Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical Composition of the Lavender Essential Oil with Gas Chromatography—Coupled to Mass Spectrometry (GC-MS)
2.2.2. Emulsions Preparation
2.2.3. Physicochemical Characterization
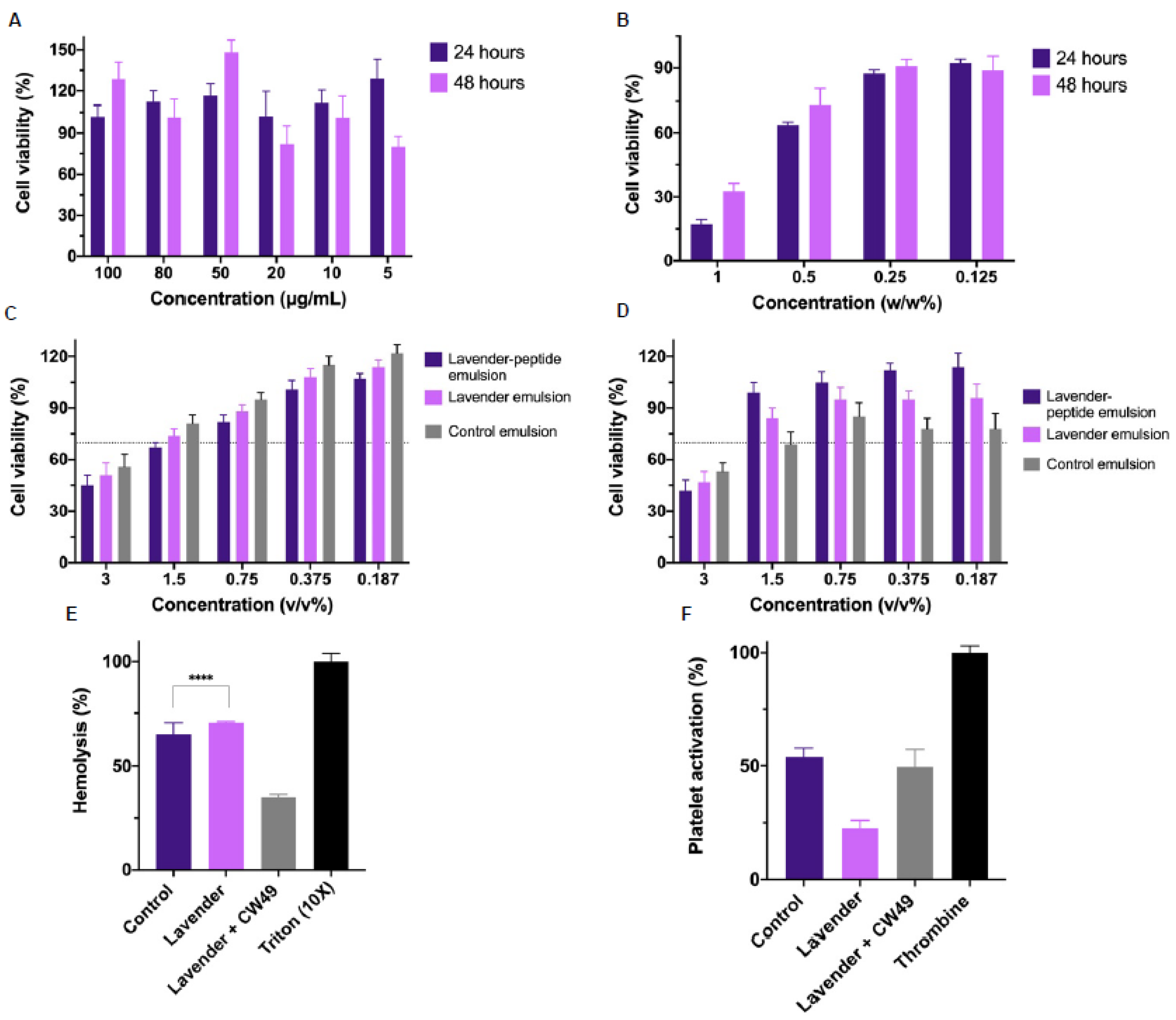
2.2.4. Biocompatibility Assays
Cell Culture
Cytotoxicity Assay
Hemolysis
Platelet Aggregation
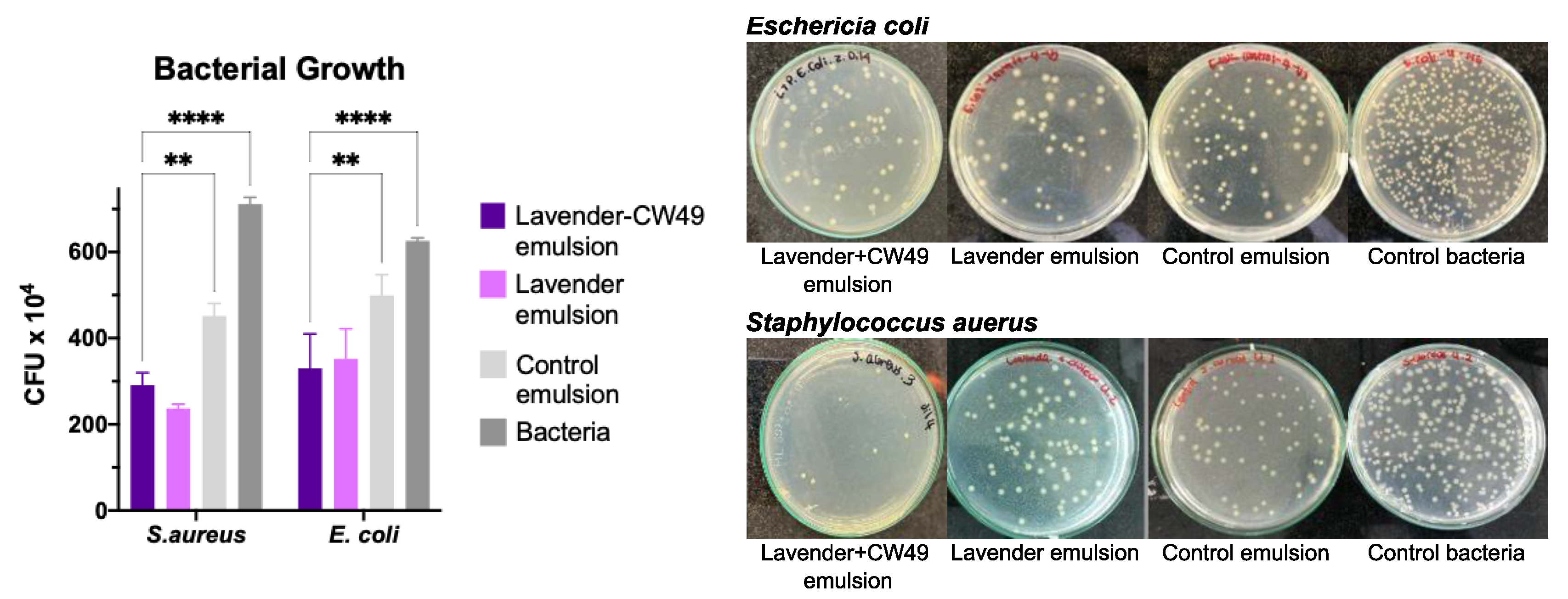
2.2.5. Antibacterial Assay
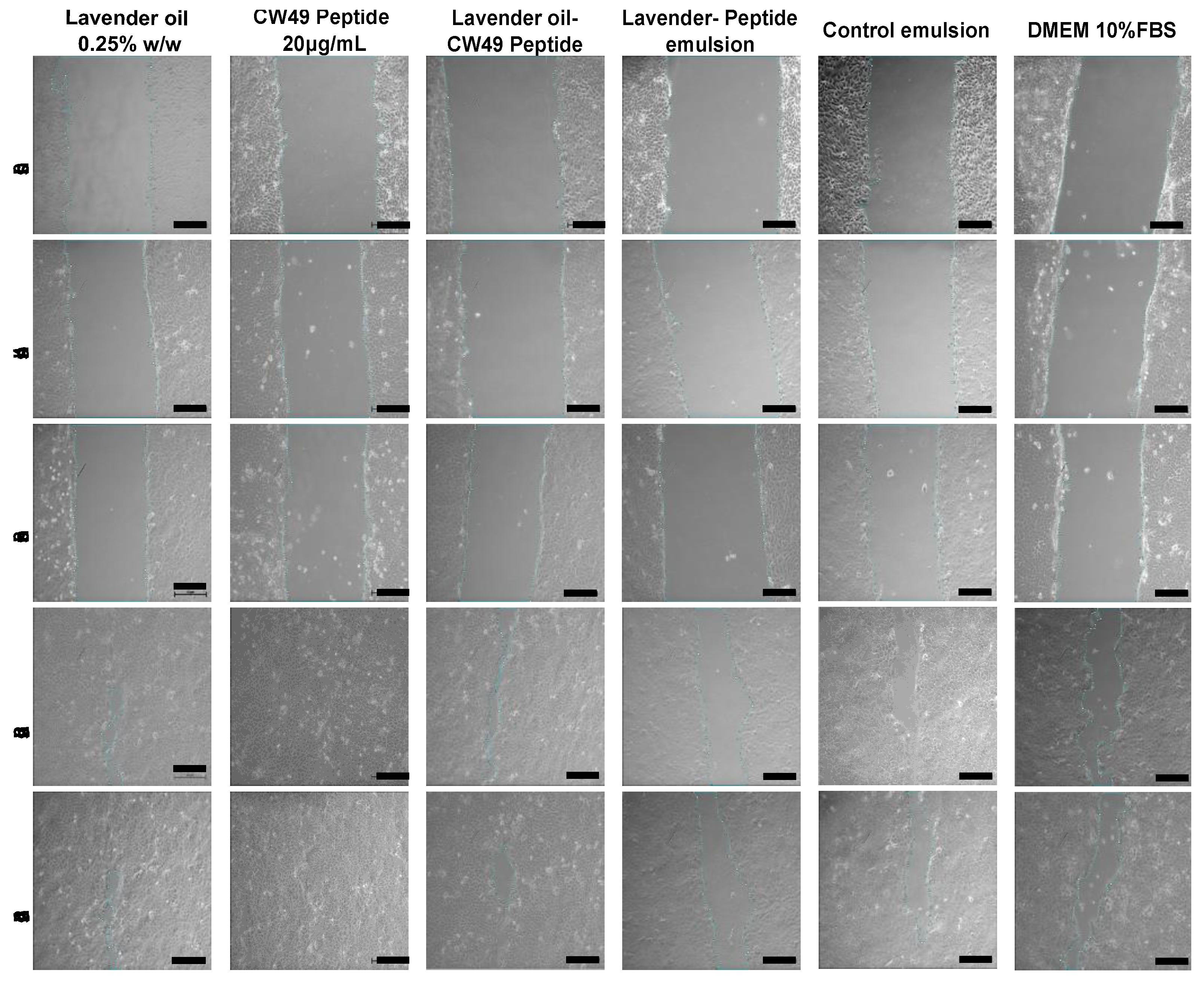
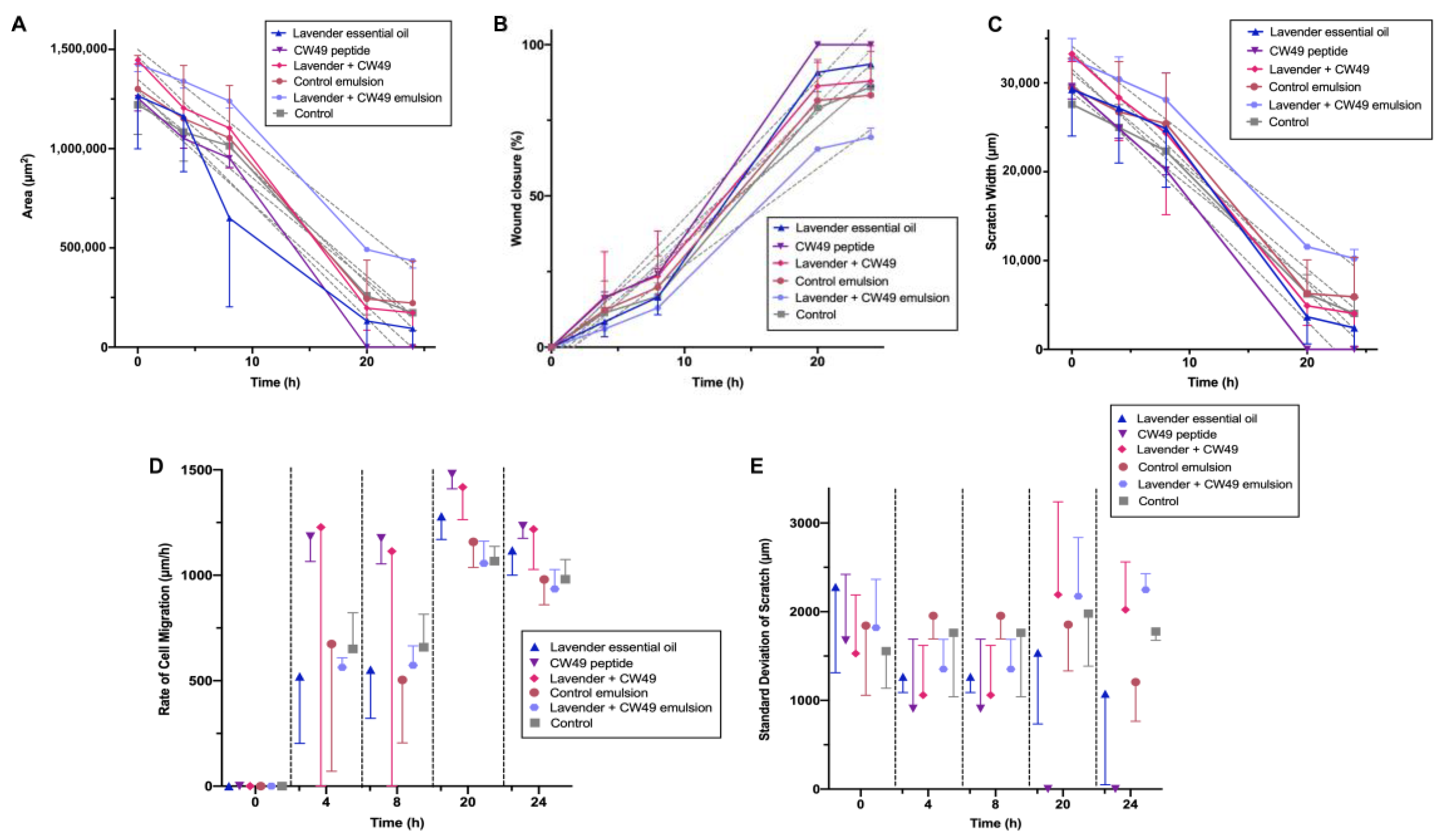
2.2.6. 2D Wound-Healing Assay
2.2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of the Lavender Essential Oil Using Gas Chromatography—Coupled to Mass Spectrometry (GC-MS)
3.2. Physicochemical Characterization of the Emulsions
3.3. Biocompatibility Assays
3.4. Assesment of Antibacterial Activity of Lavander Emulsion
3.5. Wound-Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef] [PubMed]
- ‘Izzah Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public. Health 2018, 15, 2360. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng.-Part C Methods 2019, 25, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reijnders, C.M.A.; Van Lier, A.; Roffel, S.; Kramer, D.; Scheper, R.J.; Gibbs, S. Development of a Full-Thickness Human Skin Equivalent in Vitro Model Derived from TERT-Immortalized Keratinocytes and Fibroblasts. Tissue Eng.-Part A 2015, 21, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Mellott, A.J.; Zamierowski, D.S.; Andrews, B.T. Negative pressurewound therapy in maxillofacial applications. Dent. J. 