Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
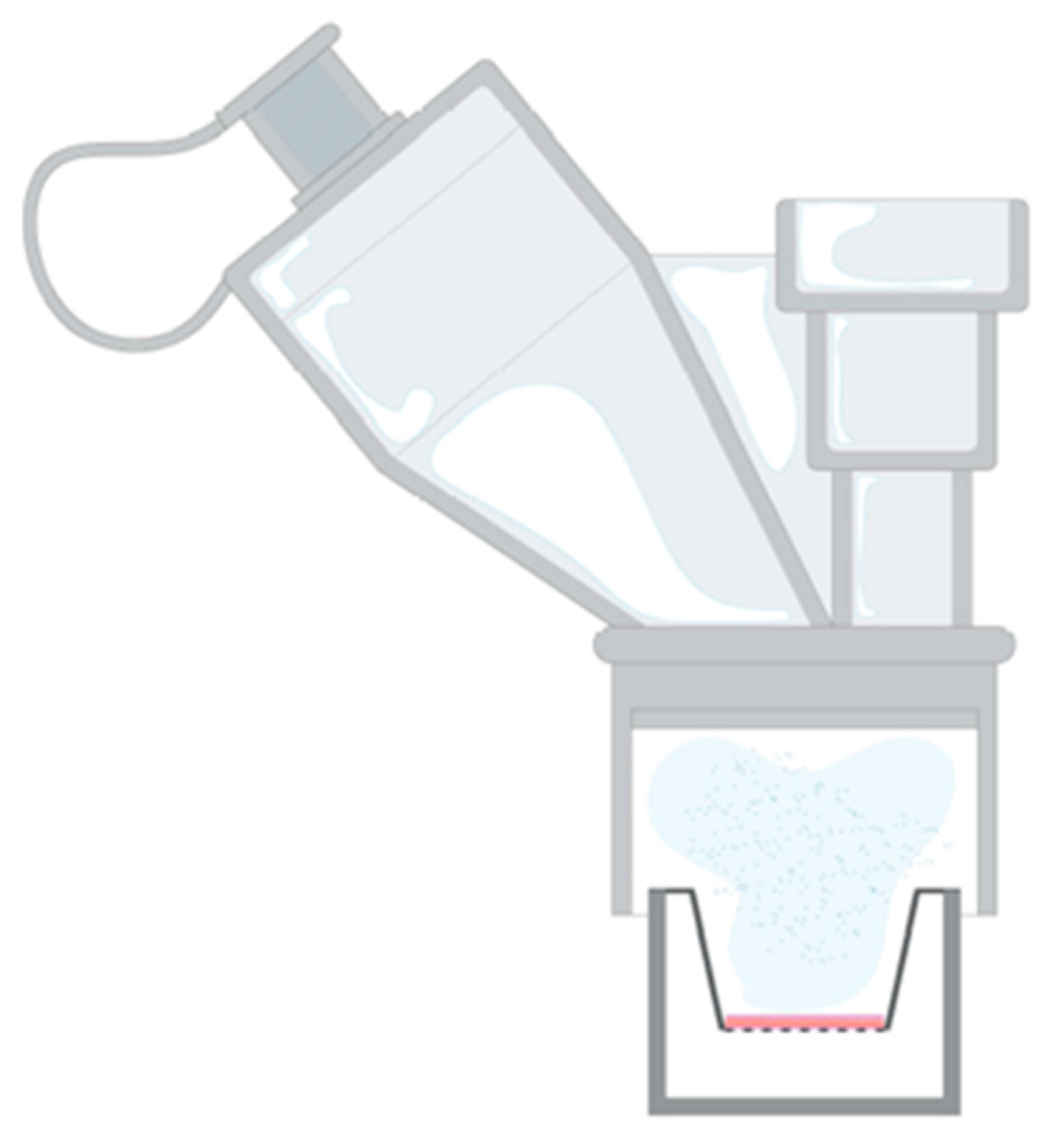
2.2. Plasminogen (PLG-OMP) Aerosolisation
2.3. Maintenance of Enzymatic Activity
2.4. Protein Characterisation
2.5. Droplet Distribution of Nebulised PLG-OMP
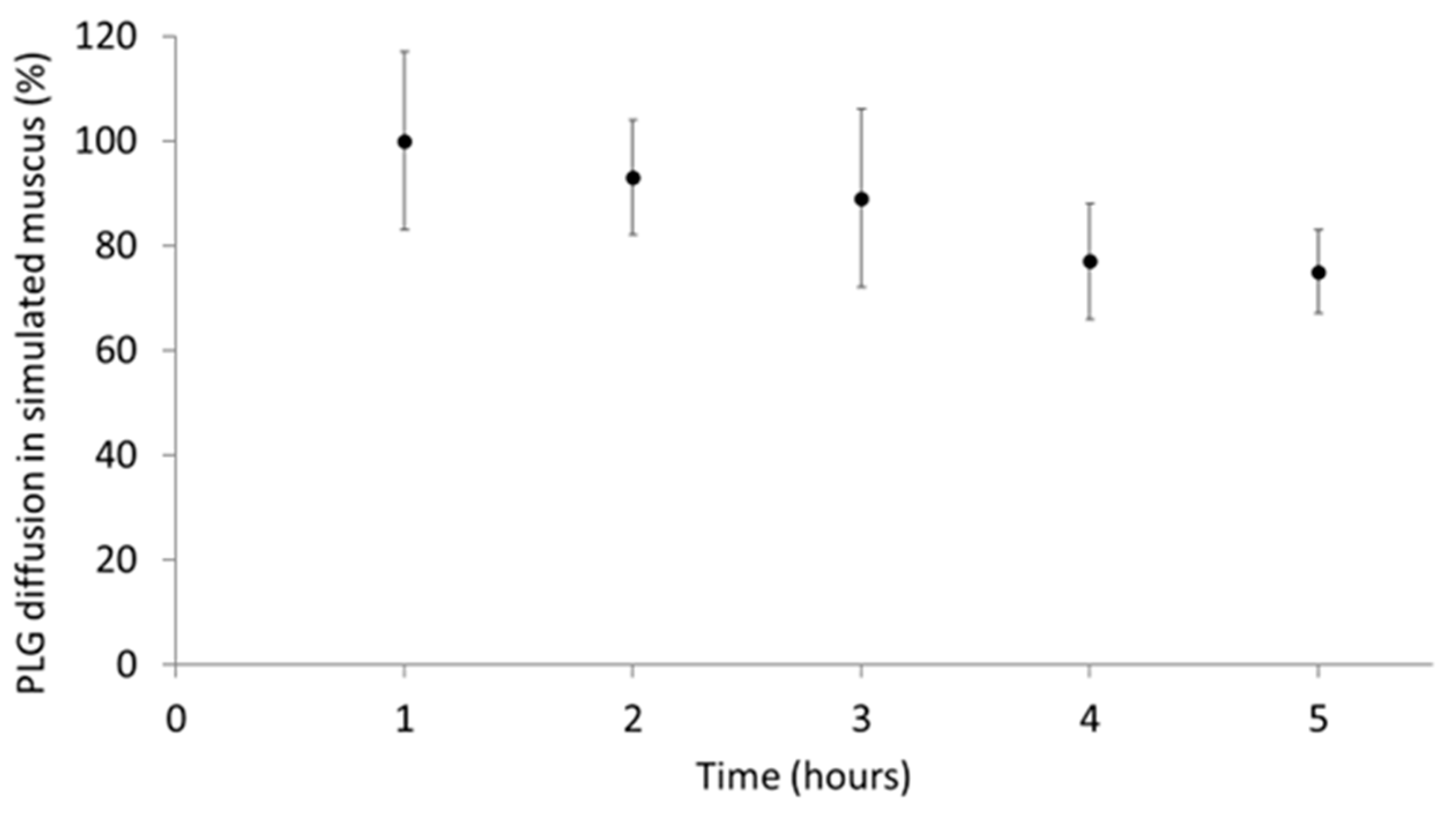
2.6. Penetration of Aerosolised PLG through Artificial Mucus
2.7. Biological Investigation
2.7.1. PLG Permeability across an In Vitro Model of Pulmonary Epithelium
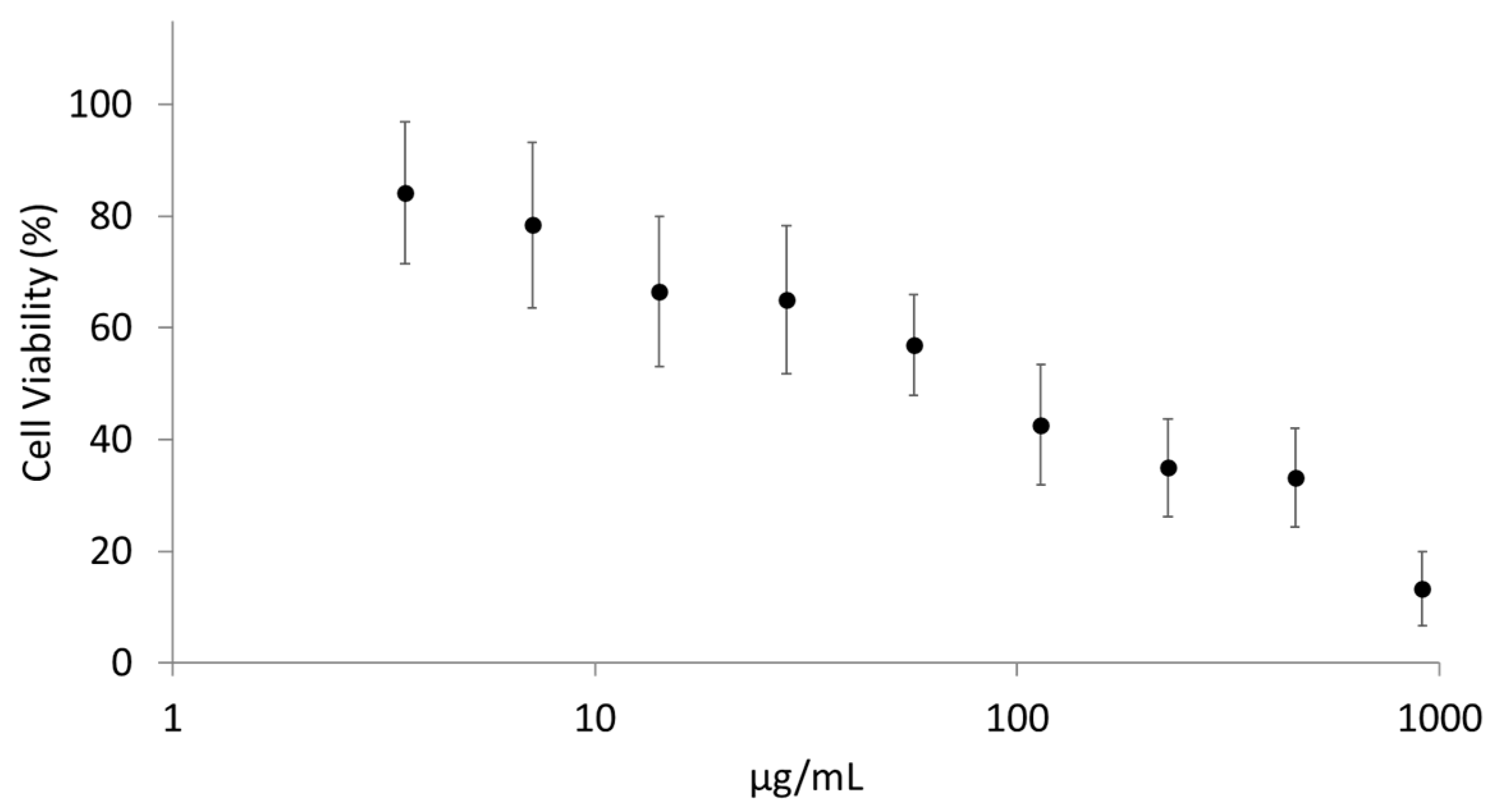
Cytotoxicity Screening on NCI-H441 Cells by Tetrazolium Salts Assay (WST-1)
In Vitro Air–Liquid Interface (ALI) Model
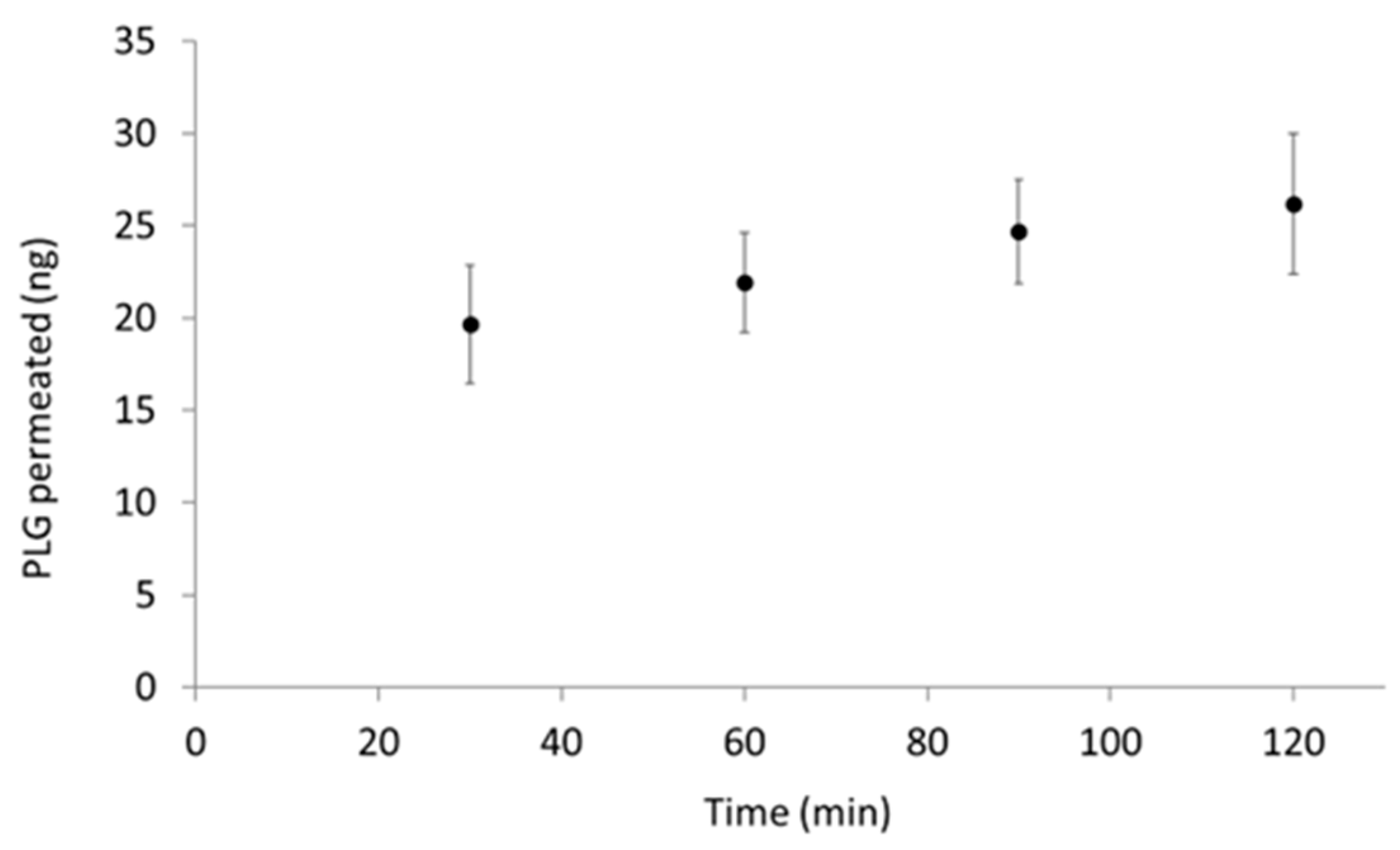
Assessment of Nebulised PLG Permeation through ALI Monolayer
2.7.2. Immunomodulating Activity of Nebulised PLG
2.8. Statistical Analysis
3. Results
3.1. PLG Aerosolisation under Air or Oxidising Condition
3.2. Aerosolised PLG Mobility in Simulated Mucus
3.3. PLG Permeability across a Monolayer
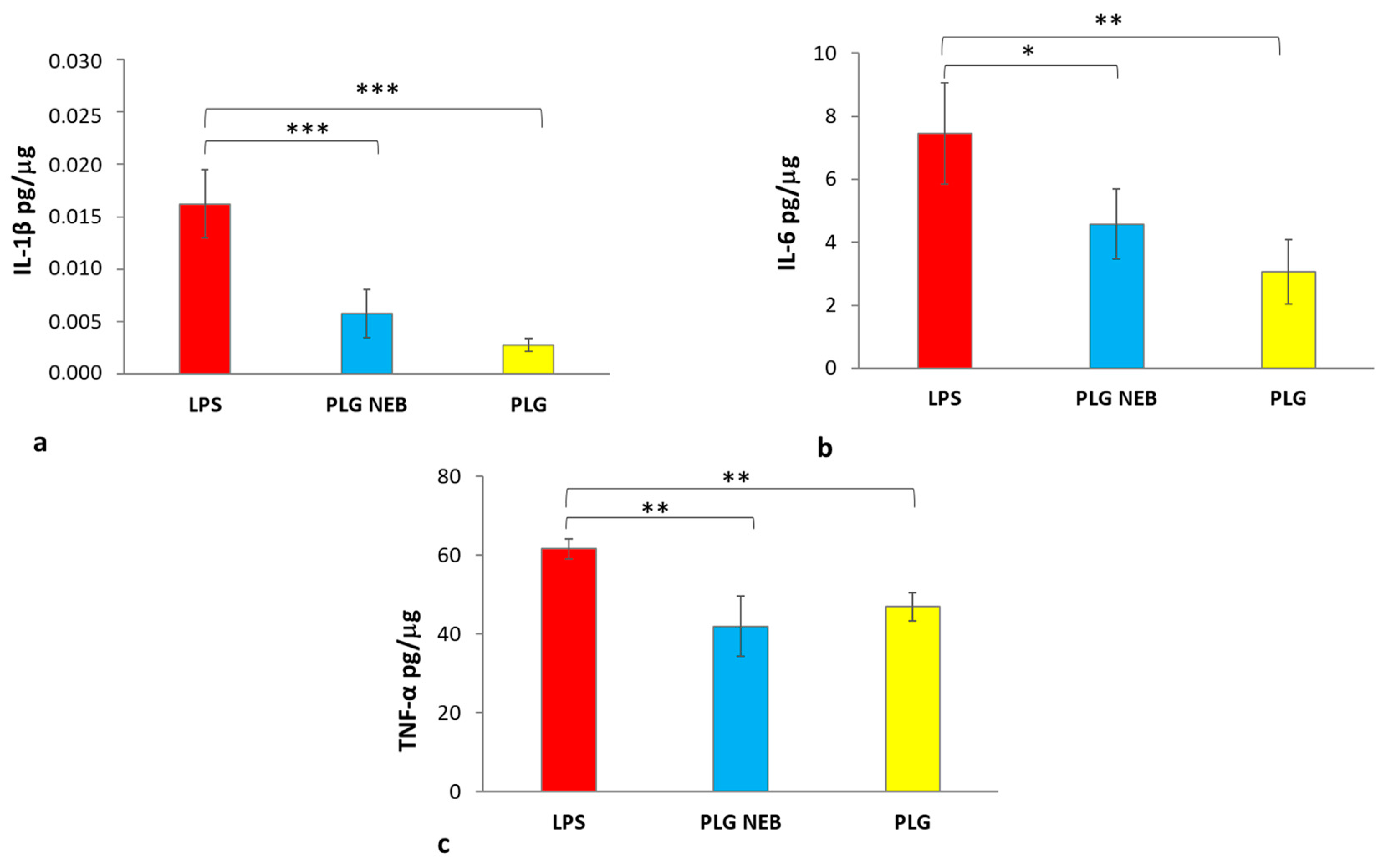
3.4. Maintenance of PLG Immunomodulatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piras, A.M.; Zambito, Y.; Lugli, M.; Ferro, B.; Roncucci, P.; Mori, F.; Salvatore, A.; Ascione, E.; Bellini, M.; Crea, R. Repurposing of Plasminogen: An Orphan Medicinal Product Suitable for SARS-CoV-2 Inhalable Therapeutics. Pharmaceuticals 2020, 13, 425. [Google Scholar] [CrossRef]
- Günther, A.; Ruppert, C.; Schmidt, R.; Markart, P.; Grimminger, F.; Walmrath, D.; Seeger, W. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir. Res. 2001, 2, 353. [Google Scholar] [CrossRef]
