Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities
Abstract
1. Introduction
2. Common Diseases of the Cornea
2.1. Dry Eye Disease
2.2. Keratitis
2.3. Corneal Ulcers
2.4. Cogan Syndrome
2.5. Corneal Neovascularization
2.6. Corneal Fibrosis
2.7. Conjunctivitis
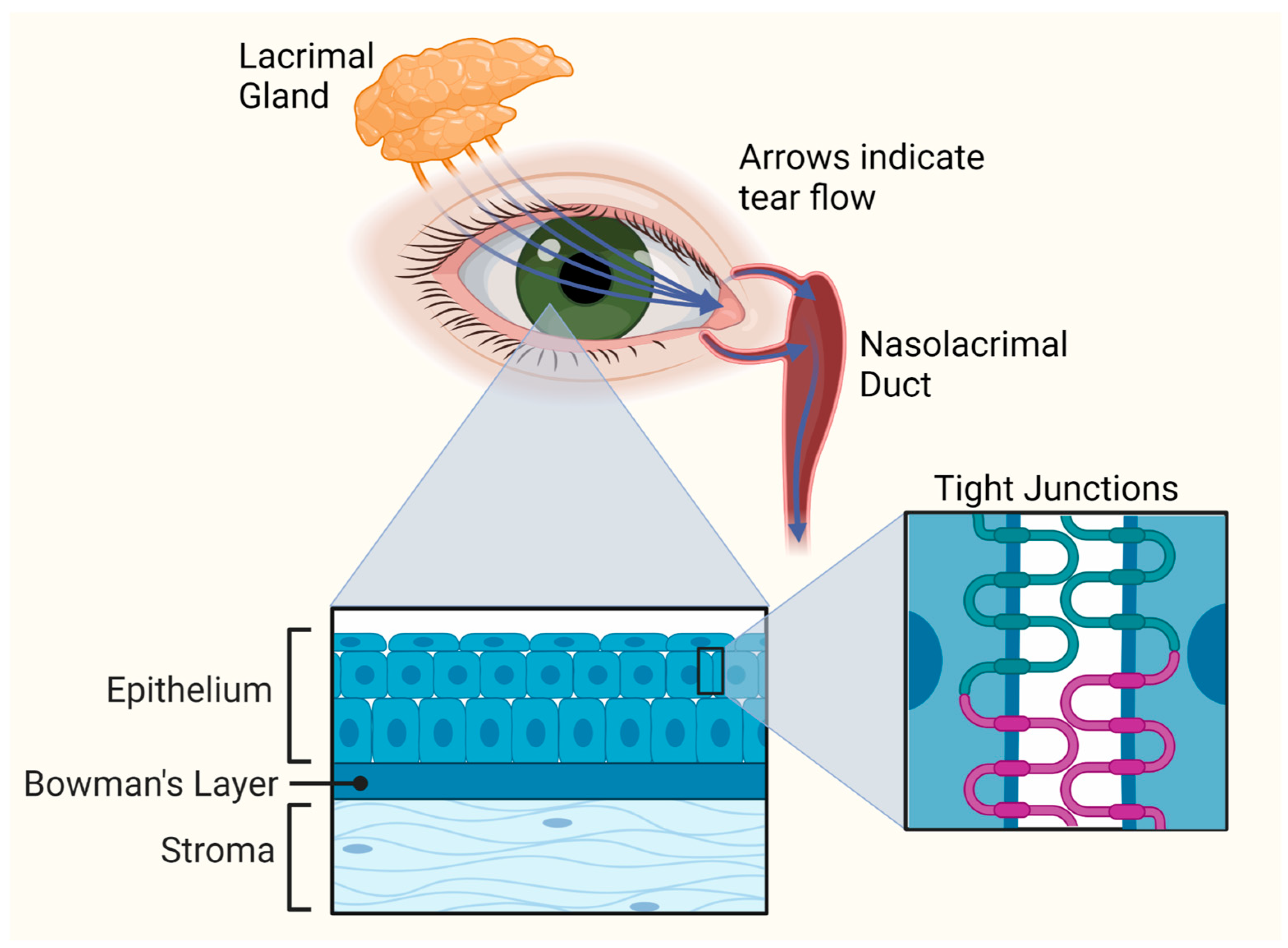
3. Corneal Anatomy and Barriers to Ocular Drug Delivery
3.1. Corneal Anatomy
3.2. Barriers to Ocular Drug Delivery
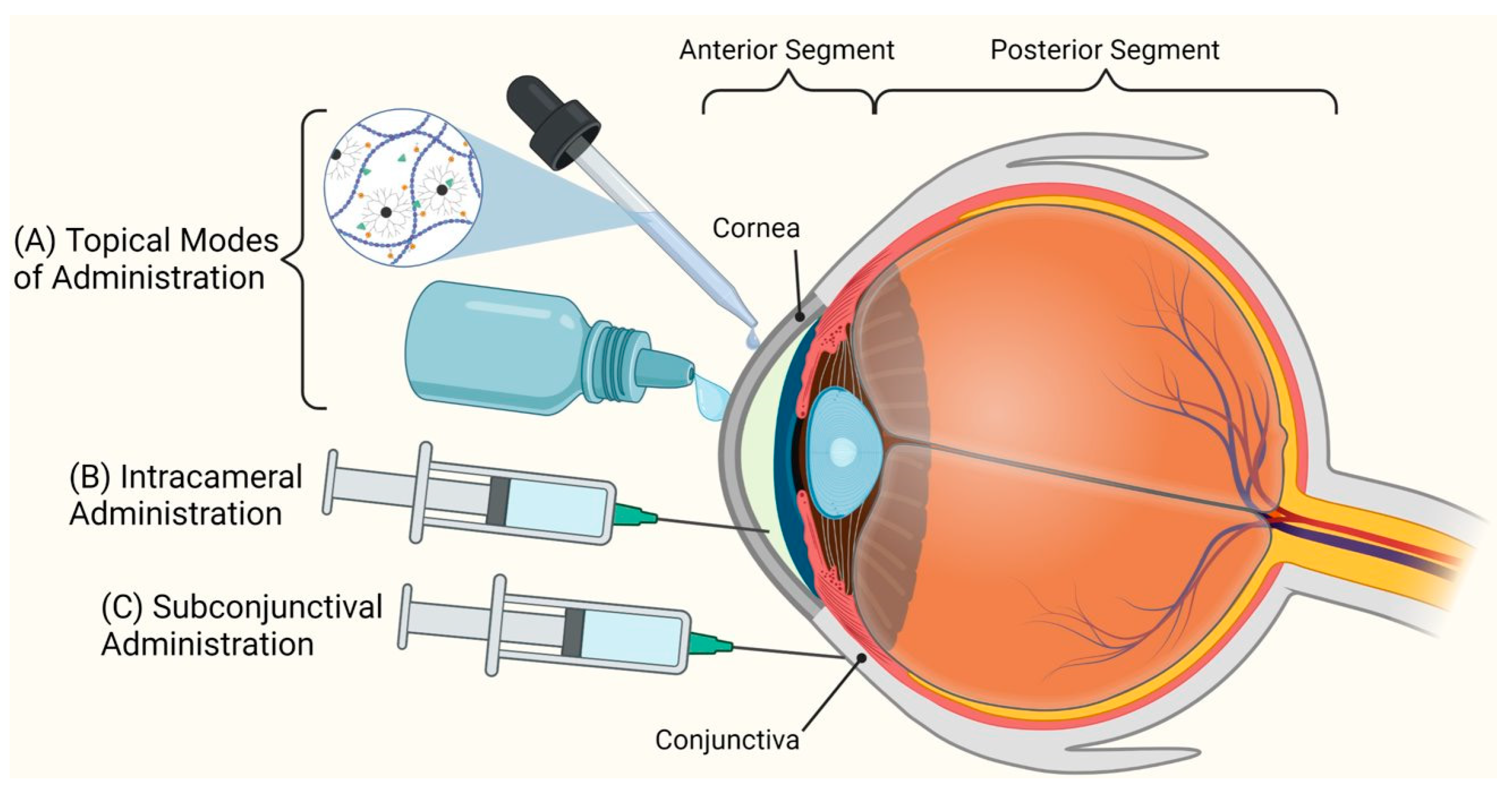
4. Routes of Administration for Corneal Drug Delivery Systems
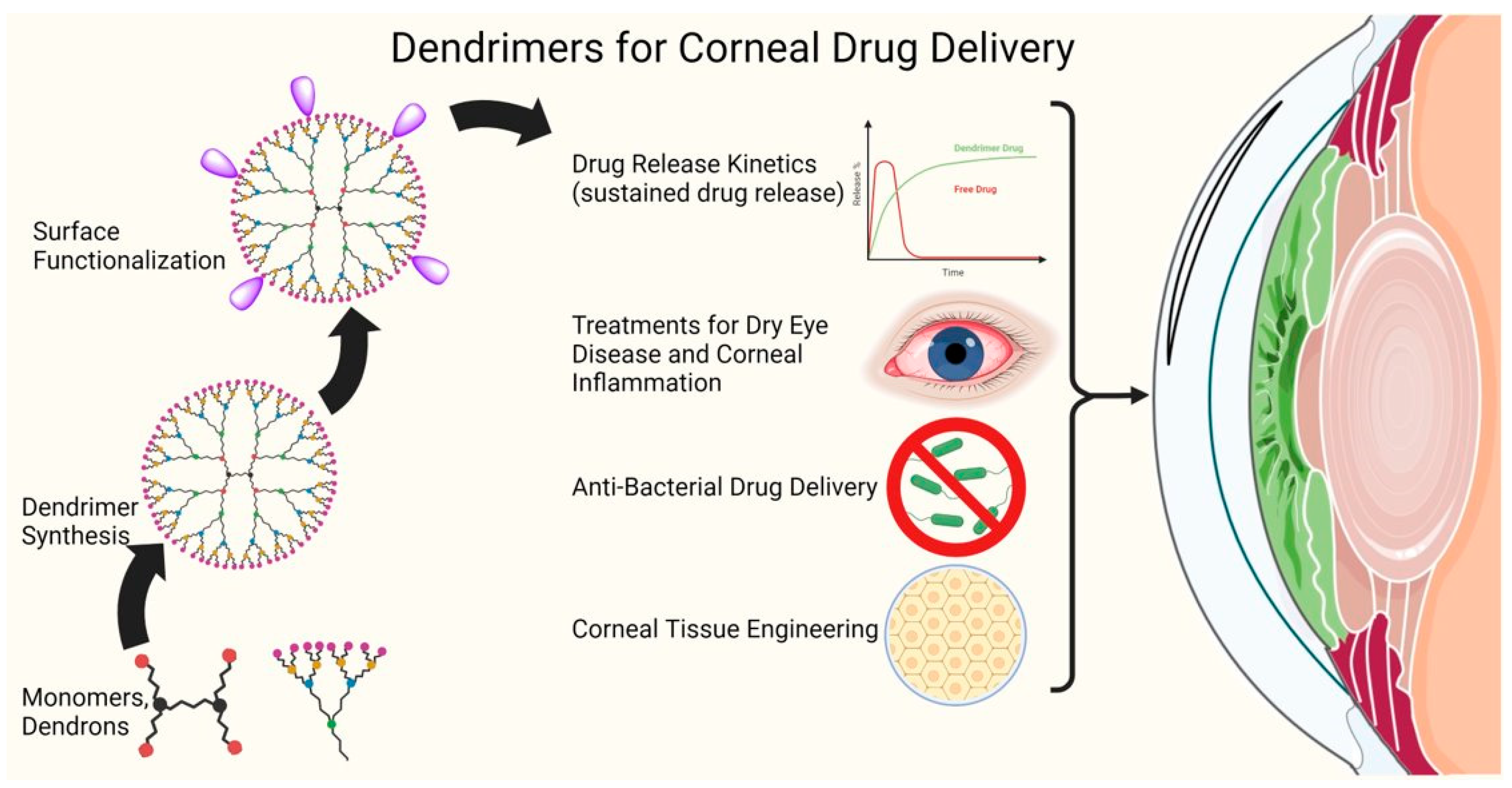
5. Dendrimers for Corneal Drug Delivery
5.1. Introduction to Dendrimers
5.2. Dendrimers for the Treatment of Corneal Diseases
5.2.1. Complexes of Dendrimers with Drugs or Genes for Corneal Delivery
5.2.2. Dendrimer Drug Covalent Conjugates for Drug Delivery to Cornea
5.2.3. Dendrimer Polymer Hydrogels for Corneal Drug Delivery and Tissue Engineering
5.2.4. Dendrimer-Based Photosensitizers for Photodynamic Therapy
6. Dendrimer Drug Delivery: Translational Opportunities and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Macwan, J.S.; Hirani, A.; Pathak, Y. Challenges in Ocular Pharmacokinetics and Drug Delivery. In Nano-Biomaterials for Ophthalmic Drug Delivery; Pathak, Y., Sutariya, V., Hirani, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 593–611. [Google Scholar]
- O’Brien Laramy, M.N.; Nagapudi, K. Long-acting ocular drug delivery technologies with clinical precedent. Expert Opin. Drug Deliv. 2022, 19, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat-Binstock, E.; Domb, A.J. Iontophoresis: A non-invasive ocular drug delivery. J. Control. Release Off. J. Control. Release Soc. 2006, 110, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat-Binstock, E.; Raiskup, F.; Stepensky, D.; Domb, A.J.; Frucht-Pery, J. Delivery of gentamicin to the rabbit eye by drug-loaded hydrogel iontophoresis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2543–2548. [Google Scholar] [CrossRef]
- Frucht-Pery, J.; Raiskup, F.; Mechoulam, H.; Shapiro, M.; Eljarrat-Binstock, E.; Domb, A. Iontophoretic treatment of experimental pseudomonas keratitis in rabbit eyes using gentamicin-loaded hydrogels. Cornea 2006, 25, 1182–1186. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Zderic, V.; Clark, J.I.; Vaezy, S. Drug delivery into the eye with the use of ultrasound. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2004, 23, 1349–1359. [Google Scholar] [CrossRef]
