In Vitro Cytotoxicity and In Vivo Antitumor Activity of Lipid Nanocapsules Loaded with Novel Pyridine Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
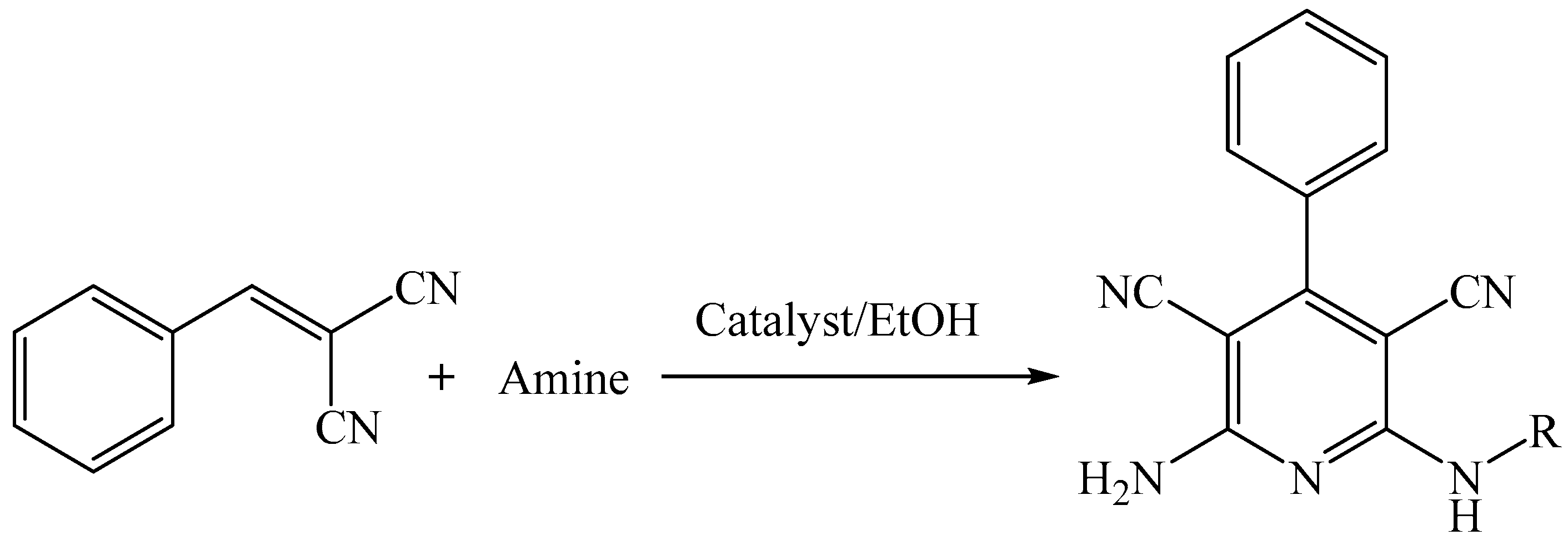
2.2. Synthesis Scheme for 2-Amino-4-aryl-6-substituted Pyridine-3,5-dicarbonitrile Derivatives
2.3. Solubility Measurement
2.4. Preparation of Lipid-Polymer Nanocapsules (LPNCs)
2.5. Physicochemical Characterization of LPNCs
2.5.1. Particle Size, Polydispersity Index, and Zeta Potential
2.5.2. Entrapment Efficiency Percentage
2.5.3. Surface Morphology
2.5.4. Differential Scanning Calorimetry (DSC)
2.6. In Vitro Release Studies
2.7. In Vitro Cytotoxicity Assay of Pyridine Derivatives-Loaded LPNCs
2.8. In Vivo Studies
2.8.1. Animals
2.8.2. Median Lethal Dose (LD50) of Test Selective Compound
2.8.3. Acute Toxicity Study
2.8.4. Induction of Solid Tumor in Mice
2.8.5. Treatment Protocol
- Group 1 (EAC control group): EAC-bearing mice were i.p. injected with 0.4 mL saline once every other day for a period of 21 days.
- Group 2: EAC-bearing mice were i.p. injected with blank LPNCs once every other day for a period of 21 days.
- Group 3: EAC-bearing mice were i.p. injected the standard anticancer drug (5-fluorouracil; 5-FU) at a dose of 10 mg/kg [28] every other day for a period of 21 days.
- Group 4: EAC-bearing mice were i.p. injected with free test compound(S4) at a dose of 10 mg/kg) once every other day for a period of 21 days.
- Group 5: EAC-bearing mice were i.p. injected with test compound (S4)-loaded LPNCs at a dose of 10 mg/kg) once every other day for a period of 21 days.
