Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Venom
2.3. Purification of Pictolysin-III
2.4. Western Blotting
2.5. Molecular Characterization
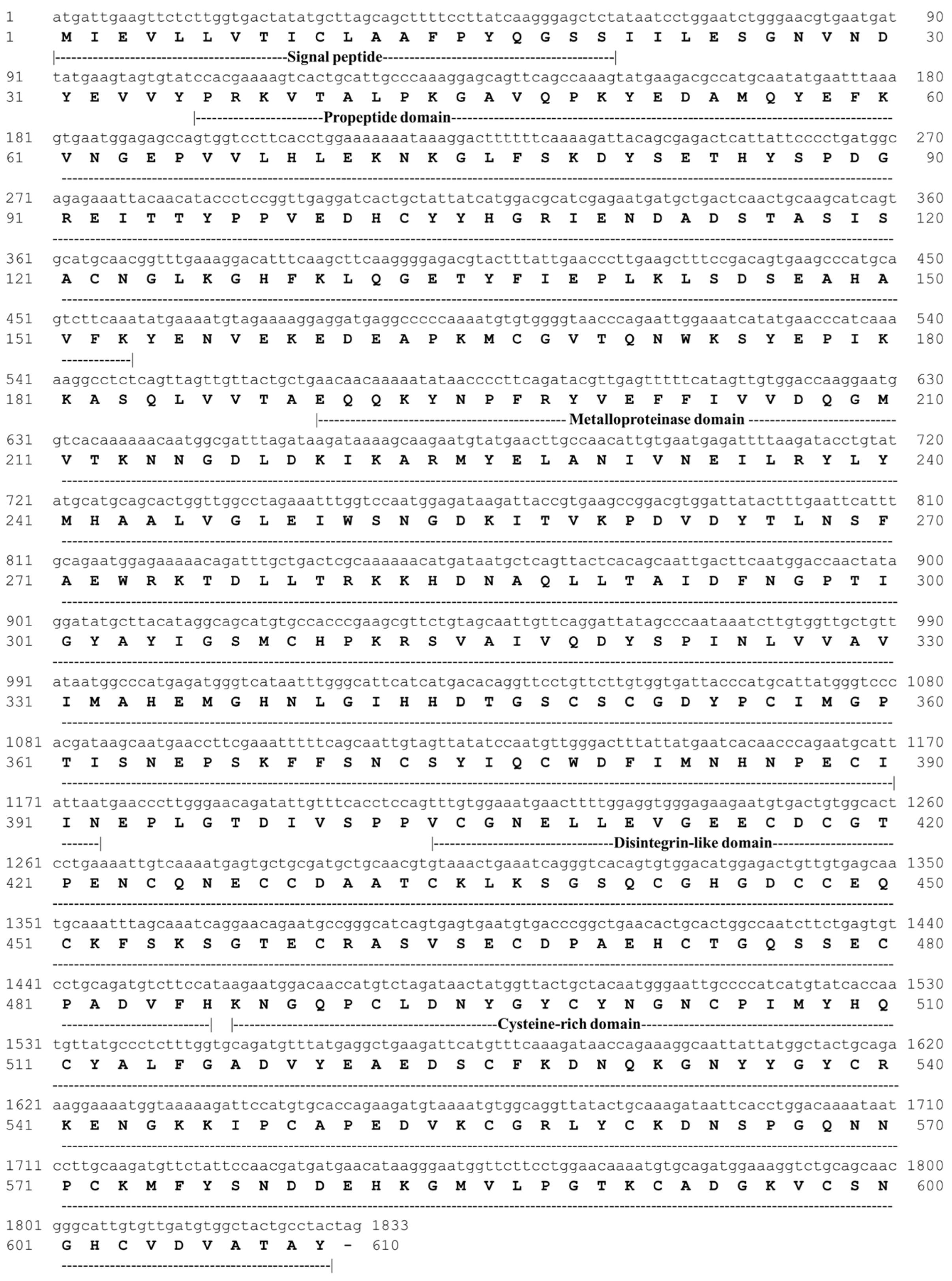
2.5.1. cDNA-Encoding Pictolysin-III and Gene Expression Analysis
2.5.2. Cloning and Nucleotide Sequencing
2.5.3. In Silico Protein Analysis
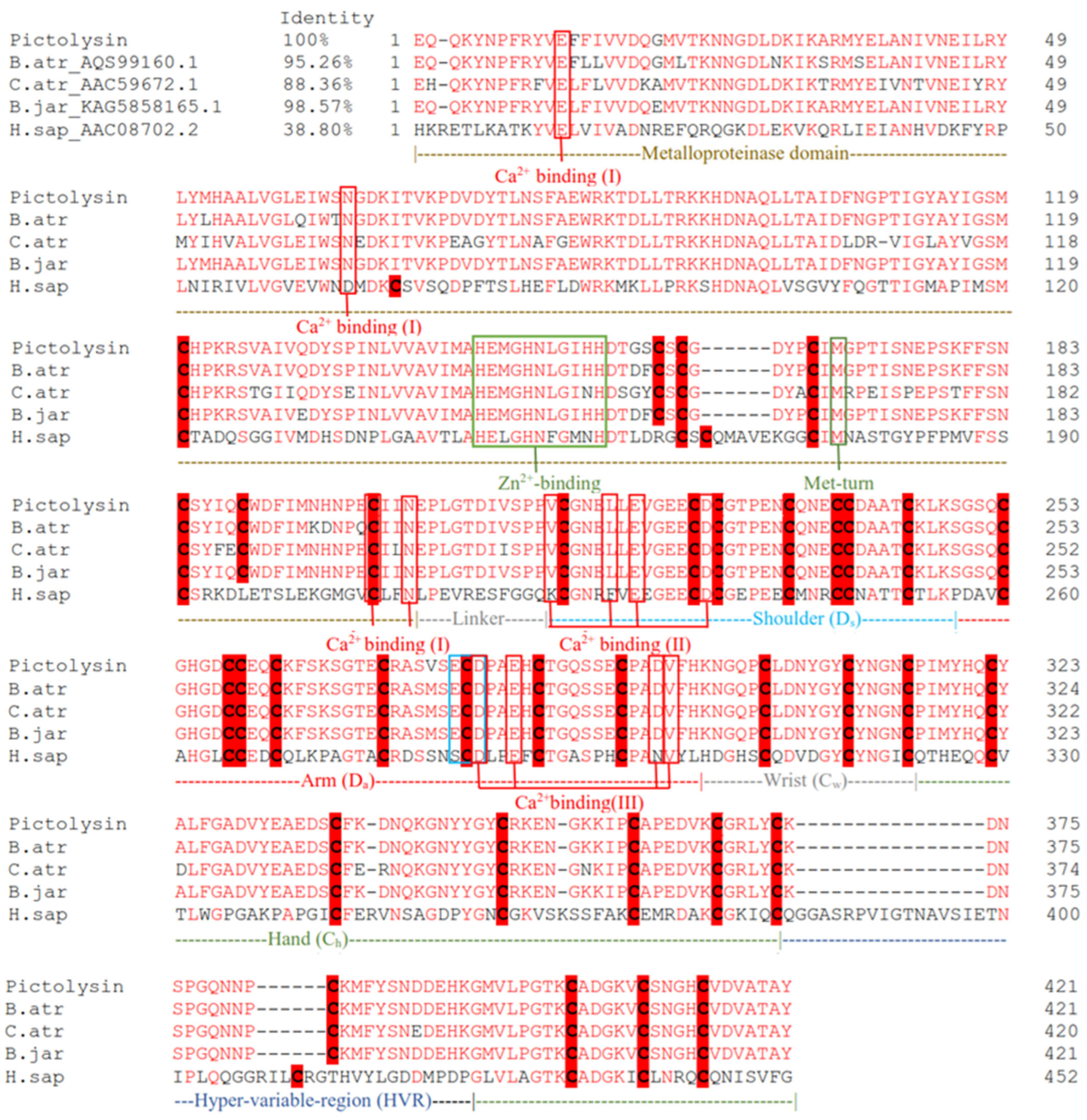
2.5.4. Sequence-Based Analysis and Phylogenetic Analysis
2.6. Biochemical Characterization
2.6.1. Enzymatic Activity
2.6.2. Interaction of Pictolysin-III with a TLE (Pictobin) and Plasma Inhibitor α2-Macroglobulin (α2-M)
2.6.3. Effect of pH, Temperature, and Inhibitors
2.6.4. Effect of Pictolysin-III Deglycosylation under no Reducing Conditions on Enzymatic Activity
2.6.5. Proteolytic Activity upon Fibrinogen and Fibrin
2.7. Biological Activities
2.7.1. Platelet Aggregation Assay
2.7.2. Hemorrhagic Activity
2.8. Studies in Cell Lines
2.8.1. Cell Culture Conditions
2.8.2. MTT Assay
2.8.3. Determination of ATP and Mitochondrial ROS (mtROS) Levels
2.8.4. Determination of NAD(P)H Levels
2.8.5. Extracellular Acidification Rate in Real Time
2.8.6. Oxygen Consumption Rate in Real Time
2.8.7. Caco-2 Morphology Analysis
2.8.8. Quantitative PCR
- GAPDH (forward): 5′-TTG CCA TCA ATG ACC CCT TC-3′
- GAPDH (reverse): 5′-ATC ATC AGC AAT GCC TCC TG-3′
- IL1β (forward): 5′-AAT CCC CAG CCC TTT TGT TG-3′
- IL1β (reverse): 5′-GTA AGC TAT GGC CCA CTC CA-3′
- TNFα (forward): 5′-CCTGGTATGAGCCCATCTATCTG-3′
- TNFα (reverse): 5′-GCAATGATCCCAAAGTAGACCTG-3′
2.8.9. Cytometric Bead Array (CBA)
2.9. Statistical Analysis
3. Results
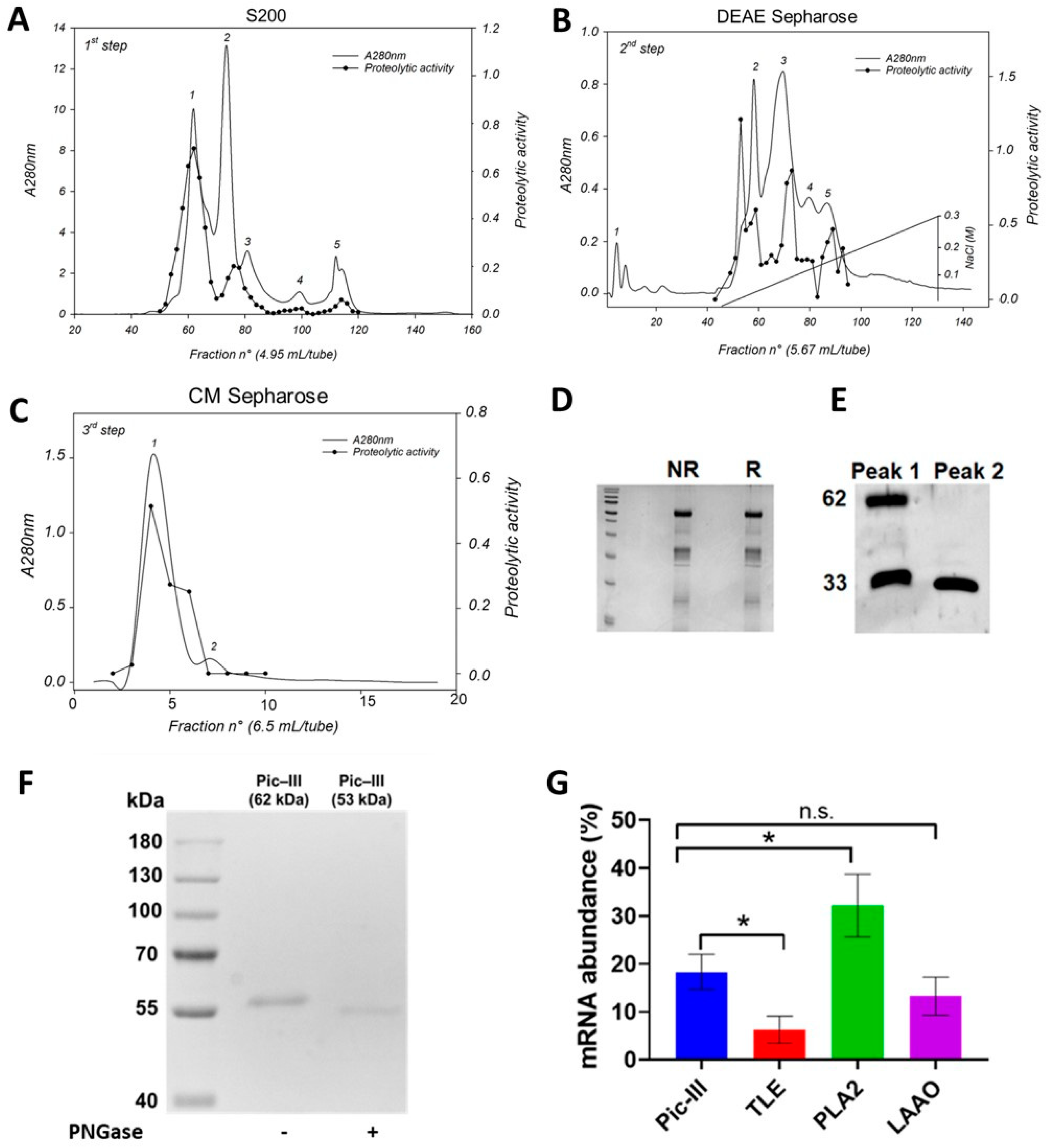
3.1. Pictolysin-III Purification and Autoproteolysis Identification
3.2. Functional Characterization of Pictolysin-III
3.3. Interaction with a TLE (Pictobin) and Plasma Inhibitor α2-Macroglobulin (α2-M)

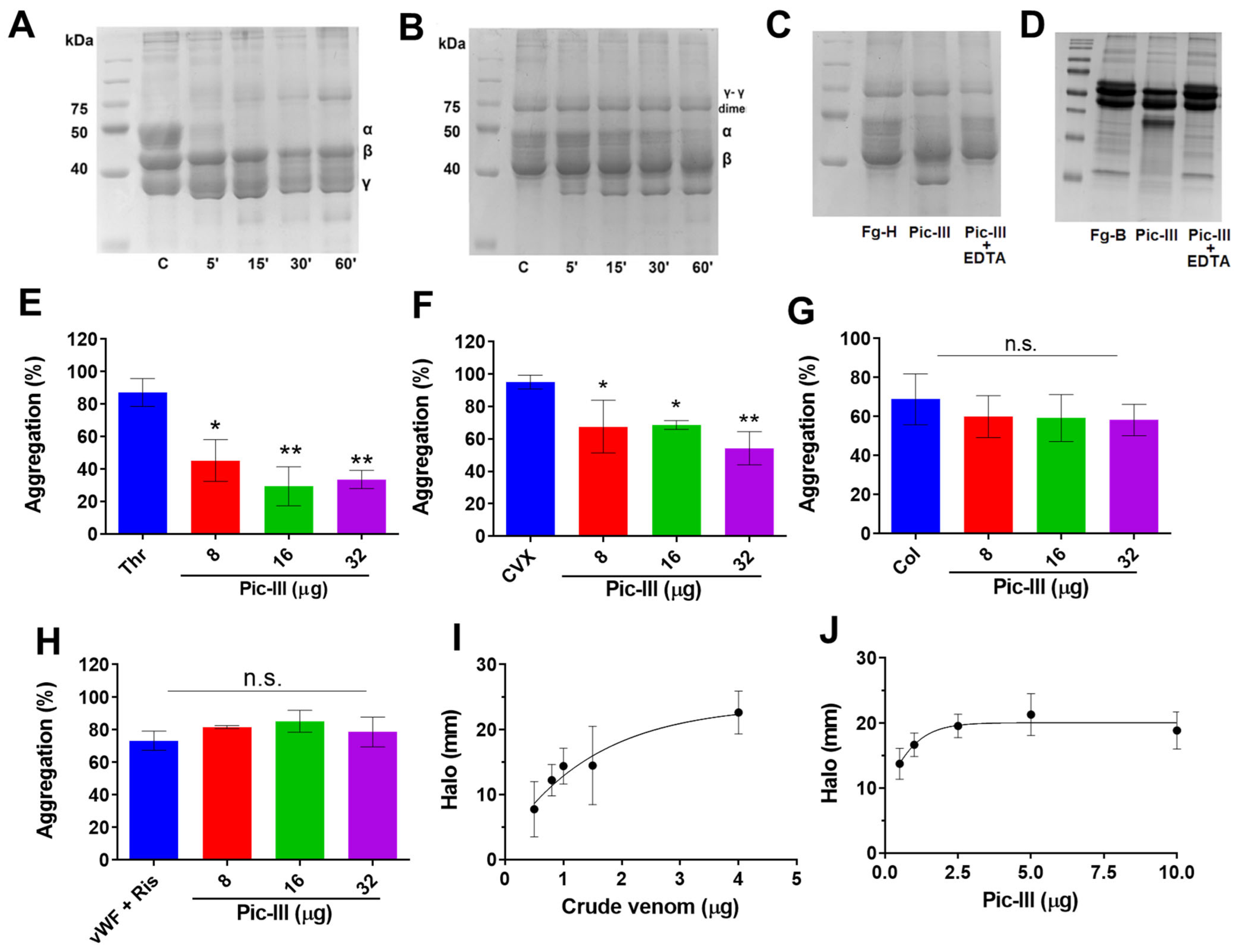
3.4. Pictolysin-III Acts as a GPVI Antagonist That Produces Hemorrhage In Vivo
3.5. Structural Characterization of Pictolysin-III
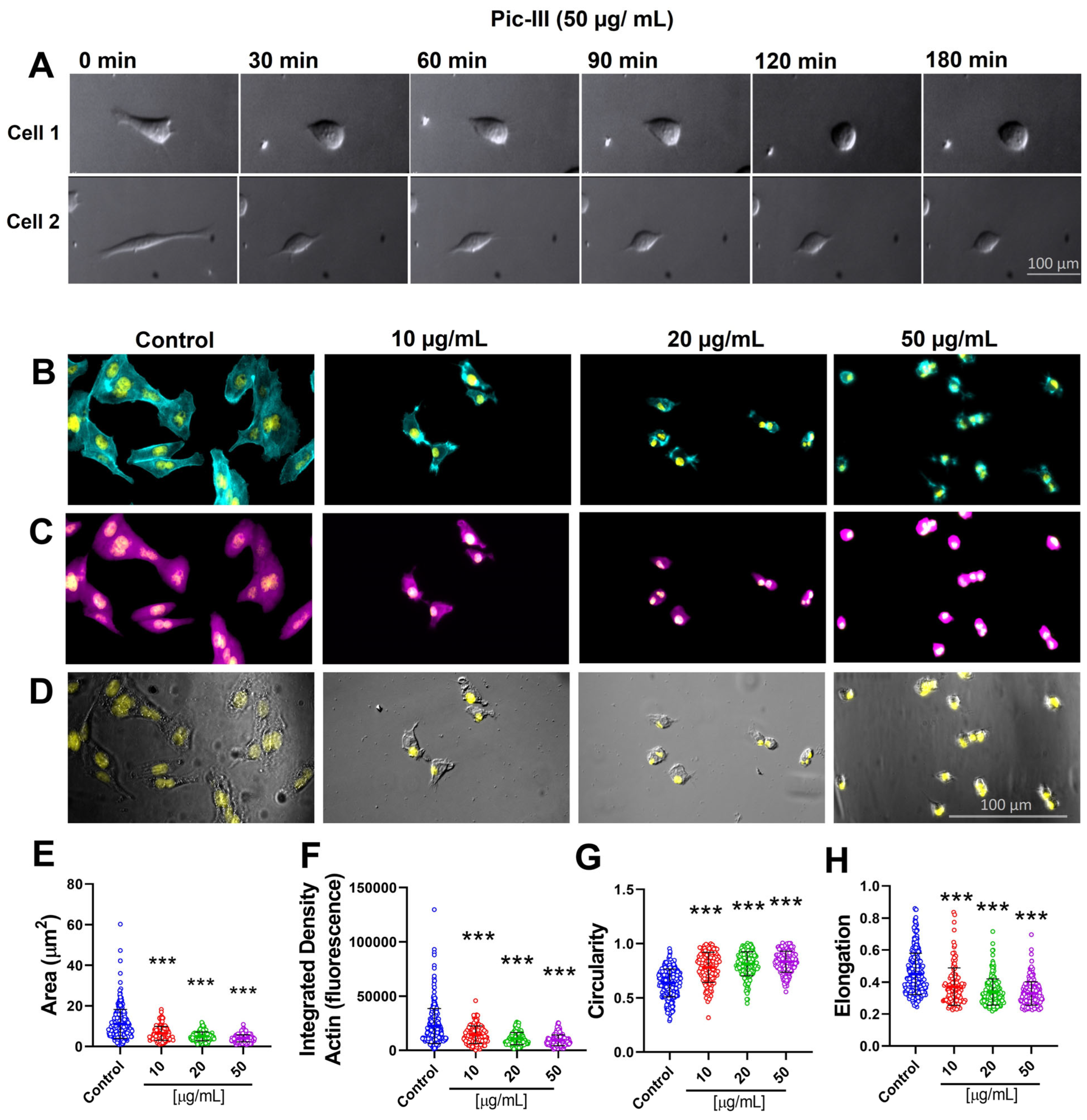
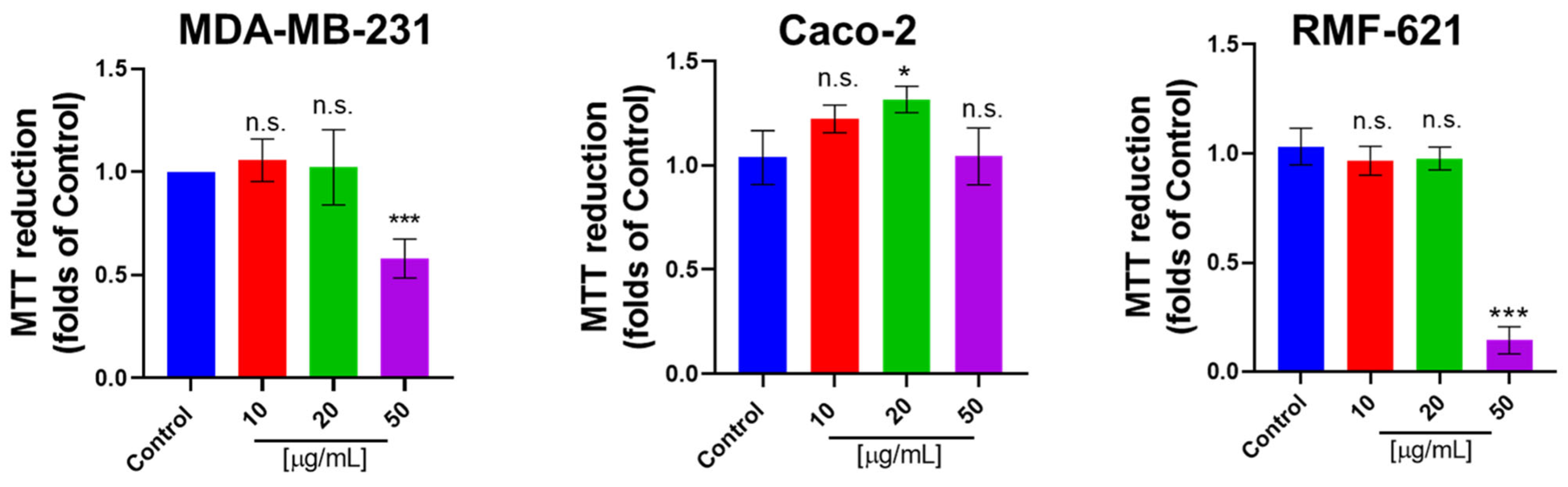
3.6. Pictolysin-III Promotes Actin Network Disruption, Reducing the Viability of MDA-MB-231 and RMF-621 Cells
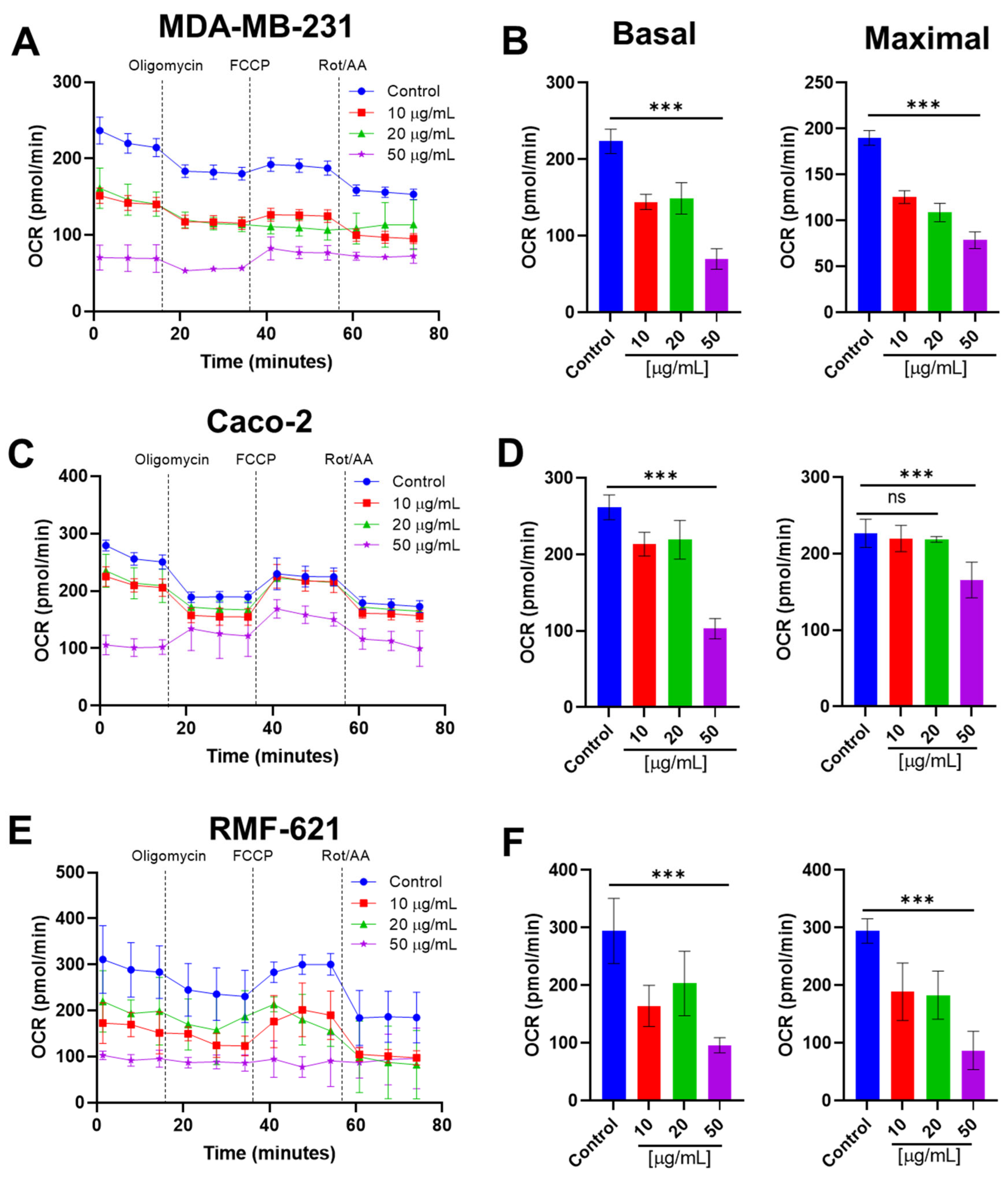
3.7. Pictolysin-III Inhibits the Basal and Maximal Mitochondrial Respiration Cell Lines
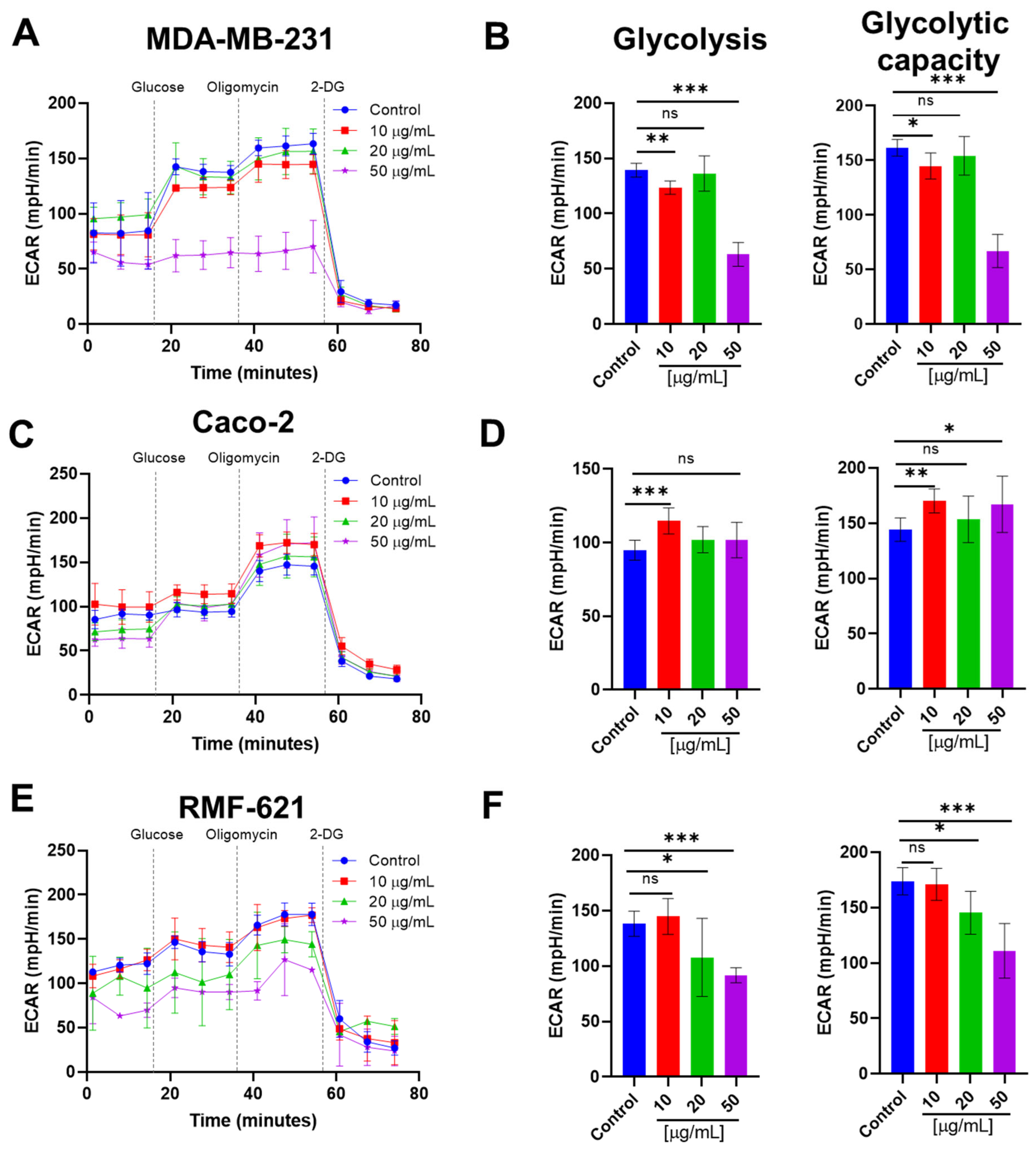
3.8. Pictolysin-III Reduces Glycolysis in MDA-MB-231 and RMF-621, but Increases It in Caco-2 Cells
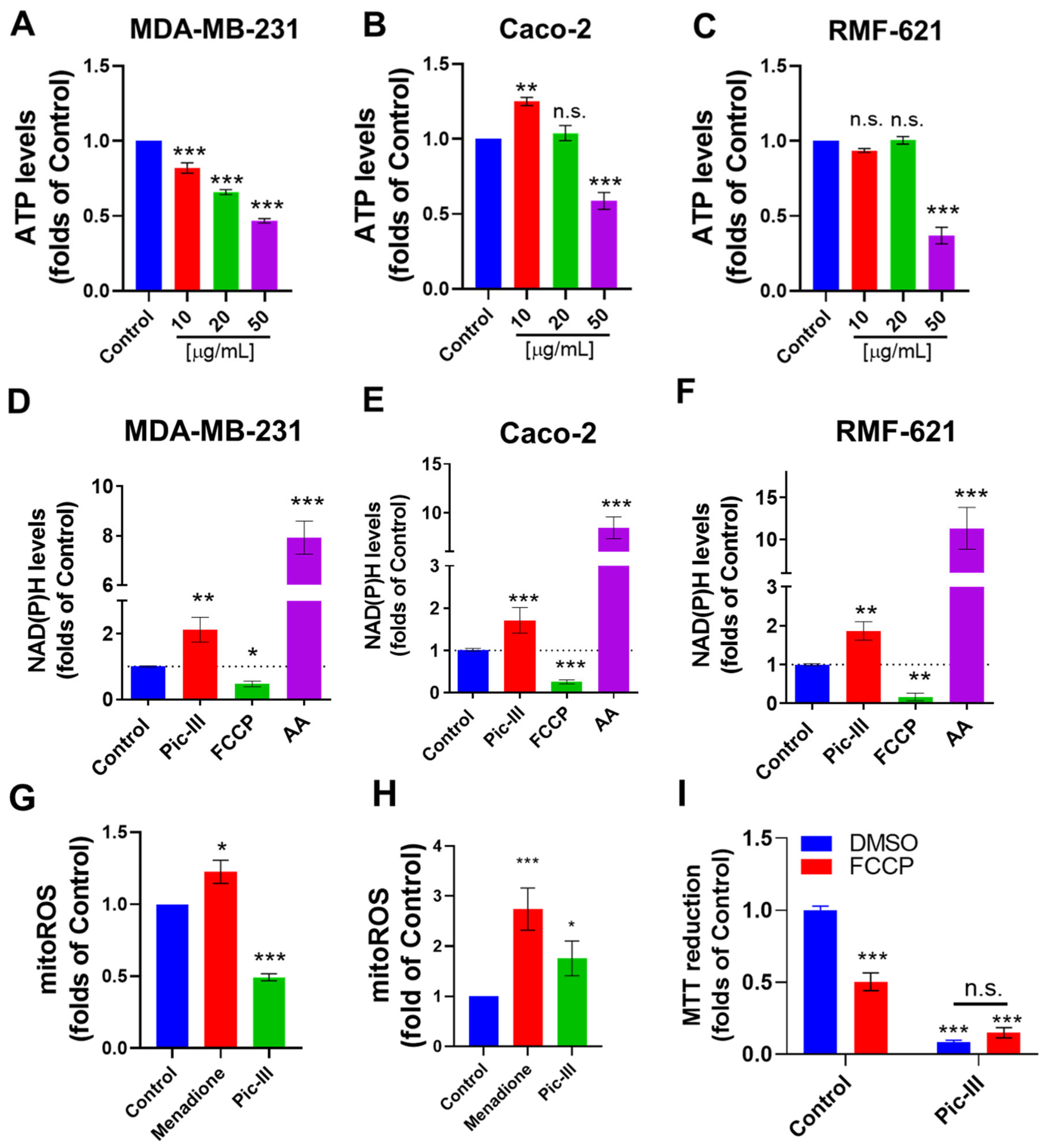
3.9. Pictolysin-III Alters the Intracellular ATP, NAD(P)H, and Mitochondrial ROS Levels
3.10. Pictolysin-III Increases the Secretion of Cytokines in Caco-2 and RMF-621 Cells
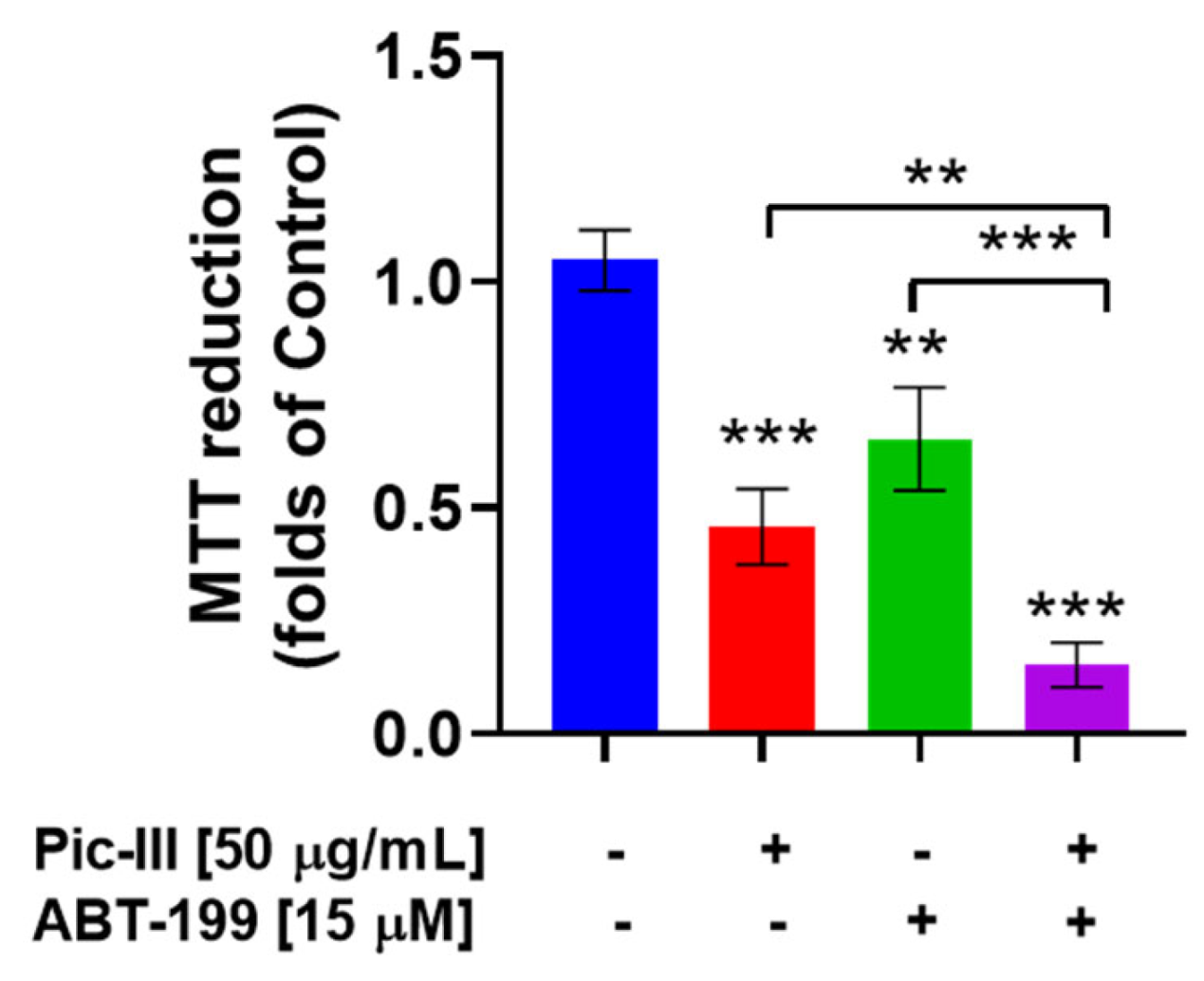
3.11. Pictolysin-III Produces Sensitization to BH3 Mimetic ABT-199 (Venetoclax) in MDA-MB-231 Cells

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Araya-Maturana, R. Targeting Metastasis with Snake Toxins: Molecular Mechanisms. Toxins 2017, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Vivas-Ruiz, D.E.; Sanchez, E.F.; Araya-Maturana, R. An Emergent Role for Mitochondrial Bioenergetics in the Action of Snake Venom Toxins on Cancer Cells. Front. Oncol. 2022, 12, 938749. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Araya-Maturana, R. Putting the brakes on tumorigenesis with snake venom toxins: New molecular insights for cancer drug discovery. Semin. Cancer Biol. 2022, 80, 195–204. [Google Scholar] [CrossRef]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front. Pharmacol. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Estevao-Costa, M.I.; Gontijo, S.S.; Correia, B.L.; Yarleque, A.; Vivas-Ruiz, D.; Rodrigues, E.; Chávez-Olortegui, C.; Oliveira, L.S.; Sanchez, E.F. Neutralization of toxicological activities of medically-relevant Bothrops snake venoms and relevant toxins by two polyvalent bothropic antivenoms produced in Peru and Brazil. Toxicon 2016, 122, 67–77. [Google Scholar] [CrossRef]