- Fujishima, S. Guideline-Based Management of Acute Respiratory Failure and Acute Respiratory Distress Syndrome. J. Intensive Care 2023, 11, 10. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Bouadma, L.; Morris, A.C.; Dhanani, J.A.; Kollef, M.; Lipman, J.; Martin-Loeches, I.; Nseir, S.; Ranzani, O.T.; Roquilly, A.; et al. Pulmonary Infections Complicating ARDS. Intensive Care Med. 2020, 46, 2168–2183. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, Mechanisms, Relevant Outcomes, and Clinical Trial Coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef]
- Eisner, M.D.; Thompson, T.; Hudson, L.D.; Luce, J.M.; Hayden, D.; Schoenfeld, D.; Matthay, M.A.; The Acute Respiratory Distress Syndrome Network. Efficacy of Low Tidal Volume Ventilation in Patients with Different Clinical Risk Factors for Acute Lung Injury and the Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 231–236. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Al Mutair, A.; Alhumaid, S.; Layqah, L.; Shamou, J.; Ahmed, G.Y.; Chagla, H.; Alsalman, K.; Alnasser, F.M.; Thoyaja, K.; Alhuqbani, W.N.; et al. Clinical Outcomes and Severity of Acute Respiratory Distress Syndrome in 1154 COVID-19 Patients: An Experience Multicenter Retrospective Cohort Study. COVID 2022, 2, 1102–1115. [Google Scholar] [CrossRef]
- Stanford, S.; Roy, A.; Rea, C.; Harris, B.; Ashton, A.; Mangles, S.; Everington, T.; Taher, R.; Burns, D.; Arbuthnot, E.; et al. Pilot Study to Evaluate Hypercoagulation and Inflammation Using Rotational Thromboelastometry and Calprotectin in COVID-19 Patients. PLoS ONE 2023, 18, e0269738. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Tusman, G.; Berra, L.; Rodríguez-Suárez, P.; Suárez-Sipmann, F. Unsuccessful and Successful Clinical Trials in Acute Respiratory Distress Syndrome: Addressing Physiology-Based Gaps. Front. Physiol. 2021, 12, 774025. [Google Scholar] [CrossRef]
- Albaiceta, G.M.; Taboada, F.; Parra, D.; Blanco, A.; Escudero, D.; Otero, J. Differences in the Deflation Limb of the Pressure-Volume Curves in Acute Respiratory Distress Syndrome from Pulmonary and Extrapulmonary Origin. Intensive Care Med. 2003, 29, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.G.; Perkins, G.D.; Gates, S.; Young, D.; McAuley, D.F.; Tunnicliffe, W.; Khan, Z.; Lamb, S.E. Effect of Intravenous β-2 Agonist Treatment on Clinical Outcomes in Acute Respiratory Distress Syndrome (BALTI-2): A Multicentre, Randomised Controlled Trial. Lancet 2012, 379, 229–235. [Google Scholar] [CrossRef]
- McAuley, D.F.; Laffey, J.G.; O’Kane, C.M.; Perkins, G.D.; Mullan, B.; Trinder, T.J.; Johnston, P.; Hopkins, P.A.; Johnston, A.J.; McDowell, C.; et al. The HARP-2 Investigators, for the Irish Critical Care Trials Group. Simvastatin in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2014, 371, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Pettilä, V.; Karvonen, M.K.; Jalkanen, J.; Nightingale, P.; Brealey, D.; Mancebo, J.; Ferrer, R.; Mercat, A.; Patroniti, N.; et al. Effect of Intravenous Interferon β-1a on Death and Days Free From Mechanical Ventilation among Patients with Moderate to Severe Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2020, 323, 725. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.A.; Kabir, A.; Abdullah, A.B.M.; Alam, M.B.; Azad, K.A.K.; Miah, M.T.; Mowla, S.G.M.; Deb, S.R.; Amin, M.R.; Asaduzzaman, M. Safety and Efficacy of Favipiravir for the Management of COVID-19 Patients: A Preliminary Randomized Control Trial. Clin. Infect. Pract. 2022, 15, 100145. [Google Scholar] [CrossRef]
- Menzella, F.; Fontana, M.; Salvarani, C.; Massari, M.; Ruggiero, P.; Scelfo, C.; Barbieri, C.; Castagnetti, C.; Catellani, C.; Gibellini, G.; et al. Efficacy of Tocilizumab in Patients with COVID-19 ARDS Undergoing Noninvasive Ventilation. Crit. Care 2020, 24, 589. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: A Multicentre, Randomised Controlled Trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical Evidence Does Not Support Corticosteroid Treatment for 2019-NCoV Lung Injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef]
- Ferro, B.; Cinelli, R.; Vegnuti, L.; Piras, A.M.; Roncucci, P. The Potential Role of Aerosolized Phosphodiesterase 3 Inhibitor Enoximone in the Management of Coronavirus Disease 2019 Hypoxemia: A Case Report. J. Aerosol Med. Pulm. Drug Deliv. 2021, 34, 262–264. [Google Scholar] [CrossRef]
- Yıldırım, F.; Yıldız Gülhan, P.; Şimşek, M. COVID-19 Related Acute Respiratory Distress Syndrome: Pathological, Radiological and Clinical Concordance. Tuberk Toraks 2021, 69, 360–368. [Google Scholar] [CrossRef]
- Belen-Apak, F.B.; Sarıalioğlu, F. Pulmonary Intravascular Coagulation in COVID-19: Possible Pathogenesis and Recommendations on Anticoagulant/Thrombolytic Therapy. J. Thromb. Thrombolysis 2020, 50, 278–280. [Google Scholar] [CrossRef]
