Design of Dual-Targeted pH-Sensitive Hybrid Polymer Micelles for Breast Cancer Treatment: Three Birds with One Stone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Cell Lines, and Animals
2.1.1. Materials
2.1.2. Cell Lines and Animals
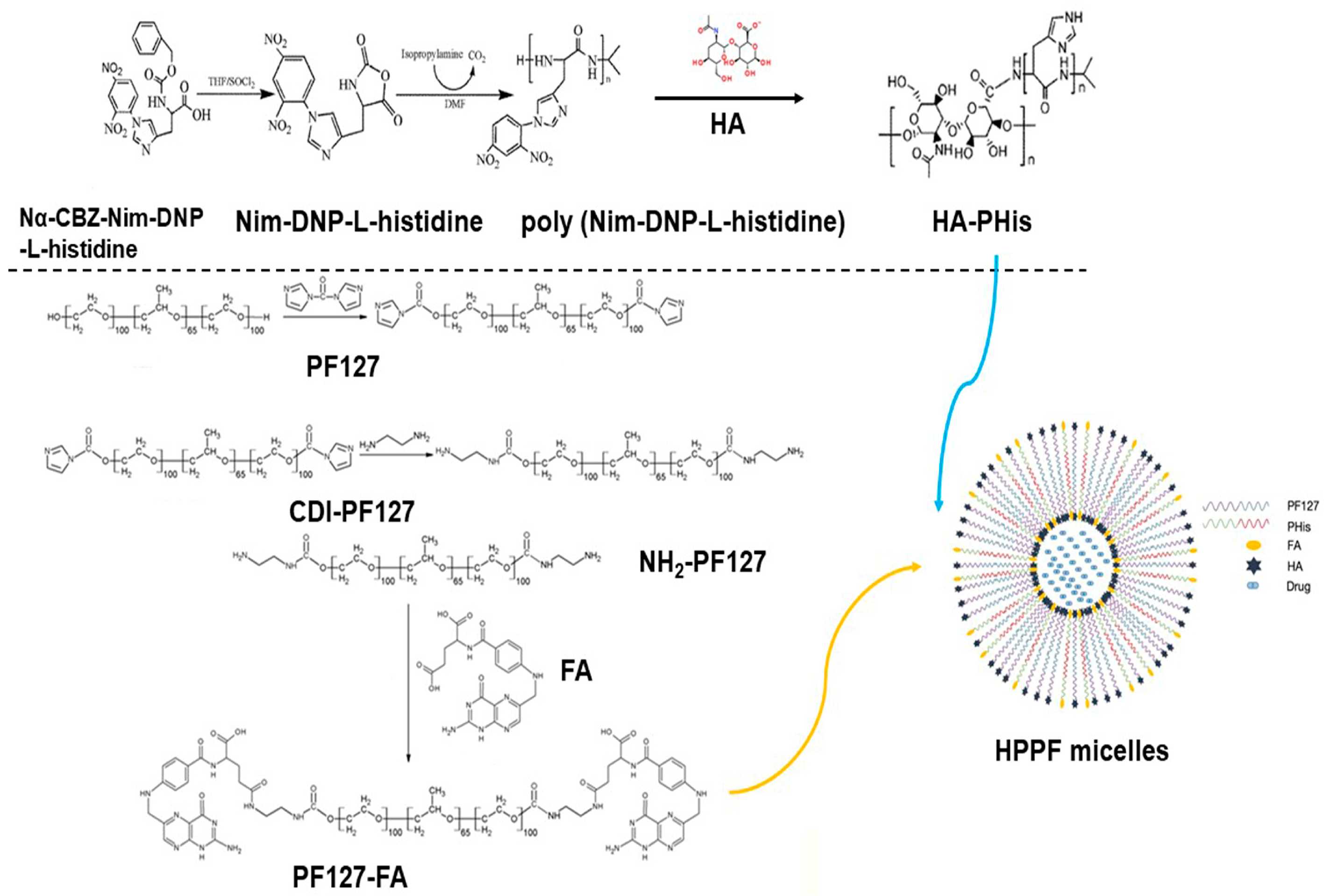
2.2. Synthesis and Characterization of HA-PHis
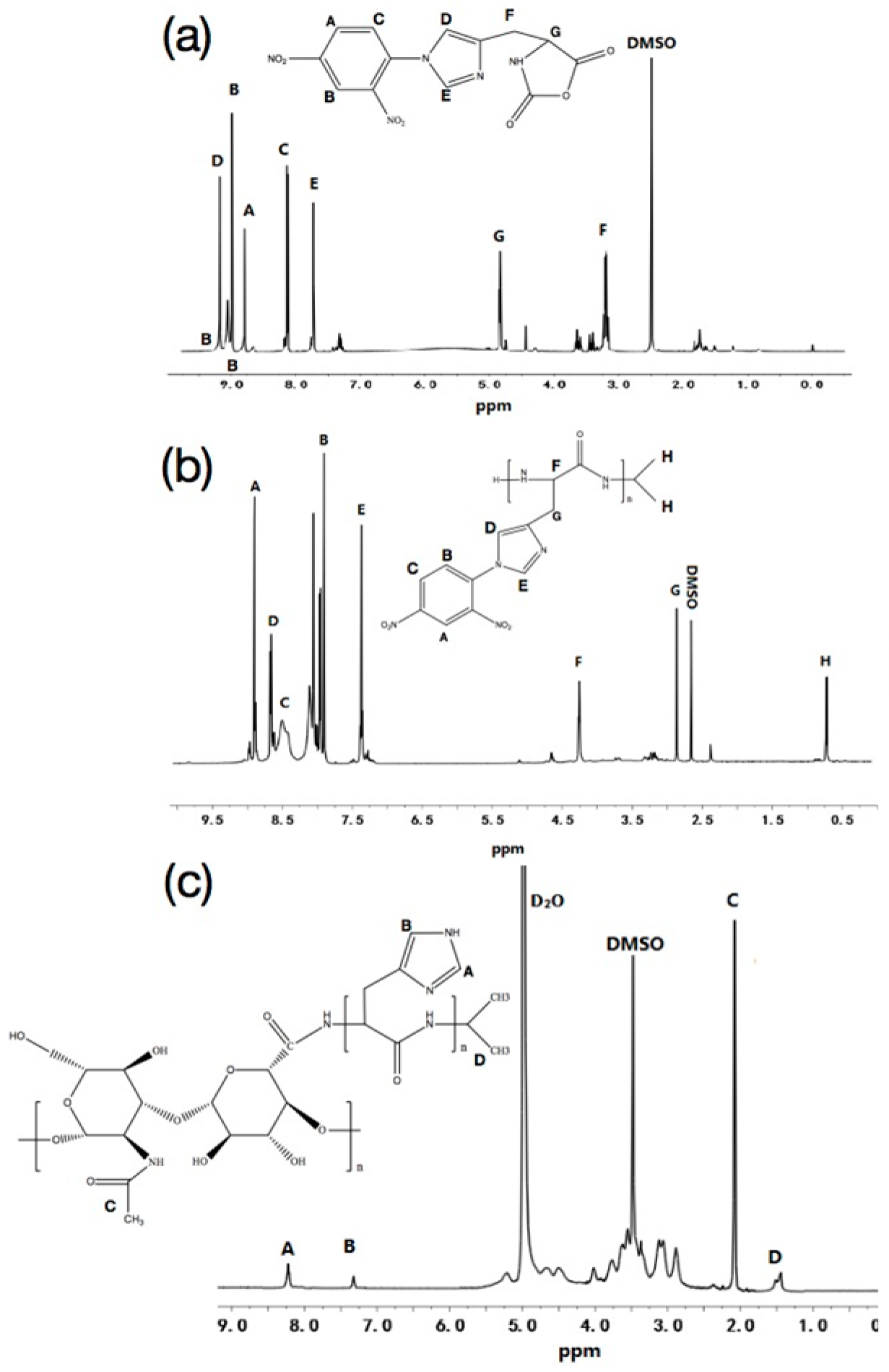
2.2.1. Synthesis and Characterization of Nim-DNP-L-Histidine
2.2.2. Synthesis and Characterization of Poly (Nim-DNP-L-Histidine)
2.2.3. Synthesis and Characterization of HA-PHis
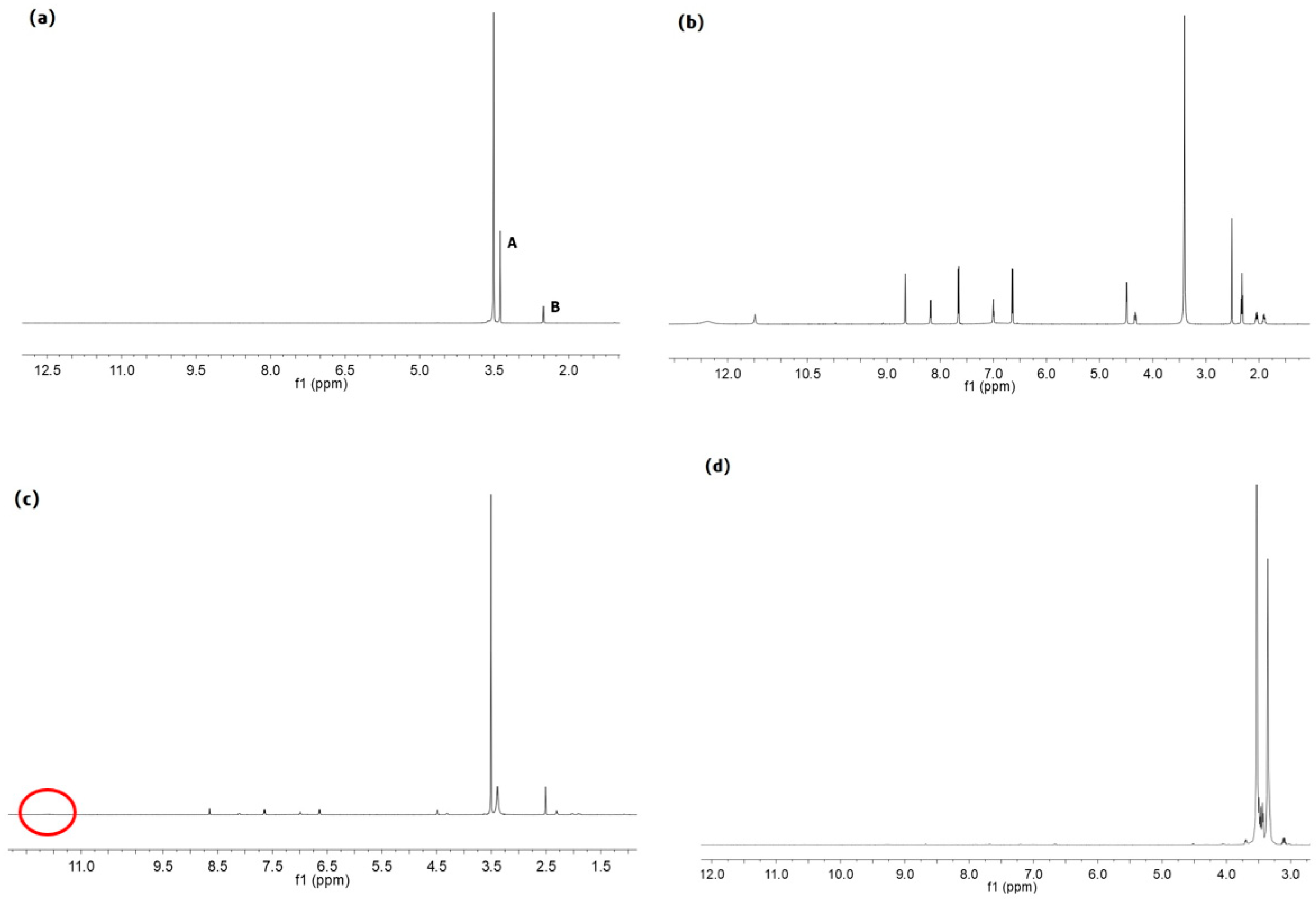
2.3. Synthesis and Characterization of PF127-FA
2.3.1. Synthesis and Characterization of CDI-PF127
2.3.2. Synthesis and Characterization of NH2-PF127
2.3.3. Synthesis and Characterization of PF127-FA
2.4. Preparation and Characterization of Micelles
2.5. In Vitro Drug Release
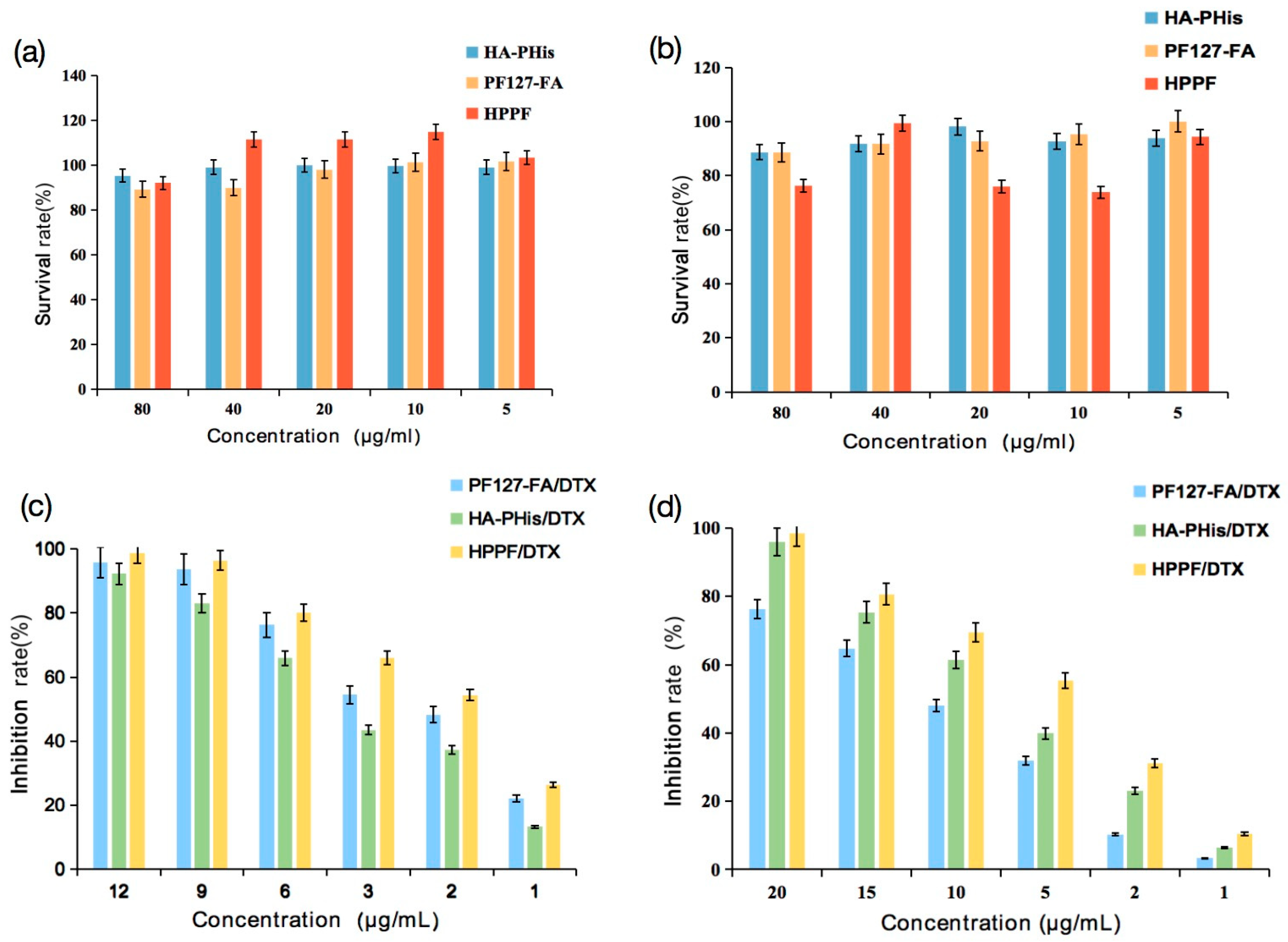
2.6. Cytotoxicity
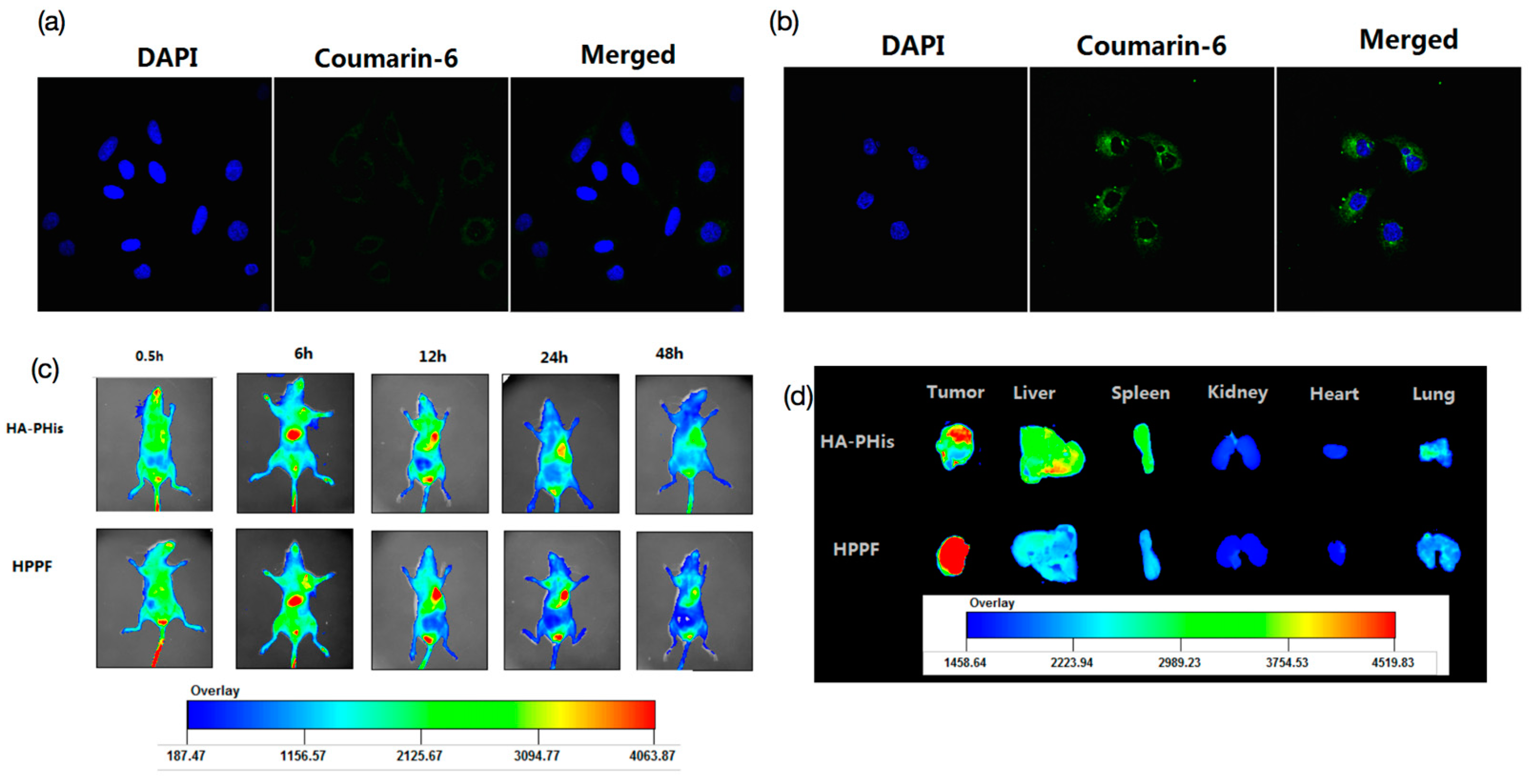
2.7. In Vitro Cellular Uptake
2.8. In Vivo Fluorescence Imaging and Tissue Distribution
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of HA-PHis and PF127-FA
3.2. Characterization of Micelles
3.2.1. Particle Size and Zeta Potential
3.2.2. Morphological Observation
3.2.3. Entrapment Efficiency and Drug Loading
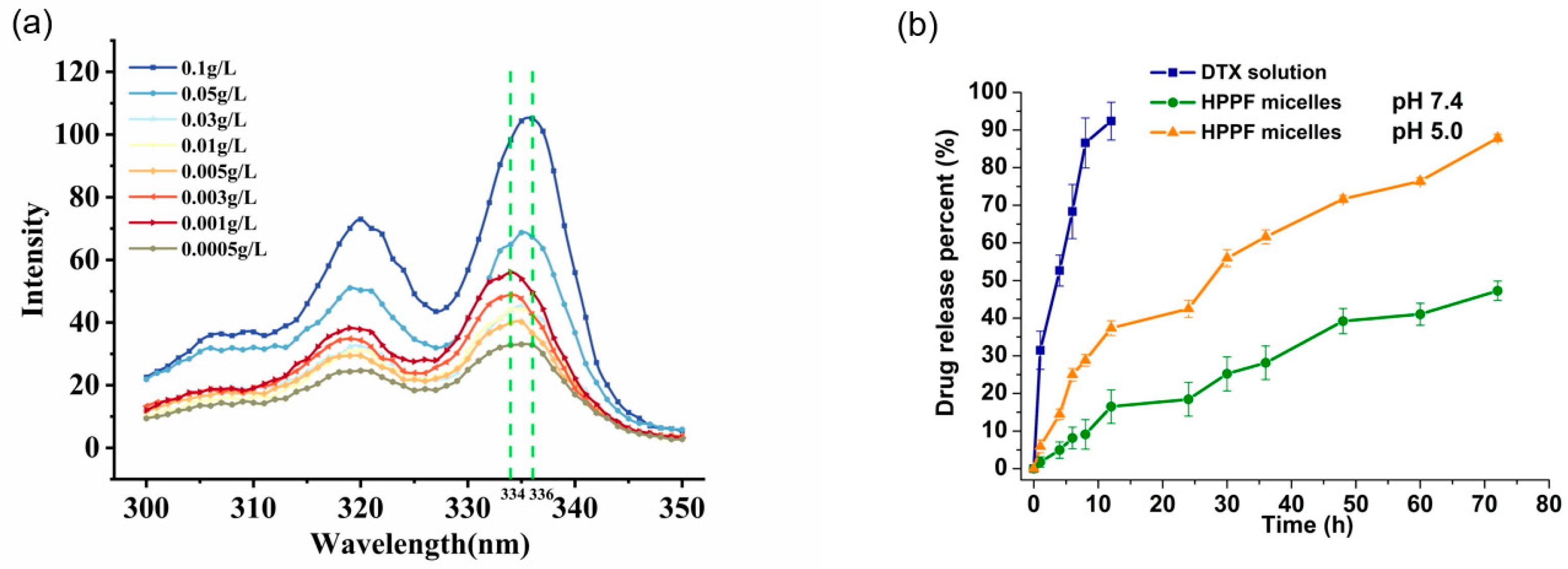
3.2.4. Determination of Critical Micelle Concentration
3.2.5. In Vitro Drug Release
3.3. Cytotoxicity
3.4. In Vitro Cellular Uptake
