Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. ASOs
2.2. Animals
2.3. NB Preparation
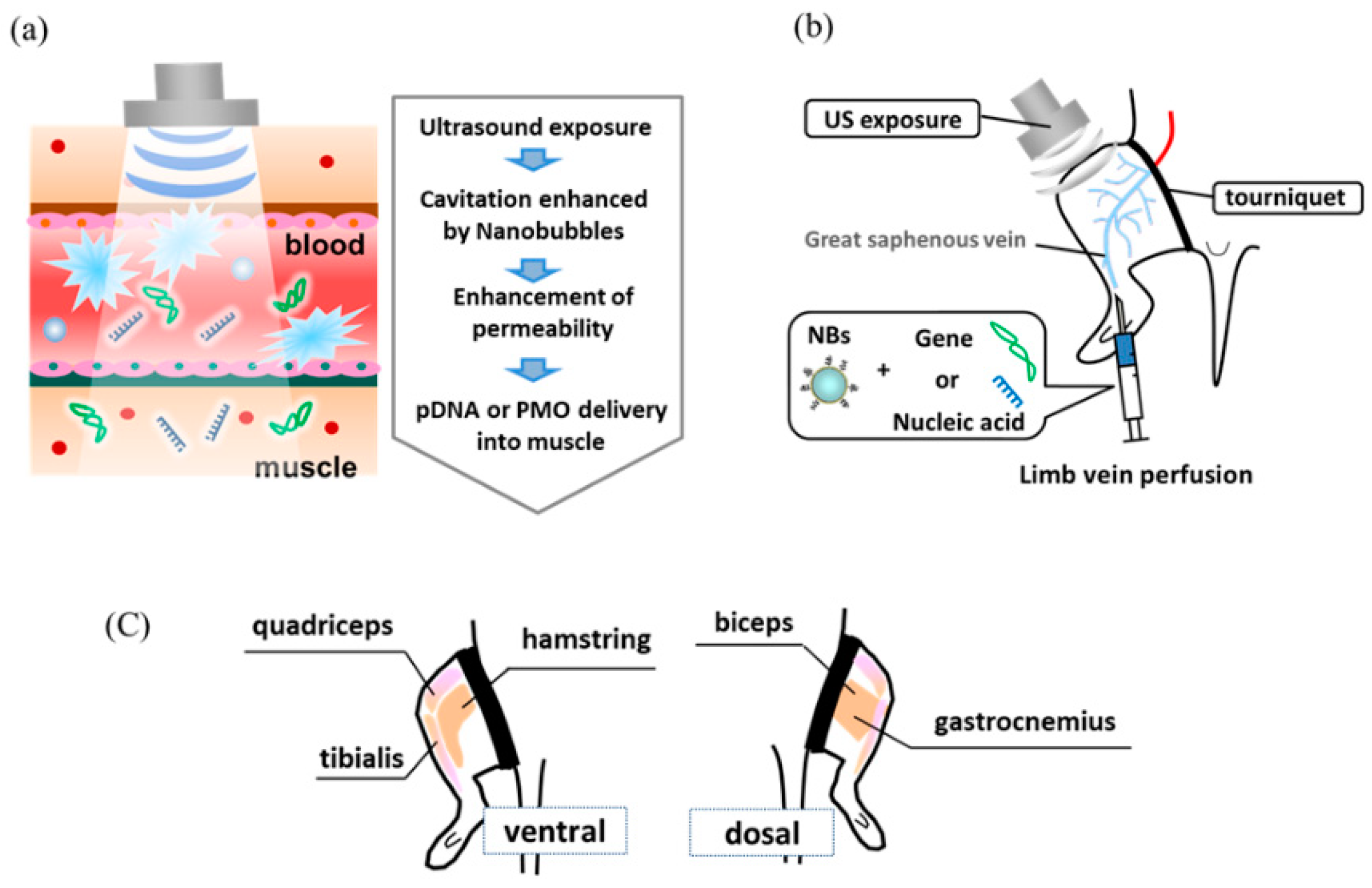
2.4. Intravenous or Intramuscular Delivery of Luciferase pDNA Vectors into the Muscle
2.5. In Vivo PMO Delivery into the Muscles of Mdx Mice Treated with NBs and US Exposure
2.6. Immunohistochemistry
2.7. Evans Blue Dye Uptake
2.8. Statistical Analyses
3. Results and Discussion
3.1. Effect of US Intensity on Luciferase Expression Following Treatment with NBs and US
3.2. Effect of US Intensity on Luciferase Expression Following Treatment with NBs and US
3.3. Comparison of Intravenous and Intramuscular Gene Delivery
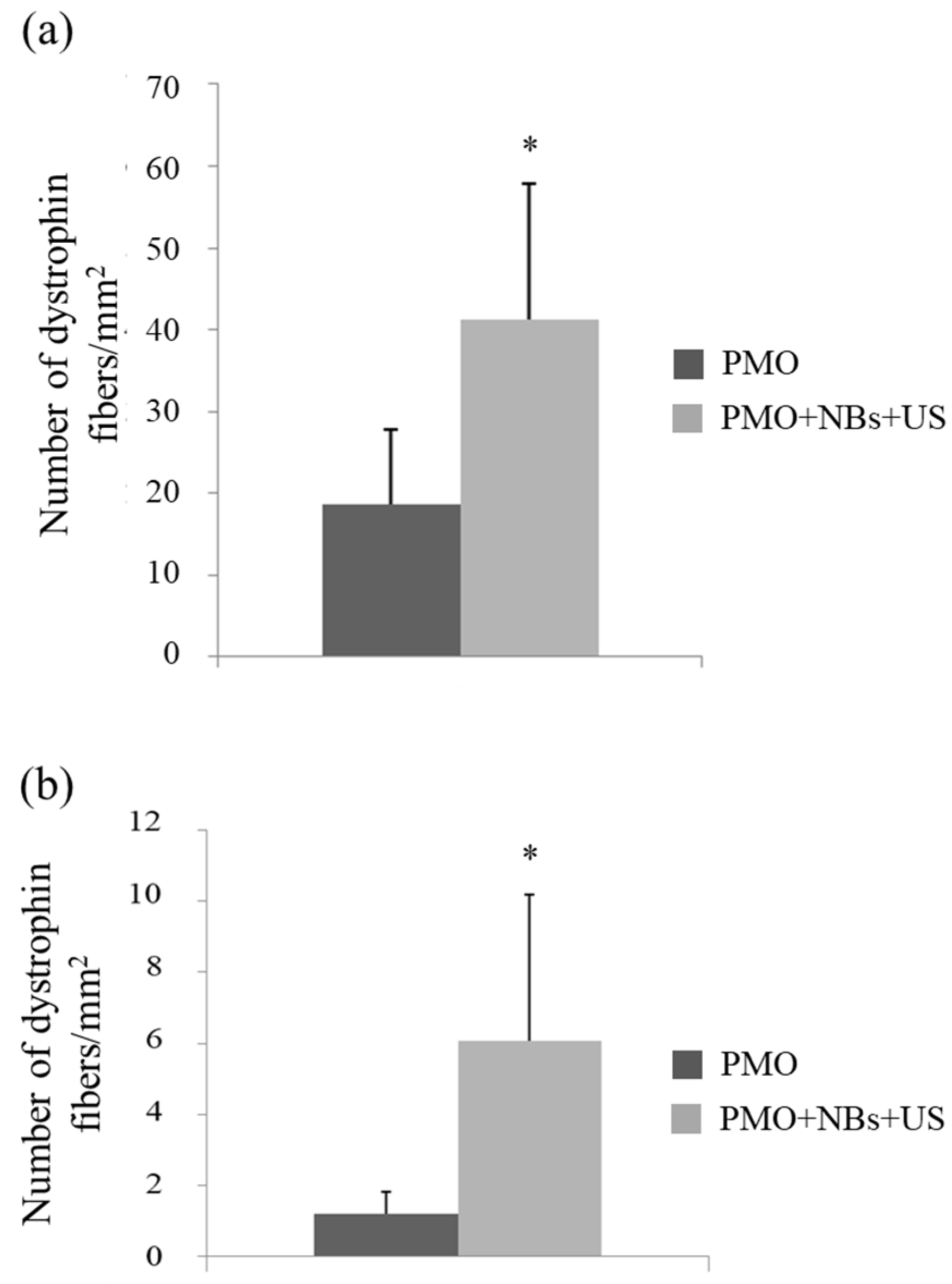
3.4. Intravenous PMO Delivery into the Muscle of DMD Model Mice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, E.P.; Brown, R.H., Jr.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Beggs, A.H.; Moyer, M.; Scherpf, S.; Heindrich, K.; Bettecken, T.; Meng, G.; Müller, C.R.; Lindlöf, M.; Kaariainen, H.; et al. The molecular basis for Duchenne versus Becker muscular dystrophy: Correlation of severity with type of deletion. Am. J. Hum. Genet. 1989, 45, 498–506. [Google Scholar]
- Monaco, A.P.; Bertelson, C.J.; Liechti-Gallati, S.; Moser, H.; Kunkel, L.M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988, 2, 90–95. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Fischbeck, K.H.; Brown, R.H.; Johnson, M.; Medori, R.; Loire, J.D.; Harris, J.B.; Waterston, R.; Brooke, M.; Specht, L.; et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N. Engl. J. Med. 1988, 318, 1363–1368. [Google Scholar] [CrossRef]
- Pramono, Z.A.; Takeshima, Y.; Alimsardjono, H.; Ishii, A.; Takeda, S.; Matsuo, M. Induction of exon skipping of the dystrophin transcript in lymphoblastoid cells by transfecting an antisense oligodeoxynucleotide complementary to an exon recognition sequence. Biochem. Biophys. Res. Commun. 1996, 226, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Honeyman, K.; Cheng, A.J.; Ly, T.; Lloyd, F.; Fletcher, S.; Morgan, J.E.; Partridge, T.A.; Wilton, S.D. Antisense induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 42–47. [Google Scholar] [CrossRef]
