Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy?
Abstract
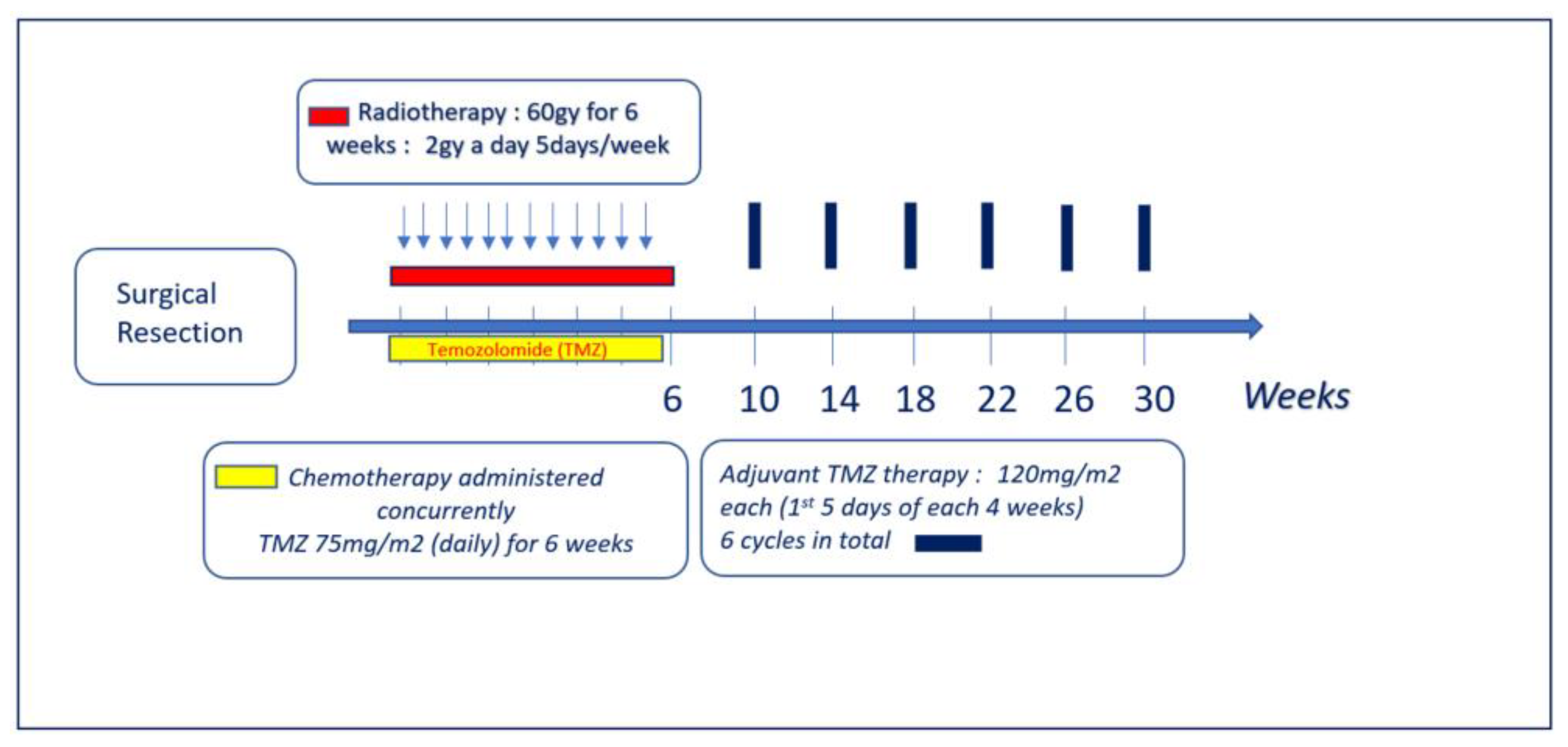
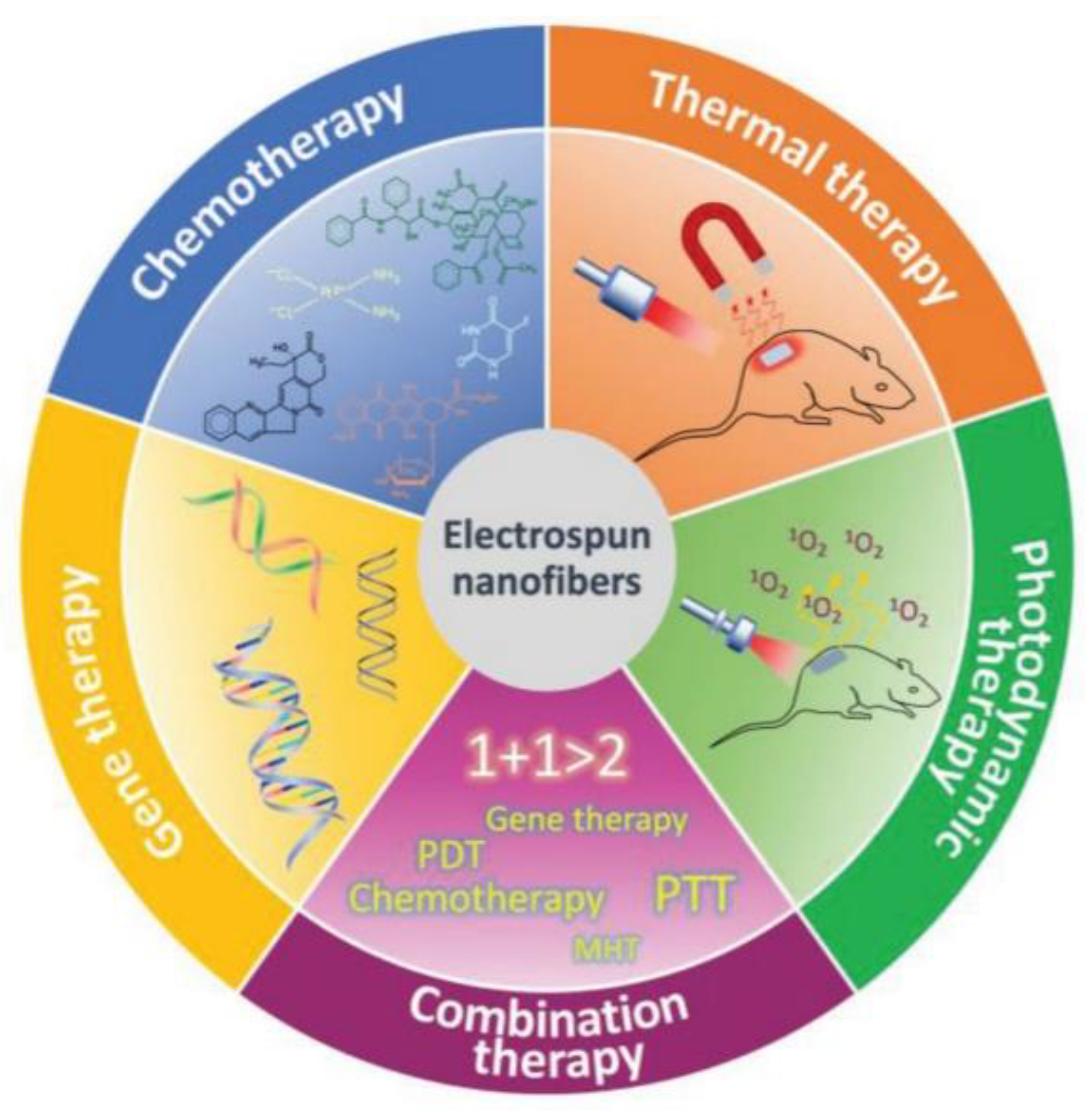
:1. Introduction
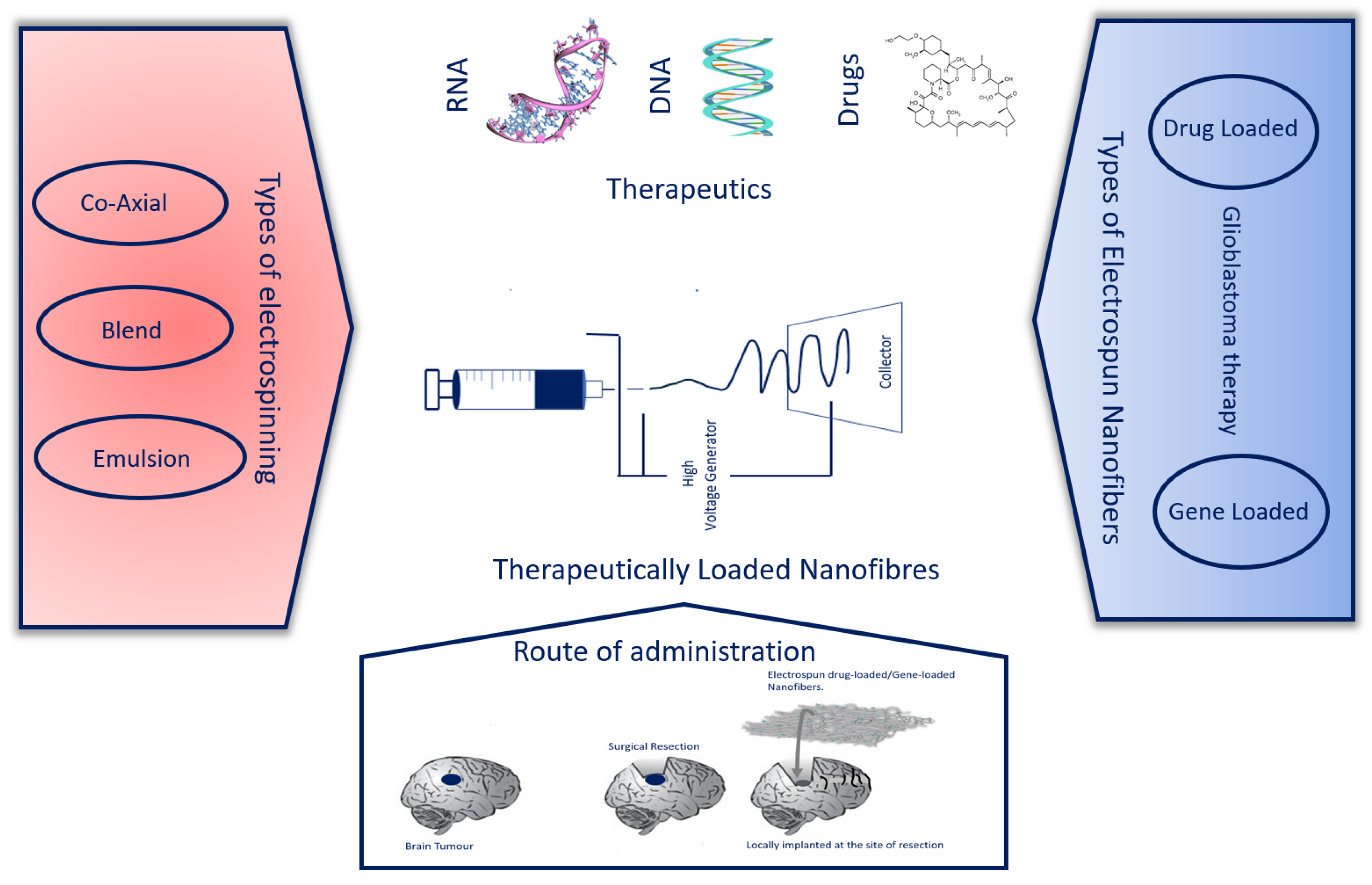
2. Electrospinning
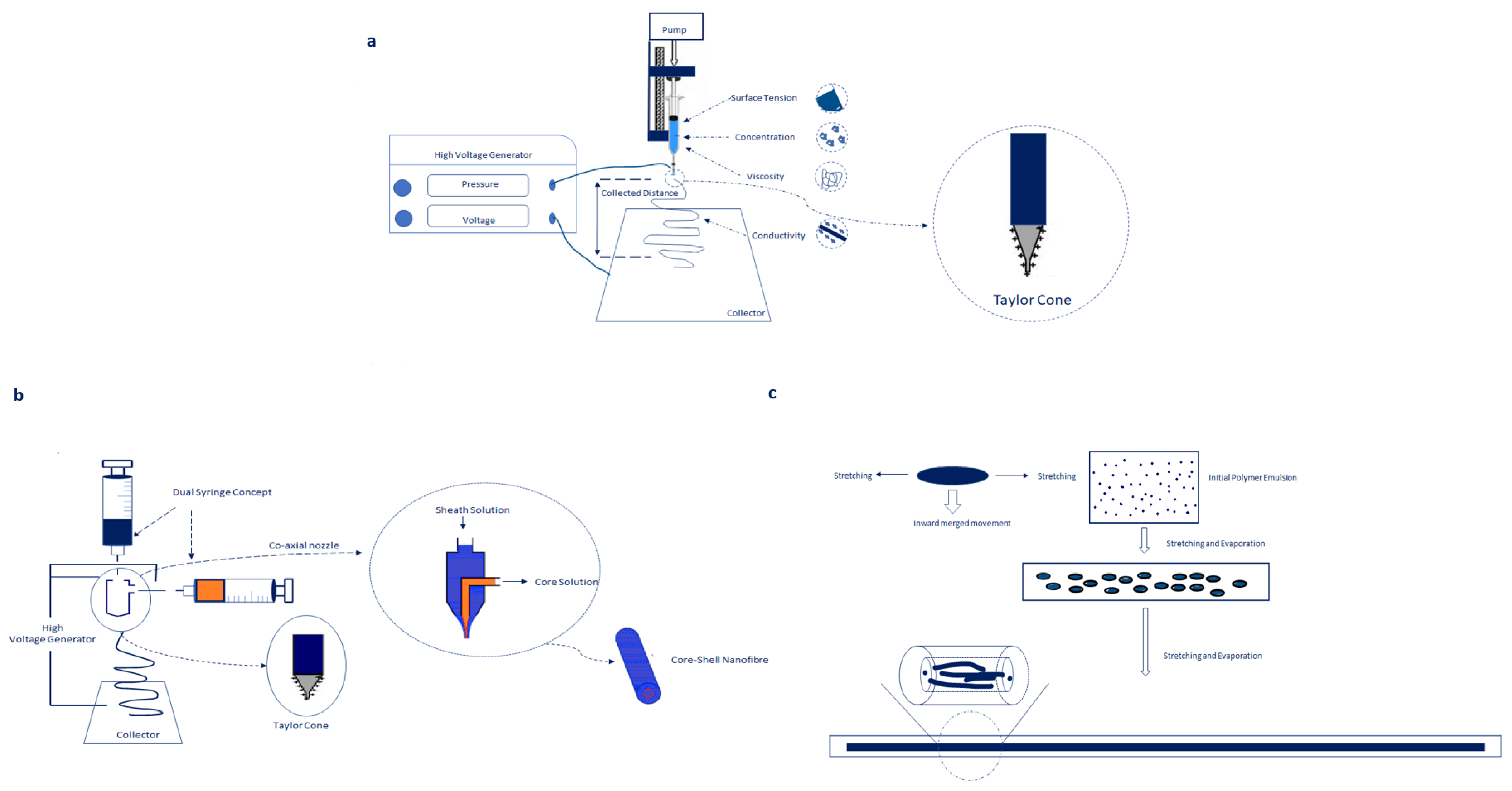
2.1. Co-Axial Electrospinning
2.2. Blend Electrospinning
2.3. Emulsion Electrospinning
3. Release Kinetics of Electrospun Nanofibres Is Dependent on Physico-Chemical Properties of Electrospun Nanofibres
3.1. Electrospun Nanofibres for Hydrophobic Drugs in Cancer Therapy
3.2. Electrospun Nanofibres for Hydrophilic Drugs in Cancer Therapy
3.3. Mechanical Properties of Engineered Electrospun Scaffolds in GBM