- Jia, L.-G.; Shimokawa, K.-I.; Bjarnason, J.B.; Fox, J.W. Snake venom metalloproteinases: Structure, function and relationship to the ADAMs family of proteins. Toxicon 1996, 34, 1269–1276. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Flores-Ortiz, R.J.; Alvarenga, V.G.; Eble, J.A. Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential. Toxins 2017, 9, 392. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Snake venom metalloproteinase containing a disintegrin-like domain, its structure-activity relationships at interacting with integrins. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 249–260. [Google Scholar] [CrossRef]
- Serrano, S.M.; Kim, J.; Wang, D.; Dragulev, B.; Shannon, J.D.; Mann, H.H.; Veit, G.; Wagener, R.; Koch, M.; Fox, J.W. The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor A domains: Role in substrate targeting. J. Biol. Chem. 2006, 281, 39746–39756. [Google Scholar] [CrossRef]
- Herrera, C.; Escalante, T.; Voisin, M.-B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M.; et al. Tissue Localization and Extracellular Matrix Degradation by PI, PII and PIII Snake Venom Metalloproteinases: Clues on the Mechanisms of Venom-Induced Hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef] [PubMed]
- Tashima, A.K.; Zelanis, A.; Kitano, E.S.; Ianzer, D.; Melo, R.L.; Rioli, V.; Sant’anna, S.S.; Schenberg, A.C.G.; Camargo, A.C.M.; Serrano, S.M.T. Peptidomics of Three Bothrops Snake Venoms: Insights Into the Molecular Diversification of Proteomes and Peptidomes. Mol. Cell. Proteom. 2012, 11, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.D.; Baramova, E.N.; Bjarnason, J.B.; Fox, J.W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J. Biol. Chem. 1989, 264, 11575–11583. [Google Scholar] [CrossRef]
- Pinto, A.F.M.; Ma, L.; Dragulev, B.; Guimaraes, J.A.; Fox, J.W. Use of SILAC for exploring sheddase and matrix degradation of fibroblasts in culture by the PIII SVMP atrolysin A: Identification of two novel substrates with functional relevance. Arch. Biochem. Biophys. 2007, 465, 11–15. [Google Scholar] [CrossRef]
- Sajevic, T.; Leonardi, A.; Kovačič, L.; Lang-Balija, M.; Kurtović, T.; Pungerčar, J.; Halassy, B.; Trampuš-Bakija, A.; Križaj, I. VaH3, one of the principal hemorrhagins in Vipera ammodytes ammodytes venom, is a homodimeric P-IIIc metalloproteinase. Biochimie 2013, 95, 1158–1170. [Google Scholar] [CrossRef]
- Asega, A.F.; Menezes, M.C.; Trevisan-Silva, D.; Cajado-Carvalho, D.; Bertholim, L.; Oliveira, A.K.; Zelanis, A.; Serrano, S.M.T. Cleavage of proteoglycans, plasma proteins and the platelet-derived growth factor receptor in the hemorrhagic process induced by snake venom metalloproteinases. Sci. Rep. 2020, 10, 12912. [Google Scholar] [CrossRef] [PubMed]