- Lu, Q.L.; Mann, C.J.; Lou, F.; Bou-Gharios, G.; Morris, G.E.; Xue, S.A.; Fletcher, S.; Partridge, T.A.; Wilton, S.D. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat. Med. 2003, 9, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Alter, J.; Lou, F.; Rabinowitz, A.; Yin, H.; Rosenfeld, J.; Wilton, S.D.; Partridge, T.A.; Lu, Q.L. Systemic delivery of morpholino oligonucleotide restores dystrophin expression body wide and improves dystrophic pathology. Nat. Med. 2006, 12, 175–177. [Google Scholar] [CrossRef]
- Gebski, B.L.; Mann, C.J.; Fletcher, S.; Wilton, S.D. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum. Mol. Genet. 2006, 12, 1801–1811. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Charleston, J.S.; Schnell, F.J.; Dworzak, J.; Donoghue, C.; Lewis, S.; Chen, L.; Young, G.D.; Milici, A.J.; Voss, J.; DeAlwis, U.; et al. Eteplirsen treatment for Duchenne muscular dystrophy: Exon skipping and dystrophin production. Neurology 2018, 90, e2146–e2154. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Nagata, T.; Saito, T.; Masuda, S.; Takeshita, E.; Sasaki, M.; Tachimori, H.; Nakamura, H.; Aoki, Y.; Takeda, S. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 2018, 10, eaan0713. [Google Scholar] [CrossRef]
- Summerton, J.; Weller, D. Morphoolino antisense oligomers design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef]
- Greenleaf, W.J.; Bolander, M.E.; Sarkar, G.; Goldring, M.B.; Greenleaf, J.F. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med. Biol. 1998, 24, 587–595. [Google Scholar] [CrossRef]
- Fechheimer, M.; Boylan, J.F.; Parker, S.; Sisken, J.E.; Patel, G.L.; Zimmer, S.G. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc. Natl. Acad. Sci. USA 1998, 84, 8463–8467. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Duvshani-Eshet, M.; Machluf, M. Therapeutic ultrasound optimization for gene delivery: A key factor achieving nuclear DNA localization. J. Control. Release 2005, 108, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Schratzberger, P.; Krainin, J.G.; Schratzberger, G.; Silver, M.; Ma, H.; Kearney, M.; Zuk, R.F.; Brisken, A.F.; Losordo, D.W.; Isner, J.M. Transcutaneous ultrasound augments naked DNA transfection of skeletal muscle. Mol. Ther. 2002, 6, 576–583. [Google Scholar] [CrossRef]
- Holmes, R.P.; Yeaman, L.D.; Taylor, R.G.; McCullough, D.L. Altered neutrophil permeability following shock wave exposure in vitro. J. Urol. 1992, 147, 733–737. [Google Scholar] [CrossRef]