- Nabili, M.; Shenoy, A.; Chawla, S.; Mahesh, S.; Liu, J.; Geist, C.; Zderic, V. Ultrasound-enhanced ocular delivery of dexamethasone sodium phosphate: An in vivo study. J. Ther. Ultrasound 2014, 2, 6. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated microneedles for drug delivery to the eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- El Chehab, H.; Le Corre, A.; Agard, E.; Ract-Madoux, G.; Coste, O.; Dot, C. Effect of topical pressure-lowering medication on prevention of intraocular pressure spikes after intravitreal injection. Eur. J. Ophthalmol. 2013, 23, 277–283. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Kannan, R.M. Dendrimer nanoparticles for ocular drug delivery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2013, 29, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Patel, P.; Patel, V.; Patel, P.M. Synthetic strategy of dendrimers: A review. J. Indian Chem. Soc. 2022, 99, 100514. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Orash Mahmoud Salehi, A.; Heidari-Keshel, S.; Poursamar, S.A.; Zarrabi, A.; Sefat, F.; Mamidi, N.; Behrouz, M.J.; Rafienia, M. Bioprinted Membranes for Corneal Tissue Engineering: A Review. Pharmaceutics 2022, 14, 2797. [Google Scholar] [CrossRef]
- Arkas, M.; Vardavoulias, M.; Kythreoti, G.; Giannakoudakis, D.A. Dendritic Polymers in Tissue Engineering: Contributions of PAMAM, PPI PEG and PEI to Injury Restoration and Bioactive Scaffold Evolution. Pharmaceutics 2023, 15, 524. [Google Scholar] [CrossRef]
- The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Lin, H.; Liu, Y.; Kambhampati, S.P.; Hsu, C.C.; Kannan, R.M.; Yiu, S.C. Subconjunctival dendrimer-drug therapy for the treatment of dry eye in a rabbit model of induced autoimmune dacryoadenitis. Ocul. Surf. 2018, 16, 415–423. [Google Scholar] [CrossRef]
- Ding, J.; Sullivan, D.A. Aging and dry eye disease. Exp. Gerontol. 2012, 47, 483–490. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Sauer, A.; Dory, A.; Denis, J.; Sabou, M. Fungal keratitis. J. Français D’ophtalmologie 2017, 40, e307–e313. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.; Arenas, E.; Ayyala, R.S.; Margo, C.E.; Espana, E.M. Fungal keratitis: Mechanisms of infection and management strategies. Surv. Ophthalmol. 2022, 67, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, U.; Sharma, S.; Garg, P.; Rao, G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthalmol. 2009, 57, 273. [Google Scholar]
- Ying, Y.L.; Hirsch, B.E. Atypical Cogan’s syndrome: A case report. Am. J. Otolaryngol. 2010, 31, 279–282. [Google Scholar] [CrossRef]
- Iliescu, D.A.; Timaru, C.M.; Batras, M.; De Simone, A.; Stefan, C. Cogan’s Syndrome. Rom. J. Ophthalmol. 2015, 59, 6–13. [Google Scholar]
