Evidence of a New Crystalline Phase of Prednisolone Obtained from the Study of the Hydration–Dehydration Mechanisms of the Sesquihydrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TGA-DSC-MS
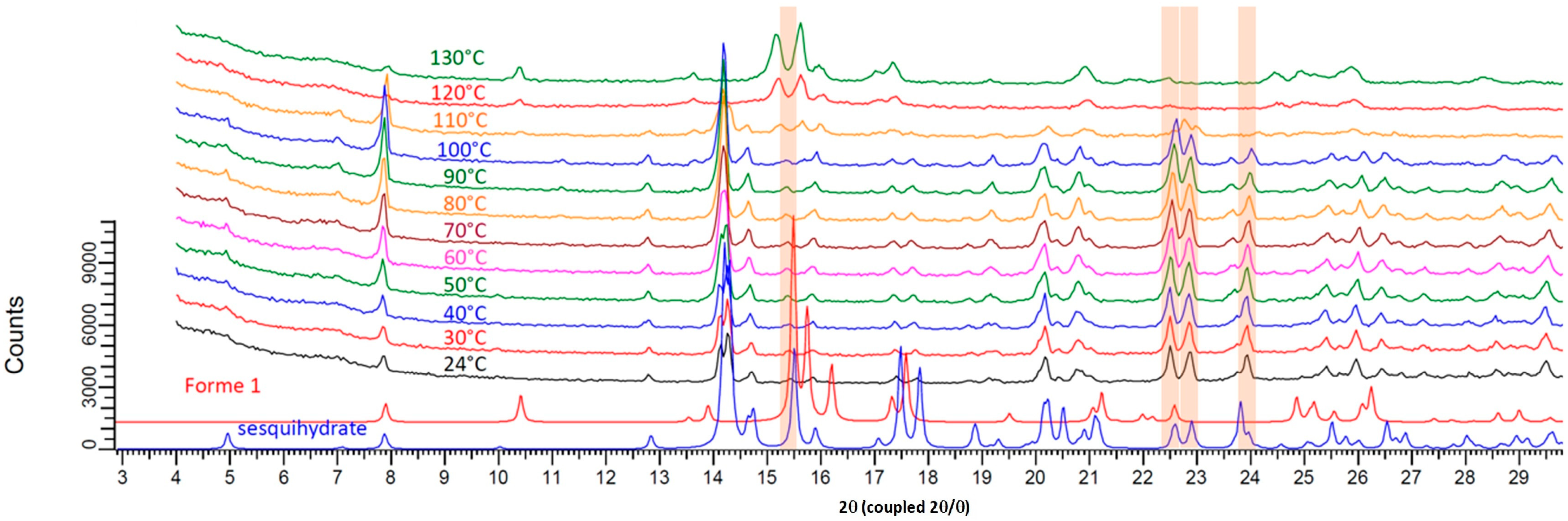
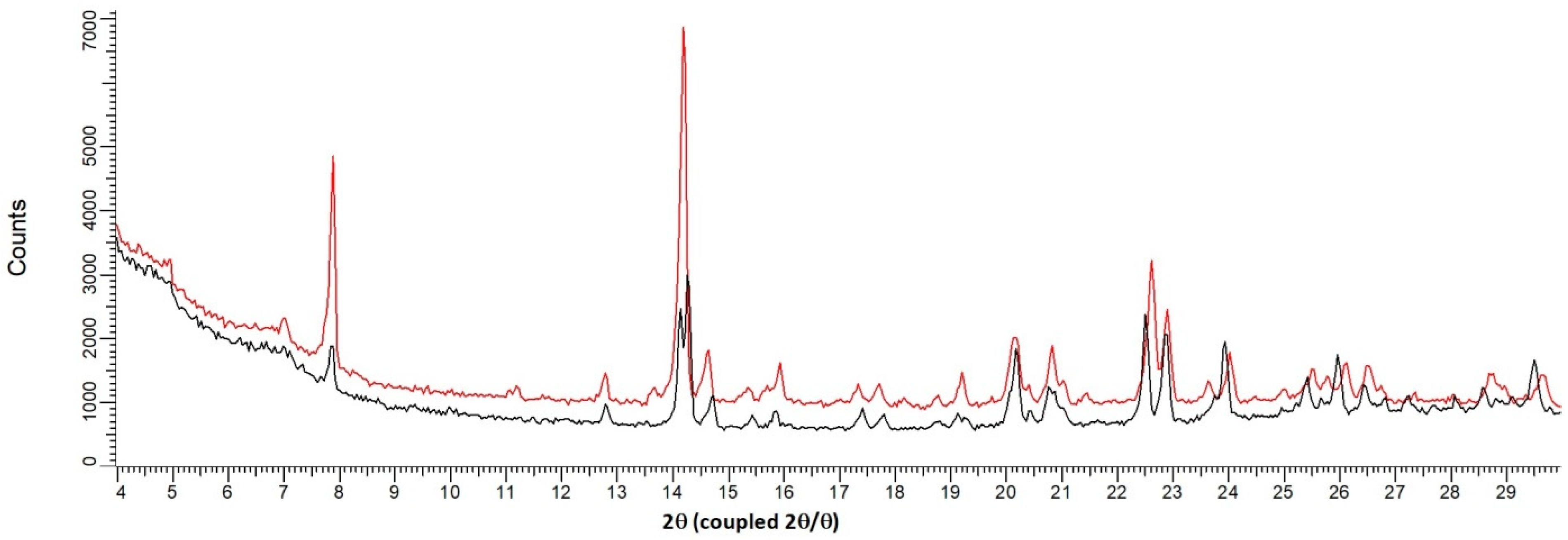
2.3. XRPD and Temperature Resolved XRPD
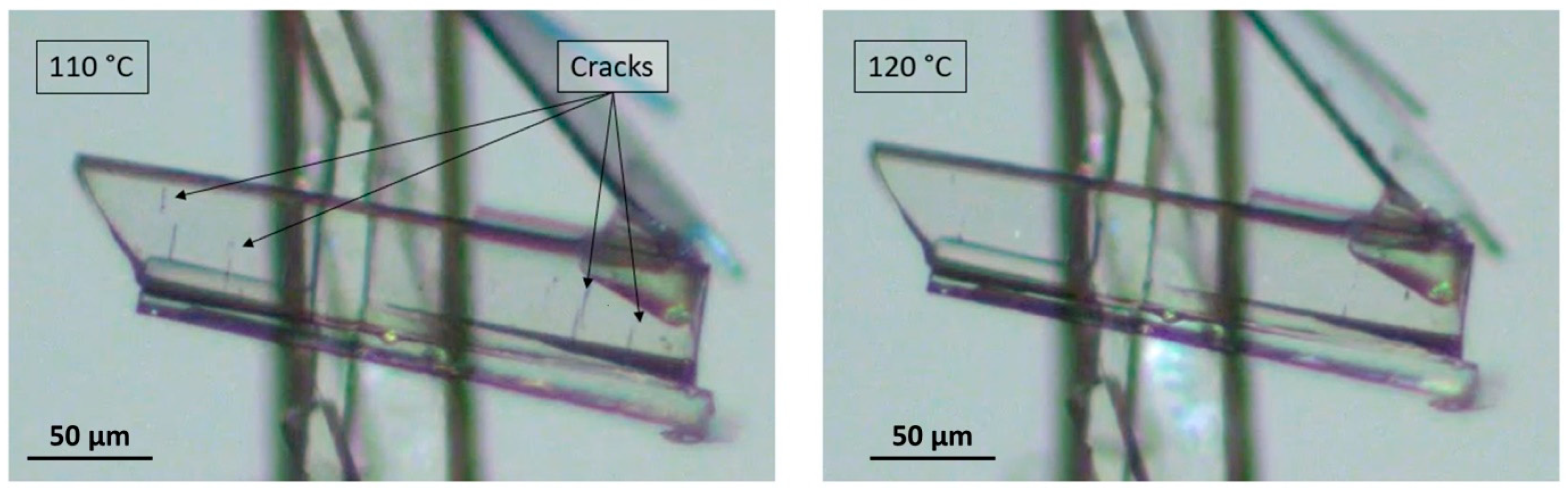
2.4. Thermal Microscopy
2.5. Dynamic Vapor Sorption