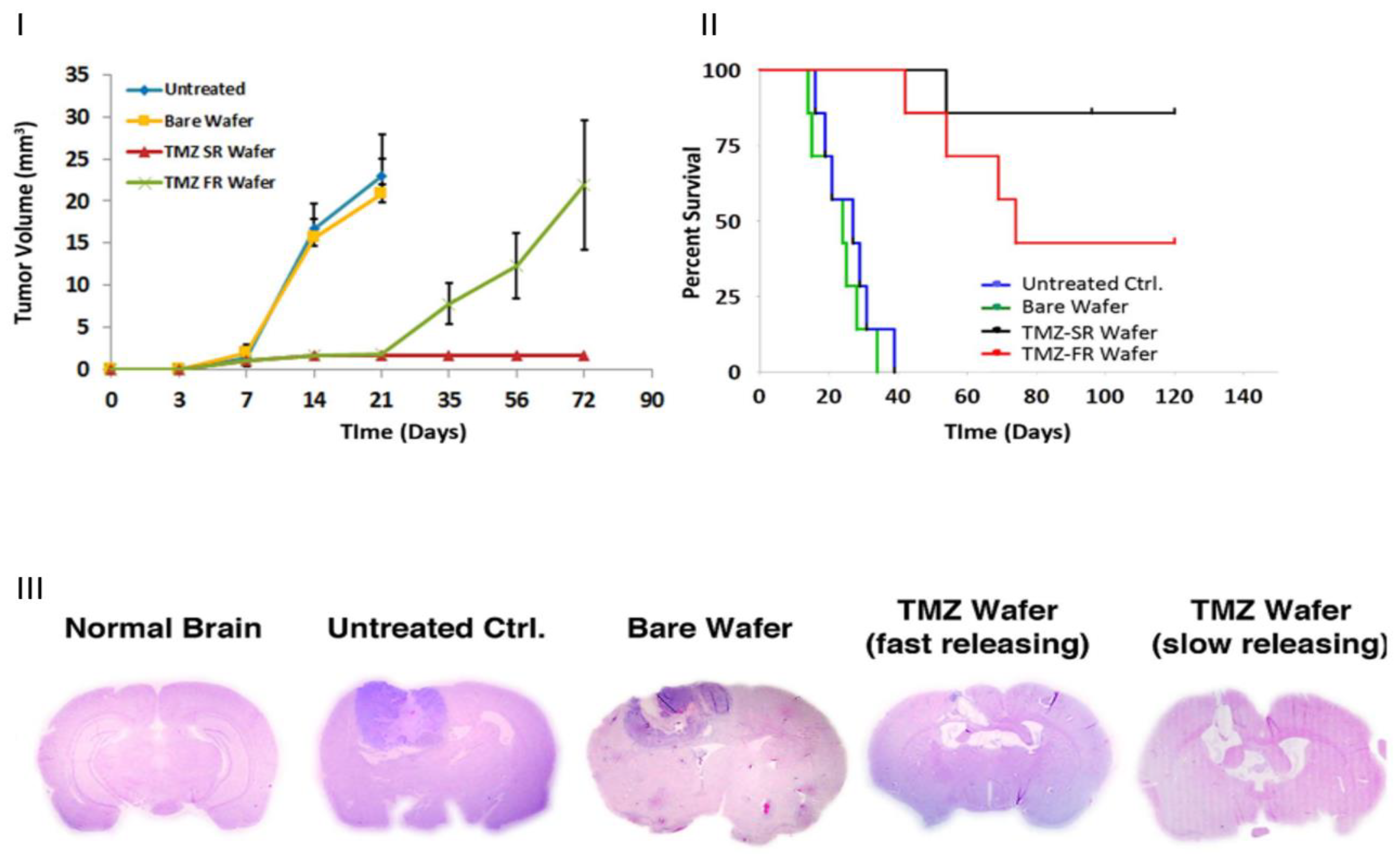
3.4. Release Kinetics of Drug-Loaded and Gene-Loaded Nanofibres for Cancer Therapy
4. Delivery of Gene Therapy Drugs Using Electrospun Polymeric Nanofibres
Encapsulation of STING Agonists for Tumour Regression in GBM
5. Conclusions, Challenges and Future Perspectives
5.1. Challenges
5.2. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ranganath, S.H.; Kee, I.; Krantz, W.B.; Chow, P.K.-H.; Wang, C.-H. Hydrogel Matrix Entrapping PLGA-Paclitaxel Microspheres: Drug Delivery with Near Zero-Order Release and Implantability Advantages for Malignant Brain Tumour Chemotherapy. Pharm. Res. 2009, 26, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Cosco, D.; Paolino, D.; Fresta, M. Nanoparticulate Devices for Brain Drug Delivery: Nanoparticulate Devices. Med. Res. Rev. 2011, 31, 716–756. [Google Scholar] [CrossRef] [PubMed]
- Short, M.P.; Choi, B.C.; Lee, J.K.; Malick, A.; Breakefield, X.O.; Martuza, R.L. Gene Delivery to Glioma Cells in Rat Brain by Grafting of a Retrovirus Packaging Cell Line. J. Neurosci. Res. 1990, 27, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Zero Order Kinetics of Drug Release. Available online: https://ukdiss.com/examples/zero-order-kinetics-drug-release.php (accessed on 16 June 2021).
