I/II Multicenter Trial of WST11 Vascular Targeted Photodynamic Therapy for Hemi-Ablation of the Prostate in Men with Unilateral Low Risk Prostate Cancer Performed in the United States. J. Urol. 2016, 196, 1096–1104. [Google Scholar] [CrossRef]
- Ito, Y.; Udo, K.; Vertosick, E.A.; Sjoberg, D.D.; Vickers, A.J.; Al-Ahmadie, H.A.; Chen, Y.B.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; et al. Clinical Usefulness of Prostate and Tumor Volume Related Parameters following Radical Prostatectomy for Localized Prostate Cancer. J. Urol. 2019, 201, 535–540. [Google Scholar] [CrossRef]
- Osuchowski, M.; Aebisher, D.; Bartusik-Aebisher, D.; Krupka-Olek, M.; Dynarowicz, K.; Przygoda, M.; Kawczyk-Krupka, A. Photodynamic Therapy-Adjunctive Therapy in the Treatment of Prostate Cancer. Diagnostics 2022, 12, 1113. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavo, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef]
- Pierrard, V.; Lebdai, S.; Kleinclauss, F.; Azzouzi, A.-R.; Terrier, J.-E.; Fortier, E.; Joniau, S.; Van Der Poel, H.; Salomon, G.; Casanova, J.; et al. Radical Prostatectomy after Vascular Targeted Photodynamic Therapy with Padeliporfin: Feasibility, and Early and Intermediate Results. J. Urol. 2019, 201, 315–321. [Google Scholar] [CrossRef]
- Lebdai, S.; Villers, A.; Barret, E.; Nedelcu, C.; Bigot, P.; Azzouzi, A.R. Feasibility, safety, and efficacy of salvage radical prostatectomy after Tookad® Soluble focal treatment for localized prostate cancer. World J. Urol. 2015, 33, 965–971. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.-S.; Liu, Y.-C.; Wang, N.; Zeng, X.-T.; Zhang, L.-L. Emerging photodynamic/sonodynamic therapies for urological cancers: Progress and challenges. J. Nanobiotechnol. 2022, 20, 437. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.; Zhang, C.; Sheng, W.; Al-Rawe, M.; Zeppernick, F.; Meinhold-Heerlein, I.; Hussain, A.F. Near Infrared Photoimmunotherapy: A Review of Recent Progress and Their Target Molecules for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2655. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) AntibodyNear-Infrared Photoimmunotherapy Targeting PSMA. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.-H.; et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; He, Z.; Yang, X.; Meng, F.; Liang, H.; Zhang, X.; Luo, L. Paclitaxel-Potentiated Photodynamic Theranostics for Synergistic Tumor Ablation and Precise Anticancer Efficacy Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 5476–5487. [Google Scholar] [CrossRef]
- Tan, H.; Hou, N.; Liu, Y.; Liu, B.; Cao, W.; Zheng, D.; Li, W.; Liu, Y.; Xu, B.; Wang, Z.; et al. CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102192. [Google Scholar] [CrossRef]
- Huang, L.; Xu, C.; Xu, P.; Qin, Y.; Chen, M.; Feng, Q.; Pan, J.; Cheng, Q.; Liang, F.; Wen, X.; et al. Intelligent Photosensitive Mesenchymal Stem Cells and Cell-Derived Microvesicles for Photothermal Therapy of Prostate Cancer. Nanotheranostics 2019, 3, 41–53. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.L.; Sutour, S.; Vergne-Salle, P.; Leger, D.Y.; Liagre, B.; Therrien, B. Evaluation of Ruthenium-Based Assemblies as Carriers of Photosensitizers to Treat Rheumatoid Arthritis by Photodynamic Therapy. Pharmaceutics 2021, 13, 2104. [Google Scholar] [CrossRef]
- Nkune, N.W.; Matlou, G.G.; Abrahamse, H. Photodynamic Therapy Efficacy of Novel Zinc Phthalocyanine Tetra Sodium 2-Mercaptoacetate Combined with Cannabidiol on Metastatic Melanoma. Pharmaceutics 2022, 14, 2418. [Google Scholar] [CrossRef]