- Olaoba, O.T.; Karina dos Santos, P.; Selistre-de-Araujo, H.S.; Ferreira de Souza, D.H. Snake Venom Metalloproteinases (SVMPs): A structure-function update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Almeida, M.T.; Portes-Junior, J.A.; Nicolau, C.A.; Gomes-Neto, F.; Valente, R.H. Processing of Snake Venom Metalloproteinases: Generation of Toxin Diversity and Enzyme Inactivation. Toxins 2016, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases:Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Ayvazyan, N.; Ghukasyan, G.; Ghulikyan, L.; Kirakosyan, G.; Sevoyan, G.; Voskanyan, A.; Karabekyan, Z. The Contribution of Phospholipase A2 and Metalloproteinases to the Synergistic Action of Viper Venom on the Bioenergetic Profile of Vero Cells. Toxins 2022, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Fuentes-Retamal, S.; Palominos, C.; Rodríguez-Lucart, Y.A.; López-Torres, C.; Araya-Maturana, R. Extracellular Matrix Signals as Drivers of Mitochondrial Bioenergetics and Metabolic Plasticity of Cancer Cells During Metastasis. Front. Cell. Dev. Biol. 2021, 9, 751301. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, S.; Van de Velde, A.C.; Matzner Perfumo, V.; Gay, C.C.; Leiva, L.C. Apoptosis induced by a snake venom metalloproteinase from Bothrops. alternatus venom in C2C12 muscle cells. Apoptosis 2017, 22, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Danilucci, T.M.; Santos, P.K.; Pachane, B.C.; Pisani, G.F.D.; Lino, R.L.B.; Casali, B.C.; Altei, W.F.; Selistre-de-Araujo, H.S. Recombinant RGD-disintegrin DisBa-01 blocks integrin αvβ3 and impairs VEGF signaling in endothelial cells. Cell Commun. Signal. 2019, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.K.; Altei, W.F.; Danilucci, T.M.; Lino, R.L.B.; Pachane, B.C.; Nunes, A.C.C.; Selistre-de-Araujo, H.S. Alternagin-C (ALT-C), a disintegrin-like protein, attenuates alpha2beta1 integrin and VEGF receptor 2 signaling resulting in angiogenesis inhibition. Biochimie 2020, 174, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.K.; Joshi, M.B.; Vasishta, S.; Jagadale, R.N.; Biligiri, S.G.; Coronado, M.A.; Arni, R.K.; Satyamoorthy, K. P-I metalloproteinases and L-amino acid oxidases from Bothrops species inhibit angiogenesis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200180. [Google Scholar] [CrossRef]
- Lazo, F.; Vivas-Ruiz, D.E.; Sandoval, G.A.; Rodríguez, E.F.; Kozlova, E.E.G.; Costal-Oliveira, F.; Chávez-Olórtegui, C.; Severino, R.; Yarlequé, A.; Sanchez, E.F. Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom. Toxicon 2017, 139, 74–86. [Google Scholar] [CrossRef]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Gonzalez-Kozlova, E.; Zarria-Romero, J.; Lazo, F.; Rodríguez, E.; Magalhães, H.P.B.; Chávez-Olortegui, C.; Oliveira, L.S.; Alvarenga, V.G.; et al. Fibrinogen-clotting enzyme, pictobin, from Bothrops pictus snake venom. Structural and functional characterization. Int. J. Biol. Macromol. 2020, 153, 779–795. [Google Scholar] [CrossRef]
- Vivas-Ruiz, D.E.; Sandoval, G.A.; Mendoza, J.; Inga, R.R.; Gontijo, S.; Richardson, M.; Eble, J.A.; Yarleque, A.; Sanchez, E.F. Coagulant thrombin-like enzyme (barnettobin) from Bothrops barnetti. venom: Molecular sequence analysis of its cDNA and biochemical properties. Biochimie 2013, 95, 1476–1486. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Estevão-Costa, M.I.; Alvarenga, V.G.; Vivas-Ruiz, D.E.; Yarleque, A.; Lima, A.M.; Cavaco, A.; Eble, J.A.; Sanchez, E.F. Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergón) Induces Glycoprotein VI Shedding and Impairs Platelet Function. Molecules 2019, 24, 3489. [Google Scholar] [CrossRef]
