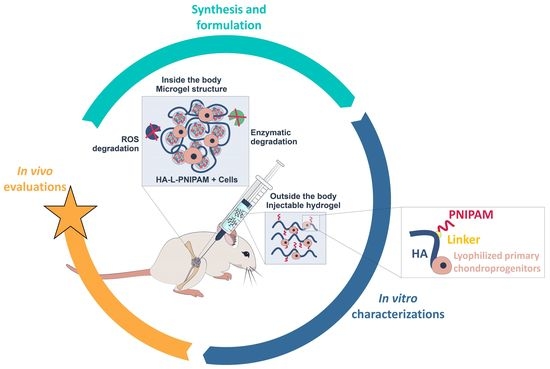
Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Consumables Used in the Study
2.2. Equipment and Instruments Used in the Study
2.3. Lyophilized FE002 Primary Chondroprogenitor Preparation and Characterization
2.4. HA-L-PNIPAM Polymer Synthesis and Characterization
2.5. HA-L-PNIPAM and HA-Based Hydrogel Formulation with Lyophilized FE002 Primary Chondroprogenitors
2.6. Determination of the Intrinsic Antioxidant Capacity of Lyophilized FE002 Primary Chondroprogenitors
2.7. Rheological Characterization of the Combination Products
2.8. Combination Product Accelerated Degradation Assays with Rheological Readouts
2.9. Combination Product Rotational Tribology Characterization
2.10. Combination Product Injectability Assessment
2.11. Combination Product In Vitro Cytocompatibility with Primary Fibroblast-like Synoviocytes
2.12. Combination Product In Vivo Efficacy Assessment in a Rodent Model of Knee Osteoarthritis
2.12.1. Knee Osteoarthritis Model and Surgical Procedure
2.12.2. Investigative Product Groups and Product Administration Modalities
2.12.3. Terminal Procedure and Sampling of Posterior Knee Joints
2.12.4. Micro-CT Data Acquisition and Data Analysis by Scoring
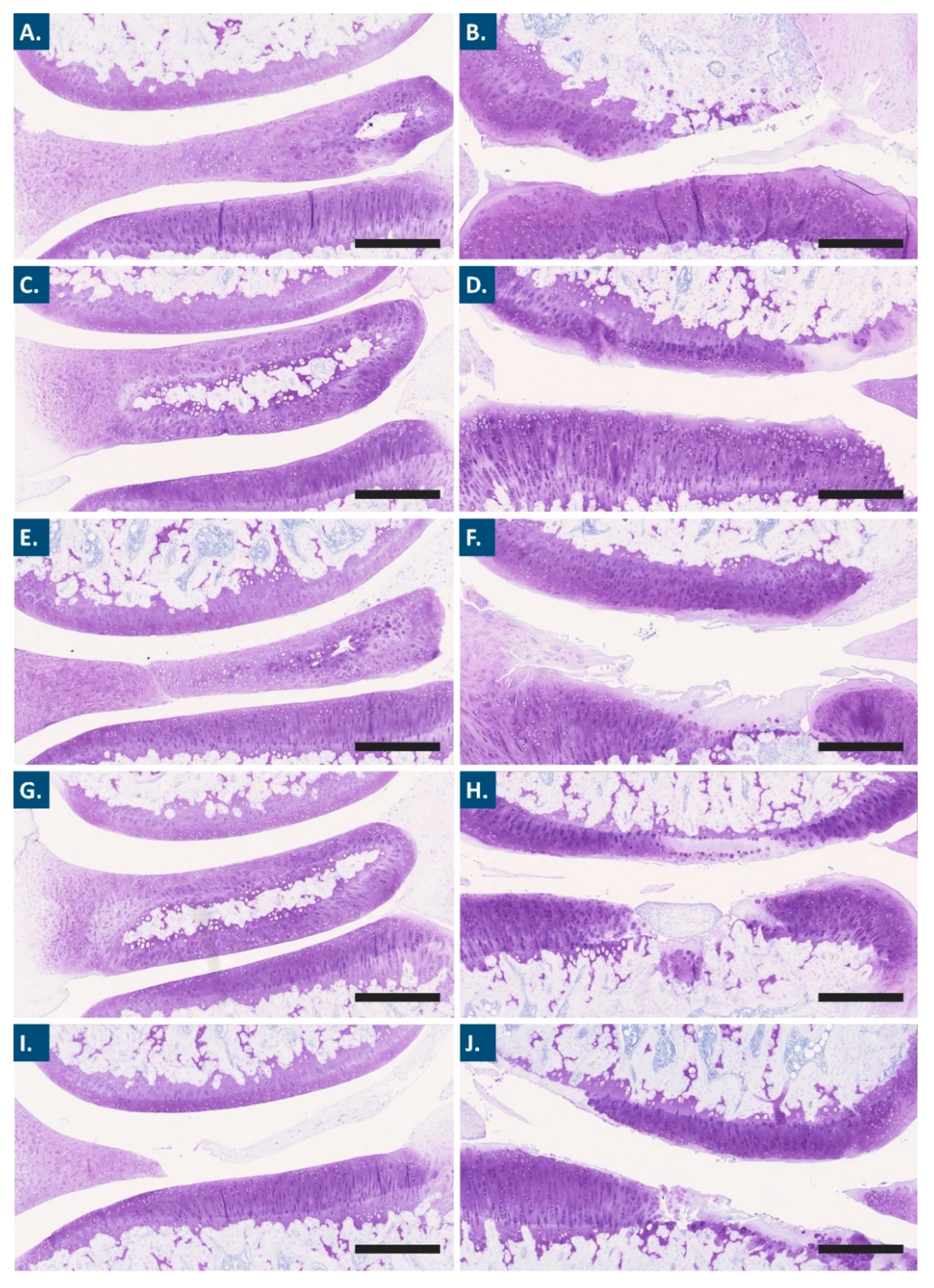
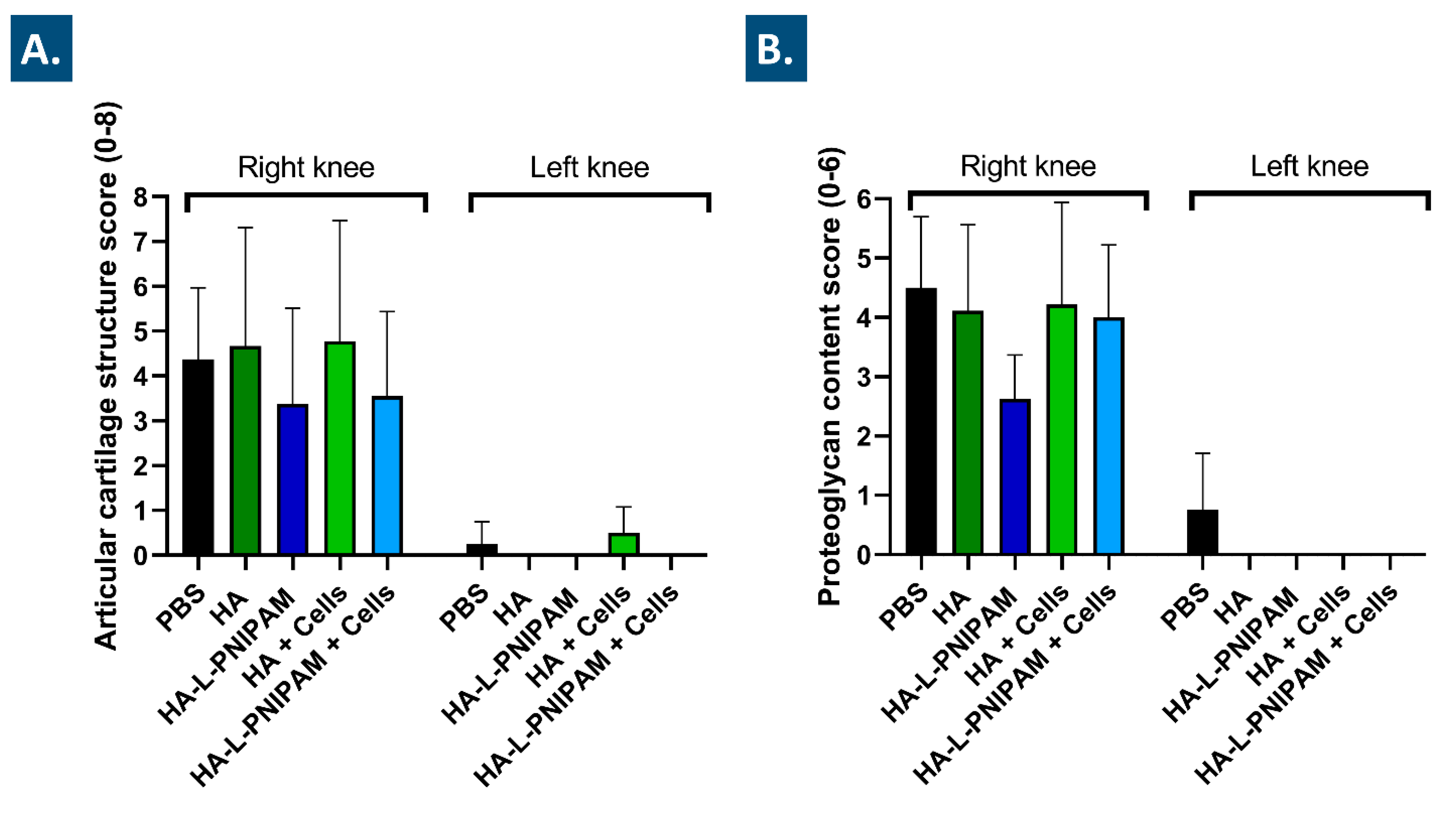
2.12.5. Histological Analyses and Histopathological Chondral Defect Grading
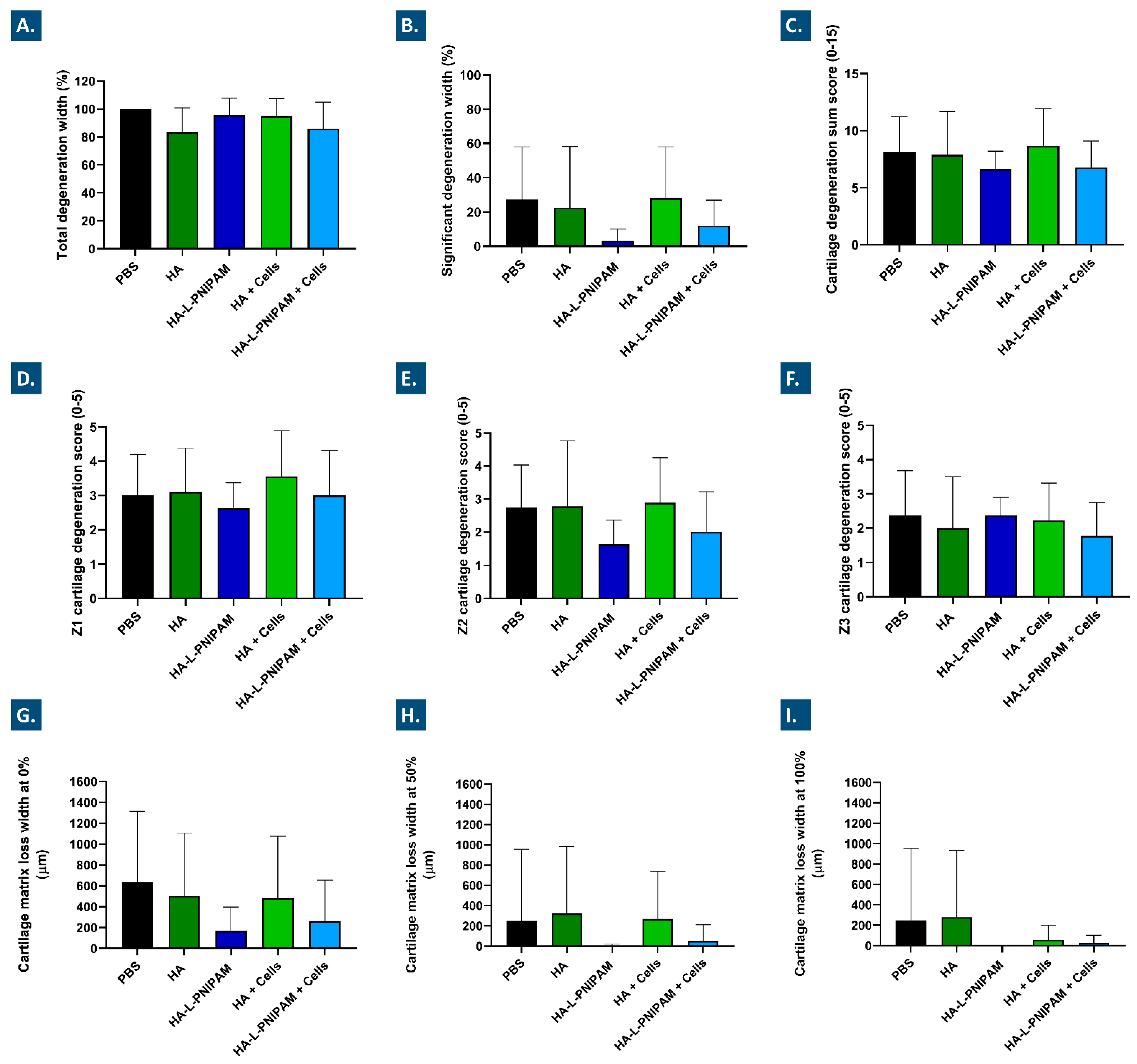
2.12.6. Histomorphometry Evaluation of Osteoarthritis Progression
2.13. Statistical Analyses and Presentation of Experimental Data
3. Results and Discussion
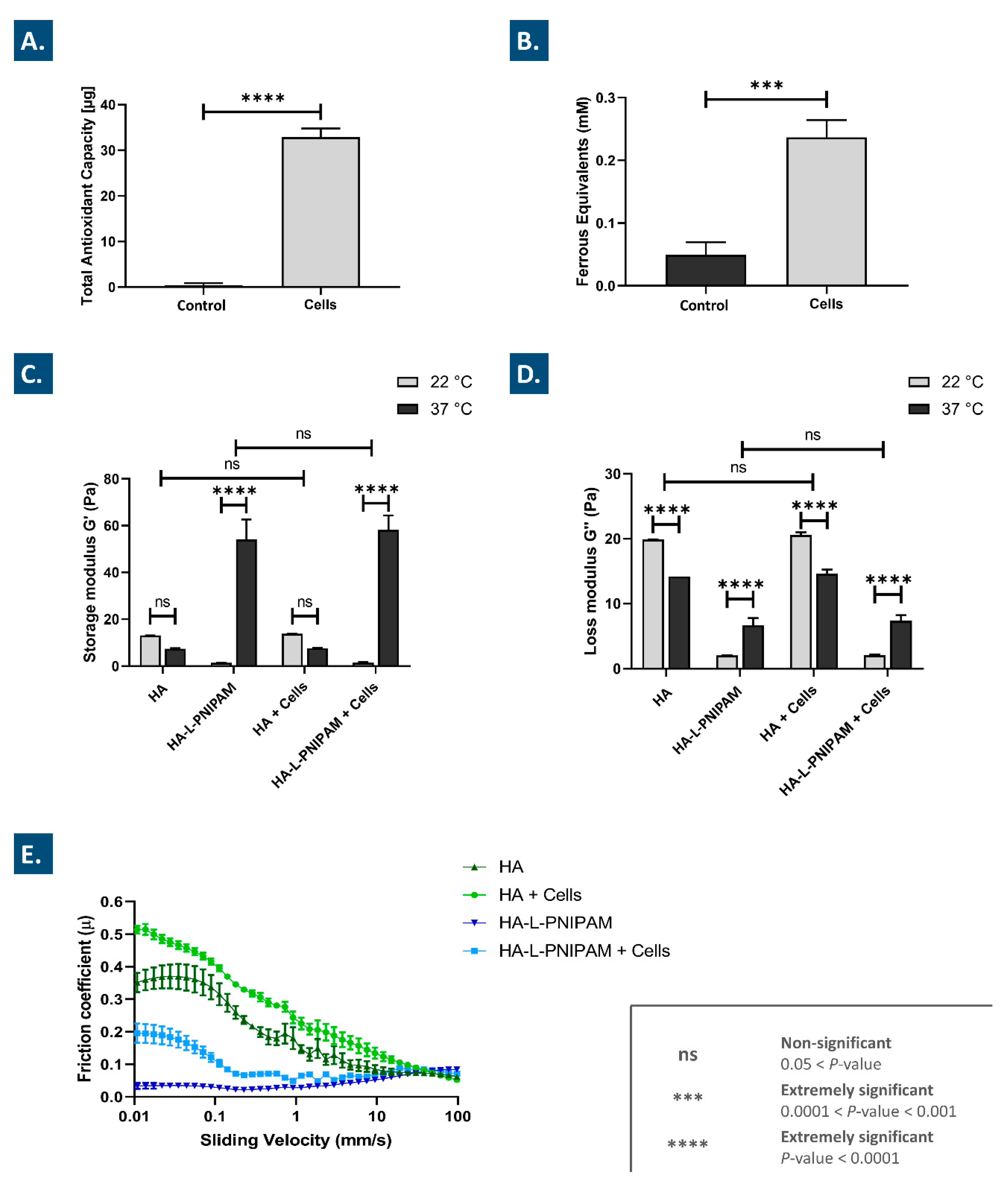
3.1. Lyophilized FE002 Chondroprogenitors Possess Intrinsic Antioxidant Properties and Functionalize Hydrogels through Stability Enhancement
3.2. Thermo-Responsive Hydrogels May Be Simply Synthetized and Autoclave-Sterilized before Inclusion of Cell Lyophilizates
3.3. Combination Products Display Optimal Thermo-Responsive Behaviors and Lubricating Effects In Vitro
3.4. Combination Products Present Significantly Improved Stability against Oxidative Degradation
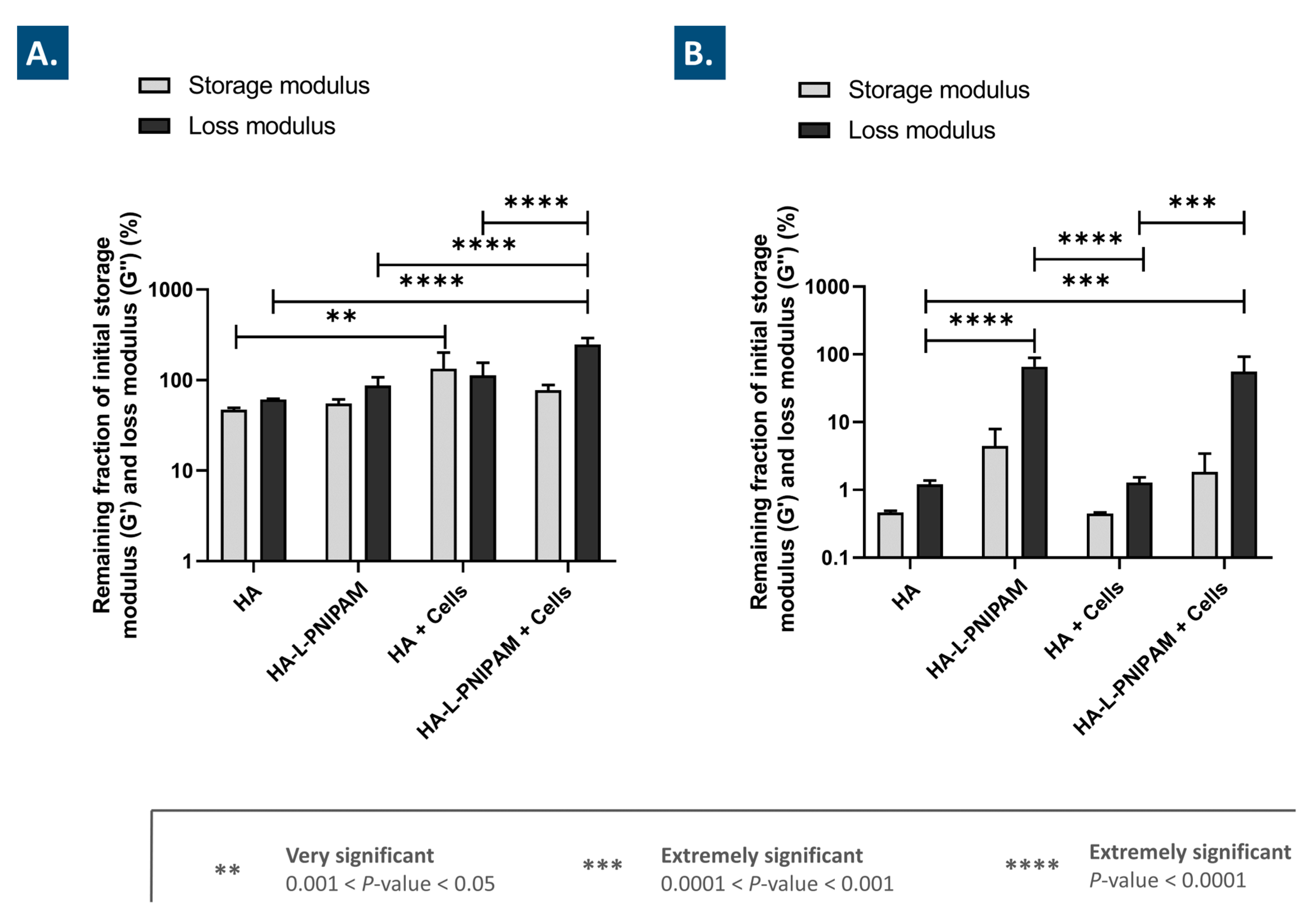