- Philipp, W.; Speicher, L.; Humpel, C. Expression of Vascular Endothelial Growth Factor and Its Receptors in Inflamed and Vascularized Human Corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2514–2522. [Google Scholar]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef]
- Azari, A.A.; Barney, N.P. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA 2013, 310, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Villegas, B.V.; Benitez-Del-Castillo, J.M. Current Knowledge in Allergic Conjunctivitis. Turk. J. Ophthalmol. 2021, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Bowman’s layer in the cornea- structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef] [PubMed]
- Hertsenberg, A.J.; Funderburgh, J.L. Chapter Three—Stem Cells in the Cornea. In Progress in Molecular Biology and Translational Science; Hejtmancik, J.F., Nickerson, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 134, pp. 25–41. [Google Scholar]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Bojic, S.; Dorgau, B.; Moyse, N.; Molina, M.M.; Yang, C.; Dey, S.; Reynolds, G.; et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul. Surf. 2021, 21, 279–298. [Google Scholar] [CrossRef]
- Hertsenberg, A.J.; Funderburgh, J.L. Stem Cells in the Cornea. Prog. Mol. Biol. Transl. Sci. 2015, 134, 25–41. [Google Scholar] [CrossRef]
- Hodges, R.R.; Dartt, D.A. Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 2013, 117, 62–78. [Google Scholar] [CrossRef]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, F.; Tabesh, H.; Farzad, F. Sustained subconjunctival drug delivery systems: Current trends and future perspectives. Int. Ophthalmol. 2020, 40, 2385–2401. [Google Scholar] [CrossRef]
- Gautam, M.; Gupta, R.; Singh, P.; Verma, V.; Verma, S.; Mittal, P.; Karkhur, S.; Sampath, A.; Mohan, R.R.; Sharma, B. Intracameral Drug Delivery: A Review of Agents, Indications, and Outcomes. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2023. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Caminade, A.M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Sharma, A.; Kakkar, A. Designing Dendrimer and Miktoarm Polymer Based Multi-Tasking Nanocarriers for Efficient Medical Therapy. Molecules 2015, 20, 16987–17015. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.D.; Voegtle, F. ‘Cascade’- and ‘nonskid-chain-like’ syntheses of molecular cavity topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K.; Russo, P.S.; Saunders, M.J. Chemistry of micelles series. Part 2. Cascade molecules. Synthesis and characterization of a benzene[9]3-arborol. J. Am. Chem. Soc. 1986, 108, 849–850. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.M.; Dewald, J.R.; Hall, M.E.; Kallos, G.; Martin, S.; Roeck, J.S.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Hawker, C.; Fréchet, J.M.J. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc. Chem. Commun. 1990, 1010–1013. [Google Scholar] [CrossRef]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, M.; Wafer, C.; Morin, J.F. Recent advances in click chemistry applied to dendrimer synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Gök, Ö.; Tomlinson, S.N.; Sharma, A.; Moser, A.B.; Kannan, S.; Kannan, R.M.; Fatemi, A. Targeted Brain Delivery of Dendrimer-4-Phenylbutyrate Ameliorates Neurological Deficits in a Long-Term ABCD1-Deficient Mouse Model of X-Linked Adrenoleukodystrophy. Neurotherapeutics 2022. [Google Scholar] [CrossRef]
- Tallon, C.; Bell, B.J.; Sharma, A.; Pal, A.; Malvankar, M.M.; Thomas, A.G.; Yoo, S.-W.; Hollinger, K.R.; Coleman, K.; Wilkinson, E.L.; et al. Dendrimer-Conjugated nSMase2 Inhibitor Reduces Tau Propagation in Mice. Pharmaceutics 2022, 14, 2066. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Satyanarayana, G.; Liu, T.; Wang, L.; Wang, J.; Cheng, J.; Itoh, K.; Sharma, A.; Bhutto, I.; et al. Mitophagy initiates retrograde mitochondrial-nuclear signaling to guide retinal pigment cell heterogeneity. Autophagy 2023, 19, 966–983. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM dendrimers significantly improves tumor macrophage targeting and specificity in glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef]
- Yavuz, B.; Kompella, U.B. Ocular Drug Delivery. Handb. Exp. Pharmacol. 2017, 242, 57–93. [Google Scholar] [CrossRef]
- Maurice, D. Review: Practical issues in intravitreal drug delivery. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2001, 17, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. Drug delivery to the posterior segment from drops. Surv. Ophthalmol. 2002, 47 (Suppl. S1), S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G., 3rd; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Krishna Venuganti, V.V.; Chauhan, S.S.; Garg, P. Polymeric Drug Delivery Devices: Role in Cornea and External Disease. Eye Contact Lens. 2022, 48, 119–126. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. BioImpacts BI 2015, 5, 159–164. [Google Scholar] [CrossRef]
- Rayner, S.A.; King, W.J.; Comer, R.M.; Isaacs, J.D.; Hale, G.; George, A.J.; Larkin, D.F. Local bioactive tumour necrosis factor (TNF) in corneal allotransplantation. Clin. Exp. Immunol. 2000, 122, 109–116. [Google Scholar] [CrossRef]