- Rombel, I.T.; Sykes, K.F.; Rayner, S.; Johnston, S.A. ORF-FINDER: A vector for high-throughput gene identification. Gene 2002, 282, 33–41. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; De Castro, E.; Langendijk-Genevaux, P.S.; Pagni, M.; Sigrist, C.J.A. The PROSITE database. Nucleic Acids Res. 2006, 34, D227–D230. [Google Scholar] [CrossRef]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.-y.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2006, 15, 5–6. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Higgins, D.G.; Thompson, J.D.; Gibson, T.J. [22] Using CLUSTAL for multiple sequence alignments. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1996; Volume 266, pp. 383–402. [Google Scholar]
- Igarashi, T.; Araki, S.; Mori, H.; Takeda, S. Crystal structures of catrocollastatin/VAP2B reveal a dynamic, modular architecture of ADAM/adamalysin/reprolysin family proteins. FEBS Lett. 2007, 581, 2416–2422. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Gomes, M.S.R.; Naves de Souza, D.L.; Guimarães, D.O.; Lopes, D.S.; Mamede, C.C.N.; Gimenes, S.N.C.; Achê, D.C.; Rodrigues, R.S.; Yoneyama, K.A.G.; Borges, M.H.; et al. Biochemical and functional characterization of Bothropoidin: The first haemorrhagic metalloproteinase from Bothrops pauloensis snake venom. J. Biochem. 2014, 157, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Freitas, T.V.; Ferreira-Alves, D.L.; Velarde, D.T.; Diniz, M.R.; Cordeiro, M.N.; Agostini-Cotta, G.; Diniz, C.R. Biological activities of venoms from South American snakes. Toxicon 1992, 30, 95–103. [Google Scholar] [CrossRef]
- Córdova-Delgado, M.; Fuentes-Retamal, S.; Palominos, C.; López-Torres, C.; Guzmán-Rivera, D.; Ramírez-Rodríguez, O.; Araya-Maturana, R.; Urra, F.A. FRI-1 Is an Anti-Cancer Isoquinolinequinone That Inhibits the Mitochondrial Bioenergetics and Blocks Metabolic Shifts by Redox Disruption in Breast Cancer Cells. Antioxidants 2021, 10, 1618. [Google Scholar] [CrossRef]
- Urra, F.A.; Muñoz, F.; Córdova-Delgado, M.; Ramírez, M.P.; Peña-Ahumada, B.; Rios, M.; Cruz, P.; Ahumada-Castro, U.; Bustos, G.; Silva-Pavez, E.; et al. FR58P1a; a new uncoupler of OXPHOS that inhibits migration in triple-negative breast cancer cells via Sirt1/AMPK/β1-integrin pathway. Sci. Rep. 2018, 8, 13190. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Córdova-Delgado, M.; Lapier, M.; Orellana-Manzano, A.; Acevedo-Arévalo, L.; Pessoa-Mahana, H.; González-Vivanco, J.M.; Martínez-Cifuentes, M.; Ramírez-Rodríguez, O.; Millas-Vargas, J.P.; et al. Small structural changes on a hydroquinone scaffold determine the complex I inhibition or uncoupling of tumoral oxidative phosphorylation. Toxicol. Appl. Pharmacol. 2016, 291, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Retamal, S.; Sandoval-Acuña, C.; Peredo-Silva, L.; Guzmán-Rivera, D.; Pavani, M.; Torrealba, N.; Truksa, J.; Castro-Castillo, V.; Catalán, M.; Kemmerling, U.; et al. Complex Mitochondrial Dysfunction Induced by TPP+-Gentisic Acid and Mitochondrial Translation Inhibition by Doxycycline Evokes Synergistic Lethality in Breast Cancer Cells. Cells 2020, 9, 407. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Lee, T.C.; Kashyap, R.L.; Chu, C.N. Building Skeleton Models via 3-D Medial Surface Axis Thinning Algorithms. CVGIP Graph. Model. Image Process. 1994, 56, 462–478. [Google Scholar] [CrossRef]
- Landskron, G.; Dubois-Camacho, K.; Orellana-Serradell, O.; De la Fuente, M.; Parada-Venegas, D.; Bitrán, M.; Diaz-Jimenez, D.; Tang, S.; Cidlowski, J.A.; Li, X.; et al. Regulation of the Intestinal Extra-Adrenal Steroidogenic Pathway Component LRH-1 by Glucocorticoids in Ulcerative Colitis. Cells 2022, 11, 1905. [Google Scholar] [CrossRef]
- Leuven, F.V.; Marynen, P.; Cassiman, J.-J.; van den Berghe, H. Receptor-mediated endocytosis of α2macroglobulin—Protease complexes by fibroblasts in culture. FEBS Lett. 1981, 134, 83–87. [Google Scholar] [CrossRef]