3.5. The Use of HA-L-PNIPAM Efficiently Improves HA-Based Hydrogel Resistance toward Enzymatic Degradation
3.6. The Studied Combination Products Display No Cytotoxicity In Vitro and Are Easily Injectable
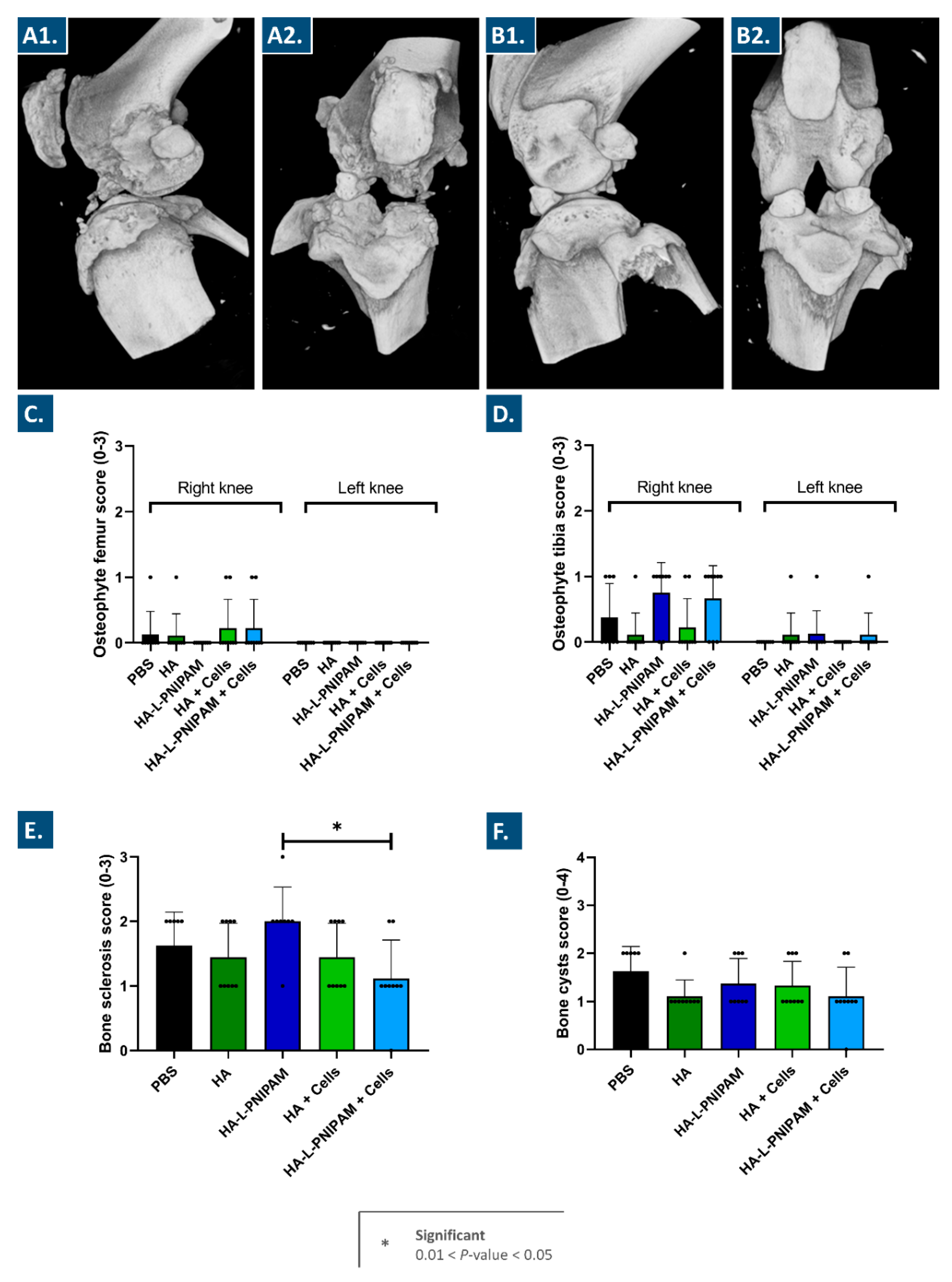
3.7. The Studied Combination Products Did Not Cause Adverse Reactions In Vivo and Procured Multi-Parameter Beneficial Trends in a Rodent Model of Knee OA
3.8. Novel Orthopedic Combination Product Preclinical Assessment: Current Insights and Bottlenecks around Efficacy Evaluation
3.9. A Historical Landmark in Clinical Allogeneic Cell-Based Management of Orthopedic Conditions: The Disruptive Case of Invossa
3.10. Study Limitations and Perspectives for Future Development Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLT | anterior cruciate ligament transection |
| Da | Daltons |
| HA | hyaluronic acid |
| H2O2 | hydrogen peroxide |
| FRAP | ferric reducing antioxidant power |
| GLP | good laboratory practices |
| HES | hematoxilin/eosin/saffron |
| hMnx | hemi-meniscectomy |
| LVE | linear viscoelastic region |
| MD | medical device |
| MW | molecular weight |
| OA | osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| Pa | Pascals |
| Pa·s | Pascal seconds |
| PBS | phosphate-buffered saline |
| Ph.Eur. | European pharmacopoeia |
| PNIPAM | poly(N-isopropylacrylamide) |
| PRP | platelet-rich plasma |
| ROS | reactive oxygen species |
| SF | synovial fluid |
| TB | Toluidine blue |
| TEAC | Trolox equivalent antioxidant capacity |
| USA | United States of America |
| UV | ultraviolet |
References
- Martell-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Etiopathogenesis of osteoarthritis. In Arthritis and Allied Conditions—A Textbook of Rheumatology, 15th ed.; Koopman, W.J., Ed.; Lippicott Williams & Wilkins: Baltimore, MA, USA, 2005; pp. 2199–2218. [Google Scholar]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Malekipour, F.; Lee, P.V. Shock absorbing ability in healthy and damaged cartilage-bone under high-rate compression. J. Mech. Behav. Biomed. Mat. 2019, 90, 388–394. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscipl. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Sah, R.L. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthr. Cartil. 2007, 15, 35–47. [Google Scholar] [CrossRef]