3.5. In Vivo Fluorescence Imaging and Tissue Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Fujimoto, H.; Kazama, T.; Nagashima, T.; Sakakibara, M.; Suzuki, T.H.; Okubo, Y.; Shiina, N.; Fujisaki, K.; Ota, S.; Miyazaki, M. Diffusion-weighted imaging reflects pathological therapeutic response and relapse in breast cancer. Breast Cancer 2014, 21, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Mazzucchelli, S.; Bonizzi, A.; Sorrentino, L.; Allevi, R.; Vanna, R.; Morasso, C.; Corsi, F. Nano-strategies to target breast cancer-associated fibroblasts: Rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci. 2019, 20, 1263. [Google Scholar] [CrossRef]
- Rajana, N.; Mounika, A.; Chary, P.S.; Bhavana, V.; Urati, A.; Khatri, D.; Singh, S.B.; Mehra, N.K. Multifunctional hybrid nanoparticles in diagnosis and therapy of breast cancer. J. Control. Release 2022, 352, 1024–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Ge, Z.S.; Toh, K.; Liu, X.Y.; Dirisala, A.; Ke, W.D.; Wen, P.Y.; Zhou, H.; Wang, Z.; Xiao, S.Y.; et al. Enzymatically transformable polymersome-based nanotherapeutics to eliminate minimal relapsable cancer. Adv. Mater. 2021, 33, e2105254. [Google Scholar] [CrossRef]
- Zhao, L.W.; Gu, C.Y.; Gan, Y.; Shao, L.L.; Chen, H.W.; Zhu, H.Y. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Fang, T.X.; Zhang, J.; Yang, J.; Xu, R.; Wang, Z.H.; Deng, H.Z.; Shen, Q. pH-sensitive molecular-switch-containing polymer nanoparticle for breast cancer therapy with ferritinophagy-cascade ferroptosis and tumor immune activation. Adv. Heal. Mater. 2021, 10, e2100683. [Google Scholar] [CrossRef]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric vesicles: From drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef]