2.6. Molecular Modeling
3. Results and Discussion
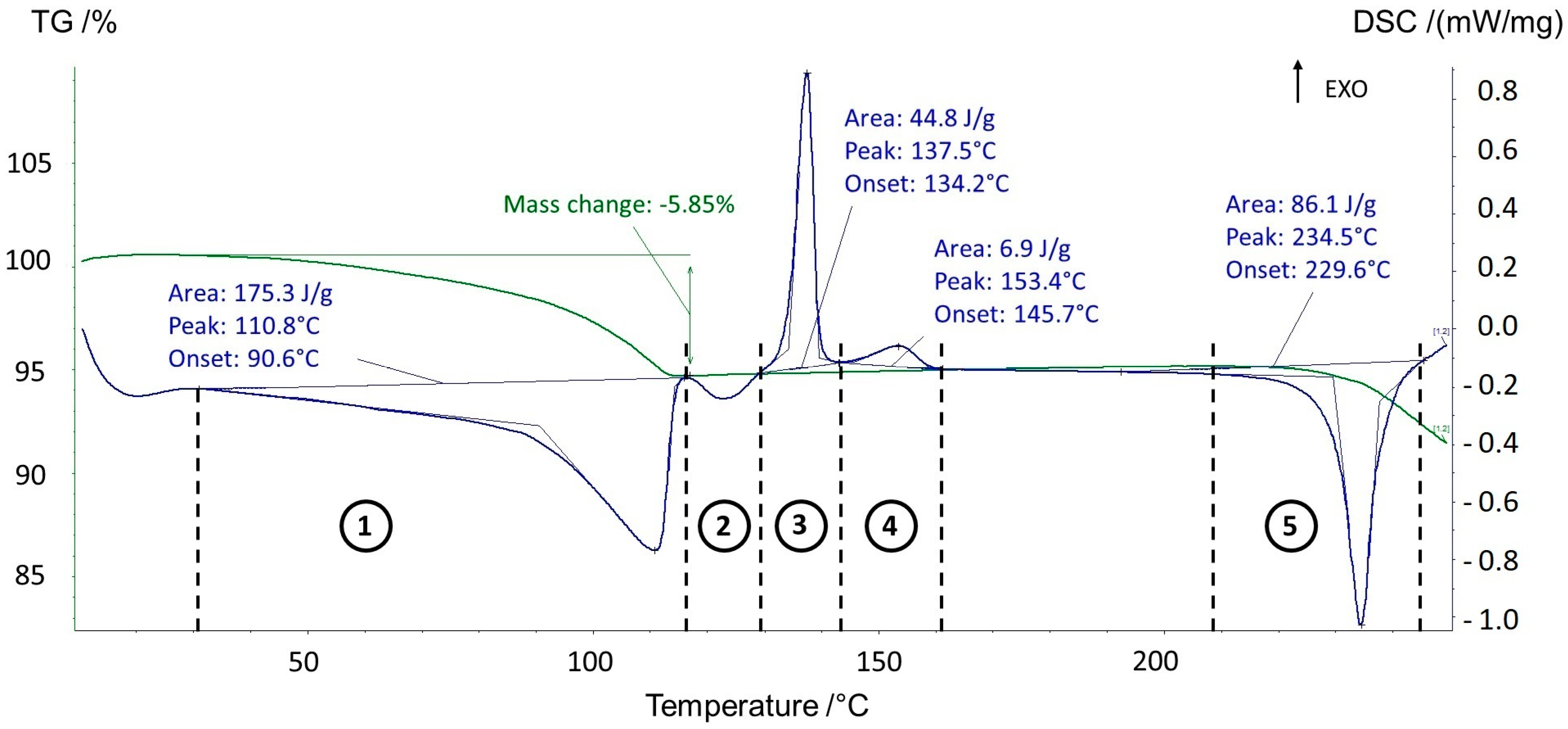
3.1. Thermal Behavior of Prednisolone Sesquihydrate during Dehydration
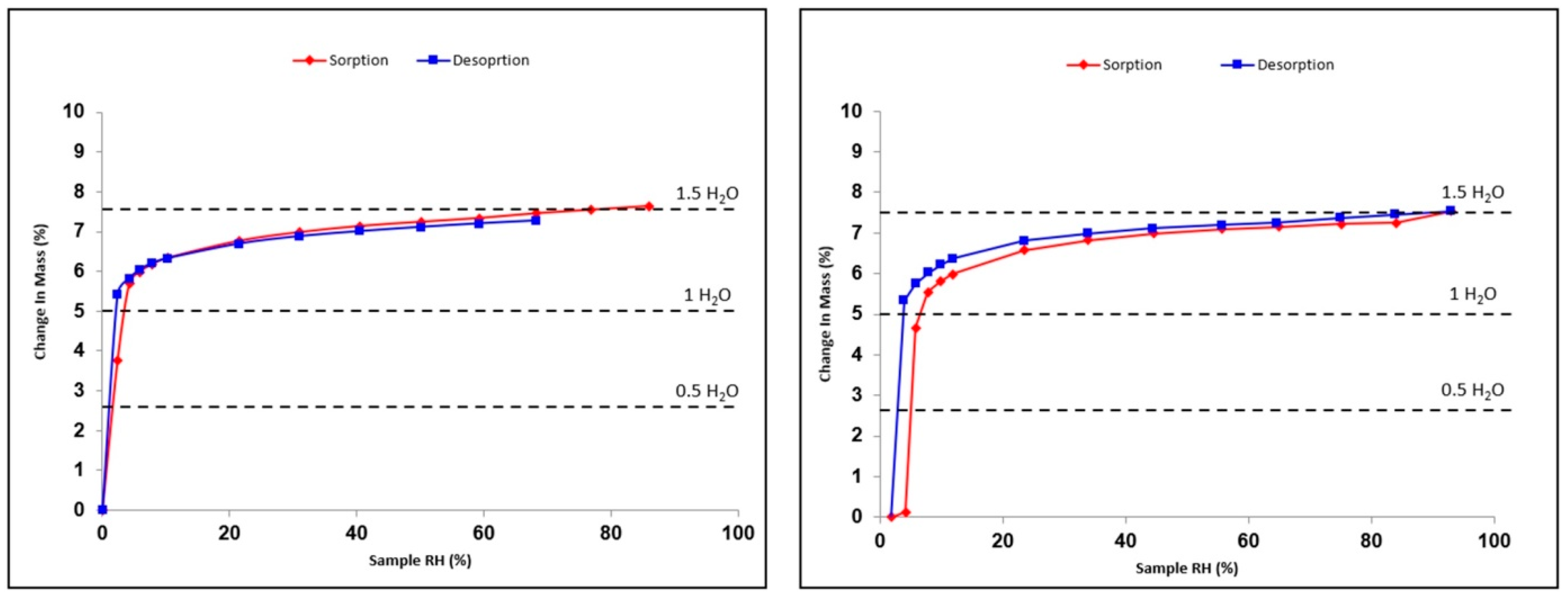
3.2. Hydration/Dehydration Behavior versus Relative Humidity of Prednisolone
- (1)
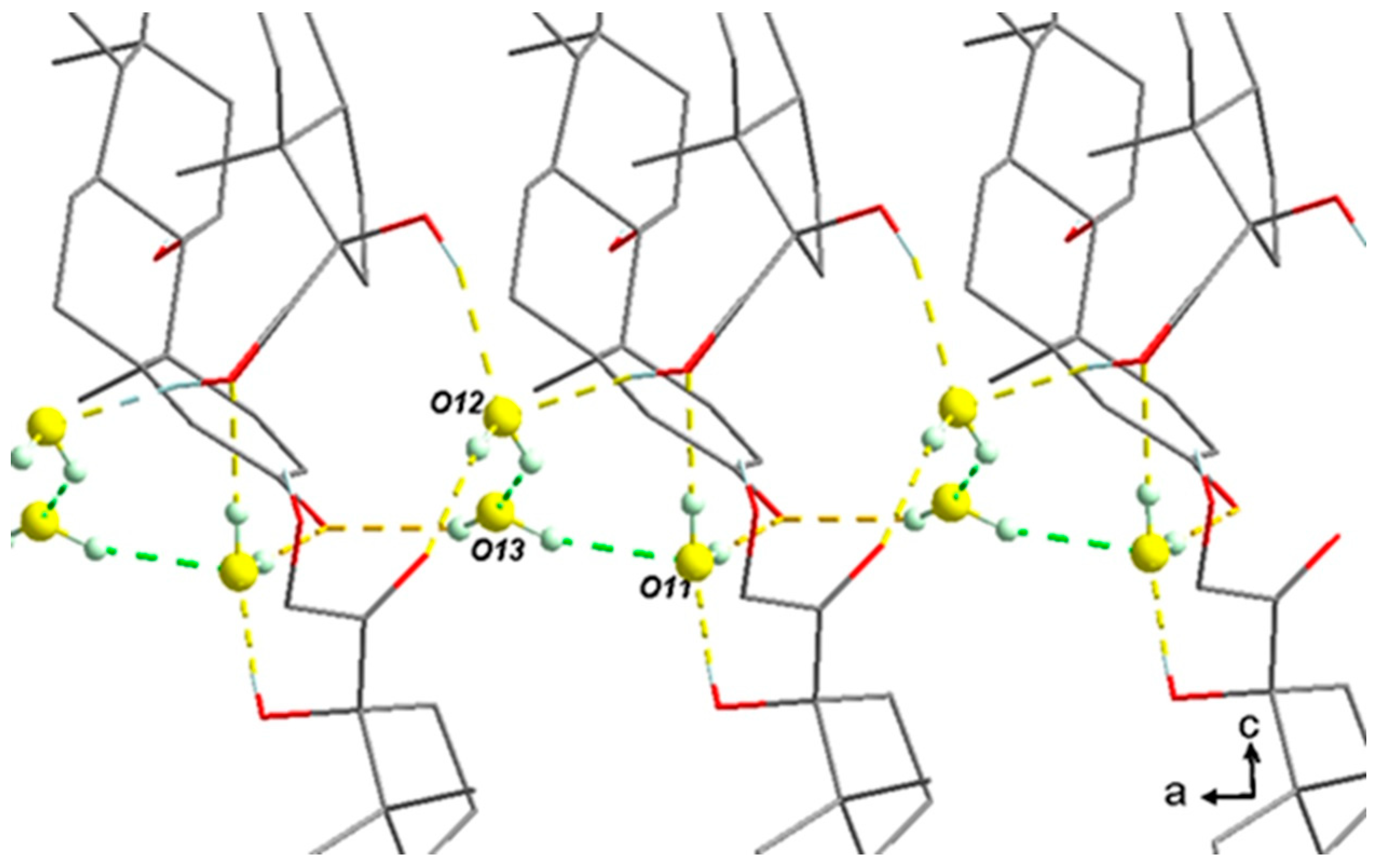
- There is a slight compensation of some degrees of vacancy for O13 by a higher