- Ching, A.T.; Rocha, M.M.; Paes Leme, A.F.; Pimenta, D.C.; de Fátima, D.F.M.; Serrano, S.M.; Ho, P.L.; Junqueira-de-Azevedo, I.L. Some aspects of the venom proteome of the Colubridae snake Philodryas olfersii revealed from a Duvernoy’s (venom) gland transcriptome. FEBS Lett. 2006, 580, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Pulgar, R.; Gutiérrez, R.; Hodar, C.; Cambiazo, V.; Labra, A. Identification and molecular characterization of five putative toxins from the venom gland of the snake Philodryas chamissonis (Serpentes: Dipsadidae). Toxicon 2015, 108, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Urra, F.A.; Weiss-López, B.; Araya-Maturana, R. Determinants of Anti-Cancer Effect of Mitochondrial Electron Transport Chain Inhibitors: Bioenergetic Profile and Metabolic Flexibility of Cancer Cells. Curr. Pharm. Des. 2016, 22, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Urra, F.A.; Muñoz, F.; Lovy, A.; Cárdenas, C. The Mitochondrial Complex(I)ty of Cancer. Front. Oncol. 2017, 7, 118. [Google Scholar] [CrossRef]
- Galemou Yoga, E.; Angerer, H.; Parey, K.; Zickermann, V. Respiratory complex I—Mechanistic insights and advances in structure determination. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148153. [Google Scholar] [CrossRef]
- Kallens, V.; Tobar, N.; Molina, J.; Bidegain, A.; Smith, P.C.; Porras, O.; Martínez, J. Glucose Promotes a Pro-Oxidant and Pro-Inflammatory Stromal Microenvironment Which Favors Motile Properties in Breast Tumor Cells. J. Cell. Biochem. 2017, 118, 994–1002. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Jonker, M.R.; Brandenburg, S.M.; de Bruin, H.G.; ten Hacken, N.H.T.; van Oosterhout, A.J.M.; Heijink, I.H. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci. Rep. 2019, 9, 15047. [Google Scholar] [CrossRef]
- ToVinh, M.; Hörr, G.; Dobrikova, K.; Gotter, C.; Rommel, C.; Hoffmeister, C.; Raabe, J.; Kaiser, K.M.; Finnemann, C.; Bischoff, J.; et al. Mitochondrial Dysfunction Contributes to Impaired Cytokine Production of CD56bright Natural Killer Cells From Human Immunodeficiency Virus-Infected Individuals Under Effective Antiretroviral Therapy. J. Infect. Dis. 2022, 226, 901–906. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; Riveiro-Naveira, R.R.; Valcárcel-Ares, M.N.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012, 64, 2927–2936. [Google Scholar] [CrossRef]
- Donoso-Bustamante, V.; Borrego, E.A.; Schiaffino-Bustamante, Y.; Gutiérrez, D.A.; Millas-Vargas, J.P.; Fuentes-Retamal, S.; Correa, P.; Carrillo, I.; Aguilera, R.J.; Miranda, D.; et al. An acylhydroquinone derivative produces OXPHOS uncoupling and sensitization to BH3 mimetic ABT-199 (Venetoclax) in human promyelocytic leukemia cells. Bioorg. Chem. 2020, 100, 103935. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qin, X.; Sun, Z.; Hou, S.; Lv, Q. Low DEDD expression in breast cancer cells indicates higher sensitivity to the Bcl-2-specific inhibitor ABT-199. Biochem. Biophys. Res. Commun. 2020, 525, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Požek, K.; Leonardi, A.; Pungerčar, J.; Rao, W.; Gao, Z.; Liu, S.; Laustsen, A.H.; Trampuš Bakija, A.; Reberšek, K.; Podgornik, H.; et al. Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein. Toxins 2022, 14, 232. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Gabriel, L.M.; Gontijo, S.; Gremski, L.H.; Veiga, S.S.; Evangelista, K.S.; Eble, J.A.; Richardson, M. Structural and functional characterization of a P-III metalloproteinase, leucurolysin-B, from Bothrops leucurus venom. Arch. Biochem. Biophys. 2007, 468, 193–204. [Google Scholar] [CrossRef]