- Lee, S.W.; Yun, M.H.; Jeong, S.W.; In, C.H.; Kim, J.Y.; Seo, M.H.; Pai, C.M.; Kim, S.O. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J. Control. Release 2011, 155, 262–271. [Google Scholar] [CrossRef]
- Li, L.B.; Tan, Y.B. Preparation and properties of mixed micelles made of Pluronic polymer and PEG-PE. Bioeng. J. Colloid. Interface Sci. 2008, 317, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.H.A.; Youssry, M. Polymeric micelles of biodegradable deblock copolymers: Enhanced encapsulation of hydrophobic drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef]
- Kaur, j.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, R.; Ishida, Y.; Sadakiyo, T.; Taniguchi, Y.; Sakurai, T.; Mitsuzumi, H.; Watanabe, H.; Fukuda, S.; Ushio, S. Effects of isomaltodextrin in postprandial lipid kinetics: Rat study and human randomized crossover study. PLoS ONE 2018, 13, e0196802. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Zhao, K.; Liu, R.; Guo, X.L.; He, B.; Yan, J.Q.; Ren, J. pH-sensitive polymeric micelles assembled by stereocomplexation between PLLA-b-PLys and PDLA-b-mPEG for drug delivery. J. Mater. Chem. B 2019, 7, 334–345. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yeh, C.F.; Mellas, M.; Oh, M.J.; Zhu, J.Y.; Li, J.; Huang, R.T.; Harrison, D.L.; Shentu, T.P.; Wu, D.; et al. Targeted polyelectrolyte complex micelles treat vascular complications in vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2114842118. [Google Scholar] [CrossRef]
- Lin, M.H.; Dai, Y.; Xia, F.; Zhang, X.J. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111626. [Google Scholar] [CrossRef]