- Camprubí-Rimblas, M.; Tantinyà, N.; Bringué, J.; Guillamat-Prats, R.; Artigas, A. Anticoagulant Therapy in Acute Respiratory Distress Syndrome. Ann. Transl. Med. 2018, 6, 36. [Google Scholar] [CrossRef]
- Aisina, R.B.; Mukhametova, L.I. Structure and Function of Plasminogen/Plasmin System. Russ. J. Bioorganic Chem. 2014, 40, 590–605. [Google Scholar] [CrossRef]
- Hertel, S.P.; Winter, G.; Friess, W. Protein Stability in Pulmonary Drug Delivery via Nebulization. Adv. Drug Deliv. Rev. 2015, 93, 79–94. [Google Scholar] [CrossRef]
- Bodier-Montagutelli, E.; Respaud, R.; Perret, G.; Baptista, L.; Duquenne, P.; Heuzé-Vourc’h, N.; Vecellio, L. Protein Stability during Nebulization: Mind the Collection Step! Eur. J. Pharm. Biopharm. 2020, 152, 23–34. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles; Council of Europe: Strasbourg, France, 2014; pp. 276–286. [Google Scholar]
- Costabile, G.; d’Angelo, I.; d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Pompili, B.; Del Porto, P.; Leoni, L.; Visca, P.; Miro, A.; Quaglia, F.; et al. Development of Inhalable Hyaluronan/Mannitol Composite Dry Powders for Flucytosine Repositioning in Local Therapy of Lung Infections. J. Control. Release 2016, 238, 80–91. [Google Scholar] [CrossRef]
- Ghani, M.; Soothill, J.S. Ceftazidime, Gentamicin, and Rifampicin, in Combination, Kill Biofilms of Mucoid Pseudomonas aeruginosa. Can. J. Microbiol. 1997, 43, 999–1004. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Chiellini, F. Perspectives on: In Vitro Evaluation of Biomedical Polymers. J. Bioact. Compat. Polym. 2006, 21, 257–271. [Google Scholar] [CrossRef]