- Nurul, A.A.; Azlan, M.; Ahmad Mohd Zain, M.R.; Sebastian, A.A.; Fan, Y.Z.; Fauzi, M.B. Mesenchymal stem cells: Current concepts in the management of inflammation in osteoarthritis. Biomedicines 2021, 9, 785. [Google Scholar] [CrossRef]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef]
- Rzhepakovsky, I.; Anusha Siddiqui, S.; Avanesyan, S.; Benlidayi, M.; Dhingra, K.; Dolgalev, A.; Enukashvily, N.; Fritsch, T.; Heinz, V.; Kochergin, S.; et al. Anti-arthritic effect of chicken embryo tissue hydrolyzate against adjuvant arthritis in rats (X-ray microtomographic and histopathological analysis). Food Sci. Nutr. 2021, 9, 5648–5669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Chen, X.; Cheng, K.; Shi, Q.; Peng, K. Anserine and glucosamine supplementation attenuates the levels of inflammatory markers in rats with rheumatoid arthritis. AMB Express 2020, 10, 57. [Google Scholar] [CrossRef]
- Plaas, A.H.K.; Moran, M.M.; Sandy, J.D.; Hascall, V.C. Aggrecan and hyaluronan: The infamous cartilage polyelectrolytes—Then and now. Adv. Exp. Med. Biol. 2023, 1402, 3–29. [Google Scholar] [CrossRef]
- Porcello, A.; Gonzalez-Fernandez, P.; Jordan, O.; Allémann, E. Nanoforming hyaluronan-based thermoresponsive hydrogels: Optimized and tunable functionality in osteoarthritis management. Pharmaceutics 2022, 14, 659. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-articular injections in knee osteoarthritis: A review of literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Wang, B.; Liu, Q.; Ke, Y.; Xu, Y.; Li, Z.; Lin, J. Intra-articular hyaluronic acid in treating knee osteoarthritis: A PRISMA-compliant systematic review of overlapping meta-analysis. Sci. Rep. 2016, 6, 32790. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef]