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, 1801183. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M. Recent Advances in Brain Tumor Therapy: Application of Electrospun Nanofibers. Drug Discov. Today 2018, 23, 912–919. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Borges, J.P. Recent Advances in Magnetic Electrospun Nanofibers for Cancer Theranostics Application. Prog. Nat. Sci. Mater. Int. 2021, 31, 835–844. [Google Scholar] [CrossRef]
- Qiu, K.; He, C.; Feng, W.; Wang, W.; Zhou, X.; Yin, Z.; Chen, L.; Wang, H.; Mo, X. Doxorubicin-Loaded Electrospun Poly(l-Lactic Acid)/Mesoporous Silica Nanoparticles Composite Nanofibers for Potential Postsurgical Cancer Treatment. J. Mater. Chem. B 2013, 1, 4601. [Google Scholar] [CrossRef]
- Jin, C.; Bai, L.; Wu, H.; Liu, J.; Guo, G.; Chen, J. Paclitaxel-Loaded Poly(D,L-Lactide-Co-Glycolide) Nanoparticles for Radiotherapy in Hypoxic Human Tumor Cells in Vitro. Cancer Biol. Ther. 2008, 7, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.A.; Liu, R.; Freedman, J.D.; Padera, R.; Schwartz, J.; Colson, Y.L.; Grinstaff, M.W. Prevention of Lung Cancer Recurrence Using Cisplatin-Loaded Superhydrophobic Nanofiber Meshes. Biomaterials 2016, 76, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, M.; Abdali, Z.; Liu, S.; Miller, D.W. Salinomycin-Loaded Nanofibers for Glioblastoma Therapy. Sci. Rep. 2018, 8, 9377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano-Feinholz, S.; Salazar-Ramiro, A.; Muñoz-Sandoval, E.; Magaña-Maldonado, R.; Pedro, N.H.; López, E.R.; Aguilar, A.G.; García, A.S.; Sotelo, J.; de la Cruz, V.P.; et al. Cytotoxicity Induced by Carbon Nanotubes in Experimental Malignant Glioma. Int. J. Nanomed. 2017, 12, 6005–6026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, Z.; Yesmurzayeva, N.; Larue, L.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. New Targeted Gold Nanorods for the Treatment of Glioblastoma by Photodynamic Therapy. J. Clin. Med. 2019, 8, 2205. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Veroniaina, H.; Su, N.; Sha, K.; Jiang, F.; Wu, Z.; Qi, X. Applications and Developments of Gene Therapy Drug Delivery Systems for Genetic Diseases. Asian J. Pharm. Sci. 2021, 16, 687–703. [Google Scholar] [CrossRef]
- Okura, H.; Smith, C.A.; Rutka, J.T. Gene Therapy for Malignant Glioma. Mol. Cell Ther. 2014, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jin, G.; Jang, J.-H. Electrospun Nanofibers as Versatile Interfaces for Efficient Gene Delivery. J. Biol. Eng. 2014, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Apoptotic Cell Signaling in Cancer Progression and Therapy. Integr. Biol. 2011, 3, 279–296. [Google Scholar] [CrossRef]
- Caffery, B.; Lee, J.; Alexander-Bryant, A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, G.H.; Lee, D.S. Stimulus-Sensitive Polymeric Nanoparticles and Their Applications as Drug and Gene Carriers. Adv. Healthc. Mater. 2013, 2, 388–417. [Google Scholar] [CrossRef]
- Cojocaru, E.; Ghitman, J.; Stan, R. Electrospun-Fibrous-Architecture-Mediated Non-Viral Gene Therapy Drug Delivery in Regenerative Medicine. Polymers 2022, 14, 2647. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.F.; Young, J.S.; Aghi, M.K. Using Viral Vectors to Deliver Local Immunotherapy to Glioblastoma. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Deldar, Y.; Zarghami, F.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Antioxidant Effects of Chrysin-Loaded Electrospun Nanofibrous Mats on Proliferation and Stemness Preservation of Human Adipose-Derived Stem Cells. Cell Tissue Bank. 2017, 18, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An Update on Clinical Applications of Electrospun Nanofibers for Skin Bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef]
- Laha, A.; Sharma, C.S.; Majumdar, S. Sustained Drug Release from Multi-Layered Sequentially Crosslinked Electrospun Gelatin Nanofiber Mesh. Mater. Sci. Eng. C 2017, 76, 782–786. [Google Scholar] [CrossRef]
- Preparation of Core-Sheath Composite Nanofibers by Emulsion Electrospinning–Xu–2006–Macromolecular Rapid Communications—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/marc.200600384 (accessed on 6 May 2021).
- Ghitman, J.; Biru, E.I.; Cojocaru, E.; Pircalabioru, G.G.; Vasile, E.; Iovu, H. Design of New Bioinspired GO-COOH Decorated Alginate/Gelatin Hybrid Scaffolds with Nanofibrous Architecture: Structural, Mechanical and Biological Investigations. RSC Adv. 2021, 11, 13653–13665. [Google Scholar] [CrossRef]
- Cavo, M.; Serio, F.; Kale, N.R.; D’Amone, E.; Gigli, G.; del Mercato, L.L. Electrospun Nanofibers in Cancer Research: From Engineering of in Vitro 3D Cancer Models to Therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic Organic-Inorganic Hybrid Hydrogel Electrospinning Periosteum for Accelerating Bone Regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef]
- Yan, E.; Fan, Y.; Sun, Z.; Gao, J.; Hao, X.; Pei, S.; Wang, C.; Sun, L.; Zhang, D. Biocompatible Core–Shell Electrospun Nanofibers as Potential Application for Chemotherapy against Ovary Cancer. Mater. Sci. Eng. C 2014, 41, 217–223. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun Fibers and Their Application in Drug Controlled Release, Biological Dressings, Tissue Repair, and Enzyme Immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Akhgari, A.; Shakib, Z.; Sanati, S. A Review on Electrospun Nanofibers for Oral Drug Delivery. Nanomed. J. 2017, 4, 197–207. [Google Scholar] [CrossRef]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent Advances in Electrospun Nanofiber-Mediated Drug Delivery Strategies for Localized Cancer Chemotherapy. J. Biomed. Mater. Res. A 2020, 108, 1444–1458. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Xu, F.; Xu, Y.; Yu, D. Zero-Order Controlled Release Nanofibers Fabricated Using Coaxial Electrospinning with Polymer Dilute Solution as a Sheath Fluid. Shanghai Ligong Daxue Xuebao/J. Univ. Shanghai Sci. Technol. 2015, 37, 165–168. [Google Scholar] [CrossRef]
- Qin, X. Coaxial Electrospinning of Nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 41–71. ISBN 978-0-08-100907-9. [Google Scholar]
- Sukumar, U.K.; Packirisamy, G. Bioactive Core–Shell Nanofiber Hybrid Scaffold for Efficient Suicide Gene Transfection and Subsequent Time Resolved Delivery of Prodrug for Anticancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 18717–18731. [Google Scholar] [CrossRef]
- Jin, Z.; Li, X.; Liu, B.; Yan, X.; Han, S.; Xu, T.; Wu, A. Coaxial Bioprinted Microfibers with Mesenchymal Stem Cells for Glioma Microenvironment Simulation. Bio-Des. Manuf. 2022, 5, 348–357. [Google Scholar] [CrossRef]