- Ren, H.; Birch, N.P.; Suresh, V. An Optimised Human Cell Culture Model for Alveolar Epithelial Transport. PLoS ONE 2016, 11, e0165225. [Google Scholar] [CrossRef]
- Hayashi, M.; Matsuzaki, Y.; Shimonaka, M. Impact of Plasminogen on an in Vitro Wound Healing Model Based on a Perfusion Cell Culture System. Mol. Cell. Biochem. 2009, 322, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hallworth, G.W.; Westmoreland, D.G. The Twin Impinger: A Simple Device for Assessing the Delivery of Drugs from Metered Dose Pressurized Aerosol Inhalers. J. Pharm. Pharmacol. 2011, 39, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Friend, J.R.; Yeo, L.Y.; Morton, D.A.V.; McIntosh, M.P.; Spiccia, L. Miniature Inhalation Therapy Platform Using Surface Acoustic Wave Microfluidic Atomization. Lab. Chip 2009, 9, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.S.; d’Angelo, I.; Maiolino, S.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Quaglia, F.; Ungaro, F. In Vitro/In Vivo Investigation on the Potential of Pluronic® Mixed Micelles for Pulmonary Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 130, 30–38. [Google Scholar] [CrossRef]
- Suresh, V. Permeability Properties of an In Vitro Model of the Alveolar Epithelium. Cell. Mol. Bioeng. 2021, 14, 653–659. [Google Scholar] [CrossRef]
- Nebulized Heparin for COVID-19-Associated Acute Respiratory Failure. Available online: https://ClinicalTrials.gov/show/NCT04842292 (accessed on 12 April 2023).