- Wang, W.J.; Huang, T.F. Purification and characterization of a novel metalloproteinase, acurhagin, from Agkistrodon acutus venom. Thromb. Haemost. 2002, 87, 641–650. [Google Scholar] [CrossRef]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Swenson, S.; Markland, F.S. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef]
- Chen, H.-S.; Tsai, H.-Y.; Wang, Y.-M.; Tsai, I.-H. P-III hemorrhagic metalloproteinases from Russell’s viper venom: Cloning, characterization, phylogenetic and functional site analyses. Biochimie 2008, 90, 1486–1498. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Saliou, B.; Leduc, M.; Carlini, C.R.; Hatmi, M.; Randon, J.; Faili, A.; Bon, C. COnvulxin, a potent platelet-aggregating protein from Crotalus durissus terrificus venom, specifically binds to platelets. Toxicon 1997, 35, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Polgár, J.; Clemetson, J.M.; Kehrel, B.E.; Wiedemann, M.; Magnenat, E.M.; Wells, T.N.; Clemetson, K.J. Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem. 1997, 272, 13576–13583. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Gutiérrez, M.; Arzanauskaite, M.; Mendieta, G.; Ben-Aicha, S.; Badimon, L. Intracellular platelet signalling as a target for drug development. Vasc. Pharmacol. 2018, 111, 22–25. [Google Scholar] [CrossRef]
- Anai, K.; Sugiki, M.; Yoshida, E.; Maruyama, M. Inhibition of a snake venom hemorrhagic metalloproteinase by human and ratα-macroglobulins. Toxicon 1998, 36, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.T.; Moura, M.B.; Magalhaes, A.; Heneine, L.G.D.; Olortegui, C.C.; Diniz, C.R.; Sanchez, E.F. Inhibition of mutalysin II, a metalloproteinase from bushmaster snake venom by human α2-macroglobulin and rabbit immunoglobulin. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 155–168. [Google Scholar] [CrossRef]
- Takeda, S. Three-dimensional domain architecture of the ADAM family proteinases. Semin. Cell Dev. Biol. 2009, 20, 146–152. [Google Scholar] [CrossRef]
- Bode, W.; Gomis-Rüth, F.-X.; Stöckler, W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Xavier Gomis-Rüth, F.; Meyer, E.F.; Kress, L.F.; Politi, V. Structures of adamalysin II with peptidic inhibitors. Implications for the design of tumor necrosis factor α convertase inhibitors. Protein Sci. 1998, 7, 283–292. [Google Scholar] [CrossRef]
- Cerretti, D.P.; DuBose, R.F.; Black, R.A.; Nelson, N. Isolation of Two Novel Metalloproteinase-Disintegrin (ADAM) cDNAs That Show Testis-Specific Gene Expression. Biochem. Biophys. Res. Commun. 1999, 263, 810–815. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Wallnoefer, H.G.; Lingott, T.; Gutiérrez, J.M.; Merfort, I.; Liedl, K.R. Backbone Flexibility Controls the Activity and Specificity of a Protein−Protein Interface: Specificity in Snake Venom Metalloproteases. J. Am. Chem. Soc. 2010, 132, 10330–10337. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.R.C.; Ambrosio, A.L.B.; Selistre-de-Araujo, H.S.; Cominetti, M.R.; Moura-da-Silva, A.M.; Oliva, G.; Garratt, R.C.; Souza, D.H.F. The three-dimensional structure of bothropasin, the main hemorrhagic factor from Bothrops jararaca venom: Insights for a new classification of snake venom metalloprotease subgroups. Toxicon 2008, 52, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Assakura, M.T.; Silva, C.A.; Mentele, R.; Camargo, A.C.M.; Serrano, S.M.T. Molecular cloning and expression of structural domains of bothropasin, a P-III metalloproteinase from the venom of Bothrops jararaca. Toxicon 2003, 41, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Astorga, J.; Gasaly, N.; Dubois-Camacho, K.; De la Fuente, M.; Landskron, G.; Faber, K.N.; Urra, F.A.; Hermoso, M.A. The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD. Front. Immunol. 2022, 13, 1028953. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell. Commun. Signal. 2019, 13, 303–318. [Google Scholar] [CrossRef]