- Coimbra, P.; Santos, P.; Alves, P.; Miguel, S.P.; Carvalho, M.P.; de Sá, K.D.; Correia, I.J.; Ferreira, P. Coaxial Electrospun PCL/Gelatin-MA Fibers as Scaffolds for Vascular Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 159, 7–15. [Google Scholar] [CrossRef]
- de Souza, S.O.L.; Guerra, M.C.A.; Heneine, L.G.D.; de Oliveira, C.R.; Junior, A.D.S.C.; Fialho, S.L.; Oréfice, R.L. Biodegradable Core-Shell Electrospun Nanofibers Containing Bevacizumab to Treat Age-Related Macular Degeneration. J. Mater. Sci. Mater. Med. 2018, 29, 173. [Google Scholar] [CrossRef]
- Iqbal, S.; Rashid, M.H.; Arbab, A.S.; Khan, M. Encapsulation of Anticancer Drugs (5-Fluorouracil and Paclitaxel) into Polycaprolactone (PCL) Nanofibers and In Vitro Testing for Sustained and Targeted Therapy. J. Biomed. Nanotechnol. 2017, 13, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, S.; Zamani, M.; Prabhakaran, M.P. Prabhakaran Advances in Drug Delivery via Electrospun and Electrosprayed Nanomaterials. IJN 2013, 8, 2997. [Google Scholar] [CrossRef] [Green Version]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. 11—Blend Electrospinning, Coaxial Electrospinning, and Emulsion Electrospinning Techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 325–347. ISBN 978-0-08-102198-9. [Google Scholar]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the Drug Compatibility with Polymer Solution on the Release Kinetics of Electrospun Fiber Formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion Electrospinning: Fundamentals, Food Applications and Prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Samanta, A.; Nandan, B.; Srivastava, R.K. Morphology of Electrospun Fibers Derived from High Internal Phase Emulsions. J. Colloid Interface Sci. 2016, 471, 29–36. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Wang, H.; Liu, C.; Yan, S.; Li, X. Antitumor Activities of Emulsion Electrospun Fibers with Core Loading of Hydroxycamptothecin via Intratumoral Implantation. Int. J. Pharm. 2012, 425, 19–28. [Google Scholar] [CrossRef]
- Prieto, E.I.; Mojares, E.B.A.; Cortez, J.J.M.; Vasquez, M.R., Jr. Electrospun Nanofiber Scaffolds for the Propagation and Analysis of Breast Cancer Stem Cells in Vitro. Biomed. Mater. 2021, 16, 035004. [Google Scholar] [CrossRef]
- Norouzi, M.; Soleimani, M.; Shabani, I.; Atyabi, F.; Ahvaz, H.H.; Rashidi, A. Protein Encapsulated in Electrospun Nanofibrous Scaffolds for Tissue Engineering Applications: Protein Encapsulated in Nanofibers. Polym. Int. 2013, 62, 1250–1256. [Google Scholar] [CrossRef]
- Jain, A.; Betancur, M.; Patel, G.D.; Valmikinathan, C.M.; Mukhatyar, V.J.; Vakharia, A.; Pai, S.B.; Brahma, B.; MacDonald, T.J.; Bellamkonda, R.V. Guiding Intracortical Brain Tumour Cells to an Extracortical Cytotoxic Hydrogel Using Aligned Polymeric Nanofibres. Nat. Mater. 2014, 13, 308–316. [Google Scholar] [CrossRef]
- Walther, M.; Rohde, F.; Kielholz, T.; Windbergs, M. Physico-Chemical Analysis of Electrospun Fibers—A Systematic Approach. Eur. J. Pharm. Biopharm. 2022, 171, 60–71. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Allen, C. Polymer–Drug Compatibility: A Guide to the Development of Delivery Systems for the Anticancer Agent, Ellipticine. J. Pharm. Sci. 2004, 93, 132–143. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Raza, Z.A.; Nazir, A. Current Applications of Electrospun Polymeric Nanofibers in Cancer Therapy. Mater. Sci. Eng. C 2019, 97, 966–977. [Google Scholar] [CrossRef]
- Pitz, M.; Elpers, M.; Nukovic, A.; Wilde, S.; Gregory, A.J.; Alexander-Bryant, A. De Novo Self-Assembling Peptides Mediate the Conversion of Temozolomide and Delivery of a Model Drug into Glioblastoma Multiforme Cells. Biomedicines 2022, 10, 2164. [Google Scholar] [CrossRef] [PubMed]
- Lebedenko, C.G.; Murray, M.E.; Goncalves, B.G.; Perez, D.S.; Lambo, D.J.; Banerjee, I.A. Interactions of Nanoscale Self-Assembled Peptide-Based Assemblies with Glioblastoma Cell Models and Spheroids. ACS Omega 2023, 8, 12124–12143. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Li, L.; Ding, S.; Zhou, S. Electrospun Micelles/Drug-Loaded Nanofibers for Time-Programmed Multi-Agent Release: Electrospun Micelles/Drug-Loaded Nanofibers. Macromol. Biosci. 2014, 14, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and Controlled Release of a Hydrophilic Antibiotic Using Poly(Lactide-Co-Glycolide)-Based Electrospun Nanofibrous Scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Sultanova, Z.; Kaleli, G.; Kabay, G.; Mutlu, M. Controlled Release of a Hydrophilic Drug from Coaxially Electrospun Polycaprolactone Nanofibers. Int. J. Pharm. 2016, 505, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, L.; Xu, X.; Wang, X.; Chen, X.; Liang, Q.; Zeng, J.; Jing, X. Ultrafine Medicated Fibers Electrospun from W/O Emulsions. J. Control. Release 2005, 108, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Laha, A.; Yadav, S.; Majumdar, S.; Sharma, C.S. In-Vitro Release Study of Hydrophobic Drug Using Electrospun Cross-Linked Gelatin Nanofibers. Biochem. Eng. J. 2016, 105, 481–488. [Google Scholar] [CrossRef]
- Li, J.-J.; Yang, Y.-Y.; Yu, D.-G.; Du, Q.; Yang, X.-L. Fast Dissolving Drug Delivery Membrane Based on the Ultra-Thin Shell of Electrospun Core-Shell Nanofibers. Eur. J. Pharm. Sci. 2018, 122, 195–204. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable Electrospun Fibers for Drug Delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.-H. Electrospun Micro- and Nanofibers for Sustained Delivery of Paclitaxel to Treat C6 Glioma in Vitro. Pharm. Res. 2006, 23, 1817–1826. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Luo, X.; Yang, Y.; Cui, W.; Zou, J.; Zhou, S. Release Modulation and Cytotoxicity of Hydroxycamptothecin-Loaded Electrospun Fibers with 2-Hydroxypropyl-β-Cyclodextrin Inoculations. Int. J. Pharm. 2010, 391, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar] [CrossRef] [Green Version]
- Ramazani, F.; Chen, W.; van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for Encapsulation of Small Hydrophilic and Amphiphilic Drugs in PLGA Microspheres: State-of-the-Art and Challenges. Int. J. Pharm. 2016, 499, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Windbergs, M. Controlled Dual Drug Release by Coaxial Electrospun Fibers—Impact of the Core Fluid on Drug Encapsulation and Release. Int. J. Pharm. 2019, 556, 363–371. [Google Scholar] [CrossRef]
- Yu, L.; Ci, T.; Zhou, S.; Zeng, W.; Ding, J. The Thermogelling PLGA–PEG–PLGA Block Copolymer as a Sustained Release Matrix of Doxorubicin. Biomater. Sci. 2013, 1, 411–420. [Google Scholar] [CrossRef]