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.S.; Mak, K.K.; Zhang, S.; Pichika, M.R.; Marappan, P.; Mohandas, K.; Balijepalli, M.K. In vitro methods used for discovering plant derived products as wound healing agents—An update on the cell types and rationale. Fitoterapia 2021, 154, 105026. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, M.; Ayub, Y.; Nazir, R.; Irshad, M.; Hussain, N.; Saleem, Y.; Ahmad, M. Garlic and ginger essential oil-based neomycin nano-emulsions as effective and accelerated treatment for skin wounds’ healing and inflammation: In-vivo and in-vitro studies. Saudi Pharm. J. 2022, 30, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Labib, R.M.; Ayoub, I.M.; Michel, H.E.; Mehanny, M.; Kamil, V.; Hany, M.; Magdy, M.; Moataz, A.; Maged, B.; Mohamed, A. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS ONE 2019, 14, e0219561. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Aderibigbe, B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers 2022, 14, 3772. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Groot, H.; Muñoz-Camargo, C.; Moscoso, J.; Riveros, G.; Salazar, V.; Kaston Florez, F.; Mitrani, E. Skin micro-organs from several frog species secrete a repertoire of powerful antimicrobials in culture. J. Antibiot. 2012, 65, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Camargo, C.; Salazar, V.; Barrero-Guevara, L.; Camargo, S.; Mosquera, A.; Groot, H.; Boix, E. Unveiling the Multifaceted Mechanisms of Antibacterial Activity of Buforin II and Frenatin 2.3S Peptides from Skin Micro-Organs of the Orinoco Lime Treefrog (Sphaenorhynchus lacteus). Int. J. Mol. Sci. 2018, 19, 2170. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Duan, Z.; Tang, J.; Lv, Q.; Rong, M.; Lai, R. A short peptide from frog skin accelerates diabetic wound healing. FEBS J. 2014, 281, 4633–4643. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Gao, C.; Mu, L.; Yang, S.; Rong, M.; Zhang, Z.; Liu, J.; Ding, Q.; Lai, R. A small peptide with potential ability to promote wound healing. PLoS ONE 2014, 9, e92082. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Wang, X.; Wei, L.; Mi, K.; Shen, Y.; Liu, T.; Yang, H.; Mu, L. A frog cathelicidin peptide effectively promotes cutaneous wound healing in mice. Biochem. J. 2018, 475, 2785–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sotto, A.; Gullì, M.; Acquaviva, A.; Tacchini, M.; Di Simone, S.C.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; et al. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022, 183, 114980. [Google Scholar] [CrossRef]
- Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics 2021, 13, 961. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; da Silva Melo, D.A.; Donadio, M.V.F.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.B.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [Green Version]
- Kokina, M.; Salevic, A.; Kaluševic, A.; Levic, S.; Pantic, M.; Pljevljakušic, D.; Šavikin, K.; Shamtsyan, M.; Nikšic, M.; Nedovic, V. Characterization, antioxidant and antibacterial activity of essential oils and their encapsulation into biodegradable material followed by freeze drying. Food Technol. Biotechnol. 2019, 57, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef] [Green Version]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef]
- Muñoz, L.N.; Jaramillo, V.; Gantiva-Diaz, M.; Cifuentes, J.; Muñoz-Camargo, C.; Cruz, J.C.; González Barrios, A.F. Formulation of a novel antibacterial topical treatment based on Magnetite-Buforin-II-silver nanobioconjugates. Front. Bioeng. Biotechnol. 2022, 10, 1003004. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Stanojevic, L.; Stankovic, M.; Cakic, M.; Nikolic, V.; Nikolic, L.; Ilic, D.; Radulovic, N. The effect of hydrodistillation techniques on yield, kinetics, composition and antimicrobial activity of essential oils from flowers of Lavandula officinalis L. Hem. Ind. 2011, 65, 455–463. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Shim, C.-H.; Lee, I.-S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Chemat, F.; Lucchesi, M.E.; Smadja, J.; Favretto, L.; Colnaghi, G.; Visinoni, F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta 2006, 555, 157–160. [Google Scholar] [CrossRef]
- Razzouk, S.; Mazri, M.A.; Jeldi, L.; Mnasri, B.; Ouahmane, L.; Alfeddy, M.N. Chemical Composition and Antimicrobial Activity of Essential Oils from Three Mediterranean Plants against Eighteen Pathogenic Bacteria and Fungi. Pharmaceutics 2022, 14, 1608. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, C.; Ng, K.M. Product-oriented process synthesis and development: Creams and pastes. AIChE J. 2001, 47, 2746–2767. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Sousa Lobo, J.M. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agents. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.R.F.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Rev. Bras. Farmacogn. 2013, 23, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Purkait, A.; Worede, R.E.; Baral, D.; Hazra, D.K.; Panja, B.N.; Biswas, P.K.; Kole, R.K. Development of nanoemulsion formulation of mustard oil, its chemical characterization and evaluation against post harvest anthracnose pathogens. Indian. Phytopathol. 2020, 73, 449–460. [Google Scholar] [CrossRef]
- Safitri, F.I.; Nawangsari, D.; Febrina, D. Overview: Application of Carbopol 940 in Gel; Atlantis Press: Paris, France, 2021. [Google Scholar] [CrossRef]
- Rawle, A.F. Basic of principles of particle-size analysis. Surf. Coat. Int. Part A Coat. J. 2003, 86, 58–65. [Google Scholar]
- Kumar, P.M.; Ghosh, A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur. J. Pharm. Sci. 2017, 96, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Gonçalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS PharmSciTech 2019, 20, 9. [Google Scholar] [CrossRef]
- Şenyiğit, T.; Tekmen, I.; Sönmez, Ü.; Santi, P.; Özer, Ö. Deoxycholate hydrogels of betamethasone-17-valerate intended for topical use: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 403, 123–129. [Google Scholar] [CrossRef]
- International Organization for Standardization; ISO: Geneva, Switzerland, 2003; p. 13.