- Skyba, D.M.; Price, R.J.; Linka, A.Z.; Skalak, T.C.; Kaul, S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 1998, 98, 290–293. [Google Scholar] [CrossRef]
- Li, T.; Tachibana, K.; Kuroki, M.; Kuroki, M. Gene transfer with echo-enhanced contrast agents: Comparison between Albunex, Optison, and Levovist in mice—Initial results. Radiology 2003, 229, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Tachibana, K.; Hiraoka, K.; Namba, T.; Yamasaki, K.; Hashiya, N.; Aoki, M.; Ogihara, T.; Yasufumi, K.; Morishita, R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 2002, 105, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.C.; Porter, T.; Culp, W.; Labell, R.; Matsunaga, T.; Zutshi, R. Therapeutic applications of lipid-coated microbubbles. Adv. Drug Deliv. Rev. 2004, 56, 1291–1314. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Hagisawa, K.; Tanaka, K.; Sawamura, K.; Utoguchi, N.; Nishioka, T.; Maruyama, K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control. Release 2007, 117, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Endo, Y.; Fukuyama, T.; Suzuki, R.; Takizawa, T.; Omata, D.; Maruyama, K.; Aramaki, Y. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J. Control. Release 2008, 132, 124–130. [Google Scholar] [CrossRef]
- Negishi, Y.; Matsuo, K.; Endo-Takahashi, Y.; Suzuki, K.; Matsuki, Y.; Takagi, N.; Suzuki, R.; Maruyama, K.; Aramaki, Y. Delivery of an angiogenic gene into ischemic muscle by novel Bubble liposomes followed by ultrasound exposure. Pharm. Res. 2011, 28, 712–719. [Google Scholar] [CrossRef]
- Negishi, Y.; Endo-Takahashi, Y.; Matsuki, Y.; Kato, Y.; Takagi, N.; Suzuki, R.; Maruyama, K.; Aramaki, Y. Systemic delivery systems of angiogenic gene by novel Bubble liposomes containing cationic lipid and ultrasound exposure. Mol. Pharm. 2012, 9, 1834–1840. [Google Scholar] [CrossRef]
- Negishi, Y.; Ishii, Y.; Shiono, H.; Akiyama, S.; Sekine, S.; Kojima, T.; Mayama, S.; Kikuchi, T.; Hamano, N.; Endo-Takahashi, Y.; et al. Bubble liposomes and ultrasound exposure improve localized morpholino oligomer delivery into the skeletal muscles of dystrophic mdx mice. Mol. Pharm. 2014, 11, 1053–1061. [Google Scholar] [CrossRef]