2.8.6. Histopathological Examination of Solid Tumors
2.8.7. Biochemical Analysis for Liver and Kidney Functions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Solubility Measurement of Various Pyridine Derivatives (S1–S4) in Different Solvents
3.2. Preparation of Lipid Polymer Nanocapsules (LPNCs)
3.2.1. Effect of Polymer Content and Oil Content on the Size Distribution of LPNCs
3.2.2. Effect of Oil Type and Surfactant Type on the Size Distribution of LPNCs
3.3. Preparation of Each Compound-Loaded LPNC
3.4. Characterization of Test Compound-Loaded LPNCs
3.4.1. Particle Size and Size Distribution
3.4.2. Zeta Potential of Test Compound-Loaded LPNCs
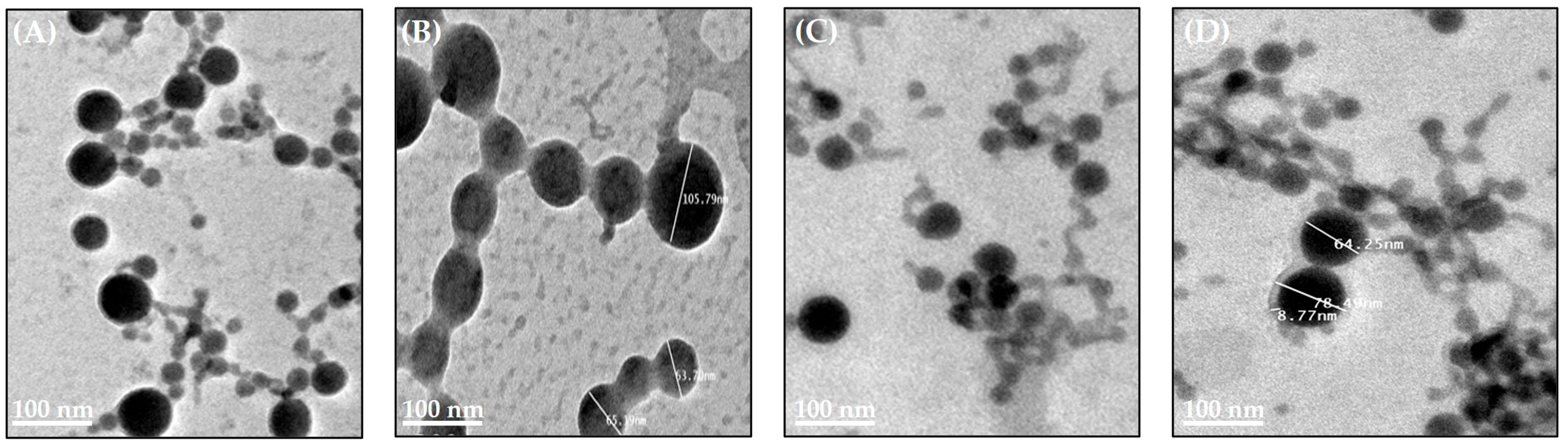
3.4.3. Morphology of Test Compound-Loaded LPNCs
3.4.4. Drug Loading and Entrapment Efficiency of Test Compound-Loaded LPNCs
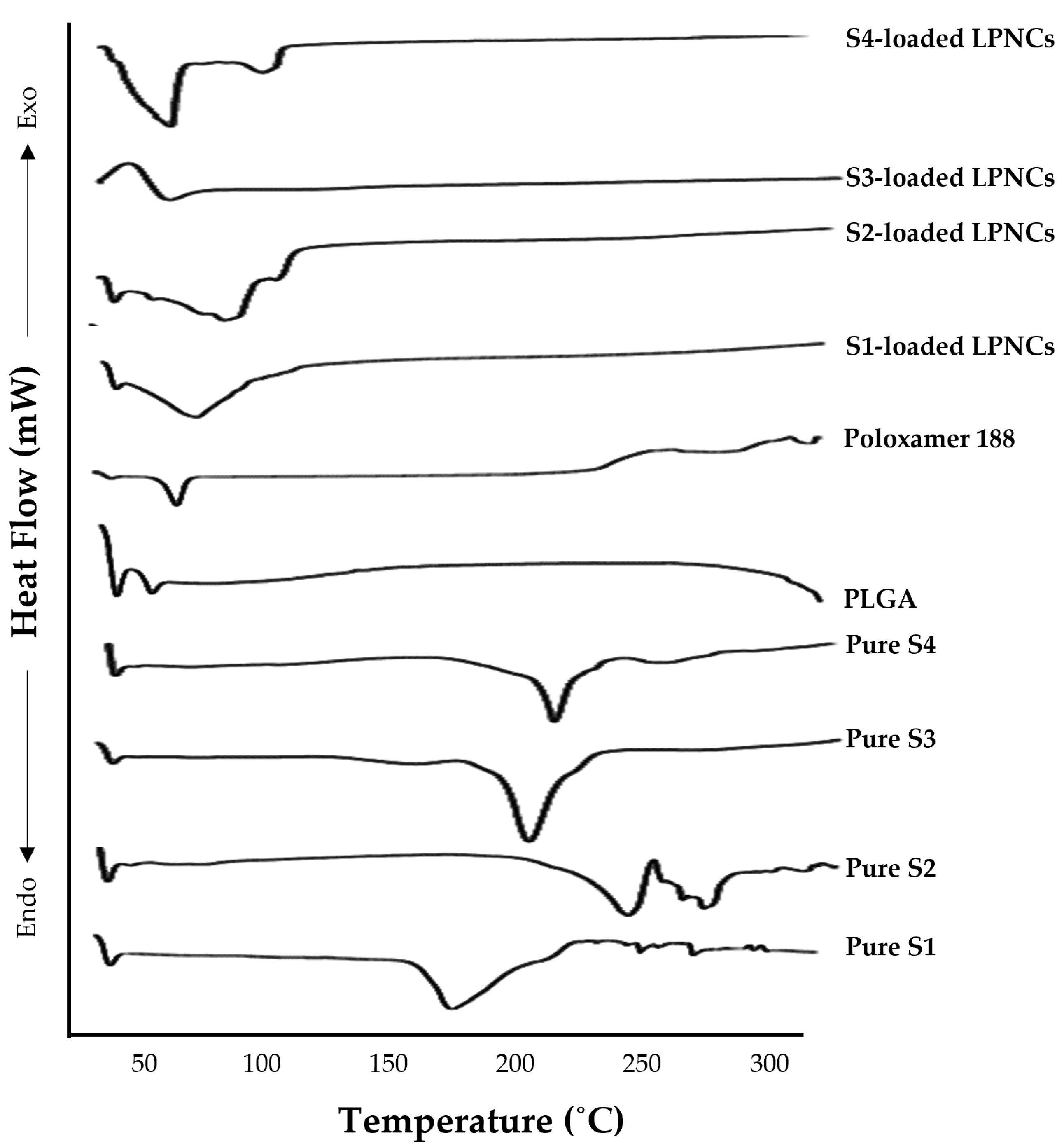
3.4.5. Differential Scanning Calorimetry Analysis
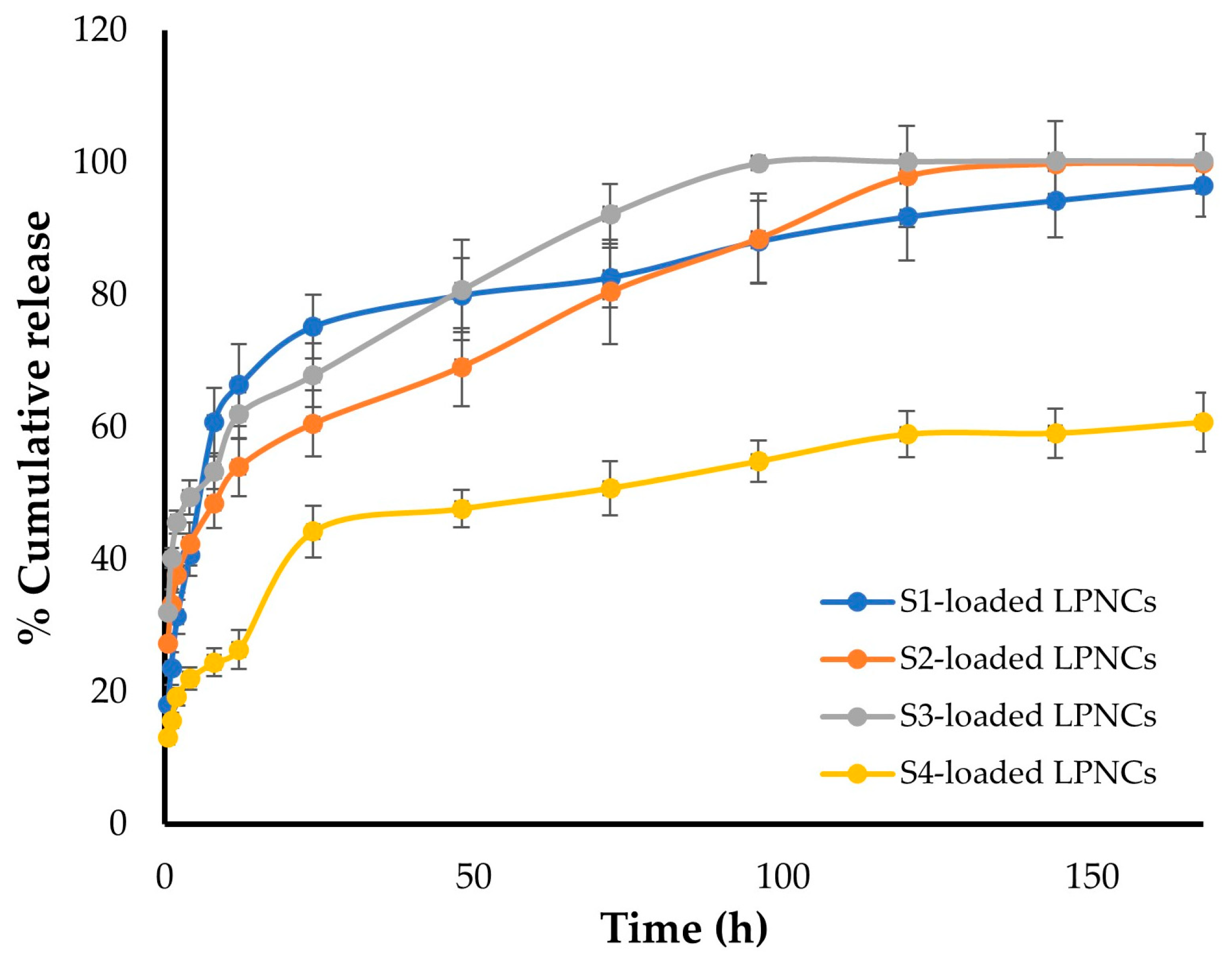
3.5. In Vitro Release of Test Compounds from LPNCs
3.6. In Vitro Cytotoxicity Assay of Test Compound-Loaded LPNCs
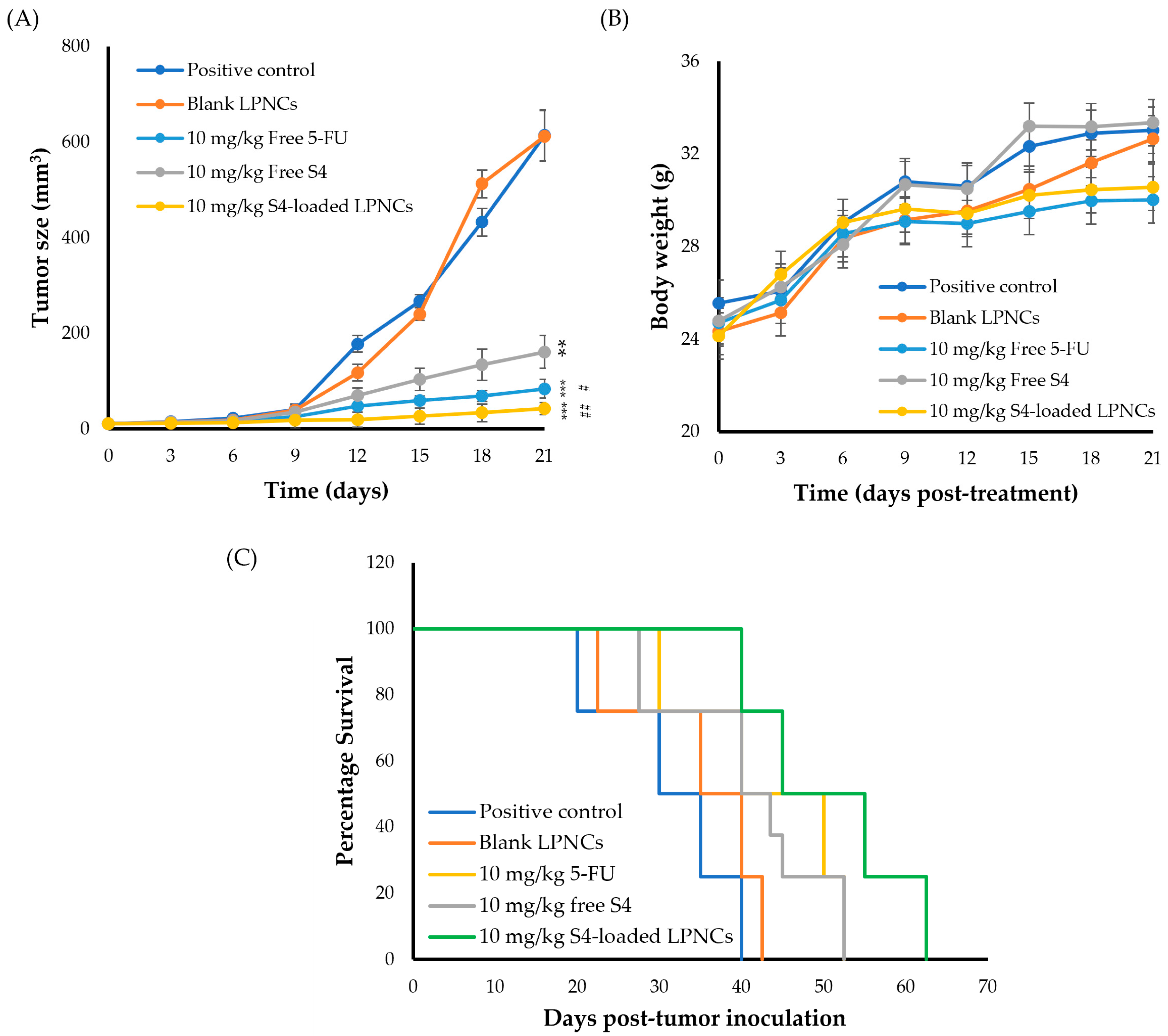
3.7. In Vivo Studies
3.7.1. Median Lethal Dose (LD50) of Compound (S4) in Female Albino Mice
3.7.2. Acute Toxicity
3.7.3. Antitumor Activity of S4-Loaded LPNCs
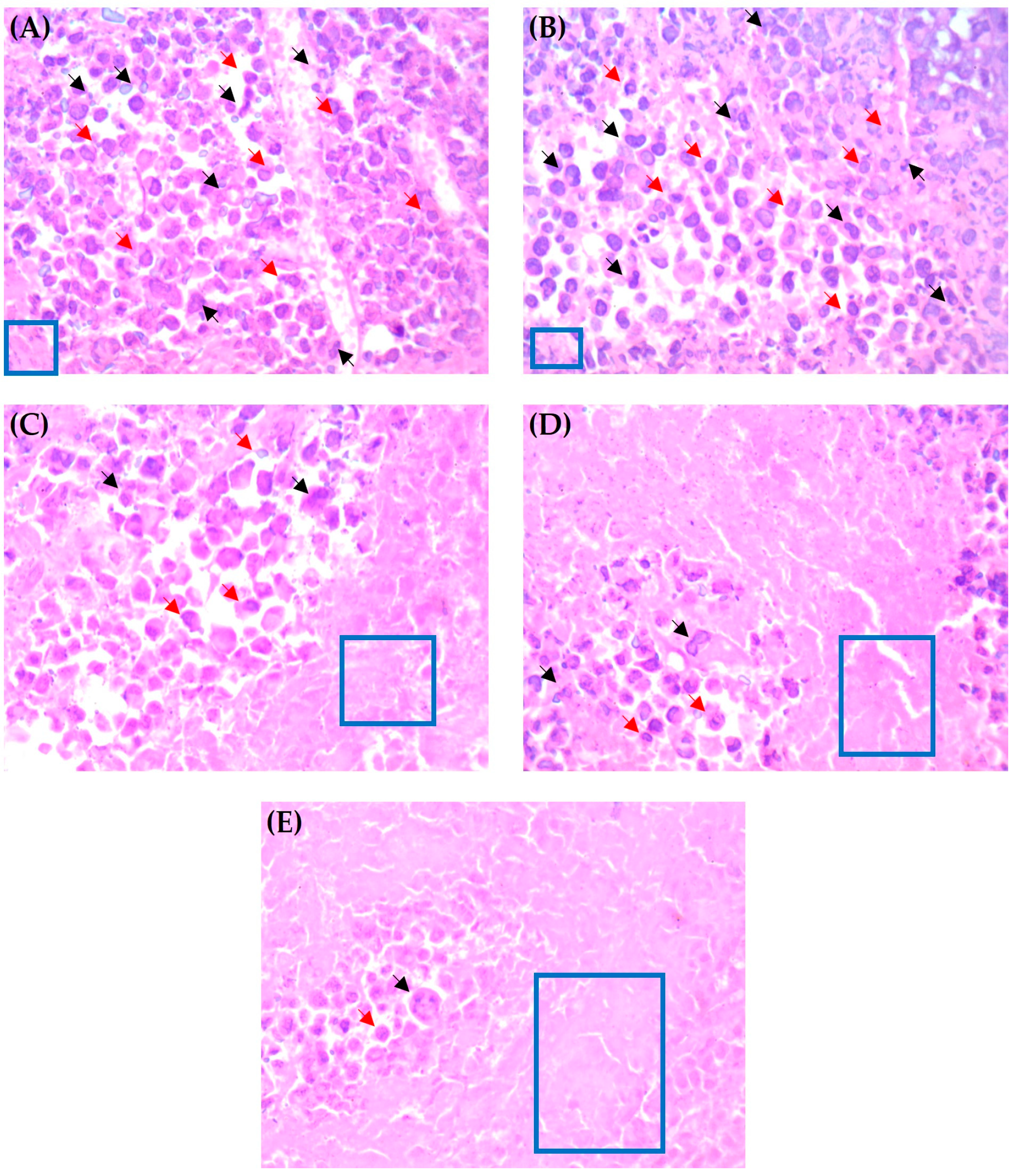
3.7.4. Histopathological Examination of Solid Tumors
3.7.5. Biochemical Analysis of Liver and Kidney Functions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Mahase, E. Cancer overtakes CVD to become leading cause of death in high income countries. BMJ 2019, 366, l5368. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Boffetta, P. Today’s lifestyles, tomorrow’s cancers: Trends in lifestyle risk factors for cancer in low- and middle-income countries. Ann. Oncol. 