- Luo, Y.L.; Yin, X.J.; Yin, X.; Chen, A.Q.; Zhao, L.L.; Zhang, G.; Liao, W.B.; Huang, X.X.; Li, J.; Zhang, C.Y. Dual pH/redox-responsive mixed polymeric micelles for anticancer drug delivery and controlled release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, D.L.; Yang, H.Y.; Wang, L.J.; Zhang, X.Y. A pH-responsive self-healing hydrogel based on multivalent coordination of Ni2+ with polyhistidine-terminated PEG and IDA-modified oligochitosan. J. Mater. Chem. B 2019, 7, 30–42. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Du, J.L.; Yu, S.P.; Yang, Y.Z.; Liu, X.G. Folic acid-conjugated magnetic ordered mesoporous carbon nanospheres for doxorubicin targeting delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109939. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Q.; Bi, H.T.; Wang, Z.; Li, C.X.; Wang, X.W.; Xu, J.T.; Zhu, H.; Zhao, R.X.; He, F.; Gai, S.L.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef]
- Gao, K.; Sun, J.; Liu, K.; Liu, X.H.; He, Z.G. Preparation and characterization of a submicron lipid emulsion of docetaxel: Submicron lipid emulsion of docetaxel. Drug Dev. Ind. Pharm. 2008, 34, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Favero, E.D.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Anson, C.E.; Briggs, J.C.; Haines, A.H.; Molinier, M. Synthesis of N-(2,4-dinitrophenyl) derivatives of D-ribosylamines; unexpected reaction and hydrolysis products. Carbohydr. Res. 2022, 516, 108564. [Google Scholar] [CrossRef]