- Negishi, Y.; Yamane, M.; Kurihara, N.; Endo-Takahashi, Y.; Sashida, S.; Takagi, N.; Suzuki, R.; Maruyama, K. Enhancement of blood–brain barrier permeability and delivery of antisense oligonucleotides or plasmid DNA to the brain by the combination of Bubble Liposomes and high-intensity focused ultrasound. Pharmaceutics 2015, 7, 344–362. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Kurokawa, R.; Sato, K.; Takizawa, N.; Katagiri, F.; Hamano, N.; Suzuki, R.; Maruyama, K.; Nomizu, M.; Takagi, N.; et al. Ternary complexes of pDNA, neuron-binding peptide, and PEGylated polyethyleneimine for brain delivery with nano-bubbles and ultrasound. Pharmaceutics 2021, 13, 1003. [Google Scholar] [CrossRef]
- Yin, H.; Saleh, A.F.; Betts, C.; Camelliti, P.; Seow, Y.; Ashraf, S.; Arzumanov, A.; Hammond, S.; Merritt, T.; Gait, M.J.; et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol. Ther. 2011, 19, 1295–1303. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Zhang, G.; Griffin, J.B.; Hegge, J.O.; Huss, T.; Wolff, J.A. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve 2010, 41, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Pajusola, K.; Künnapuu, J.; Vuorikoski, S.; Soronen, J.; André, H.; Pereira, T.; Korpisalo, P.; Ylä-Herttuala, S.; Poellinger, L.; Alitalo, K. Stabilized HIF-1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 2005, 19, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S.; Shin, J.H.; Yuasa, K.; Nishiyama, A.; Kira, J.; Okada, T.; Takeda, S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol. Ther. 2009, 17, 73–80. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Danko, I.; Williams, P.; Herweijer, H.; Zhang, G.; Latendresse, J.S.; Bock, I.; Wolff, J.A. High expression of naked plasmid DNA in muscles of young rodents. Hum. Mol. Genet. 1997, 6, 1435–1443. [Google Scholar] [CrossRef]
- Matsuda, R.; Nishikawa, A.; Tanaka, H.J. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: Evidence of apoptosis in dystrophin-deficient muscle. J. Biochem. 1995, 118, 959–964. [Google Scholar] [CrossRef]
- Straub, V.; Rafael, J.A.; Chamberlain, J.S.; Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 1997, 139, 375–385. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–994. [Google Scholar] [CrossRef]
- Wright, A.V.; Nunez, J.K.; Doudna, J.A. Biology and applications of CRISPR systems: Harnessing nature’s toolbox for genome engineering. Cell 2016, 164, 29–44. [Google Scholar] [CrossRef]