- Mkhobongo, B.; Chandran, R.; Abrahamse, H. In Vitro Photodynamic Treatment Modality for A375 Melanoma Cell Line Using a Sulphonated Aluminum Phthalocyanine Chloride-Photosensitizer-Gold Nanoparticle Conjugate. Pharmaceutics 2022, 14, 2474. [Google Scholar] [CrossRef]
- Dandash, F.; Leger, D.Y.; Diab-Assaf, M.; Sol, V.; Liagre, B. Porphyrin/Chlorin Derivatives as Promising Molecules for Therapy of Colorectal Cancer. Molecules 2021, 26, 7268. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers Used in the Photodynamic Therapy of Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef]
| Natural PSs | ||||
| Name | Natural Sources | Type of PDT * | Mechanism of Action | |
| Furanocoumarins (Fu) | Ruta graveolens, Angelicae dahuricae, Glehnia littoralis, Syzygium Sps Tetradium daniellii, and Ficus sps. | Type II | ||
| Thiophenes (Th) | Echinops, Eclipta, Pluchea, Artemisia, Atractylodes, Tagetes, Porophyllum, and Xanthium. | Type I and II |
| |
| Curcumins (Cu) | Curcuma longa (family Zingiberaceae, species Curcuma) | Type I | ||
| Flavonoids (Fl) | Herbs, Vegetables, Seeds, Cereals, Fruits, Flowers, and Nuts | Type II | ||
| Anthraquinones (An) | Polygonum cuspidatum, H. lycioides, Aloe vera, Rheum palmatu, Polyathia suberosa, Xanthoria parietina, Ramularia collo-cygni. and H. perforatum | Type I and II |
| |
| Alkaloids (Al) | Guatteria blepharophylla, Berberis vulgaris, Sanguinaria Canadensis, Peganum harmala, and Indigofera tinctoria. | Type I |
| |
| Synthetic PSs | ||||
| Name | Commercial Name/Excitation Wavelength | PS Class/Source | Type of PDT * | Mechanism of Action |
| Benzoporphyrin (Be) | Visudyne λ = 689 ± 3 nm | Porphyrin-based PS (Derived from benzoporphyrin) | Type II |
|
| Texaphyrins (Te) | Lutrin, Antrin, Optrin, Xcytrin λ = 732 nm | Texaphyrin-based PS (Derived from expanded porphyrins) | Type I |
|
| Purpurin (Pu) | Purlytin λ = 664 nm | Porphyrin-based PS (Derived from chlorophyll) | Type II |
|
| Chlorin e6 (C6) | MACEDACEPhotoditazine λ = 660 nm | Chlorin-based PS (Derived from chlorophyll) | Type I and II |
|
| Bacteriochlorin (Ba) | TOOKAD λ = 753 or 762 nm | Chlorin-based PS (Derived from bacteriochlorophyll) | Type I and II |
|
| Pyropheophorbide (Py) | Pyropheophorbide-a methylester λ = 675 nm | Chlorin-based PS (Derived from chlorophyll) | Type II | |
| Intervention/ Treatment | Phase | Actual Enrollment | Identifier | Responsible Party | Results |
|---|---|---|---|---|---|
| Drug: 1 -mediated -VTP WST 1 | Phase 1 Phase 2 Terminated | 30 | NCT00946881 | UCLA—Jonsson Comprehensive Cancer Center Los Angeles, California, United States Midtown Urology & Midtown Urology Surgical Center Atlanta, Georgia, United States Washington University School of Medicine- Barnes-Jewish Hospital Saint Louis, Missouri, United States NYU Urology Associates, New York, New York, United States Memorial Sloan-Kettering Cancer Center, New York, New York, United States | The study showed that hemi-ablation of the prostate with WST11 vascular targeted PDT could be an effective treatment for prostate cancer. Optimal dosing parameters and light dose index were found to play important roles in the tissue response, as determined by MRI and biopsy [77]. |
| Drug: WST09 | Phase 2 Completed | 28 | NCT00308919 | Princess Margaret Hospital Toronto, Ontario, Canada | No Results Posted |
| Drug: WST09 Vascular Photodynamic therapy | Phase 2 Phase 3 Terminated | 16 | NCT00312442 | Abramson Cancer Center of The University of Pennsylvania Philadelphia, Pennsylvania, United States | No Results Posted |