2011, 22, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.S.M.; de Sousa Luz, L.S.; do Nascimento, S.B.; Silva, L.R.; de Miranda Martins, N.R.; de Almeida, H.G.; de Souza Reis, V.; Maluf, S.E.C.; Budu, A.; Marinho, J.A.; et al. Improvement of antimalarial activity of a 3-alkylpiridine alkaloid analog by replacing the pyridine ring to a thiazole-containing heterocycle: Mode of action, mutagenicity profile, and Caco-2 cell-based permeability. Eur. J. Pharm. Sci. 2019, 138, 105015. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Kumar, S.K.A.; Shah, S.K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. Pyridine: The scaffolds with significant clinical diversity. RSC Adv. 2022, 12, 15385–15406. [Google Scholar] [CrossRef]
- Albratty, M.; Alhazmi, H.A. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab. J. Chem. 2022, 15, 103846. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Abdallah, M.H.; Khafagy, E.-S.; Shehata, T.M.; Soliman, M.S.; Younes, K.M.; Omran, M.M.; Gad, S. Design, synthesis and cytotoxic evaluation of 2-amino-4- aryl-6-substituted pyridine-3,5-dicarbonitrile derivatives. Trop. J. Pharm. Res. 2021, 20, 2127–2133. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T.; Kiwada, H. Recent advances in tumor vasculature targeting using liposomal drug delivery systems. Expert Opin. Drug Deliv. 2009, 6, 1297–1309. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules 2022, 27, 3237. [Google Scholar] [CrossRef]
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. Bioimpacts 2012, 2, 71–81. [Google Scholar] [CrossRef]
- Yurgel, V.; Collares, T.; Seixas, F. Developments in the use of nanocapsules in oncology. Braz. J. Med. Biol. Res. 2013, 46, 486–501. [Google Scholar] [CrossRef]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Moura, R.P.; Pacheco, C.; Pêgo, A.P.; des Rieux, A.; Sarmento, B. Lipid nanocapsules to enhance drug bioavailability to the central nervous system. J. Control. Release 2020, 322, 390–400. [Google Scholar] [CrossRef]