- Amoasii, L.; Hildyard, J.C.W.; Li, H.; Sanchez-Ortiz, E.; Mireault, A.; Caballero, D.; Harron, R.; Stathopoulou, T.R.; Massey, C.; Shelton, J.M.; et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 2018, 362, 86–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Min, Y.L.; Sanchez-Ortiz, E.; Huang, J.; Mireault, A.A.; Shelton, J.M.; Kim, J.; Mammen, P.P.A.; Bassel-Duby, R.; et al. Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci. Adv. 2020, 19, eaay6812. [Google Scholar] [CrossRef]
- Min, Y.L.; Li, H.; Rodriguez-Caycedo, C.; Mireault, A.A.; Huang, J.; Shelton, J.M.; McAnally, J.R.; Amoasii, L.; Mammen, P.P.A.; Bassel-Duby, R.; et al. CRISPRCas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci. Adv. 2019, 6, eaav4324. [Google Scholar] [CrossRef] [PubMed]
- David, R.M.; Doherty, A.T. Viral vectors: The road to reducing genotoxicity. Toxicol. Sci. 2017, 155, 315–325. [Google Scholar] [CrossRef]
- Happi Mbakam, C.; Lamothe, G.; Tremblay, G.; Tremblay, J.P. CRISPR-Cas9 Gene Therapy for Duchenne Muscular Dystrophy. Neurotherapeutics 2022, 19, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Spunde, K.; Korotkaja, K.; Zajakina, A. Recombinant Viral Vectors for Therapeutic Programming of Tumour Microenvironment: Advantages and Limitations. Biomedicines 2022, 10, 2142. [Google Scholar] [CrossRef]
- Deng, L.; Yang, P.; Li, C.; Xie, L.; Lu, W.; Zhang, Y.; Liu, M.; Wang, G. Prolonged Control of Insulin-Dependent Diabetes via Intramuscular Expression of Plasmid-Encoded Single-Strand Insulin Analogue. Genes Dis. 2023, 10, 1101–1113. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y.; Nakamura, A.; Suzuki, D.; Ukai, S.; Sugimoto, K.; Moriyasu, F.; Takagi, N.; Suzuki, R.; Maruyama, K.; et al. PDNA-Loaded Bubble Liposomes as Potential Ultrasound Imaging and Gene Delivery Agents. Biomaterials 2013, 34, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Endo-Takahashi, Y.; Negishi, Y.; Nakamura, A.; Ukai, S.; Ooaku, K.; Oda, Y.; Sugimoto, K.; Moriyasu, F.; Takagi, N.; Suzuki, R.; et al. Systemic Delivery of MiR-126 by MiRNA-Loaded Bubble Liposomes for the Treatment of Hindlimb Ischemia. Sci. Rep. 2014, 4, 3883. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y. Microbubbles and Nanobubbles with Ultrasound for Systemic Gene Delivery. Pharmaceutics 2020, 12, 964. [Google Scholar] [CrossRef] [PubMed]
