Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Dhvar5 Synthesis and Characterization
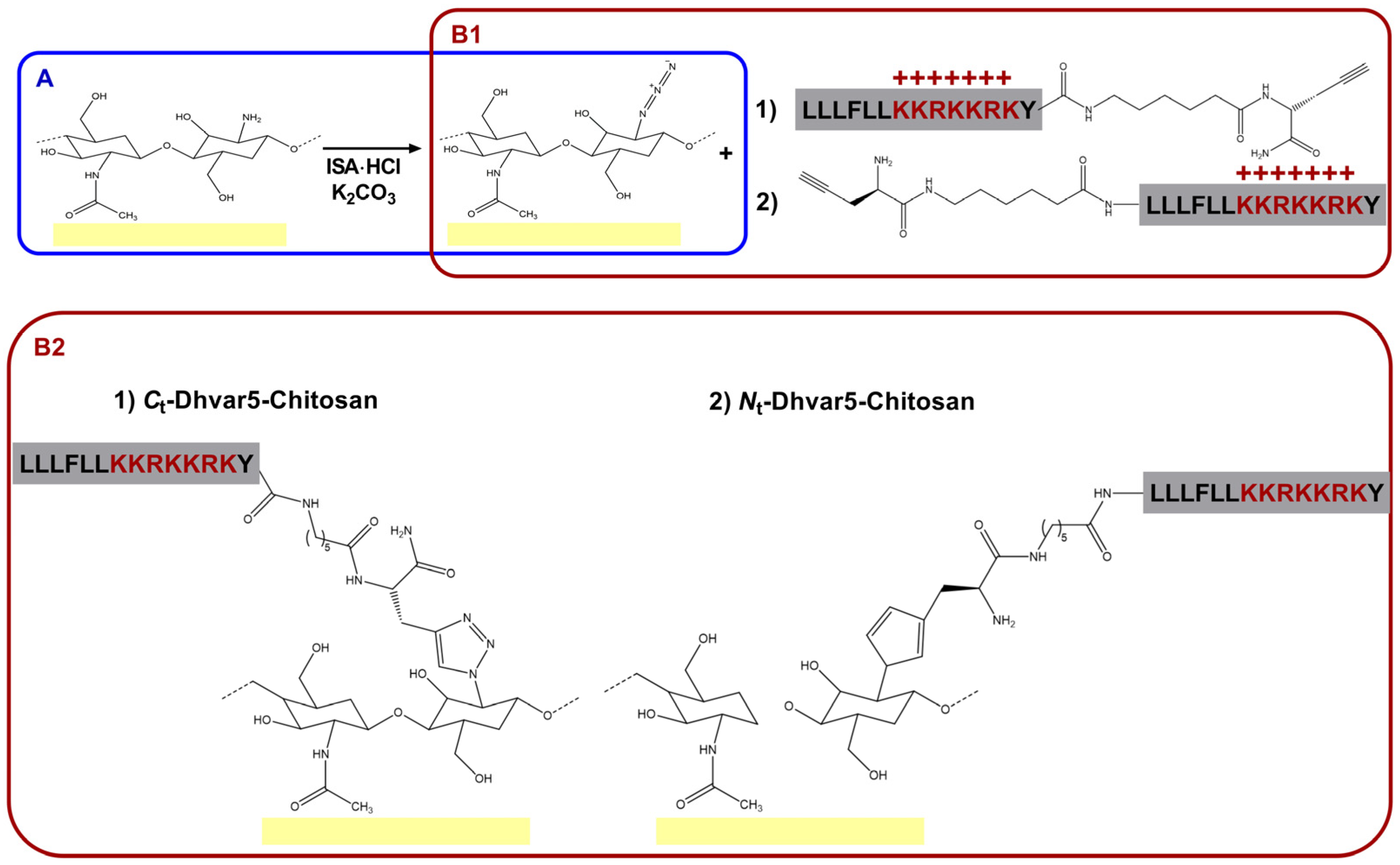
2.2. Dhvar5-Chitosan (Film) Coatings (Immobilization on Film)
2.2.1. Chitosan Coatings on Gold Substrates
2.2.2. Conversion of Chitosan Amines into Azides (N3-Chitosan)
2.2.3. Tethering of Alkyne-Modified Dhvar5 onto N3-Chitosan Coatings (Dhvar5-Chitosan Films)
2.3. Dhvar5-Chitosan (Bulk) Coatings (Immobilization in Bulk)
2.4. Surface Characterization of Dhvar5-Chitosan Coatings
2.4.1. Ellipsometry
2.4.2. Water Contact Angle Measurements (WCA)
2.4.3. Fourier Transform Infrared Reflection-Absorption Spectroscopy (IRRAS)
2.4.4. X-ray Photoelectron Spectroscopy (XPS)
2.4.5. Fluorescence Spectroscopy
2.4.6. Atomic Force Microscopy (AFM)
2.4.7. Electrokinetic Analysis (EKA)
2.4.8. Quartz Crystal Microbalance with Dissipation (QCM-D)
2.5. Antibacterial Activity Assays
2.5.1. Bacterial Strains, Media, and Growth Conditions
2.5.2. Surface Antimicrobial Activity Evaluation
3. Results
3.1. Characterization of Dhvar5-Chitosan Coatings (Film and Bulk)
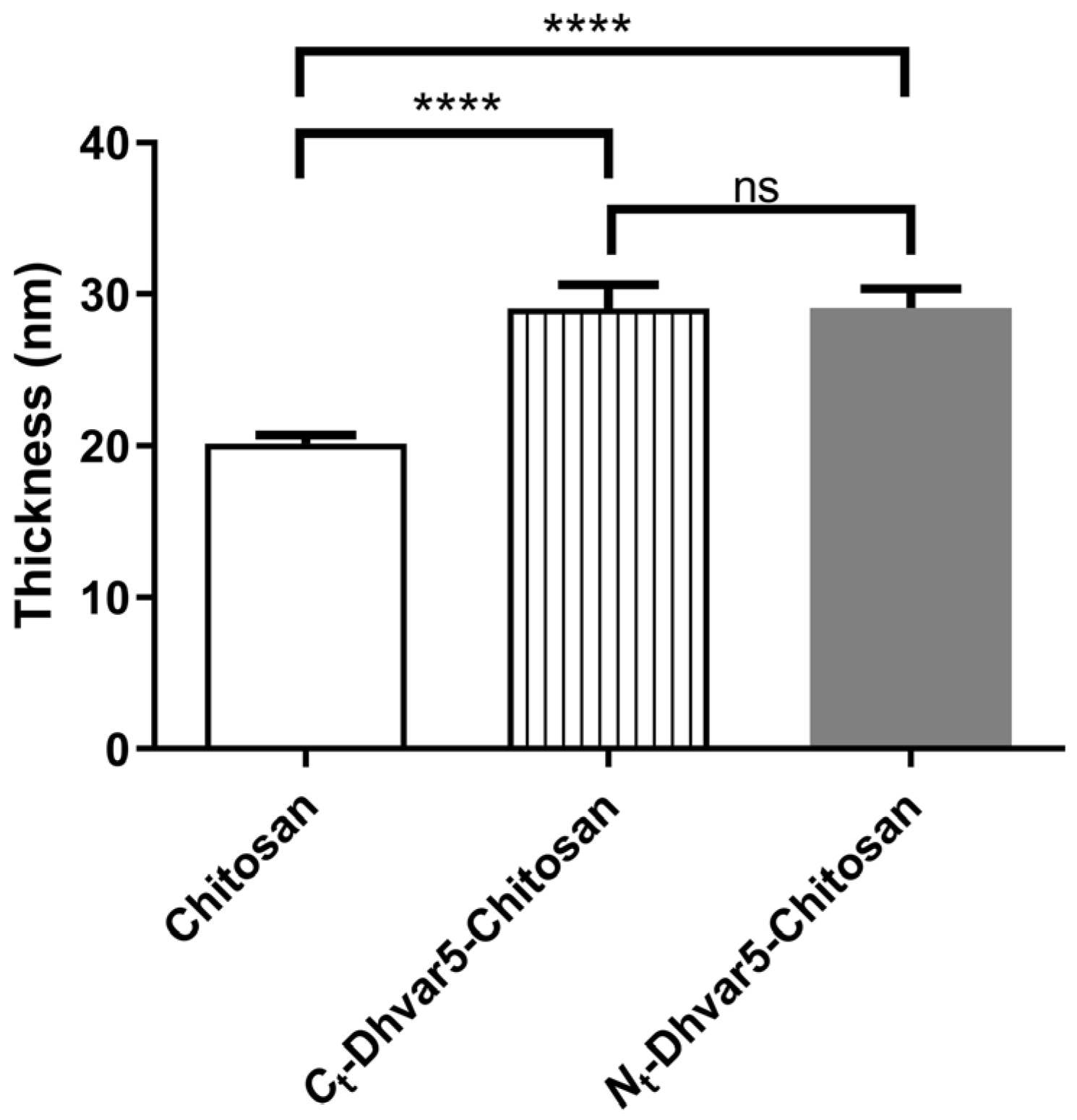
3.1.1. Ellipsometry
3.1.2. Water Contact Angles (WCA) Analysis
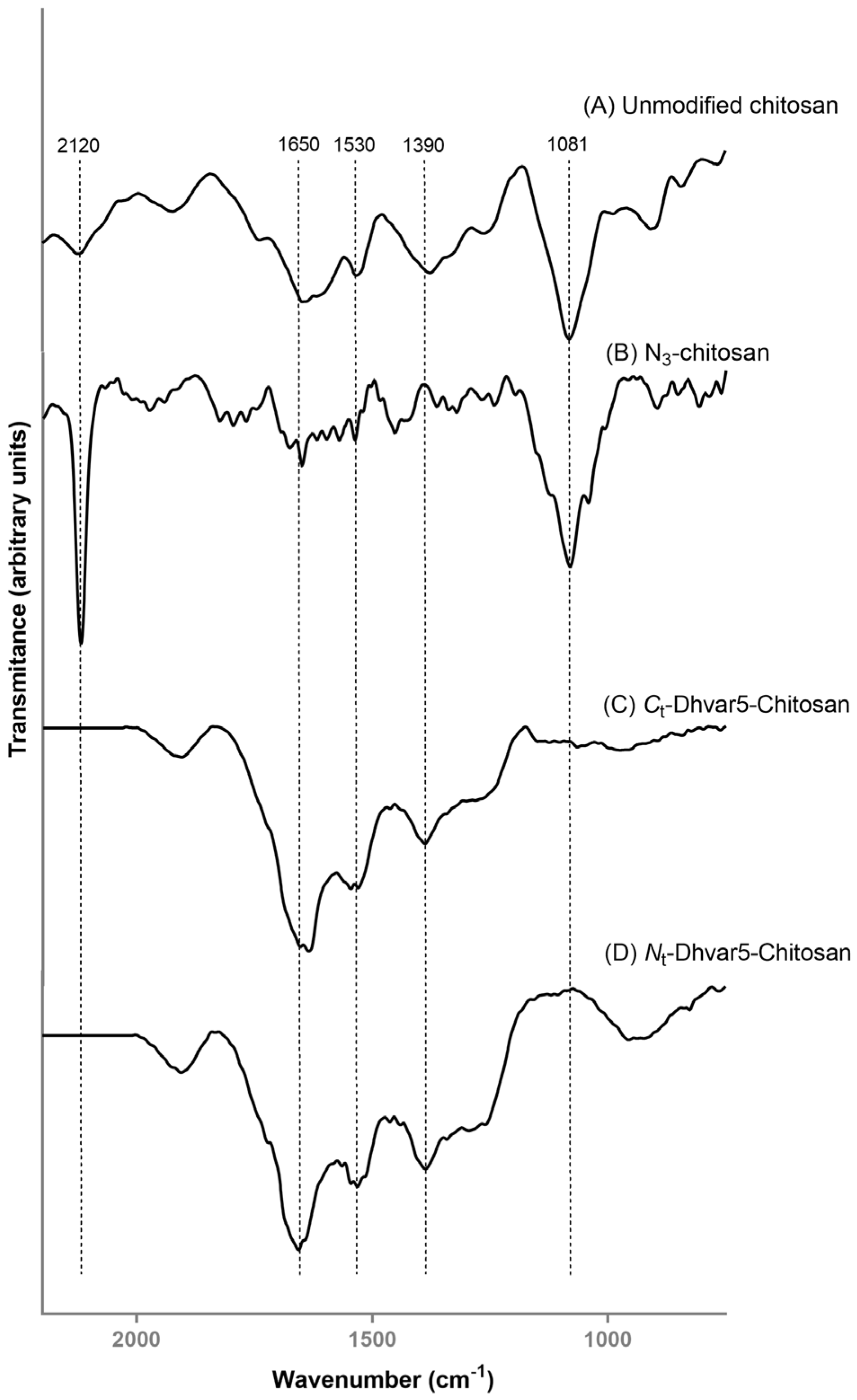
3.1.3. Fourier Transform Infrared Reflection-Absorption Spectroscopy (IRRAS)
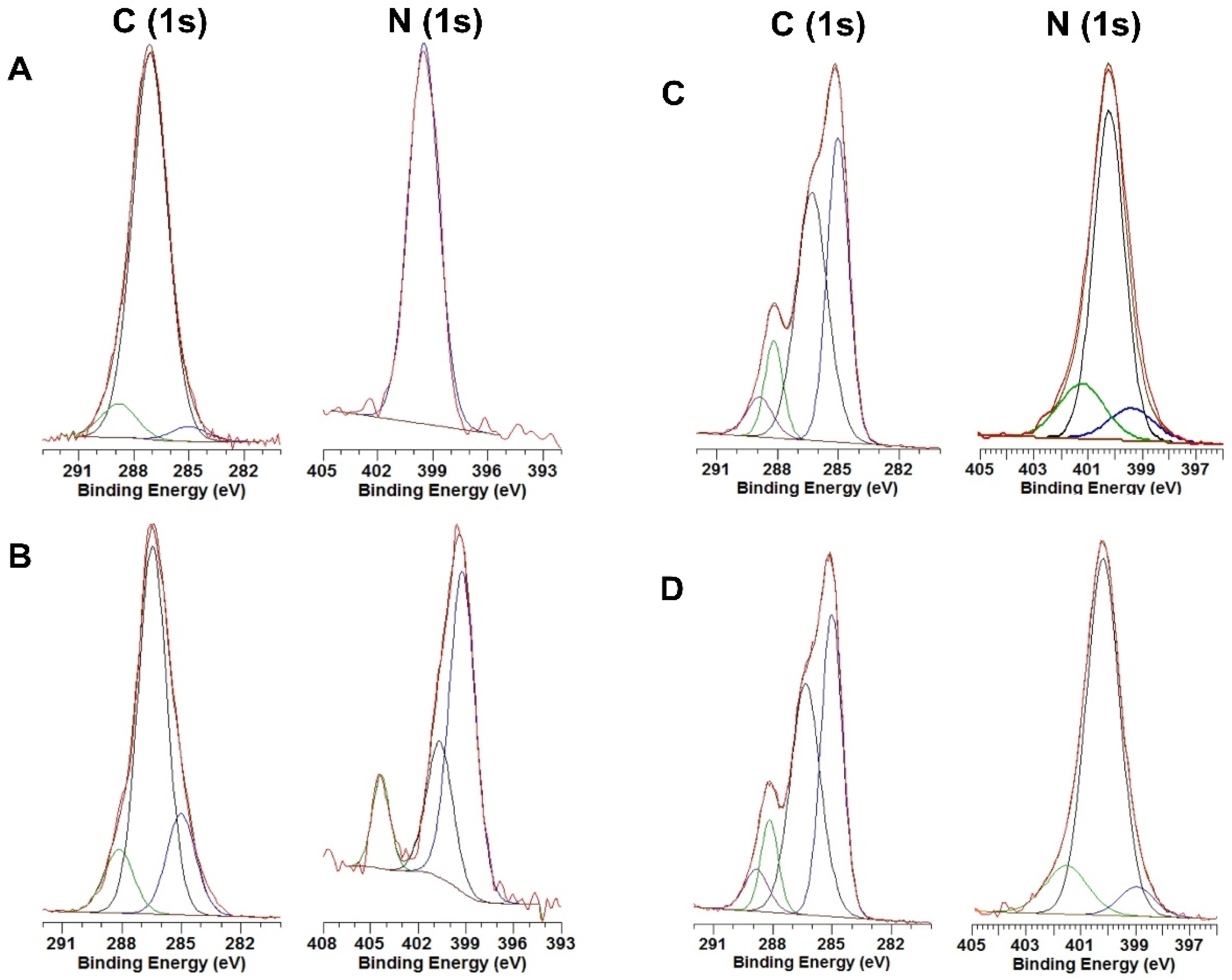
3.1.4. X-ray Photoelectron Spectroscopy (XPS)
3.1.5. Peptide Surface Density Determination
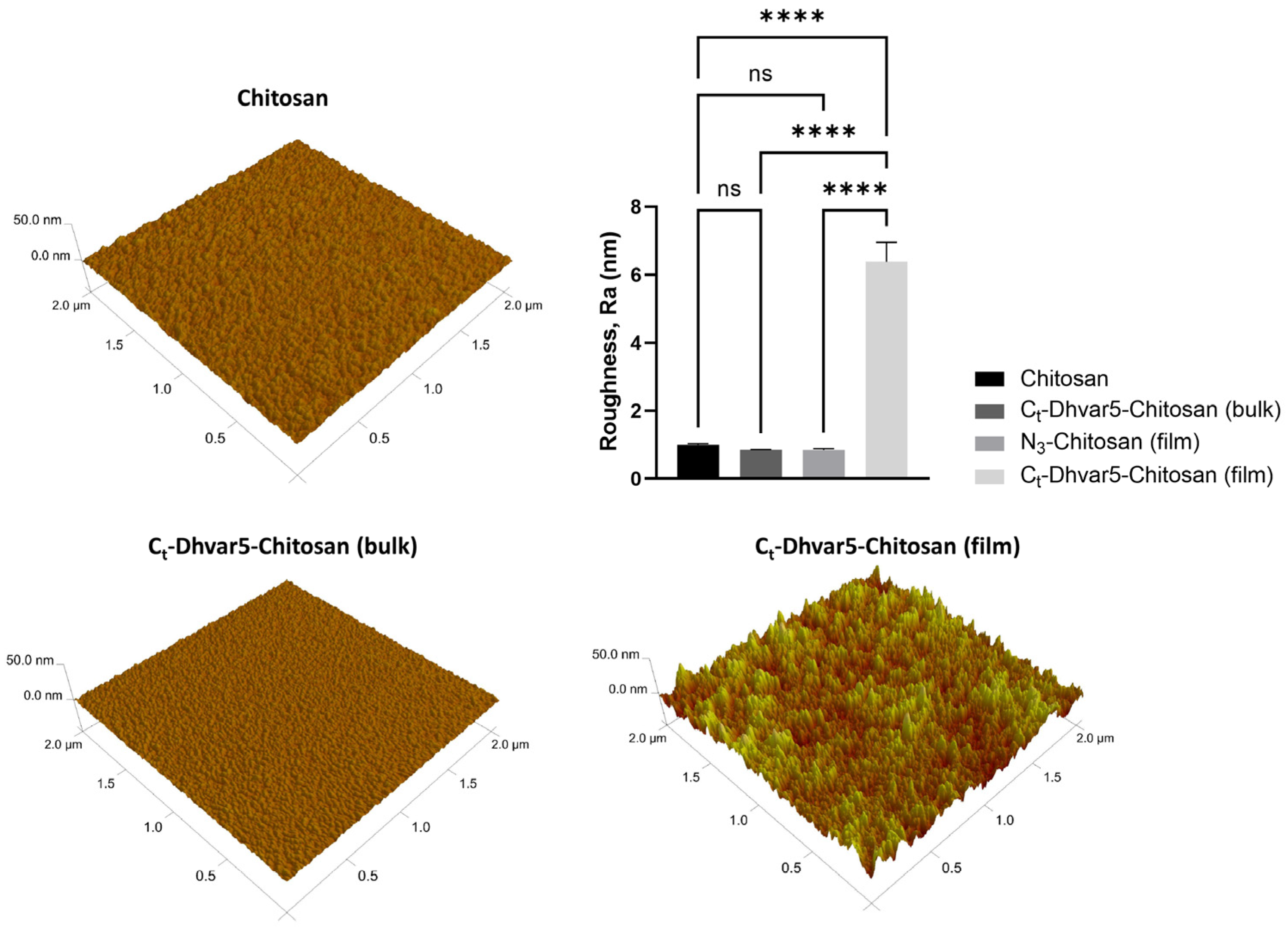