- Béduneau, A.; Saulnier, P.; Hindré, F.; Clavreul, A.; Leroux, J.-C.; Benoit, J.-P. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’ fragments. Biomaterials 2007, 28, 4978–4990. [Google Scholar] [CrossRef]
- Figueiró, F.; de Oliveira, C.P.; Rockenbach, L.; Mendes, F.B.; Bergamin, L.S.; Jandrey, E.H.; Edelweiss, M.I.; Guterres, S.S.; Pohlmann, A.R.; Battastini, A.M. Pharmacological Improvement and Preclinical Evaluation of Methotrexate-Loaded Lipid-Core Nanocapsules in a Glioblastoma Model. J. Biomed. Nanotechnol. 2015, 11, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Frozza, R.L.; Hoppe, J.B.; Salbego, C.; Pohlmann, A.R.; Battastini, A.M.; Guterres, S.S. The antiproliferative effect of indomethacin-loaded lipid-core nanocapsules in glioma cells is mediated by cell cycle regulation, differentiation, and the inhibition of survival pathways. Int. J. Nanomed. 2013, 8, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Molaahmadi, M.R.; Varshosaz, J.; Taymouri, S.; Akbari, V. Lipid Nanocapsules for Imatinib Delivery: Design, Optimization and Evaluation of Anticancer Activity against Melanoma Cell Line. Iran. J. Pharm. Res. 2019, 18, 1676–1693. [Google Scholar] [CrossRef]
- Moin, A.; Wani, S.U.D.; Osmani, R.A.; Abu Lila, A.S.; Khafagy, E.S.; Arab, H.H.; Gangadharappa, H.V.; Allam, A.N. Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv. 2021, 28, 1626–1636. [Google Scholar] [CrossRef]
- Akhila, J.S.; Shyamjith, D.; Alwar, M.C. Acute toxicity studies and determination of median lethal dose. Curr. Sci. 2007, 93, 917–920. [Google Scholar]
- Chinedu, E.; Arome, D.; Ameh, F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef]
- He, Y.C.; Chen, J.W.; Cao, J.; Pan, D.Y.; Qiao, J.G. Toxicities and therapeutic effect of 5-fluorouracil controlled release implant on tumor-bearing rats. World J. Gastroenterol. 2003, 9, 1795–1798. [Google Scholar] [CrossRef]