- Arpicco, S.; Battaglia, L.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Milla, P.; Peira, E.; Rocco, F.; et al. Recent Studies on the Delivery of Hydrophilic Drugs in Nanoparticulate Systems. J. Drug Deliv. Sci. Technol. 2016, 32, 298–312. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of Encapsulating Hydrophobic Drugs in PLA/PLGA Microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Luo, H.; Jie, T.; Zheng, L.; Huang, C.; Chen, G.; Cui, W. Electrospun Nanofibers for Cancer Therapy. In Bio-Nanomedicine for Cancer Therapy; Fontana, F., Santos, H.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1295, pp. 163–190. ISBN 978-3-030-58173-2. [Google Scholar]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current Strategies for Sustaining Drug Release from Electrospun Nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.F.; Suarez, D.; Rocha, J.C.B.; de Carvalho Teixeira, A.V.N.; Cortés, M.E.; De Sousa, F.B.; Sinisterra, R.D. Electrospun Nanofibers of PolyCD/PMAA Polymers and Their Potential Application as Drug Delivery System. Mater. Sci. Eng. C 2015, 54, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Viry, L.; Moulton, S.E.; Romeo, T.; Suhr, C.; Mawad, D.; Cook, M.; Wallace, G.G. Emulsion-Coaxial Electrospinning: Designing Novel Architectures for Sustained Release of Highly Soluble Low Molecular Weight Drugs. J. Mater. Chem. 2012, 22, 11347. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Ortega, M.M.; Montaño-Figueroa, A.G.; Rodríguez-Félix, D.E.; Munive, G.T.; Herrera-Franco, P.J. Amoxicillin Embedded in Cellulose Acetate-Poly (Vinyl Pyrrolidone) Fibers Prepared by Coaxial Electrospinning: Preparation and Characterization. Mater. Lett. 2012, 76, 250–254. [Google Scholar] [CrossRef]
- Zupančič, Š.; Casula, L.; Rijavec, T.; Lapanje, A.; Luštrik, M.; Fadda, A.M.; Kocbek, P.; Kristl, J. Sustained Release of Antimicrobials from Double-Layer Nanofiber Mats for Local Treatment of Periodontal Disease, Evaluated Using a New Micro Flow-through Apparatus. J. Control. Release 2019, 316, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, L.; Mueller, A.; Carlson, M.A.; Teusink, M.J.; Shuler, F.D.; Xie, J. Twisting Electrospun Nanofiber Fine Strips into Functional Sutures for Sustained Co-Delivery of Gentamicin and Silver. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1435–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohrabi, A.; Shaibani, P.M.; Etayash, H.; Kaur, K.; Thundat, T. Sustained Drug Release and Antibacterial Activity of Ampicillin Incorporated Poly(Methyl Methacrylate)–Nylon6 Core/Shell Nanofibers. Polymer 2013, 54, 2699–2705. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, S.; Xia, T.; Yao, Q.; Li, H.; Wang, B.; Wang, J.; Li, X.; Su, W. Anti-Neoplastic Cytotoxicity of SN-38-Loaded PCL/Gelatin Electrospun Composite Nanofiber Scaffolds against Human Glioblastoma Cells In Vitro. J. Pharm. Sci. 2015, 104, 4345–4354. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, X.; Lu, T.; Wang, X.; Yang, L.; Jing, X. BCNU-Loaded PEG–PLLA Ultrafine Fibers and Their In Vitro Antitumor Activity against Glioma C6 Cells. J. Control. Release 2006, 114, 307–316. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.-S.; Chow, P.K.-H.; Wang, C.-H. The Use of Submicron/Nanoscale PLGA Implants to Deliver Paclitaxel with Enhanced Pharmacokinetics and Therapeutic Efficacy in Intracranial Glioblastoma in Mice. Biomaterials 2010, 31, 5199–5207. [Google Scholar] [CrossRef]
- Xie, J.; Tan, R.S.; Wang, C.-H. Biodegradable Microparticles and Fiber Fabrics for Sustained Delivery of Cisplatin to Treat C6 Glioma in Vitro. J. Biomed. Mater. Res. Part A 2008, 85A, 897–908. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG–PLA Fibers Loaded with Both Paclitaxel and Doxorubicin Hydrochloride and Their in Vitro Cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef]
- Ni, S.; Fan, X.; Wang, J.; Qi, H.; Li, X. Biodegradable Implants Efficiently Deliver Combination of Paclitaxel and Temozolomide to Glioma C6 Cancer Cells In Vitro. Ann. Biomed. Eng. 2014, 42, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Sadeghi, G.M.M.; Haririan, I. Electrospun Biocompatible Poly (ε-Caprolactonediol)-Based Polyurethane Core/Shell Nanofibrous Scaffold for Controlled Release of Temozolomide. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 361–366. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.K.; Panikar, D.; Nair, S.V.; et al. Theranostic 3-Dimensional Nano Brain-Implant for Prolonged and Localized Treatment of Recurrent Glioma. Sci. Rep. 2017, 7, 43271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, H.; Yao, Q.; Zhang, Y.; Zhu, X.; Xia, T.; Wang, J.; Li, G.; Li, X.; Ni, S. Local in Vitro Delivery of Rapamycin from Electrospun PEO/PDLLA Nanofibers for Glioblastoma Treatment. Biomed. Pharmacother. 2016, 83, 1345–1352. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Liao, J.-Y.; Chen, W.-A.; Kao, Y.-C.; Liu, S.-J. Sustainable Release of Carmustine from Biodegradable Poly[((D,L))-Lactide-Co-Glycolide] Nanofibrous Membranes in the Cerebral Cavity: In Vitro and in Vivo Studies. Expert Opin. Drug Deliv. 2013, 10, 879–888. [Google Scholar] [CrossRef]
- Han, D.; Sasaki, M.; Yoshino, H.; Kofuji, S.; Sasaki, A.T.; Steckl, A.J. In-Vitro Evaluation of MPA-Loaded Electrospun Coaxial Fiber Membranes for Local Treatment of Glioblastoma Tumor Cells. J. Drug Deliv. Sci. Technol. 2017, 40, 45–50. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Melt Electrospinning of Daunorubicin Hydrochloride-Loaded Poly (ε-Caprolactone) Fibrous Membrane for Tumor Therapy. Bioact. Mater. 2017, 2, 96–100. [Google Scholar] [CrossRef]
- Guo, G.; Fu, S.; Zhou, L.; Liang, H.; Fan, M.; Luo, F.; Qian, Z.; Wei, Y. Preparation of Curcumin Loaded Poly(ε-Caprolactone)-Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Nanofibers and Their in Vitro Antitumor Activity against Glioma 9L Cells. Nanoscale 2011, 3, 3825–3832. [Google Scholar] [CrossRef]