3.1.6. Atomic Force Microscopy (AFM)
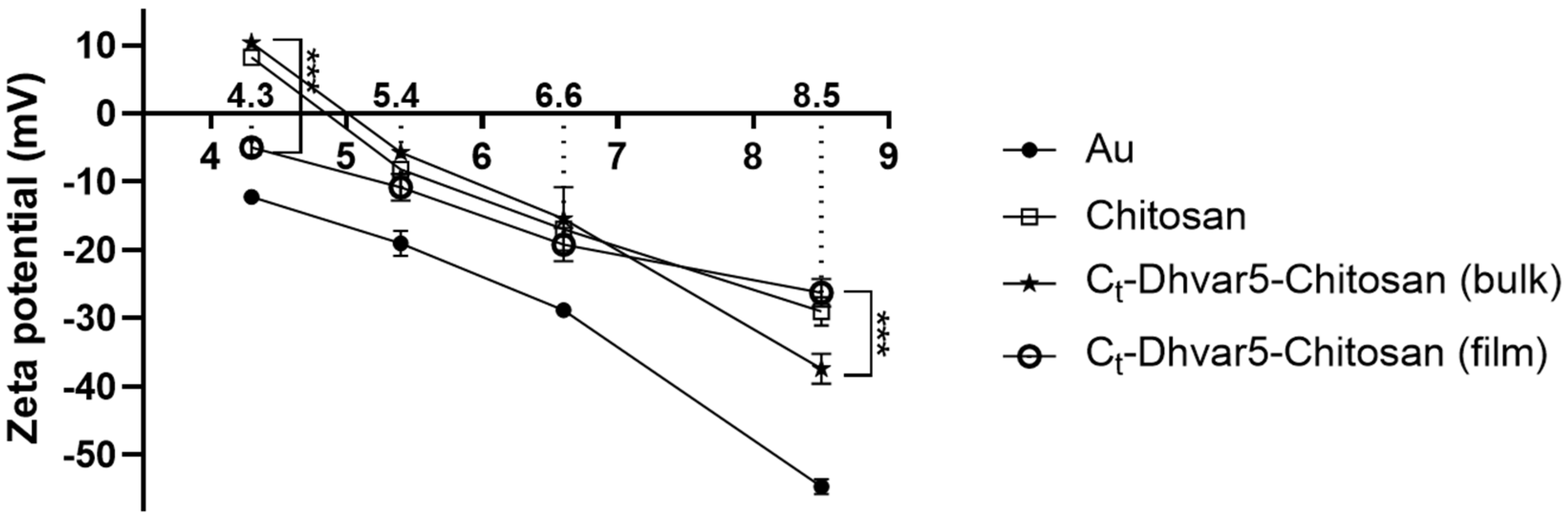
3.1.7. Electrokinetic Analysis (EKA)
3.1.8. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
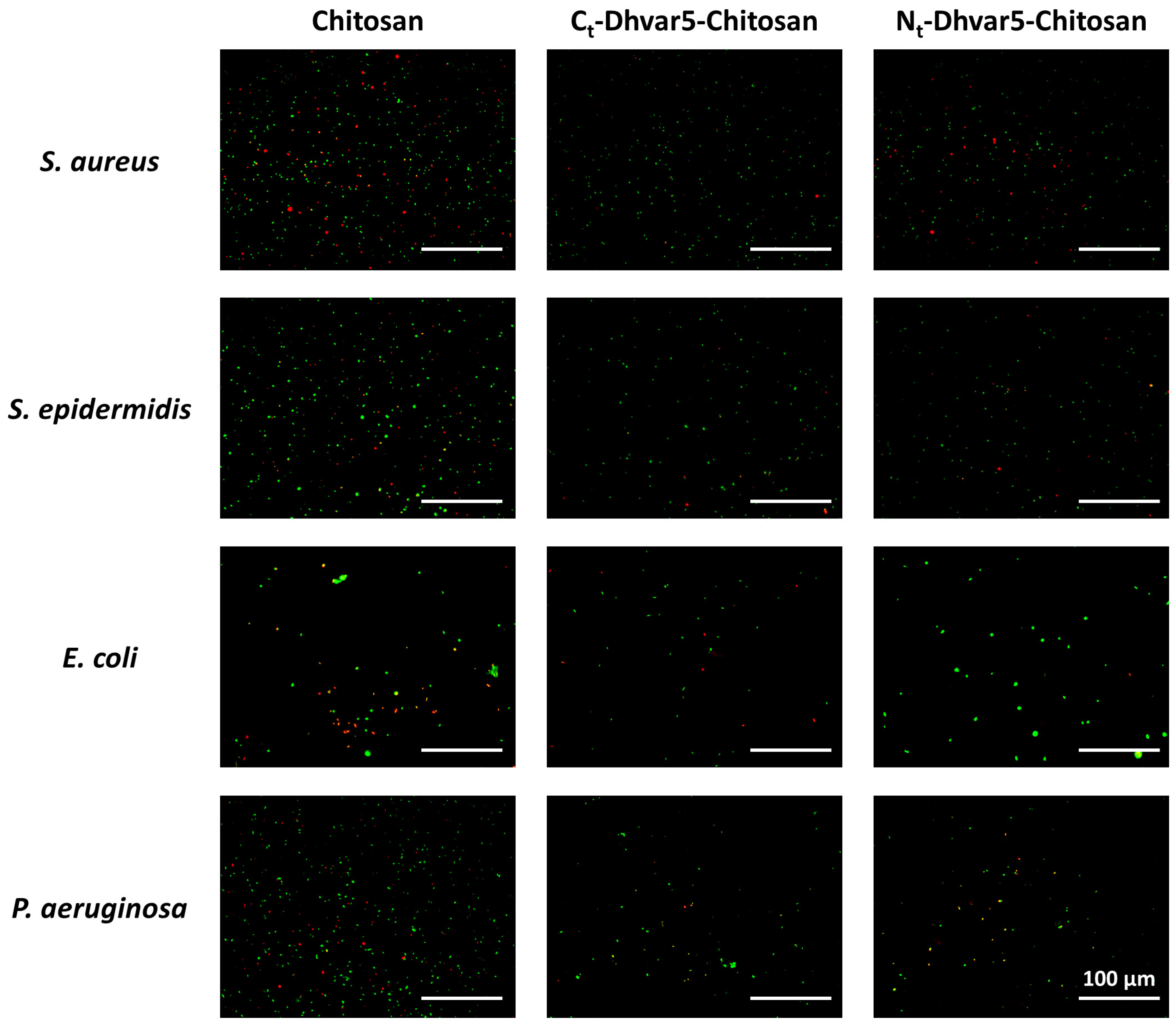
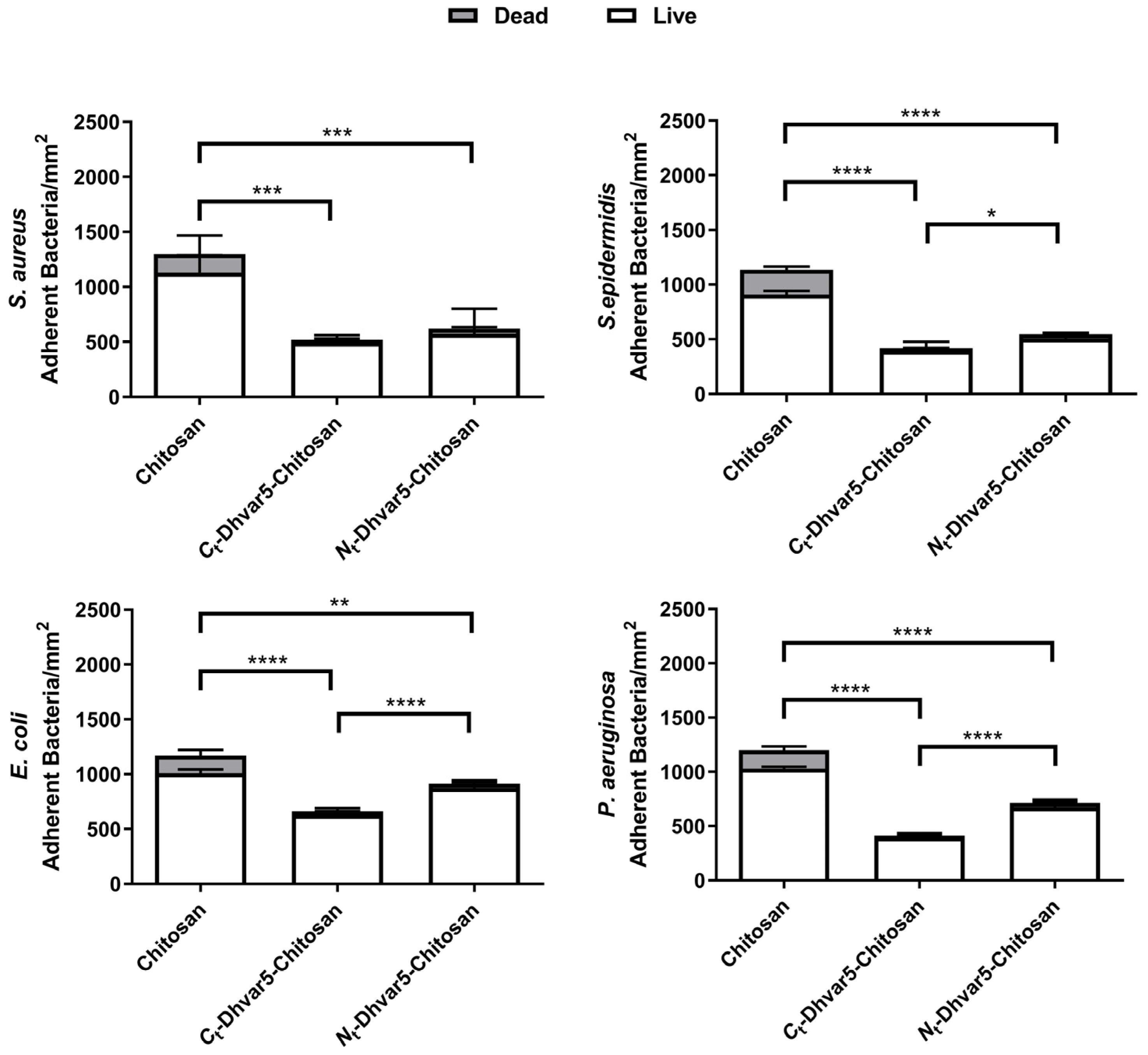
3.2. Antibacterial Activity Assays
Surface Antimicrobial Activity Mechanism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, F.; Maia, S.R.; Gomes, P.A.C.; Martins, M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef]
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices: Infection and Control; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–95. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y.; Galantini, L.; Schillén, K.; Del Giudice, A.; Du, G.; Wang, Y. Noncovalent Bile Acid Oligomers as Facial Amphiphilic Antimicrobials. Langmuir 2023, 39, 495–506. [Google Scholar] [CrossRef]
- Dižová, S.; Bujdáková, H. Properties and role of the quorum sensing molecule Farnesol in relation to the yeast Candida albicans. Pharmazie 2017, 72, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhou, Z.; Li, Q.; Tang, X.; Chen, X.; Ge, Y.; Ling, J. Light-Triggered Adhesive Silk-Based Film for Effective Photodynamic Antibacterial Therapy and Rapid Hemostasis. Front. Bioeng. Biotechnol. 2022, 9, 820434. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Wang, D.; Chen, X.; Ferreira, L.; Martins, M.C.L.; Wang, Y.; Jin, Q.; Wang, D.; Tang, B.Z.; et al. A NIR-II emissive polymer AIEgen for imaging-guided photothermal elimination of bacterial infection. Biomaterials 2022, 286, 121579. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; de la Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. 2020, 41, 94–120. [CrossRef]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef]
- Barbosa, M.; Costa, F.; Monteiro, C.; Duarte, F.; Martins, M.C.L.; Gomes, P. Antimicrobial coatings prepared from Dhvar-5-click-grafted chitosan powders. Acta Biomater. 2019, 84, 242–256. [Google Scholar] [CrossRef]
- Silva, R.R.; Avelino, K.Y.P.S.; Ribeiro, K.L.; Franco, O.L.; Oliveira, M.D.L.; Andrade, C.A.S. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci. Sch. 2016, 8, 129–142. [Google Scholar] [CrossRef]
- Costa, B.; Martínez-De-tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial peptides in the battle against orthopedic implant-related infections: A review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Barrias, C.C.; Gomes, P.; Martins, M.C.L. Smart biomaterial-based systems for intrinsic stimuli-responsive chronic wound management. Mater. Today Chem. 2021, 22, 100623. [Google Scholar] [CrossRef]
- Tang, X.; Gu, X.; Wang, Y.; Chen, X.; Ling, J.; Yang, Y. Stable antibacterial polysaccharide-based hydrogels as tissue adhesives for wound healing. RSC Adv. 2020, 10, 17280–17287. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Pereira, R.F.; Costa, B.; Tassi, N.; Teixeira, C.; Leiro, V.; Monteiro, C.; Gomes, P.; Costa, F.; Martins, M.C.L. Thiol-Norbornene Photoclick Chemistry for Grafting Antimicrobial Peptides onto Chitosan to Create Antibacterial Biomaterials. ACS Appl. Polym. Mater. 2022, 4, 5012–5026. [Google Scholar] [CrossRef]
- Ruissen, A.L.A.; Groenink, J.; Van’t Hof, W.; Walgreen-Weterings, E.; Van Marle, J.; Van Veen, H.A.; Voorhout, W.F.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Histatin 5 and derivatives: Their localization and effects on the ultra-structural level. Peptides 2002, 23, 1391–1399. [Google Scholar] [CrossRef]
- Den Hertog, A.L.; Wong Fong Sang, H.W.; Kraayenhof, R.; Bolscher, J.G.M.; Van’t Hof, W.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Interactions of histatin 5 and histatin 5-derived peptides with liposome membranes: Surface effects, translocation and permeabilization. Biochem. J. 2004, 379 Pt 3, 665. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tomøe, C.W. Cu-catalyzed azide–Alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Iha, R.K.; Wooley, K.L.; Nyström, A.M.; Burked, D.J.; Kade, M.J.; Hawker, C.J. Applications of orthogonal “Click” Chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Vale, N.; Costa, F.M.T.A.; Martins, M.C.L.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Martins, M.C.L.; Mafra, L.; Gomes, P. Synthesis of an O-alkynyl-chitosan and its chemoselective conjugation with a PEG-like amino-azide through click chemistry. Carbohydr. Polym. 2012, 87, 240–249. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methyl-terminated alkanethiols self-assembled monolayers. J. Biomed. Mater. Res. Part A 2003, 67, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.; Macakova, L.; Dedinaite, A.; Claesson, P. Interactions between chitosan and SDS at a low-charged silica substrate compared to interactions in the bulk—The effect of ionic strength. Langmuir 2008, 24, 3814–3827. [Google Scholar] [CrossRef]
- Hashizume, M.; Ohashi, M.; Kobayashi, H.; Tsuji, Y.; Iijima, K. Free-standing polysaccharide composite films: Improved preparation and physical properties. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 18–24. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.W.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; MacQuarrie, R. A sensitive fluorometric method for the determination of arginine using 9,10-phenanthrenequinone. Anal. Biochem. 1978, 90, 246–255. [Google Scholar] [CrossRef]
- Nogueira, F.; Gonçalves, I.C.; Martins, M.C.L. Effect of gastric environment on Helicobacter pylori adhesion to a mucoadhesive polymer. Acta Biomater. 2013, 9, 5208–5215. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ochoa-Mendes, V.; Ferreira, G.; Barbosa, J.N.; Curtin, S.A.; Ratner, B.D.; Barbosa, M.A. Interactions of leukocytes and platelets with poly(lysine/leucine) immobilized on tetraethylene glycol-terminated self-assembled monolayers. Acta Biomater. 2011, 7, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; Martins, M.C.L.