Beq. Consistently, several TGA analyses tend to converge to 1.3 equivalents of water molecules starting from a sample conditioned at room temperature and under an ambient atmosphere. This could therefore indicate the non-stoichiometric behavior of the sesquihydrate. The maximum stoichiometry (1.5 water molecules) is reached only with high RH (cf. DVS data).
- (2)
- The three water molecules O12-O13-O11 form a cluster, as shown in Figure 11. The slight global deficiency in water molecules under ambient conditions should not be considered for a single molecule only but rather for the whole cluster. At room temperature for a RH higher than 2%, there is a strong trend toward approximately occupying 2/3 of the three positions. For higher RH, the last third is progressively filled.
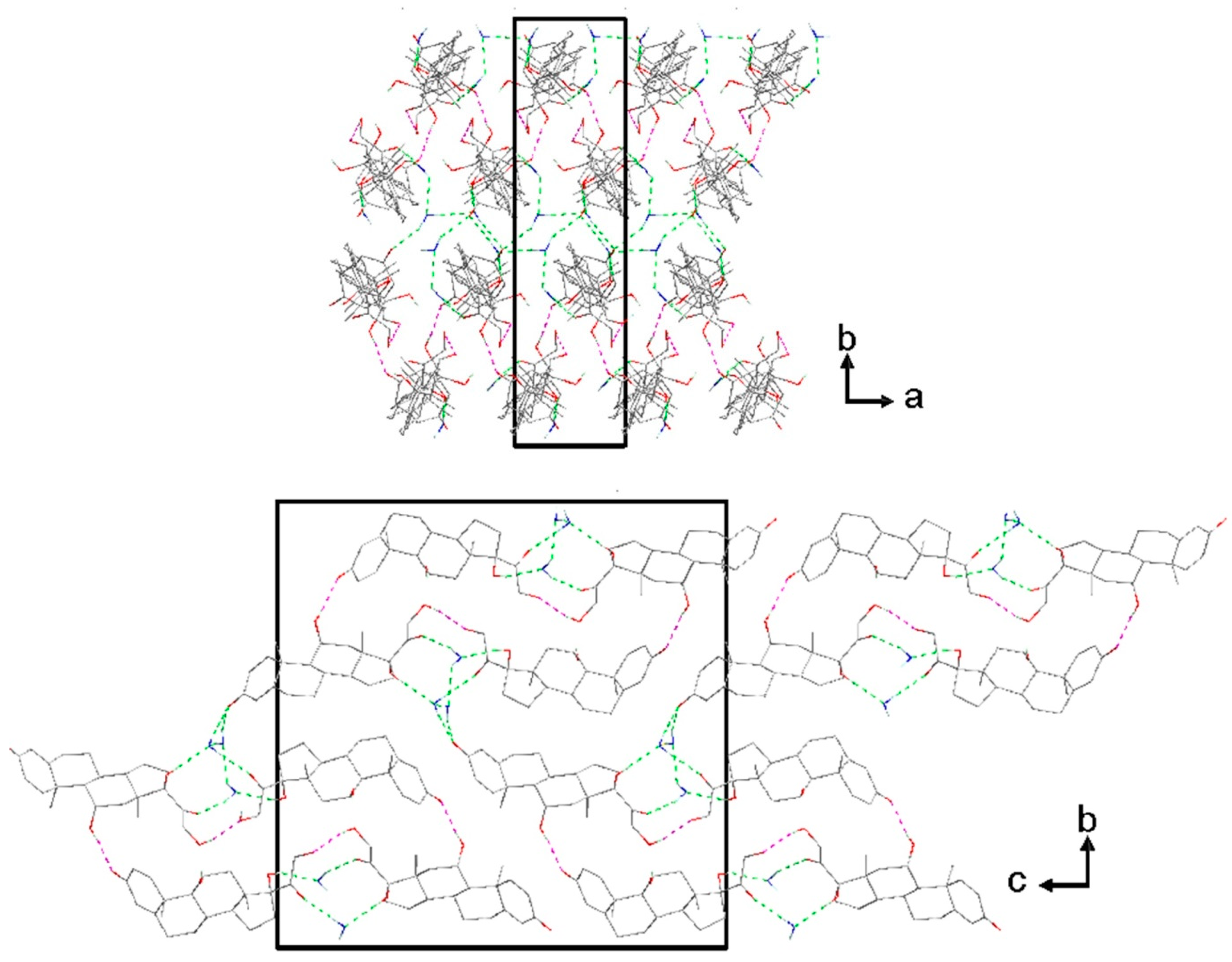