| Drug: WST11 | Phase 2 Completed | 86 | NCT00975429 | Centre Hospitalier Universitaire (CHU) Angers, France Hôpital Claude Huriez Lille, France Institut Mutualiste Montsouris (IMM) Paris, France University College London Hospital (UCLH) Kings College Hospital (KCH) Frimley Park Hospital NHS Trust Catharina Ziekenhuis | No Results Posted |
| Drug: Motexafin lutetium | Phase 1 Terminated | 24 | NCT00005067 | Abramson Cancer Center of The University of Pennsylvania Philadelphia, Pennsylvania, United States | No Results Posted |
| Drug: TOOKAD® Soluble 4 mg/kg | Phase 2 Active | 50 | NCT03315754 | Memorial Sloan-Kettering Cancer Center New York, New York, United States | No Results Posted |
| Focal therapies including PDT (PS undetermined) | Recruiting | 200 | NCT03492424 | Weill Cornell Medicine New York, New York, United States | No Results Posted |
| Drug: WST11 | Phase 2 Completed | 42 | NCT00707356 | University Health Network-Princess Margaret Hospital Toronto, Canada Centre Hospitalier Universitaire Angers, France Hôpital Bocage-CHU Dijon, France Hôpital Claude Huriez, Lille, France Institut Mutualiste Montsouris (IMM), Paris, France Frimley Park Hospital NHS Trust, Frimley, United Kingdom | No Results Posted |
| Drug: TOOKAD® Soluble | Phase 2 Completed | 8 | NCT00305929 | The Prostate Centre Princess Margaret Hospital Toronto, Ontario, Canada | No Results Posted |
| Drug: TOOKAD® Soluble | Phase 3 Completed | 81 | NCT01875393 | Hospital General Tlahuac Mexico DF, Mexico Pan-American Medical Research Institute (PAMRI) then moved to Consultario del Dr Rodriguez Panama city, Panama Hospital Nacional Cayetano Heredia San Martin de Porres, Peru | No Results Posted |
| Drug: TOOKAD® Soluble | Phase 3 Withdrawn | 0 | NCT04225299 | Memorial Sloan-Kettering Cancer Center New York, New York, United States | No Results Posted |
| Drug: TOOKAD® Soluble | Phase 4 Terminated | 23 | NCT03849365 | Centre Hospitalier Universitaire Angers, France | No Results Posted |
| Drug: Visudyne® Device: SpectraCure P18 System | Phase 1 Phase 2 Recruiting | 66 | NCT03067051 | Memorial Sloan Kettering Cancer Center New York, New York, United States Keith Cengel Philadelphia, Pennsylvania, United States Princess Margaret Cancer Centre Toronto, Ontario, Canada | No Results Posted |
| Drug: TOOKAD® Soluble | Phase 3 Completed | 413 | NCT01310894 | Dept. of Urology-University Hospitals Leuven Leuven, Belgium Department of Urology-Tampere University Hospital- Tampere, Finland Service d’Urologie—Centre Hospitalier Universitaire Angers, France | No Results Posted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahnou, H.; Youlyouz-Marfak, I.; Liagre, B.; Sol, V.; Oudghiri, M.; Duval, R.E.; Limami, Y. Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics 2023, 15, 1767. https://doi.org/10.3390/pharmaceutics15061767
Wahnou H, Youlyouz-Marfak I, Liagre B, Sol V, Oudghiri M, Duval RE, Limami Y. Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics. 2023; 15(6):1767. https://doi.org/10.3390/pharmaceutics15061767
Chicago/Turabian StyleWahnou, Hicham, Ibtissam Youlyouz-Marfak, Bertrand Liagre, Vincent Sol, Mounia Oudghiri, Raphaël Emmanuel Duval, and Youness Limami. 2023. "Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches" Pharmaceutics 15, no. 6: 1767. https://doi.org/10.3390/pharmaceutics15061767
APA StyleWahnou, H., Youlyouz-Marfak, I., Liagre, B., Sol, V., Oudghiri, M., Duval, R. E., & Limami, Y. (2023). Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics, 15(6), 1767. https://doi.org/10.3390/pharmaceutics15061767