- Amr, A.E.E.; Ibrahimd, A.A.; El-Shehry, M.F.; Hosni, H.M.; Fayed, A.A.; Elsayed, E.A. In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates. Molecules 2019, 24, 2255. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Hosni, H.M.; Amr, A.E.-G.E.; Abdalla, M.M. Synthesis of novel substituted pyridines from 1-(3-aminophenyl)-3-(1H-indol-3-yl)prop-2-en-1-one and their anticancer activity. Russ. J. Gen. Chem. 2016, 86, 672–680. [Google Scholar] [CrossRef]
- Hather, G.; Liu, R.; Bandi, S.; Mettetal, J.; Manfredi, M.; Shyu, W.C.; Donelan, J.; Chakravarty, A. Growth rate analysis and efficient experimental design for tumor xenograft studies. Cancer Inf. 2014, 13, 65–72. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kizuki, S.; Doi, Y.; Suzuki, T.; Ishida, T.; Kiwada, H. Oxaliplatin encapsulated in PEG-coated cationic liposomes induces significant tumor growth suppression via a dual-targeting approach in a murine solid tumor model. J. Control. Release 2009, 137, 8–14. [Google Scholar] [CrossRef]
- Alotaibi, B.; Tousson, E.; El-Masry, T.A.; Altwaijry, N.; Saleh, A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environ. Toxicol. 2021, 36, 105–113. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Nicolas, S.; Bolzinger, M.A.; Jordheim, L.P.; Chevalier, Y.; Fessi, H.; Almouazen, E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 2018, 550, 170–179. [Google Scholar] [CrossRef]
- Moinard-Chécot, D.; Chevalier, Y.; Briançon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion–diffusion process. J. Colloid Interface Sci. 2008, 317, 458–468. [Google Scholar] [CrossRef]
- Kim, M.W.; Kwon, S.-H.; Choi, J.H.; Lee, A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Nicolete, R.; dos Santos, D.F.; Faccioli, L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011, 11, 1557–1563. [Google Scholar] [CrossRef]