- Lei, C.; Cui, Y.; Zheng, L.; Chow, P.K.-H.; Wang, C.-H. Development of a Gene/Drug Dual Delivery System for Brain Tumor Therapy: Potent Inhibition via RNA Interference and Synergistic Effects. Biomaterials 2013, 34, 7483–7494. [Google Scholar] [CrossRef]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Kazan, D.; Oktar, F.N.; Gunduz, O. Glioblastoma Cell Adhesion Properties through Bacterial Cellulose Nanocrystals in Polycaprolactone/Gelatin Electrospun Nanofibers. Carbohydr. Polym. 2020, 233, 115820. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Orive, G.; Franze, K.; Appel, E.A. Towards Brain-Tissue-like Biomaterials. Nat. Commun. 2020, 11, 3423. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.G.; Domino, J.S.; Chamoun, R.; Thomas, S.M. Mechanical Properties in the Glioma Microenvironment: Emerging Insights and Theranostic Opportunities. Front. Oncol. 2022, 11, 805628. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerke, A.; Bussink, J.; Rowan, A.E.; Span, P.N. The Mechanical Microenvironment in Cancer: How Physics Affects Tumours. Semin. Cancer Biol. 2015, 35, 62–70. [Google Scholar] [CrossRef]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The Role of Myeloid Cells in Cancer Therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical Traits of Cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Kalli, M.; Stylianopoulos, T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front. Oncol. 2018, 8, 55. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sahai, E. Mechanisms and Impact of Altered Tumour Mechanics. Nat. Cell Biol. 2018, 20, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Katira, P.; Bonnecaze, R.T.; Zaman, M.H. Modeling the Mechanics of Cancer: Effect of Changes in Cellular and Extra-Cellular Mechanical Properties. Front. Oncol. 2013, 3, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, S.Y.; Hufnagel, T.C.; Lim, C.T.; Leong, K.W. Mechanical Properties of Single Electrospun Drug-Encapsulated Nanofibres. Nanotechnology 2006, 17, 3880–3891. [Google Scholar] [CrossRef]
- Kaphle, P.; Li, Y.; Yao, L. The Mechanical and Pharmacological Regulation of Glioblastoma Cell Migration in 3D Matrices. J. Cell. Physiol. 2019, 234, 3948–3960. [Google Scholar] [CrossRef]
- Doustgani, A.; Vasheghani-Farahani, E.; Soleimani, M.; Hashemi-Najafabadi, S. Optimizing the Mechanical Properties of Electrospun Polycaprolactone and Nanohydroxyapatite Composite Nanofibers. Compos. Part B Eng. 2012, 43, 1830–1836. [Google Scholar] [CrossRef]
- Sarma, S.; Verma, A.K.; Phadkule, S.S.; Saharia, M. Towards an Interpretable Machine Learning Model for Electrospun Polyvinylidene Fluoride (PVDF) Fiber Properties. Comput. Mater. Sci. 2022, 213, 111661. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Xia, Y. Perspective: Aligned Arrays of Electrospun Nanofibers for Directing Cell Migration. APL Mater. 2018, 6, 120902. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.; Nelson, M.T.; Xue, R.; DeJesus, J.K.; Viapiano, M.S.; Lannutti, J.J.; Sarkar, A.; Winter, J.O. Mimicking White Matter Tract Topography Using Core–Shell Electrospun Nanofibers to Examine Migration of Malignant Brain Tumors. Biomaterials 2013, 34, 5181–5190. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Shin, H.J.; Yang, D.-H.; Koh, Y.-J.; Shin, H.; Chun, H.J. Advanced Capability of Radially Aligned Fibrous Scaffolds Coated with Polydopamine for Guiding Directional Migration of Human Mesenchymal Stem Cells. J. Mater. Chem. B 2017, 5, 8725–8737. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.D.; Kinsella, J.M. Directing the Self-Assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly(Ethylene-Co-Vinylacetate), Poly(Lactic Acid), and a Blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, N.; Glitza Oliva, I.C.; O’Brien, B.J.; Parker Kerrigan, B.C.; Heimberger, A.B.; Ferguson, S.D. Targeting the Tumor Microenvironment in Brain Metastasis. Neurosurg. Clin. N. Am. 2020, 31, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun Nanofibers for Drug Delivery and Biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Thews, O.; Riemann, A. Tumor PH and Metastasis: A Malignant Process beyond Hypoxia. Cancer Metastasis Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef]
- Agudelo-Garcia, P.A.; De Jesus, J.K.; Williams, S.P.; Nowicki, M.O.; Chiocca, E.A.; Liyanarachchi, S.; Li, P.-K.; Lannutti, J.J.; Johnson, J.K.; Lawler, S.E.; et al. Glioma Cell Migration on Three-Dimensional Nanofiber Scaffolds Is Regulated by Substrate Topography and Abolished by Inhibition of STAT3 Signaling. Neoplasia 2011, 13, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Cui, W. Functional Electrospun Fibers for Local Therapy of Cancer. Adv. Fiber Mater. 2020, 2, 229–245. [Google Scholar] [CrossRef]
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging Roles of Electrospun Nanofibers in Cancer Research. Adv. Healthc. Mater. 2018, 7, 1701024. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Wang, C.-H. Biodegradable Microfiber Implants Delivering Paclitaxel for Post-Surgical Chemotherapy against Malignant Glioma. Biomaterials 2008, 29, 2996–3003. [Google Scholar] [CrossRef]
- Dai, J.; Jin, J.; Yang, S.; Li, G. Doxorubicin-Loaded PLA/Pearl Electrospun Nanofibrous Scaffold for Drug Delivery and Tumor Cell Treatment. Mater. Res. Express 2017, 4, 075403. [Google Scholar] [CrossRef]
- Pompos, A.; Durante, M.; Choy, H. Heavy Ions in Cancer Therapy. JAMA Oncol. 2016, 2, 1539. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Han, Y.; Zhang, X.; Ma, H.; Li, L.; Yu, R.; Liu, H. Application of New Radiosensitizer Based on Nano-Biotechnology in the Treatment of Glioma. Front. Oncol. 2021, 11, 633827. [Google Scholar] [CrossRef] [PubMed]
- De Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of Sterilization by Electron Beam, Gamma Radiation and X-Rays on Electrospun Poly-(ε-Caprolactone) Fiber Mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Chen, C.-C.; Lin, L.-T.; Chang, C.-H.; Chen, L.-C.; Wang, H.-E.; Lee, T.-W.; Lee, Y.-J. PEGylated Liposome-Encapsulated Rhenium-188 Radiopharmaceutical Inhibits Proliferation and Epithelial–Mesenchymal Transition of Human Head and Neck Cancer Cells in Vivo with Repeated Therapy. Cell Death Discov. 2018, 4, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, W.; Li, B.; Long, L.; Chen, L.; Huang, Q.; Liang, Z. Induction of Autophagy Promotes Differentiation of Glioma-Initiating Cells and Their Radiosensitivity. Int. J. Cancer 2011, 129, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mi, X.; Su, H.; Yang, J.; Gu, Y.; Zhang, L.; Sun, W.; Liang, X.; Zhang, C. GE11-PDA-Pt@USPIOs Nano-Formulation for Relief of Tumor Hypoxia and MRI/PAI-Guided Tumor Radio-Chemotherapy. Biomater. Sci. 2019, 7, 2076–2090. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Shi, M.; Li, D.; Lu, C.; Cao, X.; Peng, C.; Mignani, S.; Majoral, J.-P.; Shi, X. Poly(Amidoamine) Dendrimer-Coordinated Copper(II) Complexes as a Theranostic Nanoplatform for the Radiotherapy-Enhanced Magnetic Resonance Imaging and Chemotherapy of Tumors and Tumor Metastasis. Nano Lett. 2019, 19, 1216–1226. [Google Scholar] [CrossRef]
- Luu, Y.K.; Kim, K.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Development of a Nanostructured DNA Delivery Scaffold via Electrospinning of PLGA and PLA–PEG Block Copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Nie, H.; Wang, C.-H. Fabrication and Characterization of PLGA/HAp Composite Scaffolds for Delivery of BMP-2 Plasmid DNA. J. Control. Release 2007, 120, 111–121. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Krishnaswamy, V.R.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Fabrication of Electrospun Zein Nanofibers for the Sustained Delivery of SiRNA. J. Mater. Sci. Mater. Med. 2015, 26, 101. [Google Scholar] [CrossRef]