- Hudde, T.; Rayner, S.A.; Comer, R.M.; Weber, M.; Isaacs, J.D.; Waldmann, H.; Larkin, D.F.; George, A.J. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther. 1999, 6, 939–943. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vicario-de-la-Torre, M.; Andrés-Guerrero, V.; Sánchez-Nieves, J.; Guzmán-Navarro, M.; de la Mata, F.J.; Gómez, R.; de Las Heras, B.; Argüeso, P.; Ponchel, G.; et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol. Pharm. 2016, 13, 2966–2976. [Google Scholar] [CrossRef]
- Yao, C.; Wang, W.; Xiudi, Z.; Qu, T.; Mu, H.; Rongcai, L.; Aiping, W.; Sun, K. Effects of Poly(amidoamine) Dendrimers on Ocular Absorption of Puerarin Using Microdialysis. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2011, 27, 565–569. [Google Scholar] [CrossRef]
- Yao, W.J.; Sun, K.X.; Liu, Y.; Liang, N.; Mu, H.J.; Yao, C.; Liang, R.C.; Wang, A.P. Effect of poly(amidoamine) dendrimers on corneal penetration of puerarin. Biol. Pharm. Bull. 2010, 33, 1371–1377. [Google Scholar] [CrossRef]
- Souza, J.G.; Dias, K.; Silva, S.A.; de Rezende, L.C.; Rocha, E.M.; Emery, F.S.; Lopez, R.F. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 200, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Richichi, B.; Baldoneschi, V.; Burgalassi, S.; Fragai, M.; Vullo, D.; Akdemir, A.; Dragoni, E.; Louka, A.; Mamusa, M.; Monti, D.; et al. A Divalent PAMAM-Based Matrix Metalloproteinase/Carbonic Anhydrase Inhibitor for the Treatment of Dry Eye Syndrome. Chemistry 2016, 22, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Skalka, H.W.; Prchal, J.T. Effect of Corticosteroids on Cataract Formation. Arch. Ophthalmol. 1980, 98, 1773–1777. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef]
- Bertram, J.P.; Saluja, S.S.; McKain, J.; Lavik, E.B. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J. Microencapsul. 2009, 26, 18–26. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.S.; Lancina Iii, M.G.; Yang, H. Mildly Cross-Linked Dendrimer Hydrogel Prepared via Aza-Michael Addition Reaction for Topical Brimonidine Delivery. J. Biomed. Nanotechnol. 2017, 13, 1089–1096. [Google Scholar] [CrossRef]
- Pagano, L.; Shah, H.; Al Ibrahim, O.; Gadhvi, K.A.; Coco, G.; Lee, J.W.; Kaye, S.B.; Levis, H.J.; Hamill, K.J.; Semeraro, F.; et al. Update on Suture Techniques in Corneal Transplantation: A Systematic Review. J. Clin. Med. 2022, 11, 1078. [Google Scholar] [CrossRef]
- Wathier, M.C.; Carnahan, M.A.; Jung, P.J.; Kim, T.; Grinstaff, M.W. Dendrimer Hydrogels as New Alternative for the Repair of Clear Corneal Incisions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4993. [Google Scholar]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef]