- Juhaščik, M.; Kováčik, A.; Huerta-Ángeles, G. Recent advances of hyaluronan for skin delivery: From structure to fabrication strategies and applications. Polymers 2022, 14, 4833. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Mathieu, P.; Rinaudo, M. Mannitol preserves the viscoelastic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol. Ther. 2014, 1, 45–54. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-articular drug delivery for osteoarthritis treatment. Pharmaceutics 2021, 13, 2166. [Google Scholar] [CrossRef]
- Larsen, C.; Ostergaard, J.; Larsen, S.W.; Jensen, H.; Jacobsen, S.; Lindegaard, C.; Andersen, P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008, 97, 4622–4654. [Google Scholar] [CrossRef]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The degradation of hyaluronan in the skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Fernandez, P.G.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.; et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics 2021, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef] [PubMed]
- Schanté, C.; Zubera, G.; Herlinb, C.; Vandammea, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Maudens, P.; Meyer, S.; Seemayer, C.A.; Jordan, O.; Allémann, E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale 2018, 10, 1845–1854. [Google Scholar] [CrossRef]
- Zhou, T.; Ran, J.; Xu, P.; Shen, L.; He, Y.; Ye, J.; Wu, L.; Gao, C. A hyaluronic acid/platelet-rich plasma hydrogel containing MnO2 nanozymes efficiently alleviates osteoarthritis in vivo. Carbohydr. Polym. 2022, 292, 119667. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Targeted and responsive biomaterials in osteoarthritis. Theranostics 2023, 13, 931–954. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Roman, J.S. Introduction to smart polymers and their applications. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., Roman, J.S., Eds.; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mat. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Chiang, E.R.; Ma, H.L.; Wang, J.P.; Liu, C.L.; Chen, T.H.; Hung, S.C. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS ONE 2016, 11, e0149835. [Google Scholar] [CrossRef]