- Nebulized Heparin for the Treatment of COVID-19. Available online: https://ClinicalTrials.gov/show/NCT04723563 (accessed on 12 April 2023).
- Serisier, D.J.; Shute, J.K.; Hockey, P.M.; Higgins, B.; Conway, J.; Carroll, M.P. Carroll. Inhaled Heparin in Cystic Fibrosis. Eur. Respir. J. 2006, 27, 354. [Google Scholar] [CrossRef]
- van Haren, F.M.P.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J.; et al. Nebulised Heparin as a Treatment for COVID-19: Scientific Rationale and a Call for Randomised Evidence. Crit. Care 2020, 24, 454. [Google Scholar] [CrossRef]
- Abdelaal Ahmed Mahmoud, A.; Mahmoud, H.E.; Mahran, M.A.; Khaled, M. Streptokinase Versus Unfractionated Heparin Nebulization in Patients with Severe Acute Respiratory Distress Syndrome (ARDS): A Randomized Controlled Trial with Observational Controls. J. Cardiothorac. Vasc. Anesth. 2020, 34, 436–443. [Google Scholar] [CrossRef]
- Matthews, A.A.; Ee, P.L.R.; Ge, R. Developing Inhaled Protein Therapeutics for Lung Diseases. Mol. Biomed. 2020, 1, 11. [Google Scholar] [CrossRef]
- Najlah, M.; Parveen, I.; Alhnan, M.A.; Ahmed, W.; Faheem, A.; Phoenix, D.A.; Taylor, K.M.G.; Elhissi, A. The Effects of Suspension Particle Size on the Performance of Air-Jet, Ultrasonic and Vibrating-Mesh Nebulisers. Int. J. Pharm. 2014, 461, 234–241. [Google Scholar] [CrossRef]
- Dallas, R.H.; Rains, J.K.; Wilder, K.; Humphrey, W.; Cross, S.J.; Ghafoor, S.; Brazelton de Cardenas, J.N.; Hayden, R.T.; Hijano, D.R. The Aerogen® Solo Is an Alternative to the Small Particle Aerosol Generator (SPAG-2) for Administration of Inhaled Ribavirin. Pharmaceutics 2020, 12, 1163. [Google Scholar] [CrossRef]
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Oxidation of Proteins. In Chemical Changes During Processing and Storage of Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–123. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein Oxidation-Formation Mechanisms, Detection and Relevance as Biomarkers in Human Diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Gupta, A.; Yadav, V.; Yadav, J.S.; Rawat, S. Method Development and Photolytic Degradation Study of Doxofylline by RP-HPLC and LC-MS/MS. Asian J. Pharm. Anal. 2011, 1, 14–18. [Google Scholar]
- Sayed, N.E.E.; Abdelrahman, M.A.; Abdelrahim, M.E.A. Effect of Functional Principle, Delivery Technique, and Connection Used on Aerosol Delivery from Different Nebulizers: An In-Vitro Study. Pulm. Pharmacol. Ther. 2021, 70, 102054. [Google Scholar] [CrossRef]
- Sweeney, L.; McCloskey, A.P.; Higgins, G.; Ramsey, J.M.; Cryan, S.-A.; MacLoughlin, R. Effective Nebulization of Interferon-γ Using a Novel Vibrating Mesh. Respir. Res. 2019, 20, 66. [Google Scholar] [CrossRef]
- McDermott, K.; Oakley, J.G. Droplet Size and Distribution of Nebulized 3% Sodium Chloride, Albuterol, and Epoprostenol by Phase Doppler Particle Analyzer. Curr. Ther. Res. 2021, 94, 100623. [Google Scholar] [CrossRef]
- Salomon, J.J.; Muchitsch, V.E.; Gausterer, J.C.; Schwagerus, E.; Huwer, H.; Daum, N.; Lehr, C.-M.; Ehrhardt, C. The Cell Line NCl-H441 Is a Useful in Vitro Model for Transport Studies of Human Distal Lung Epithelial Barrier. Mol. Pharm. 2014, 11, 995–1006. [Google Scholar] [CrossRef]
- Guo, Y.; Bera, H.; Shi, C.; Zhang, L.; Cun, D.; Yang, M. Pharmaceutical Strategies to Extend Pulmonary Exposure of Inhaled Medicines. Acta Pharm. Sin. B 2021, 11, 2565–2584. [Google Scholar] [CrossRef]
- Baker, S.K.; Strickland, S. A Critical Role for Plasminogen in Inflammation. J. Exp. Med. 2020, 217, e20191865. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Ribeiro, A.L.C.; Costa, B.R.C.; Vago, J.P.; Lima, K.M.; Carneiro, F.S.; Ortiz, M.M.O.; Lima, G.L.N.; Carmo, A.A.F.; Rocha, R.M.; et al. Plasmin and Plasminogen Induce Macrophage Reprogramming and Regulate Key Steps of Inflammation Resolution via Annexin A1. Blood 2017, 129, 2896–2907. [Google Scholar] [CrossRef]