- Masila, V.M.; Ndakala, A.J.; Byamukama, R.; Midiwo, J.O.; Kamau, R.W.; Wang, M.; Kumarihamy, M.; Zhao, J.P.; Heydreich, M.; Muhammad, I. Synthesis, structural assignments and antiinfective activities of 3-O-benzyl-carvotacetone and 3-hydroxy-2-isopropyl-5-methyl- p-benzoquinone. Nat. Prod. Res. 2021, 35, 3599–3607. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, M.; Gowda, N.M.N.; Murthy, K.G.S.; Nagasundara, K.R. Activation of -N=CH- bond in a Schiff base by divalent nickel monitored by NMR evidence. Magn. Reason. Chem. 2012, 50, 335–340. [Google Scholar] [CrossRef]
- Rana, A.; Bhatnagar, S. Advancements in folate receptor targeting for anti-cancer therapy: A small molecule-drug conjugate approach. Bioorg. Chem. 2021, 112, 104946. [Google Scholar] [CrossRef]
- Bae, Y.; Alani, A.W.G.; Rockich, N.C.; Chung Lai, T.S.Z.; Kwon, G.S. Mixed pH-sensitive polymeric micelles for combination drug delivery. Pharm. Res. 2010, 27, 2421–2432. [Google Scholar] [CrossRef]
- Bhut, P.R.; Pal, N.; Mandal, A. Characterization of hydrophobically modified polyacrylamide in mixed polymer-gemini surfactant systems for enhanced oil recovery application. ACS Omega 2019, 4, 20164–20177. [Google Scholar] [CrossRef]
- Naharros-Molinero, A.; Caballo-González, M.A.; de la Mata, F.J.; García-Gallego, S. Direct and reverse pluronic micelles: Design and characterization of promising drug delivery nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, R.; Fan, Y. Encapsulation and release of drug nanoparticles in functional polymeric vesicles. Soft Matter. 2020, 16, 3088–3095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yue, Z.G.; Xie, J.B.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z.Q. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef]