- Duan, X.; McLaughlin, C.; Griffith, M.; Sheardown, H. Biofunctionalization of collagen for improved biological response: Scaffolds for corneal tissue engineering. Biomaterials 2007, 28, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Carlsson, D.; Lohmann, C.; Suuronen, E.; Vascotto, S.; Kobuch, K.; Sheardown, H.; Munger, R.; Nakamura, M.; Griffith, M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl. Acad. Sci. USA 2003, 100, 15346–15351. [Google Scholar] [CrossRef] [PubMed]
- Grinstaff, M.W. Designing hydrogel adhesives for corneal wound repair. Biomaterials 2007, 28, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Long, Y.; Liu, Y.; Long, K.; Liu, S.; Wang, Z.; Wang, Y.; Ren, L. Fabrication and characterization of chitosan–collagen crosslinked membranes for corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 1962–1972. [Google Scholar] [CrossRef]
- Grolik, M.; Szczubiałka, K.; Wowra, B.; Dobrowolski, D.; Orzechowska-Wylęgała, B.; Wylęgała, E.; Nowakowska, M. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Princz, M.A.; Sheardown, H. Heparin-modified dendrimer crosslinked collagen matrices for the delivery of heparin-binding epidermal growth factor. J. Biomed. Mater. Res. Part A 2012, 100, 1929–1937. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Daftarian, P.; Kovalski, L.; Wang, B.; Tian, R.; Castilla, D.M.; Dikici, E.; Perez, V.L.; Deo, S.; Daunert, S.; et al. Directing and Potentiating Stem Cell-Mediated Angiogenesis and Tissue Repair by Cell Surface E-Selectin Coating. PLoS ONE 2016, 11, e0154053. [Google Scholar] [CrossRef]
- Nair, A.; Javius-Jones, K.; Bugno, J.; Poellmann, M.J.; Mamidi, N.; Kim, I.-S.; Kwon, I.C.; Hong, H.; Hong, S. Hybrid Nanoparticle System Integrating Tumor-Derived Exosomes and Poly(amidoamine) Dendrimers: Implications for an Effective Gene Delivery Platform. Chem. Mater. 2023, 35, 3138–3150. [Google Scholar] [CrossRef]
- Sugisaki, K.; Usui, T.; Nishiyama, N.; Jang, W.D.; Yanagi, Y.; Yamagami, S.; Amano, S.; Kataoka, K. Photodynamic therapy for corneal neovascularization using polymeric micelles encapsulating dendrimer porphyrins. Investig. Ophthalmol. Vis. Sci. 2008, 49, 894–899. [Google Scholar] [CrossRef]
- Epstein, R.J.; Stulting, R.D.; Hendricks, R.L.; Harris, D.M. Corneal neovascularization. Pathogenesis and inhibition. Cornea 1987, 6, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, C.; Kadam, R.; Chandler, J.; Hutcherson, S.; Kompella, U. Nanosized Dendritic Polyguanidilyated Translocators for Enhanced Solubility, Permeability, and Delivery of Gatifloxacin. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5804–5816. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Zhang, Z.; Liaw, K.; Kambhampati, S.P.; Porterfield, J.E.; Lin, K.C.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020, 6, eaay8514. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef]