- Bahat, A.; MacVicar, T.; Langer, T. Metabolism and Innate Immunity Meet at the Mitochondria. Front. Cell Dev. Biol. 2021, 9, 720490. [Google Scholar] [CrossRef]
- Zornetta, I.; Caccin, P.; Fernandez, J.; Lomonte, B.; Gutierrez, J.M.; Montecucco, C. Envenomations by Bothrops and Crotalus snakes induce the release of mitochondrial alarmins. PLoS Negl. Trop. Dis. 2012, 6, e1526. [Google Scholar] [CrossRef]
- Cano-Sanchez, M.; Ben-Hassen, K.; Louis, O.P.; Dantin, F.; Gueye, P.; Roques, F.; Mehdaoui, H.; Resiere, D.; Neviere, R. Bothrops lanceolatus snake venom impairs mitochondrial respiration and induces DNA release in human heart preparation. PLoS Negl. Trop. Dis. 2022, 16, e0010523. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Neviere, R. Inflammation and Oxidative Stress in Snakebite Envenomation: A Brief Descriptive Review and Clinical Implications. Toxins 2022, 14, 802. [Google Scholar] [CrossRef]
- Šribar, J.; Kovačič, L.; Oberčkal, J.; Ivanušec, A.; Petan, T.; Fox, J.W.; Križaj, I. The neurotoxic secreted phospholipase A2 from the Vipera a. ammodytes. venom targets cytochrome c oxidase in neuronal mitochondria. Sci. Rep. 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.S.; Faquim-Mauro, E.; Magalhães, G.S.; Lima, I.C.; Baldo, C.; Fox, J.W.; Moura-da-Silva, A.M.; Clissa, P.B. Gene expression of inflammatory mediators induced by jararhagin on endothelial cells. Toxicon 2012, 60, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Lisita, K.; Silva, M.D.S.; Santana, H.M.; Ikenohuchi, Y.J.; Paloschi, M.V.; Rego, C.M.A.; Serrath, S.N.; Lima, A.M.; Sousa, M.N.; Soares, A.M.; et al. Action of BjussuMP-II, a snake venom metalloproteinase isolated from Bothrops jararacussu venom, on human neutrophils. Toxicon 2023, 222, 106992. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef]
- Bartsch, J.E.; Staren, E.D.; Appert, H.E. Adhesion and migration of extracellular matrix-stimulated breast cancer. J. Surg. Res. 2003, 110, 287–294. [Google Scholar] [CrossRef]
- Rathinam, R.; Alahari, S.K. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010, 29, 223–237. [Google Scholar] [CrossRef]
- Cunniff, B.; McKenzie, A.J.; Heintz, N.H.; Howe, A.K. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol. Biol. Cell 2016, 27, 2662–2674. [Google Scholar] [CrossRef]
- Wan, S.-G.; Jin, Y.; Lee, W.-H.; Zhang, Y. A snake venom metalloproteinase that inhibited cell proliferation and induced morphological changes of ECV304 cells. Toxicon 2006, 47, 480–489. [Google Scholar] [CrossRef]
- Gabriel, L.; Sanchez, E.; Silva, S.; Santos, R. Tumor cytotoxicity of leucurolysin-B, a P-III snake venom metalloproteinase from Bothrops leucurus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 24–33. [Google Scholar] [CrossRef]