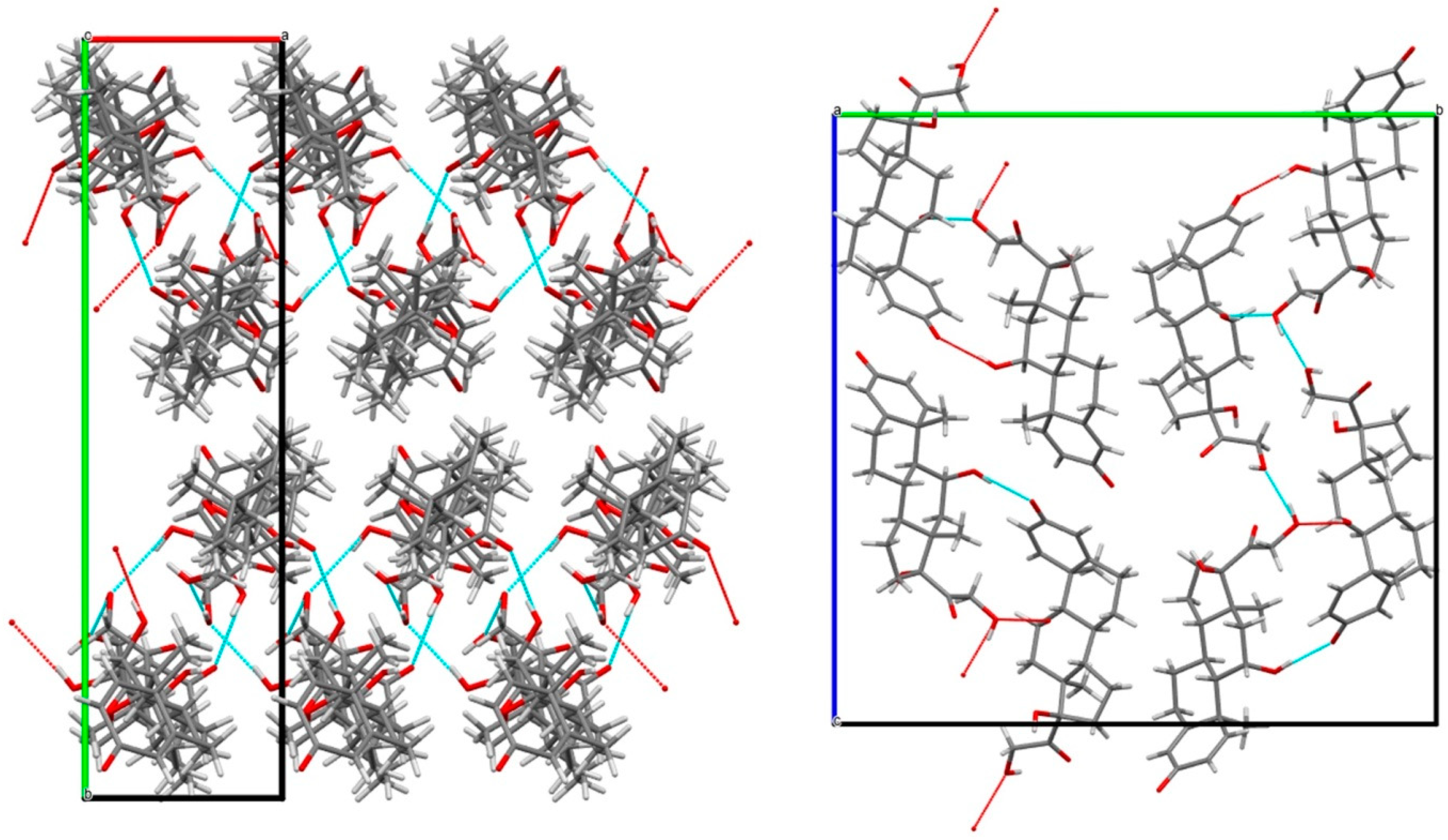
3.3. Classification of the Sesquihydrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Healy, A.M.; Worku, Z.A.; Kumar, D.M.; Madi, A. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Láng, P.; Kiss, V.; Ambrus, R.; Farkas, G.; Szabó-Révész, P.; Aigner, Z.; Várkonyi, E. Polymorph screening of an active material. J. Pharm. Biomed. Anal. 2013, 84, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Suitchmezian, V.; Jess, I.; Sehnert, J.; Seyfarth, L.; Senker, J.; Näther, C. Structural, Thermodynamic, and Kinetic Aspects of the Polymorphism and Pseudopolymorphism of Prednisolone (11,17α,21-Trihydroxy-1,4-pregnadien-3,20-dion). Cryst. Growth Des. 2008, 8, 98–107. [Google Scholar] [CrossRef]
- Veiga, M.D.; Cadorniga, R.; Lozano, R. Thermal study of prednisolone polymorphs. Thermochim. Acta 1985, 96, 111–115. [Google Scholar] [CrossRef]
- Corvis, Y.; Négrier, P.; Soulestin, J.; Espeau, P. New Melting Data of the Two Polymorphs of Prednisolone. J. Phys. Chem. B 2016, 120, 10839–10843. [Google Scholar] [CrossRef]
- BIOVIA Material Studio (19.1.0.2553) Software, Dassault System. 2019. Available online: https://www.3ds.com/products-services/biovia/ (accessed on 25 May 2023).