- Yu, W.; Xu, P.; Huang, G.; Liu, L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, E.; Jiménez, G.; Álvarez de Cienfuegos, L.; Antic, C.; Sabata, R.; Marchal, J.A.; Gálvez-Martín, P. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur. Cells Mater. 2019, 37, 186–213. [Google Scholar] [CrossRef]
- Li, L.; Duan, X.; Fan, Z.; Chen, L.; Xing, F.; Xu, Z.; Chen, Q.; Xiang, Z. Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci. Rep. 2018, 8, 9900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Gu, X.; Hao, Y.; Liu, X.; Lin, J.; Chen, J.; Chen, S. Conditioned medium of mesenchymal stem cells delays osteoarthritis progression in a rat model by protecting subchondral bone, maintaining matrix homeostasis, and enhancing autophagy. J. Tissue Eng. Regen. Med. 2019, 13, 1618–1628. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Z.; Li, D.; Wang, X.; Dai, D.; Fu, H. Effect study of exosomes derived from platelet-rich plasma in the treatment of knee cartilage defects in rats. J. Orthop. Surg. Res. 2023, 18, 160. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Si, H.B.; Tang, L.; Xie, H.Q.; Shen, B. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 2022, 50, 1088–1105. [Google Scholar] [CrossRef]
- Huang, G.S.; Peng, Y.J.; Hwang, D.W.; Lee, H.S.; Chang, Y.C.; Chiang, S.W.; Hsu, Y.C.; Liu, Y.C.; Lin, M.H.; Wang, C.Y. Assessment of the efficacy of intra-articular platelet rich plasma treatment in an ACLT experimental model by dynamic contrast enhancement MRI of knee subchondral bone marrow and MRI T2∗ measurement of articular cartilage. Osteoarthr. Cartil. 2021, 29, 718–727. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; Roessingh, A.; Raffoul, W.; Pioletti, D.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal models of osteoarthritis: Challenges of model selection and analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef]
- Ginesin, E.; Chari, N.S.; Barnhart, J.; Wojnowski, N.; Patel, R.M. Cartilage restoration for isolated patellar chondral defects: An updated systematic review. Orthop. J. Sports Med. 2023, 11, 23259671231153422. [Google Scholar] [CrossRef]