- Patil, S.; Gao, Y.-G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.-J.; Jiang, S.-F.; Qadir, A.; Qian, A.-R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, M.L.; Logan, A.; Seymour, L.W. Barriers to Gene Delivery Using Synthetic Vectors. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2005; Volume 53, pp. 19–46. ISBN 978-0-12-017653-3. [Google Scholar]
- Lee, S.; Kim, J.-S.; Chu, H.S.; Kim, G.-W.; Won, J.-I.; Jang, J.-H. Electrospun Nanofibrous Scaffolds for Controlled Release of Adeno-Associated Viral Vectors. Acta Biomater. 2011, 7, 3868–3876. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by PCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399. [Google Scholar] [CrossRef]
- Berger, G.; Knelson, E.H.; Jimenez-Macias, J.L.; Nowicki, M.O.; Han, S.; Panagioti, E.; Lizotte, P.H.; Adu-Berchie, K.; Stafford, A.; Dimitrakakis, N.; et al. STING Activation Promotes Robust Immune Response and NK Cell–Mediated Tumor Regression in Glioblastoma Models. Proc. Natl. Acad. Sci. USA 2022, 119, e2111003119. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Jiang, C.; Andriessen, A.S.; Wang, K.; Wang, Z.; Ding, H.; Zhao, J.; Luo, X.; Lee, M.S.; Lei, Y.L.; et al. STING Controls Nociception via Type I Interferon Signalling in Sensory Neurons. Nature 2021, 591, 275–280. [Google Scholar] [CrossRef]
- Li, S.; Luo, M.; Wang, Z.; Feng, Q.; Wilhelm, J.; Wang, X.; Li, W.; Wang, J.; Cholka, A.; Fu, Y.; et al. Prolonged Activation of Innate Immune Pathways by a Polyvalent STING Agonist. Nat. Biomed. Eng. 2021, 5, 455–466. [Google Scholar] [CrossRef]
- Wang, F.; Su, H.; Xu, D.; Dai, W.; Zhang, W.; Wang, Z.; Anderson, C.F.; Zheng, M.; Oh, R.; Wan, F.; et al. Tumour Sensitization via the Extended Intratumoural Release of a STING Agonist and Camptothecin from a Self-Assembled Hydrogel. Nat. Biomed. Eng. 2020, 4, 1090–1101. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, R.M.; De Vlaeminck, Y.; Maebe, J.; Goyvaerts, C.; Breckpot, K. Turn Back the TIMe: Targeting Tumor Infiltrating Myeloid Cells to Revert Cancer Progression. Front. Immunol. 2018, 9, 1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Rashidi, A.; Zhao, J.; Silvers, C.; Wang, H.; Castro, B.; Ellingwood, A.; Han, Y.; Lopez-Rosas, A.; Zannikou, M.; et al. STING Agonist-Loaded, CD47/PD-L1-Targeting Nanoparticles Potentiate Antitumor Immunity and Radiotherapy for Glioblastoma. Nat. Commun. 2023, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.E.; Najem, H.; Ott, M.; Horbinski, C.; Fang, D.; DeRay, C.M.; Levine, J.M.; Curran, M.A.; Heimberger, A.B. Intratumoral Delivery of STING Agonist Results in Clinical Responses in Canine Glioblastoma. Clin. Cancer Res. 2021, 27, 5528–5535. [Google Scholar] [CrossRef]
- Wilson, D.R.; Sen, R.; Sunshine, J.C.; Pardoll, D.M.; Green, J.J.; Kim, Y.J. Biodegradable STING Agonist Nanoparticles for Enhanced Cancer Immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 237–246. [Google Scholar] [CrossRef]
- Yang, K.; Han, W.; Jiang, X.; Piffko, A.; Bugno, J.; Han, C.; Li, S.; Liang, H.; Xu, Z.; Zheng, W.; et al. Zinc Cyclic Di-AMP Nanoparticles Target and Suppress Tumours via Endothelial STING Activation and Tumour-Associated Macrophage Reinvigoration. Nat. Nanotechnol. 2022, 17, 1322–1331. [Google Scholar] [CrossRef]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The Approved Gene Therapy Drugs Worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
| Polymer Drug Delivery System | Drug | Mechanism of Drug Release | Cancer Cell Type | Ref. |
|---|---|---|---|---|
| Poly(ε-caprolactone) (PCL)/ gelatin (GT) | SN-38 97-ethyl-10-hydroxy camptothecin) | Diffusion and anomalous transport | Human glioblastoma 251 and U87 cells | [81] |
| Poly(ethylene glycol)– poly(l-lactic acid) (PEG–PLLA) | 1,3-bis(2- chloroethyl)-1-nitrosourea (BCNU) | Diffusion/Degradation of polymer matrix | Glioma C6 | [82] |
| Poly-(d,l-lactide-co-glycolide) (PLGA) | Paclitaxel (PTX) | Polymer matrix degradation | Glioma C6 cells in rats | [83] |
| Poly(l-actide) (PLA)/poly- (d,l-lactide-co-glucolic acid) (PLGA) | Cisplatin (CP) | Diffusion | Rat C6 glioma cells | [84] |
| Poly(ethylene glycol)-(llactic acid) (PEG–PLA) | Paclitaxcel (PTX) and Doxorubicin Hydrochloride | Diffusion | Murine glioma c6 cells | [85] |
| Polypropylene carbonate Ca Alginate MPs | Paclitaxcel (PTX) and Temozolomide (TMZ) | Prolonged release/Polymer matrix degradation | Glioma C6 cells in rats | [86] |
| poly (ε-caprolactonediol) (PCL)/Polyurethane(PU) | Temozolomide (TMZ) | 1 Diffusion | U87 Cells | [87] |
| PLGA-PLA-PCL blends | Temozolomide (TMZ) | Sustained Release/Polymer matrix degradation | U87 cells and rat c6 glioma cells | [88] |
| poly(lactic acid) (PLA)/polyethylene oxide (PEO) | Rapamycin | Sustained Release/Polymer matrix degradation | Human glioblastoma 251 and U87 cells | [89] |
| Poly-(d,l-lactide-co-glycolide) (PLGA) | 1,3-bis(2- chloroethyl)-1-nitrosou)rea (BCNU) | Polymer matrix degradation | Wistar Rats | [90] |
| poly(ε-caprolactone) (PCL)/polyvinylpyrrolidone (PVP) | Mycophenolic Acid | N/A | U87 cells | [91] |
| poly (ε-caprolactone) | Daunorubicin | Polymer matrix degradation | U87 cells and Hela | [92] |
| poly(L-lactic acid) (PLLA) | Doxorubicin | Initial Rapid Release followed by sustained release | Hela Cells | [9] |
| poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) | Curcumin | Diffusion | Glioma 9 L | [93] |
| Poly-(d,l-lactide-co-glycolide) (PLGA)/polyethylenimine (PEI) | Paclitaxcel (PTX) | Sustained Release | BALB/c nude mice | [94] |
| polycaprolactone (PCL)/gelatin (Gel) | bacterial cellulose nano-crystal (BCNC) | N/A | U251 MG | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louis, L.; Chee, B.s.; McAfee, M.; Nugent, M. Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy? Pharmaceutics 2023, 15, 1649. https://doi.org/10.3390/pharmaceutics15061649
Louis L, Chee Bs, McAfee M, Nugent M. Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy? Pharmaceutics. 2023; 15(6):1649. https://doi.org/10.3390/pharmaceutics15061649
Chicago/Turabian StyleLouis, Lynn, Bor shin Chee, Marion McAfee, and Michael Nugent. 2023. "Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy?" Pharmaceutics 15, no. 6: 1649. https://doi.org/10.3390/pharmaceutics15061649
APA StyleLouis, L., Chee, B. s., McAfee, M., & Nugent, M. (2023). Electrospun Drug-Loaded and Gene-Loaded Nanofibres: The Holy Grail of Glioblastoma Therapy? Pharmaceutics, 15(6), 1649. https://doi.org/10.3390/pharmaceutics15061649