- Budker, V.; Zhang, G.; Danko, I.; Williams, P.; Wolff, J. The Efficient Expression of Intravascularly Delivered DNA in Rat Muscle. Gene Ther. 1998, 5, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wooddell, C.I.; Hegge, J.O.; Griffin, J.B.; Huss, T.; Braun, S.; Wolff, J.A. Functional Efficacy of Dystrophin Expression from Plasmids Delivered to Mdx Mice by Hydrodynamic Limb Vein Injection. Hum. Gene Ther. 2010, 21, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, Y.T.; Le Gall, T.; Midoux, P.; Guégan, P.; Braun, S.; Montier, T. Gene Transfer to Skeletal Muscle Using Hydrodynamic Limb Vein Injection: Current Applications, Hurdles and Possible Optimizations. J. Gene Med. 2020, 22, e3150. [Google Scholar] [CrossRef]
- Walsh, A.P.G.; Gordon, H.N.; Peter, K.; Wang, X. Ultrasonic Particles: An Approach for Targeted Gene Delivery. Adv. Drug Deliv. Rev. 2021, 179, 113998. [Google Scholar] [CrossRef]
- Miller, D.L.; Quddus, J. Diagnostic Ultrasound Activation of Contrast Agent Gas Bodies Induces Capillary Rupture in Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 10179–10184. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, S.; Mayama, S.; Nishijima, N.; Kojima, T.; Endo-Takahashi, Y.; Ishii, Y.; Shiono, H.; Akiyama, S.; Sakurai, A.; Sashida, S.; et al. Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound. Pharmaceutics 2023, 15, 1665. https://doi.org/10.3390/pharmaceutics15061665
Sekine S, Mayama S, Nishijima N, Kojima T, Endo-Takahashi Y, Ishii Y, Shiono H, Akiyama S, Sakurai A, Sashida S, et al. Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound. Pharmaceutics. 2023; 15(6):1665. https://doi.org/10.3390/pharmaceutics15061665
Chicago/Turabian StyleSekine, Shohko, Sayaka Mayama, Nobuaki Nishijima, Takuo Kojima, Yoko Endo-Takahashi, Yuko Ishii, Hitomi Shiono, Saki Akiyama, Akane Sakurai, Sanae Sashida, and et al. 2023. "Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound" Pharmaceutics 15, no. 6: 1665. https://doi.org/10.3390/pharmaceutics15061665
APA StyleSekine, S., Mayama, S., Nishijima, N., Kojima, T., Endo-Takahashi, Y., Ishii, Y., Shiono, H., Akiyama, S., Sakurai, A., Sashida, S., Hamano, N., Tada, R., Suzuki, R., Maruyama, K., & Negishi, Y. (2023). Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound. Pharmaceutics, 15(6), 1665. https://doi.org/10.3390/pharmaceutics15061665