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; Roessingh, A.B.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, J.; Harrysson, O.; Shirwaiker, R.; Starly, B.; Wysk, R.; Cohen, P.; Allickson, J.; Yoo, J.; Atala, A. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl. Med. 2015, 4, 130–135. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, B.; Alonso, P.; Rodriguez, A.; La Nuez, M.; Marzo, F.; Prieto, J.G. Characterization of the visco-elastic properties of hyaluronic acid. Biorheology 2018, 55, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human synovial fluid. Adv. Ther. 2018, 35, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751622. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar] [CrossRef]
- Schanté, C.; Zuber, G.; Herlin, C.; Vandamme, T.F. Synthesis of N-alanyl-hyaluronamide with high degree of substitution for enhanced resistance to hyaluronidase-mediated digestion. Carbohydr. Polym. 2011, 86, 747–752. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability evaluation: An open issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Hugues, E.A.; Bose, A.; Cornish, E.A.; Teo, J.Y.; Eisenstein, E.M.; Grover, L.M.; Cox, S.C. Filling the gap: A correlation between objective and subjective measures of injectability. Adv. Health Mater. 2020, 9, 1901521. [Google Scholar] [CrossRef] [PubMed]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Pickarski, M.; Hayami, T.; Zhuo, Y.; Duong, L.T. Molecular changes in articular cartilage and subchondral bone in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. BMC Musculoskelet. Disord. 2011, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal models of osteoarthritis: Comparisons and key considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Nielsen, R.H.; Bay-Jensen, A.C.; Byrjalsen, I.; Karsdal, M.A. Oral salmon calcitonin reduces cartilage and bone pathology in an osteoarthritis rat model with increased subchondral bone turnover. Osteoarthr. Cartil. 2011, 19, 466–473. [Google Scholar] [CrossRef]
- Nordberg, R.C.; Otarola, G.A.; Wang, D.; Hu, J.C.; Athanasiou, K.A. Navigating regulatory pathways for translation of biologic cartilage repair products. Sci. Transl. Med. 2022, 14, eabp8163. [Google Scholar] [CrossRef]