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell. Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Behling-Kelly, E.L.; Wakshlag, J. A commercial soy-based phospholipid emulsion accelerates clot formation in normal canine whole blood and induces hemolysis in whole blood from normal and dogs with inflammatory leukograms. J. Vet. Emerg. Crit. Care 2018, 28, 252–260. [Google Scholar] [CrossRef]
- Oliveira de Veras, B.; Melo de Oliveira, M.B.; Granja da Silva Oliveira, F.; Queiroz dos Santos, Y.; Saturnino de Oliveira, J.R.; Lúcia de Menezes Lima, V.; Guedes da Silva Almeida, J.R.; Maria do Amaral Ferraz Navarro, D.; Ribeiro de Oliveira Farias de Aguiar, J.C.; Aguiar, J.d.S.; et al. Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: A natural medicine. J. Ethnopharmacol. 2020, 247, 112265. [Google Scholar] [CrossRef]
- Pimentel, K.G.B.; da Fônseca, N.F.; da Silva, J.V.B.; de Medeiros, A.C.D.; Fernandes, F.H.A. Evaluation of non-isothermal thermal degradation kinetics of lavender essential oil (Lavandula angustifólia). J. Therm. Anal. Calorim. 2023, 148, 169–175. [Google Scholar] [CrossRef]
- Jumaa, M.; Kleinebudde, P.; Müller, B.W. Physicochemical properties and hemolytic effect of different lipid emulsion formulations using a mixture of emulsifiers. Pharm. Acta Helv. 1999, 73, 293–301. [Google Scholar] [CrossRef]
- Souza, S.O.L.; Cotrim, M.A.P.; Oréfice, R.L.; Carvalho, S.G.; Dutra, J.A.P.; de Paula Careta, F.; Resende, J.A.; Villanova, J.C.O. Electrospun poly(ε-caprolactone) matrices containing silver sulfadiazine complexed with β-cyclodextrin as a new pharmaceutical dosage form to wound healing: Preliminary physicochemical and biological evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 67. [Google Scholar] [CrossRef]
- Oaks, R.J.; Cindass, R. Silver Sulfadiazine; StatPearls: Tampa, FL, USA, 2023. [Google Scholar]
- Liu, C.; Rinderknecht, H.; Histing, T.; Kolbenschlag, J.; Nussler, A.K.; Ehnert, S. Establishment of an In Vitro Scab Model for Investigating Different Phases of Wound Healing. Bioengineering 2022, 9, 191. [Google Scholar] [CrossRef]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J. Biomed. Sci. 2017, 24, 16. [Google Scholar] [CrossRef] [Green Version]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D.; Zych, S. Antibacterial activity of lavender essential oil and linalool combined with gentamicin on selected bacterial strains. Med. Weter. 2020, 76, 115–118. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug. Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koca Kutlu, A.; Çeçen, D.; Gürgen, S.G.; Sayın, O.; Çetin, F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evidence-Based Complement. Altern. Med. 2013, 2013, 361832. [Google Scholar] [CrossRef] [Green Version]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns 2014, 40, 1562–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Emulsion | Control | Lavender | Lavender–CW49 Peptide |