- Kaur, K.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Beakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, W.; Cheng, L.Z.; Zhou, Y.; Fang, A.P.; Jin, C.H.; Zeng, J.; Song, X.R.; Guo, X. Novel polymeric hybrid nanocarrier for curcumin and survivin shRNA co-delivery augments tumor penetration and promotes synergistic tumor suppression. Front. Chem. 2020, 8, 762. [Google Scholar] [CrossRef]
- Di, Y.; Li, T.; Zhu, Z.H.; Chen, F.; Jia, L.Q.; Liu, W.B.; Gai, X.M.; Wang, Y.Y.; Pan, W.S.; Yang, X.G. pH-sensitive and folic acid-targeted MPeg-PhIs/Fa-Peg-Ve mixed micelles for the delivery of PTX-Ve and their antitumor activity. Int. J. Nanomed. 2017, 12, 5863–5877. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef]
- Khosroushahi, A.Y.; Naderi-Manesh, H.; Yeganeh, H.; Barar, J.; Omidi, Y. Novel water-soluble polyurethane nanomicelles for cancer chemotherapy: Physicochemical characterization and cellular activities. J. Nanobiotechnology 2012, 10, 2. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.L.; Ma, J.L.; Zhou, T.P.; Wu, Z.; Ding, P.; Sun, N.; Liu, L.F.; Pei, R.J.; Zhu, W.P. Folic acid-modified fluorescent-magnetic nanoparticles for efficient isolation and identification of circulating tumor cells in ovarian cancer. Biosensors 2022, 12, 184. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; Altawabeyeh, R.M.; Darweesh, R.S. Preparation and characterization of docetaxel-PLGA nanoparticles coated with folic acid-chitosan conjugate for cancer treatment. J. Pharm Sci. 2022, 111, 485–494. [Google Scholar] [CrossRef]