- Singh, E.; Osmani, R.A.M.; Banerjee, R.; Abu Lila, A.S.; Moin, A.; Almansour, K.; Arab, H.H.; Alotaibi, H.F.; Khafagy, E.S. Poly ε-Caprolactone Nanoparticles for Sustained Intra-Articular Immune Modulation in Adjuvant-Induced Arthritis Rodent Model. Pharmaceutics 2022, 14, 519. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.J.M.; Araújo, D.R.d.; Fraceto, L.F. Ivermectin-loaded polymeric nanoparticles: Screening the effects of polymers, methods, and the usefulness of mathematical models. J. Nanosci. Nanotechnol. 2017, 17, 4218–4234. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.F.; Grillo, R.; Guilherme, V.A.; de Araujo, D.R.; de Paula, E.; Rosa, A.H.; Fraceto, L.F. Poly(lactide-co-glycolide) nanocapsules containing benzocaine: Influence of the composition of the oily nucleus on physico-chemical properties and anesthetic activity. Pharm. Res. 2011, 28, 1984–1994. [Google Scholar] [CrossRef]
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef]
- Chen, C.K.; Law, W.C.; Aalinkeel, R.; Yu, Y.; Nair, B.; Wu, J.; Mahajan, S.; Reynolds, J.L.; Li, Y.; Lai, C.K.; et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug-gene co-delivery to cancer cells. Nanoscale 2014, 6, 1567–1572. [Google Scholar] [CrossRef]
- Sethi, M.; Sukumar, R.; Karve, S.; Werner, M.E.; Wang, E.C.; Moore, D.T.; Kowalczyk, S.R.; Zhang, L.; Wang, A.Z. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale 2014, 6, 2321–2327. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef]
| Compound Code | Amine | R | Chemical Formula | M·wt |
|---|---|---|---|---|
| S1 | CH3CH2NH2 | CH3CH2 | C15H13N5 | 263 |
| S2 | 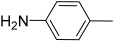 |  | C20H15N5 | 325 |
| S3 | NH4OAc | H | C15H9N5 | 235 |
| S4 | 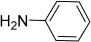 |  | C19H15N5 | 311 |
| Formula | PLGA (mg) | CCT (mg) | Oleic Acid (mg) | Olive Oil (mg) | Poloxamer 188 (%w/v) | Tween 80 (%w/v) |
|---|---|---|---|---|---|---|
| F1 | 10 | 100 | --- | --- | 0.25 | --- |
| F2 | 10 | 150 | --- | --- | 0.25 | --- |
| F3 | 10 | 200 | --- | --- | 0.25 | --- |