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications—Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- De Saint Jores, C.; Brandel, C.; Gharbi, N.; Sanselme, M.; Cardinael, P.; Coquerel, G. Limitations of Preferential Enrichment: A Case Study on Tryptophan Ethyl Ester Hydrochloride. Chem. Eng. Technol. 2019, 42, 1500–1504. [Google Scholar] [CrossRef]
- Fours, B.; Cartigny, Y.; Petit, S.; Coquerel, G. Formation of New Polymorphs without Any Nucleation Step. Desolvation of the Rimonabant Monohydrate: Directional Crystallisation Concomitant to Smooth Dehydration. Faraday Discuss. 2015, 179, 475–488. [Google Scholar] [CrossRef]
- Stephenson, G.A.; Groleau, E.G.; Kleemann, R.L.; Xu, W.; Rigsbee, D.R. Formation of Isomorphic Desolvates: Creating a Molecular Vacuum. J. Pharm. Sci. 1998, 87, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Variation of Peak Temperature with Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Shimanovich, R.; Cooke, M.; Peterson, M.L. A Rapid Approach to the Preliminary Assessment of the Physical Stability of Pharmaceutical Hydrates. J. Pharm. Sci. 2012, 101, 4013–4017. [Google Scholar] [CrossRef] [PubMed]
- Ravikiran, A.; Arthanareeswari, M.; Kamaraj, P.; Praveen, C. Nonisothermal kinetics analysis of the dehydration of ziprasidone hydrochloride monohydrate by thermogravimetry. Indian J. Pharm. Sci. 2013, 75, 361–364. [Google Scholar] [CrossRef]
- Neglur, R.; Grooff, D.; Hosten, E.; Aucamp, M.; Liebenberg, W. Approximation-based integral versus differential isoconversional approaches to the evaluation of kinetic parameters from thermogravimetry. J. Therm. Anal. Calorim. 2016, 123, 2599–2610. [Google Scholar] [CrossRef]
- Petit, S.; Coquerel, G. Mechanism of Several Solid−Solid Transformations between Dihydrated and Anhydrous Copper(II) 8-Hydroxyquinolinates. Proposition for a Unified Model for the Dehydration of Molecular Crystals. Chem. Mater. 1996, 8, 2247–2258. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformation Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Nanghia, A. Conformational Polymorphism in Organic Crystals. Acc. Chem. Res. 2008, 41, 595–604. [Google Scholar] [CrossRef]
- Brittain, H.G.; Morris, K.R.; Boerrigter, S.X.M. Structural Aspects of Solvatomorphic Systems. In Polymorphism in Pharmaceutical Solids, 2nd ed.; Brittain, H.G., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 233–281. [Google Scholar]
- Mimura, H.; Kitamura, S.; Kitagawa, T.; Kohda, S. Characterization of the Non-Stoichiometric and Isomorphic Hydration and Solvation in FK041 Clathrate. Colloids Surf. B Biointerfaces 2002, 26, 397–406. [Google Scholar] [CrossRef]
- Takahashi, M.; Uekusa, H. Dehydration and Rehydration Mechanisms of Pharmaceutical Crystals: Classification of Hydrates by Activation Energy. J. Pharm. Sci. 2022, 111, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Galwey, A.K. Structure and Order in Thermal Dehydrations of Crystalline Solids. Thermochim. Acta 2000, 355, 181–238. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemercier, A.; Couvrat, N.; Cartigny, Y.; Sanselme, M.; Corvis, Y.; Espeau, P.; Coquerel, G. Evidence of a New Crystalline Phase of Prednisolone Obtained from the Study of the Hydration–Dehydration Mechanisms of the Sesquihydrate. Pharmaceutics 2023, 15, 1694. https://doi.org/10.3390/pharmaceutics15061694
Lemercier A, Couvrat N, Cartigny Y, Sanselme M, Corvis Y, Espeau P, Coquerel G. Evidence of a New Crystalline Phase of Prednisolone Obtained from the Study of the Hydration–Dehydration Mechanisms of the Sesquihydrate. Pharmaceutics. 2023; 15(6):1694. https://doi.org/10.3390/pharmaceutics15061694
Chicago/Turabian StyleLemercier, Aurélien, Nicolas Couvrat, Yohann Cartigny, Morgane Sanselme, Yohann Corvis, Philippe Espeau, and Gérard Coquerel. 2023. "Evidence of a New Crystalline Phase of Prednisolone Obtained from the Study of the Hydration–Dehydration Mechanisms of the Sesquihydrate" Pharmaceutics 15, no. 6: 1694. https://doi.org/10.3390/pharmaceutics15061694
APA StyleLemercier, A., Couvrat, N., Cartigny, Y., Sanselme, M., Corvis, Y., Espeau, P., & Coquerel, G. (2023). Evidence of a New Crystalline Phase of Prednisolone Obtained from the Study of the Hydration–Dehydration Mechanisms of the Sesquihydrate. Pharmaceutics, 15(6), 1694. https://doi.org/10.3390/pharmaceutics15061694