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Gomes, C.A.R.; Batista, M.K.S.; Pinto, L.F.; Silva, P.A.P. Synthesis, structural characterization and properties of water-soluble N-(γ-propanoyl-amino acid)-chitosans. Carbohydr. Polym. 2008, 71, 54–65. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Castro, V.; Blanco-Canosa, J.B.; Rodriguez, H.; Albericio, F. Imidazole-1-sulfonyl Azide-Based Diazo-Transfer Reaction for the Preparation of Azido Solid Supports for Solid-Phase Synthesis. 2013; 15, 331–334. [Google Scholar] [CrossRef]
- Sahariah, P.; Sørensen, K.K.; Hjálmarsdóttir, M.A.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, P.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. Electrical signal guided click coating of chitosan hydrogel on conductive surface. RSC Adv. 2014, 4, 13477–13480. [Google Scholar] [CrossRef]
- Henriques, P.C.; Pereira, A.T.; Bogas, D.; Fernandes, J.R.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Graphene films irradiated with safe low-power NIR-emitting diodes kill multidrug resistant bacteria. Carbon 2021, 180, 10–21. [Google Scholar] [CrossRef]
- Marrani, A.G.; Motta, A.; Amato, F.; Schrebler, R.; Zanoni, R.; Dalchiele, E.A. Effect of electrolytic medium on the electrochemical reduction of graphene oxide on Si(111) as probed by XPS. Nanomaterials 2022, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Shakiba, A.; Jamison, A.C.; Lee, T.R. Poly(L-lysine) Interfaces via Dual Click Reactions on Surface-Bound Custom-Designed Dithiol Adsorbates. Langmuir 2015, 31, 6154–6163. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Willcox, M.D.P.; Cole, N.; Ho, K.K.K.; Rasul, R.; Denman, J.A.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef]
- Hilpert, K.; Elliott, M.; Jenssen, H.; Kindrachuk, J.; Fjell, C.D.; Körner, J.; Winkler, D.F.H.; Weaver, L.L.; Henklein, P.; Ulrich, A.S.; et al. Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity. Chem. Biol. 2009, 16, 58–69. [Google Scholar] [CrossRef]
- Veiga, A.S.; Sinthuvanich, C.; Gaspar, D.; Franquelim, H.G.; Castanho, M.A.R.B.; Schneider, J.P. Arginine-rich self-assembling peptides as potent antibacterial gels. Biomaterials 2012, 33, 8907–8916. [Google Scholar] [CrossRef]
- Strøm, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The pharmacophore of short cationic antibacterial peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.M.; Pradier, C.M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The Effect of Surface Nanometre-Scale Morphology on Protein Adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of protein adsorption induced by surface roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef] [PubMed]


| Polymer | Atomic Composition (%) | ||
|---|---|---|---|
| C1s | N1s | O1s | |
| Chitosan | 56.9 | 8.3 | 34.8 |
| N3-chitosan | 56.5 | 10.4 | 33.1 |
| Ct-Dhvar5-Chitosan (film) | 64.7 | 16.4 | 18.9 |