| F4 | 20 | 100 | --- | --- | 0.25 | --- |
| F5 | 30 | 100 | --- | --- | 0.25 | --- |
| F6 | 20 | --- | 100 | --- | 0.25 | --- |
| F7 | 20 | --- | --- | 100 | 0.25 | --- |
| F8 | 20 | 100 | --- | --- | --- | 0.25 |
| F9 | 20 | --- | 100 | --- | --- | 0.25 |
| F10 | 20 | --- | --- | 100 | --- | 0.25 |
| Formula | Dv0.1 | Dv0.5 | Dv0.9 | D[4,3] | Span |
|---|---|---|---|---|---|
| F1 | 73.67 ± 0.58 | 126.67 ± 0.58 | 210.00 ± 1 | 135.33 ± 0.58 | 1.07 ± 0.01 |
| F2 | 91.00 ± 6.08 | 172.00 ± 3 | 326.67 ± 16.07 | 193.33 ± 2.08 | 1.37 ± 0.15 |
| F3 | 80.67 ± 4.62 | 187.00 ± 9.17 | 420.67 ± 35.1 | 225.00 ± 10.54 | 1.82 ± 0.23 |
| F4 | 78.67 ± 1.53 | 128.00 ± 2.65 | 205.33 ± 2.31 | 136.00 ± 1.73 | 0.99 ± 0.01 |
| F5 | 76.00 ± 3.61 | 158.00 ± 4.36 | 334.00 ± 21.63 | 184.67 ± 6.43 | 1.63 ± 0.12 |
| F6 | 86.00 ± 4.36 | 203.33 ± 10.26 | 461.67 ± 80.39 | 255.00 ± 40.63 | 1.85 ± 0.37 |
| F7 | 102.33 ± 6.11 | 253.00 ± 11.53 | 693.33 ± 73.38 | 346.67 ± 26.03 | 2.33 ± 0.17 |
| F8 | 79.33 ± 4.73 | 138.67 ± 3.51 | 238.33 ± 28.31 | 153.67 ± 11.55 | 1.14 ± 0.22 |
| F9 | 80.67 ± 5.03 | 172.00 ± 9.54 | 362.67 ± 56.89 | 201.67 ± 19.73 | 1.63 ± 0.30 |
| F10 | 84.33 ± 2.89 | 201.67 ± 5.77 | 472.00 ± 22.52 | 256.00 ± 15.59 | 1.92 ± 0.19 |
| Formula | Size Distribution (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Loading (%) |
|---|---|---|---|---|---|
| S1-LPNCs | 185.0 ± 17.4 | 0.098 ± 0.01 | −7.55 ± 1.7 | 92.67 ± 0.84 | 1.99 ± 0.02 |
| S2-LPNCs | 187.5 ± 15.2 | 0.141 ± 0.02 | −11.60 ± 1.8 | 90.62 ± 0.52 | 2.39 ± 0.01 |
| S3-LPNCs | 218.9 ± 10.9 | 0.200 ± 0.02 | −11.90 ± 1.5 | 91.20 ± 1.70 | 1.75 ± 0.03 |
| S4-LPNCs | 223.0 ± 15.3 | 0.218 ± 0.03 | −13.4 ± 2.1 | 93.72 ± 0.66 | 2.37 ± 0.02 |
| Formula | IC50 (µM) against MCF-7 | IC50 (µM) against A549 |
|---|---|---|
| Pure S1 | >100 | >100 |
| Pure S2 | >100 | >100 |
| Pure S3 | >100 | >100 |
| Pure S4 | >100 | >100 |
| S1-loaded LPNCs | 21.9 ± 1.3 | 63.1 ± 3.2 |