- Tikiz, C.; Unlü, Z.; Sener, A.; Efe, M.; Tüzün, C. Comparison of the efficacy of lower and higher molecular weight viscosupplementation in the treatment of hip osteoarthritis. Clin. Rheumatol. 2005, 24, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Blicharski, T.; Łukasik, P.; Plebanski, R.; Żęgota, Z.; Szuścik, M.; Moster, E.; Pavelka, K.; Jeon, S.; Park, S. Efficacy and safety of intra-articular cross-linked sodium hyaluronate for the treatment of knee osteoarthritis: A prospective, active-controlled, randomized, parallel-group, double-blind, multicenter study. J. Clin. Med. 2023, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P. Intra-articular hyaluronic acid in knee osteoarthritis: Clinical data for a product family (ARTHRUM), with comparative meta-analyses. Curr. Ther. Res. Clin. Exp. 2021, 95, 100637. [Google Scholar] [CrossRef]
- Baron, D.; Flin, C.; Porterie, J.; Despaux, J.; Vincent, P. Hyaluronic acid single intra-articular injection in knee osteoarthritis: A multicenter open prospective study (ART-ONE 75) with placebo post hoc comparison. Curr. Ther. Res. Clin. Exp. 2018, 88, 35–46. [Google Scholar] [CrossRef]
- Vincent, P.; Lucas de Couville, T.; Thomas, T. Intra-articular hyaluronic acid for knee osteoarthritis: A postmarket, open-label, long-term historical control study with analysis detailed per Krellgren-Lawrence radiologic osteoarthritis scale grade. Curr. Ther. Res. Clin. Exp. 2020, 92, 100575. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and effectiveness of intra-articular injection of hyaluronic acid in the treatment of knee osteoarthritis: A systematic review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: A systematic review. Semin. Arthritis Rheum. 2018, 48, 168–175. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef]
- Noh, M.J.; Copeland, R.O.; Yi, Y.; Choi, K.B.; Meschter, C.; Hwang, S.; Lim, C.L.; Yip, V.; Hyun, J.P.; Lee, H.Y.; et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 2010, 12, 384–393. [Google Scholar] [CrossRef]
- Lee, B. INVOSSA, a first-in-class of cell and gene therapy for osteoarthritis treatment: The phase III trial. Osteoarthr. Cartil. 2018, 26, S43–S44. [Google Scholar] [CrossRef]