- Yang, H.Y.; Du, J.M.; Jang, M.S.; Mo, X.W.; Sun, X.S.; Lee, D.S.; Lee, J.H.; Fu, Y. CD44-targeted and enzyme-responsive photo-cross-linked nanogels with enhanced stability for in vivo protein delivery. Biomacromolecules 2021, 22, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, S.; Baruha, B.; Smith, C.; Bellerby, R.; Goddard, L.; Jordan, N.; Poghosyan, Z.; Nicholson, R.; Barrett-Lee, P.; Gee, J. Overexpression of CD44 accompanies acquired tamoxifen resistance in MCF7 cells and augments their sensitivity to the stromal factors, heregulin and hyaluronan. BMC Cancer 2012, 12, 458. [Google Scholar] [CrossRef]
- Butt, A.M.; Abdullah, N.; Mat Rani, N.N.I.; Ahmad, N.; Mohd Amin, M.C.I. Endosomal escape of bioactives deployed via nanocarriers: Insights into the design of polymeric micelles. Pharm. Res. 2022, 39, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Miclea, L.C.; Mihailescu, M.; Tarba, N.; Brezoiu, A.M.; Sandu, A.M.; Mitran, R.A.; Berger, D.; Matei, C.; Moisescu, M.G.; Savopol, T. Evaluation of intracellular distribution of folate functionalized silica nanoparticles using fluorescence and hyperspectral enhanced dark field microscopy. Nanoscale 2022, 14, 12744–12756. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.L.; Dong, L.H.; Zhang, P.; Mu, Y.; Cui, X.Y.; Zhou, J.P.; Huo, M.R.; Yin, T.J. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef]
- Xiao, H.F.; Yu, H.; Wang, D.Q.; Liu, X.Z.; Sun, W.R.; Li, Y.J.; Sun, G.B.; Liang, Y.; Sun, H.F.; Wang, P.Y.; et al. Dual-Targeted Fe₃O₄@MnO₂ nanoflowers for magnetic resonance imaging-guided photothermal-enhanced chemodynamic/chemotherapy for tumor. J. Biomed. Nanotechnol. 2022, 18, 352–368. [Google Scholar] [CrossRef]

| Sample | HA-PHis:PF127-FA |
|---|---|
| A | 9:1 |
| B | 8:2 |