- Vago, J.P.; Sugimoto, M.A.; Lima, K.M.; Negreiros-Lima, G.L.; Baik, N.; Teixeira, M.M.; Perretti, M.; Parmer, R.J.; Miles, L.A.; Sousa, L.P. Plasminogen and the Plasminogen Receptor, Plg-RKT, Regulate Macrophage Phenotypic, and Functional Changes. Front. Immunol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Grassi, L.; Pompilio, A.; Kaya, E.; Rinaldi, A.C.; Sanjust, E.; Maisetta, G.; Crabbé, A.; Di Bonaventura, G.; Batoni, G.; Esin, S. The Anti-Microbial Peptide (Lin-SB056-1)2-K Reduces Pro-Inflammatory Cytokine Release through Interaction with Pseudomonas Aeruginosa Lipopolysaccharide. Antibiotics 2020, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Kaspi, H.; Semo, J.; Abramov, N.; Dekel, C.; Lindborg, S.; Kern, R.; Lebovits, C.; Aricha, R. MSC-NTF (NurOwn®) Exosomes: A Novel Therapeutic Modality in the Mouse LPS-Induced ARDS Model. Stem Cell Res. Ther. 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Burke, T.; Plow, E.F. Histone H2B as a Functionally Important Plasminogen Receptor on Macrophages. Blood 2007, 110, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y. The Role of Fibrinolytic Regulators in Vascular Dysfunction of Systemic Sclerosis. Int. J. Mol. Sci. 2019, 20, 619. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Hu, Y.-F.; Wu, J.-S.; Wang, L.; Huang, W.-G.; Xu, C.-S.; Meng, X.-L.; Wang, P. Semi-Mechanism-Based Pharmacodynamic Model for the Anti-Inflammatory Effect of Baicalein in LPS-Stimulated RAW264.7 Macrophages. Front. Pharmacol. 2018, 9, 793. [Google Scholar] [CrossRef]
- Tumolo, T.; Angnes, L.; Baptista, M.S. Determination of the Refractive Index Increment (Dn/Dc) of Molecule and Macromolecule Solutions by Surface Plasmon Resonance. Anal. Biochem. 2004, 333, 273–279. [Google Scholar] [CrossRef]
- Barlow, G.H.; Summaria, L.; Robbins, K.C. Molecular Weight Studies on Human Plasminogen and Plasmin at the Microgram Level. J. Biol. Chem. 1969, 244, 1138–1141. [Google Scholar] [CrossRef]
| Samples | BAND% ± SD% | |||
|---|---|---|---|---|
| Glu-I | Glu-II | Lys-I | Lys-II | |
| STD Glu PLG | 66 | 35 | - | - |
| STD Lys PLG | - | - | 62 | 38 |
| CTRL | 65 | 35 | - | - |
| Neb PLG air | 64 ± 4.5 | 36 ± 7.9 | - | - |
| Neb PLG ox | 59 ± 8.8 | 39 ± 10.3 | - | - |
| Simulation of the Nebulised Protein Distribution | |
|---|---|
| TSI Portion | % ± SD |
| Junction (T-piece) | 11.5 ± 9.7 |
| Throat | 0.2 ± 0.2 |
| Lower respiratory tract | 80.8 ± 5.7 |
| Exhaled | 10.2 ± 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizzoni, L.; Migone, C.; Grassiri, B.; Zambito, Y.; Ferro, B.; Roncucci, P.; Mori, F.; Salvatore, A.; Ascione, E.; Crea, R.; et al. Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment. Pharmaceutics 2023, 15, 1618. https://doi.org/10.3390/pharmaceutics15061618
Vizzoni L, Migone C, Grassiri B, Zambito Y, Ferro B, Roncucci P, Mori F, Salvatore A, Ascione E, Crea R, et al. Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment. Pharmaceutics. 2023; 15(6):1618. https://doi.org/10.3390/pharmaceutics15061618
Chicago/Turabian StyleVizzoni, Lucia, Chiara Migone, Brunella Grassiri, Ylenia Zambito, Baldassare Ferro, Paolo Roncucci, Filippo Mori, Alfonso Salvatore, Ester Ascione, Roberto Crea, and et al. 2023. "Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment" Pharmaceutics 15, no. 6: 1618. https://doi.org/10.3390/pharmaceutics15061618
APA StyleVizzoni, L., Migone, C., Grassiri, B., Zambito, Y., Ferro, B., Roncucci, P., Mori, F., Salvatore, A., Ascione, E., Crea, R., Esin, S., Batoni, G., & Piras, A. M. (2023). Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment. Pharmaceutics, 15(6), 1618. https://doi.org/10.3390/pharmaceutics15061618