- Ha, C.W.; Noh, M.J.; Choi, K.B.; Lee, K.H. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ha, C.W.; In, Y.; Cho, S.D.; Choi, E.S.; Ha, J.K.; Lee, J.H.; Yoo, J.D.; Bin, S.I.; Choi, C.H.; et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum. Gene Ther. 2018, 29, 48–59. [Google Scholar] [CrossRef]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthop. Rev. 2021, 13, 25549. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
| Formulation Type | Gross (Co-)Polymer Concentration (mg/mL) | Net HA Concentration (mg/mL) 2 | Lyophilized FE002 Chondroprogenitor Presence (Y/N) |
|---|---|---|---|
| HA | 10 | 10 | N |
| HA-L-PNIPAM | 37 | 10 | N |
| HA + Cells | 10 | 10 | Y (3 × 106 cells/vial) |
| HA-L-PNIPAM + Cells | 37 | 10 | Y (3 × 106 cells/vial) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcello, A.; Gonzalez-Fernandez, P.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Allémann, E.; et al. Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis. Pharmaceutics 2023, 15, 1528. https://doi.org/10.3390/pharmaceutics15051528
Porcello A, Gonzalez-Fernandez P, Jeannerat A, Peneveyre C, Abdel-Sayed P, Scaletta C, Raffoul W, Hirt-Burri N, Applegate LA, Allémann E, et al. Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis. Pharmaceutics. 2023; 15(5):1528. https://doi.org/10.3390/pharmaceutics15051528
Chicago/Turabian StylePorcello, Alexandre, Paula Gonzalez-Fernandez, Annick Jeannerat, Cédric Peneveyre, Philippe Abdel-Sayed, Corinne Scaletta, Wassim Raffoul, Nathalie Hirt-Burri, Lee Ann Applegate, Eric Allémann, and et al. 2023. "Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis" Pharmaceutics 15, no. 5: 1528. https://doi.org/10.3390/pharmaceutics15051528
APA StylePorcello, A., Gonzalez-Fernandez, P., Jeannerat, A., Peneveyre, C., Abdel-Sayed, P., Scaletta, C., Raffoul, W., Hirt-Burri, N., Applegate, L. A., Allémann, E., Laurent, A., & Jordan, O. (2023). Thermo-Responsive Hyaluronan-Based Hydrogels Combined with Allogeneic Cytotherapeutics for the Treatment of Osteoarthritis. Pharmaceutics, 15(5), 1528. https://doi.org/10.3390/pharmaceutics15051528