| C | 7:3 |
| D | 6:4 |
| E | 5:5 |
| Sample | Particle Size (nm) | Zeta Potential (mV) | Single/Double Peak |
|---|---|---|---|
| HA-PHis | 119.1 ± 7.7 | −13.2 ± 7.8 | Single peak |
| PF127-FA | 40.0 ± 1.2 | −8.5 ± 1.1 | Single peak |
| A (9:1) | 115.3 ± 6.1 | −16.2 ± 0.8 | Single peak |
| B (8:2) | 119.6 ± 6.3 | −17.4 ± 0.9 | Single peak |
| C (7:3) | 143.0 ± 6.3 | −17.1 ± 3.2 | Irregular single peak |
| D (6:4) | 131.7 ± 7.0 | −21.0 ± 2.5 | Double peak |
| E (5:5) | 122.3 ± 8.4 | −19.2 ± 3.1 | Double peak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Li, Z.; Zhang, Y.; Chen, X.; Liu, M.; Yang, C. Design of Dual-Targeted pH-Sensitive Hybrid Polymer Micelles for Breast Cancer Treatment: Three Birds with One Stone. Pharmaceutics 2023, 15, 1580. https://doi.org/10.3390/pharmaceutics15061580
Yang D, Li Z, Zhang Y, Chen X, Liu M, Yang C. Design of Dual-Targeted pH-Sensitive Hybrid Polymer Micelles for Breast Cancer Treatment: Three Birds with One Stone. Pharmaceutics. 2023; 15(6):1580. https://doi.org/10.3390/pharmaceutics15061580
Chicago/Turabian StyleYang, Degong, Ziqing Li, Yinghui Zhang, Xuejun Chen, Mingyuan Liu, and Chunrong Yang. 2023. "Design of Dual-Targeted pH-Sensitive Hybrid Polymer Micelles for Breast Cancer Treatment: Three Birds with One Stone" Pharmaceutics 15, no. 6: 1580. https://doi.org/10.3390/pharmaceutics15061580
APA StyleYang, D., Li, Z., Zhang, Y., Chen, X., Liu, M., & Yang, C. (2023). Design of Dual-Targeted pH-Sensitive Hybrid Polymer Micelles for Breast Cancer Treatment: Three Birds with One Stone. Pharmaceutics, 15(6), 1580. https://doi.org/10.3390/pharmaceutics15061580