- Porterfield, J.E.; Sharma, R.; Jimenez, A.S.; Sah, N.; McCracken, S.; Zhang, L.; An, H.-T.; Lee, S.; Kannan, S.; Sharma, A.; et al. Galactosylated hydroxyl-polyamidoamine dendrimer targets hepatocytes and improves therapeutic outcomes in a severe model of acetaminophen poisoning-induced liver failure. Bioeng. Transl. Med. 2023, 8, e10486. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Bhutto, I.A.; Wu, T.; Ho, K.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release Off. J. Control. Release Soc. 2021, 335, 527–540. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Millwood, I.Y.; McDonald, H.M.; Price, C.F.; Kaldor, J.M.; Paull, J.R. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): A dose ranging, phase I study. Sex. Transm. Dis. 2010, 37, 100–104. [Google Scholar] [CrossRef]
- Cohen, C.R.; Brown, J.; Moscicki, A.B.; Bukusi, E.A.; Paull, J.R.; Price, C.F.; Shiboski, S. A phase I randomized placebo controlled trial of the safety of 3% SPL7013 Gel (VivaGel®) in healthy young women administered twice daily for 14 days. PLoS ONE 2011, 6, e16258. [Google Scholar] [CrossRef]
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS 2011, 25, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Holmes, W.R.; Maher, L.; Rosenthal, S.L. Attitudes of men in an Australian male tolerance study towards microbicide use. Sex. Health 2008, 5, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, A.; Kambhampati, S.P.; Reddy, R.R.; Zhang, Z.; Cleland, J.L.; Kannan, S.; Kannan, R.M. Scalable synthesis and validation of PAMAM dendrimer-N-acetyl cysteine conjugate for potential translation. Bioeng. Transl. Med. 2018, 3, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra146. [Google Scholar] [CrossRef]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng. Transl. Med. 2020, 5, e10160. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Mishra, M.K.; Jyoti, A.; Balakrishnan, B.; Zhang, F.; Nance, E.; Romero, R.; Kannan, S.; Kannan, R.M. Biodistribution of Fluorescently Labeled PAMAM Dendrimers in Neonatal Rabbits: Effect of Neuroinflammation. Mol. Pharm. 2013, 10, 4560–4571. [Google Scholar] [CrossRef]
- Sadekar, S.; Ghandehari, H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef]