|---|---|---|---|
| Composition (% w/w) | |||
| Oily phase | |||
| Lavender oil | - | 0.25 | 0.25 |
| Span 80 | 1.60 | 1.60 | 1.60 |
| Mineral oil | 8.40 | 8.15 | 8.15 |
| Aqueous phase | |||
| CW49 peptide | - | - | 0.20 |
| Tween 20 | 2.40 | 2.40 | 2.40 |
| Carbopol | 0.3 | 0.3 | 0.3 |
| Triethanolamine | 0.6 | 0.6 | 0.6 |
| Water | 86.7 | 86.7 | 86.5 |
| tR (min) | Tentative Identification | Relative Amount | Range of Relative Amount Reported [34,35,36,37,38] |
|---|---|---|---|
| 16.3 | α-Pinene | 0.3 | 0.19–0.9 |
| 17.1 | Camphene | 0.2 | 0.32–0.6 |
| 18.3 | β-Pinene | 0.4 | 0.76–0.82 |
| 18.7 | β-Myrcene | 0.4 | 0.12–1.8 |
| 19.7 | Hexyl acetate | 0.4 | 0.3–0.4 |
| 20.3 | p-Cymene | 1.9 | 0.6–5.15 |
| 20.5 | Limonene | 1.5 | 0.15–8.5 |
| 20.7 | 1,8-Cineole | 8.5 | 2.2–13.74 |
| 22.3 | Cis-Linalool oxide | 0.1 | 0.83–6.63 |
| 22.9 | Trans-Linalool oxide | 0.1 | 2.1–5.34 |
| 23.5 | Linalool | 32.6 | 17.10–49.50 |
| 24.3 | Fenchol | 0.2 | - |
| 24.8 | 1,2-Dihydrolinalool | 0.3 | - |
| 25 | p-Menth-3-en-1-ol | 0.2 | - |
| 25.5 | Camphor | 9.5 | 0.9–19.9 |
| 26.1 | Isoborneol | 0.3 | 2.57–6.9 |
| 26.2 | p-Menth-8-en-1-ol | 0.1 | 0.06–5.21 |
| 26.4 | Endo-Borneol | 0.6 | - |
| 27.3 | α-Terpineol | 2.8 | 1.16–4.20 |
| 27.4 | γ-Terpineol | 1.1 | - |
| 28.1 | Fenchyl acetate | 0.2 | - |
| 29.2 | Linalyl acetate | 27.8 | 11.23–28.20 |
| 32.7 | α-Terpinyl acetate | 0.1 | - |
| 33 | Neryl acetate | 0.2 | 0.38–2.02 |
| 33.8 | Geranyl acetate | 3.5 | 0.26–2.85 |
| 35.8 | trans-β-Caryophyllene | 2.7 | 0.21–6.85 |
| 37 | α-Humelene | 0.2 | - |
| 41 | Caryophyllene oxide | 0.3 | 0.19–5.12 |
| Emulsion | m | n |
|---|---|---|
| CW49 peptide–lavender | 53.17 | 0.263 |
| Lavender | 31.24 | 0.244 |
| Control | 34.67 | 0.263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, V.; Díaz, E.; Muñoz, L.N.; González-Barrios, A.F.; Rodríguez-Cortina, J.; Cruz, J.C.; Muñoz-Camargo, C. Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection. Pharmaceutics 2023, 15, 1739. https://doi.org/10.3390/pharmaceutics15061739
Jaramillo V, Díaz E, Muñoz LN, González-Barrios AF, Rodríguez-Cortina J, Cruz JC, Muñoz-Camargo C. Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection. Pharmaceutics. 2023; 15(6):1739. https://doi.org/10.3390/pharmaceutics15061739
Chicago/Turabian StyleJaramillo, Valentina, Erika Díaz, Laura N. Muñoz, Andrés Fernando González-Barrios, Jader Rodríguez-Cortina, Juan C. Cruz, and Carolina Muñoz-Camargo. 2023. "Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection" Pharmaceutics 15, no. 6: 1739. https://doi.org/10.3390/pharmaceutics15061739
APA StyleJaramillo, V., Díaz, E., Muñoz, L. N., González-Barrios, A. F., Rodríguez-Cortina, J., Cruz, J. C., & Muñoz-Camargo, C. (2023). Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection. Pharmaceutics, 15(6), 1739. https://doi.org/10.3390/pharmaceutics15061739