- Guimaraes, D.O.; Lopes, D.S.; Azevedo, F.V.; Gimenes, S.N.; Silva, M.A.; Ache, D.C.; Gomes, M.S.; Vecchi, L.; Goulart, L.R.; Yoneyama, K.A.; et al. In vitro antitumor and antiangiogenic effects of Bothropoidin, a metalloproteinase from Bothrops pauloensis snake venom. Int. J. Biol. Macromol. 2017, 97, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.C.; Mosier, J.A.; Padmanabhan, R.S.; White, A.; Xing, Q.; Hapach, L.A.; Taufalele, P.V.; Ortiz, I.; Reinhart-King, C.A. Link between glucose metabolism and epithelial-to-mesenchymal transition drives triple-negative breast cancer migratory heterogeneity. iScience 2022, 25, 105190. [Google Scholar] [CrossRef] [PubMed]
- Lucantoni, F.; Düssmann, H.; Llorente-Folch, I.; Prehn, J.H.M. BCL2 and BCL(X)L selective inhibitors decrease mitochondrial ATP production in breast cancer cells and are synthetically lethal when combined with 2-deoxy-D-glucose. Oncotarget 2018, 9, 26046–26063. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef]
- Jacque, N.; Ronchetti, A.M.; Larrue, C.; Meunier, G.; Birsen, R.; Willems, L.; Saland, E.; Decroocq, J.; Maciel, T.T.; Lambert, M.; et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 2015, 126, 1346–1356. [Google Scholar] [CrossRef]
- Sharon, D.; Cathelin, S.; Mirali, S.; Di Trani, J.M.; Yanofsky, D.J.; Keon, K.A.; Rubinstein, J.L.; Schimmer, A.D.; Ketela, T.; Chan, S.M. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci. Transl. Med. 2019, 11, eaax2863. [Google Scholar] [CrossRef]
- Proleón, A.; Torrejón, D.; Urra, F.A.; Lazo, F.; López-Torres, C.; Fuentes-Retamal, S.; Quispe, E.; Bautista, L.; Agurto, A.; Gavilan, R.G.; et al. Functional, immunological characterization, and anticancer activity of BaMtx: A new Lys49- PLA2 homologue isolated from the venom of Peruvian Bothrops atrox snake (Serpentes: Viperidae). Int. J. Biol. Macromol. 2022, 206, 990–1002. [Google Scholar] [CrossRef]
- Chen, K.C.; Chiou, Y.L.; Kao, P.H.; Lin, S.R.; Chang, L.S. Taiwan cobra cardiotoxins induce apoptotic death of human neuroblastoma SK-N-SH cells mediated by reactive oxygen species generation and mitochondrial depolarization. Toxicon 2008, 51, 624–634. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Chen, Z.; Shrivastava, I.H.; Gasanoff, E.S.; Dagda, R.K. Naja mossambica mossambica cobra cardiotoxin targets mitochondria to disrupt mitochondrial membrane structure and function. Toxins 2019, 11, 152. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivas-Ruiz, D.E.; Rosas, P.; Proleón, A.; Torrejón, D.; Lazo, F.; Tenorio-Ricca, A.B.; Guajardo, F.; Almarza, C.; Andrades, V.; Astorga, J.; et al. Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines. Pharmaceutics 2023, 15, 1533. https://doi.org/10.3390/pharmaceutics15051533
Vivas-Ruiz DE, Rosas P, Proleón A, Torrejón D, Lazo F, Tenorio-Ricca AB, Guajardo F, Almarza C, Andrades V, Astorga J, et al. Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines. Pharmaceutics. 2023; 15(5):1533. https://doi.org/10.3390/pharmaceutics15051533
Chicago/Turabian StyleVivas-Ruiz, Dan E., Paola Rosas, Alex Proleón, Daniel Torrejón, Fanny Lazo, Ana Belén Tenorio-Ricca, Francisco Guajardo, Cristopher Almarza, Víctor Andrades, Jessica Astorga, and et al. 2023. "Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines" Pharmaceutics 15, no. 5: 1533. https://doi.org/10.3390/pharmaceutics15051533
APA StyleVivas-Ruiz, D. E., Rosas, P., Proleón, A., Torrejón, D., Lazo, F., Tenorio-Ricca, A. B., Guajardo, F., Almarza, C., Andrades, V., Astorga, J., Oropesa, D., Toledo, J., Vera, M. J., Martínez, J., Araya-Maturana, R., Dubois-Camacho, K., Hermoso, M. A., Alvarenga, V. G., Sanchez, E. F., ... Urra, F. A. (2023). Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines. Pharmaceutics, 15(5), 1533. https://doi.org/10.3390/pharmaceutics15051533