- Tallon, C.; Sharma, A.; Zhang, Z.; Thomas, A.G.; Ng, J.; Zhu, X.; Donoghue, A.; Schulte, M.; Joe, T.R.; Kambhampati, S.P.; et al. Dendrimer-2PMPA Delays Muscle Function Loss and Denervation in a Murine Model of Amyotrophic Lateral Sclerosis. Neurotherapeutics 2022, 19, 274–288. [Google Scholar] [CrossRef]
- Sharma, R.; Porterfield, J.E.; An, H.-T.; Jimenez, A.S.; Lee, S.; Kannan, S.; Sharma, A.; Kannan, R.M. Rationally Designed Galactose Dendrimer for Hepatocyte-Specific Targeting and Intracellular Drug Delivery for the Treatment of Liver Disorders. Biomacromolecules 2021, 22, 3574–3589. [Google Scholar] [CrossRef] [PubMed]



| S. No. | Study | References | Type of Dendrimers Used |
|---|---|---|---|
| 1. | Increased penetration of drugs through cornea using dendrimers | Brobeck et al. [94] | PAMAM dendrimers of various generations. |
| Lopez et al. [73] | Generation 3.5 and generation 4 PAMAM dendrimers. | ||
| Kompella et al. [77] | PLGA-PAMAM hydrogel dendrimer Complex. | ||
| Yang et al. [79] | Generation 5 PAMAM dendrimer. | ||
| 2. | Treatment of dry eye disease (DED) | Nativi etal. [74] | Generation 2 PAMAM dendrimer. |
| 3. | Anti-bacterial drug delivery to cornea | Kompella et al. [95] | Generation 6 dendrimeric polyguanidilyated translocators (DPTs) |
| 4. | Treatment of corneal inflammation | Sun et al. [71] | Generation 3 PAMAM dendrimers with Puerarin |
| Kannan et al. [75] | Generation 4-hydroxyl terminating PAMAM dendrimer. | ||
| 5. | Corneal tissue engineering | Sheardown et al. [82] | Generation 2 polypropylene octamine dendrimers |
| Grinstaff et al. [85] | (PGLSA-MA)2-PEG dendrimer | ||
| Sheardown et al. [83] | Generation 2 polypropyleneimine dendrimer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhull, A.; Yu, C.; Wilmoth, A.H.; Chen, M.; Sharma, A.; Yiu, S. Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities. Pharmaceutics 2023, 15, 1591. https://doi.org/10.3390/pharmaceutics15061591
Dhull A, Yu C, Wilmoth AH, Chen M, Sharma A, Yiu S. Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities. Pharmaceutics. 2023; 15(6):1591. https://doi.org/10.3390/pharmaceutics15061591
Chicago/Turabian StyleDhull, Anubhav, Carson Yu, Alex Hunter Wilmoth, Minjie Chen, Anjali Sharma, and Samuel Yiu. 2023. "Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities" Pharmaceutics 15, no. 6: 1591. https://doi.org/10.3390/pharmaceutics15061591
APA StyleDhull, A., Yu, C., Wilmoth, A. H., Chen, M., Sharma, A., & Yiu, S. (2023). Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities. Pharmaceutics, 15(6), 1591. https://doi.org/10.3390/pharmaceutics15061591