| S2-loaded LPNCs | 28.3 ± 1.7 | 51.3 ± 2.9 |
| S3-loaded LPNCs | 28.2 ± 1.4 | 58.9 ± 2.6 |
| S4-loaded LPNCs | 9.33 ± 0.9 | 28.8 ± 1.1 |
| Groups | AST (IU/L) | ALT (IU/L) | Creatinine (mg/dL) |
|---|---|---|---|
| EAC control | 36.06 ± 1.72 | 17.45 ± 1.31 | 0.49 ± 0.09 |
| EAC + blank LPNCs | 34.61 ± 1.33 | 16.55 ± 0.71 | 0.54 ± 0.03 |
| EAC + 5-FU | 74.23 ± 3.84 * | 51.87 ± 1.29 * | 1.05 ± 0.15 * |
| EAC + Free S4 (10 mg/kg) | 36.66 ± 1.87 | 20.50 ± 1.28 | 0.54 ± 0.02 |
| EAC + S4-loaded LPNCs (10 mg/kg) | 36.13 ± 2.15 | 18.96 ± 1.56 | 0.51 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Lila, A.S.; Amran, M.; Tantawy, M.A.; Moglad, E.H.; Gad, S.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. In Vitro Cytotoxicity and In Vivo Antitumor Activity of Lipid Nanocapsules Loaded with Novel Pyridine Derivatives. Pharmaceutics 2023, 15, 1755. https://doi.org/10.3390/pharmaceutics15061755
Abu Lila AS, Amran M, Tantawy MA, Moglad EH, Gad S, Alotaibi HF, Obaidullah AJ, Khafagy E-S. In Vitro Cytotoxicity and In Vivo Antitumor Activity of Lipid Nanocapsules Loaded with Novel Pyridine Derivatives. Pharmaceutics. 2023; 15(6):1755. https://doi.org/10.3390/pharmaceutics15061755
Chicago/Turabian StyleAbu Lila, Amr Selim, Mohammed Amran, Mohamed A. Tantawy, Ehssan H. Moglad, Shadeed Gad, Hadil Faris Alotaibi, Ahmad J. Obaidullah, and El-Sayed Khafagy. 2023. "In Vitro Cytotoxicity and In Vivo Antitumor Activity of Lipid Nanocapsules Loaded with Novel Pyridine Derivatives" Pharmaceutics 15, no. 6: 1755. https://doi.org/10.3390/pharmaceutics15061755
APA StyleAbu Lila, A. S., Amran, M., Tantawy, M. A., Moglad, E. H., Gad, S., Alotaibi, H. F., Obaidullah, A. J., & Khafagy, E.-S. (2023). In Vitro Cytotoxicity and In Vivo Antitumor Activity of Lipid Nanocapsules Loaded with Novel Pyridine Derivatives. Pharmaceutics, 15(6), 1755. https://doi.org/10.3390/pharmaceutics15061755