| Nt-Dhvar5-Chitosan (film) | 65.3 | 16.4 | 18.3 |
| Polymer | Atomic% N1s | |||
|---|---|---|---|---|
| C–N/CO–N | CO–N–CO/N–CO–O | NH3+ | N=N+=N− | |
| 399.5 eV | 400.2 eV | 401.3 eV | 404.3 eV | |
| Chitosan | 100 | - | - | - |
| N3-chitosan | 61.7 | - | 26.2 | 12.1 |
| Ct-Dhvar5-Chitosan (film) | 32.9 | 50.1 | 17.0 | - |
| Nt-Dhvar5-Chitosan (film) | 6.70 | 79.8 | 13.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.; Alves, P.M.; Costa, F.; Monteiro, C.; Parreira, P.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings. Pharmaceutics 2023, 15, 1510. https://doi.org/10.3390/pharmaceutics15051510
Barbosa M, Alves PM, Costa F, Monteiro C, Parreira P, Teixeira C, Gomes P, Martins MCL. Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings. Pharmaceutics. 2023; 15(5):1510. https://doi.org/10.3390/pharmaceutics15051510
Chicago/Turabian StyleBarbosa, Mariana, Pedro M. Alves, Fabíola Costa, Cláudia Monteiro, Paula Parreira, Cátia Teixeira, Paula Gomes, and Maria Cristina L. Martins. 2023. "Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings" Pharmaceutics 15, no. 5: 1510. https://doi.org/10.3390/pharmaceutics15051510
APA StyleBarbosa, M., Alves, P. M., Costa, F., Monteiro, C., Parreira, P., Teixeira, C., Gomes, P., & Martins, M. C. L. (2023). Influence of Immobilization Strategies on the Antibacterial Properties of Antimicrobial Peptide-Chitosan Coatings. Pharmaceutics, 15(5), 1510. https://doi.org/10.3390/pharmaceutics15051510









