Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial
Abstract
1. Introduction
2. Structural Features and Classification
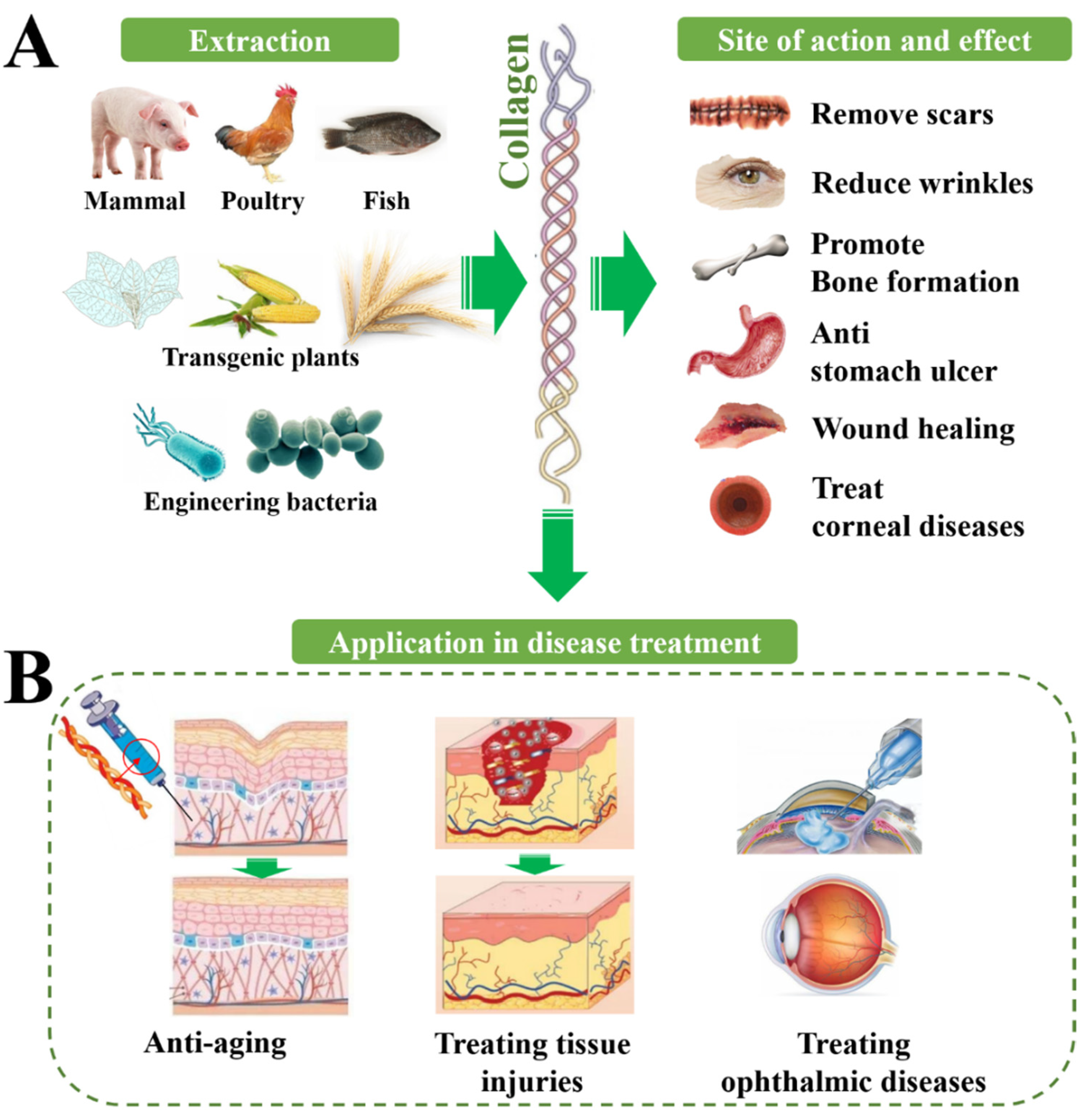
3. Physiological and Therapeutic Effects of Collagens
3.1. Collagen for Treating Skin Injuries
| Diseases | Therapeutic Effects | Refs. | |
|---|---|---|---|
| Treating skin injuries | Wound healing | Wound closure; anti-bacterial activity | [14,15,16,17,18,19] |
| Burn healing | Accelerated healing and skin appendage generation | [20,21,22,23] | |
| Chronic wound | Faster wound healing | [24,25,26] | |
| Treating orthopedic diseases | Osteoporosis | Improved bone mineral density; increased bone hydroxyproline content; enhanced alkaline phosphatase level | [27] |
| Bone defect healing | New bone tissue forming; guided bone regeneration | [28,29,30,31] | |
| Treating ophthalmic diseases | Corneal defects | Filled corneal defects; restored corneal curvature | [29,32] |
| Keratoconus | Increased corneal rigidity; decreased interfibrillar Bragg spacing | [33,34,35,36] | |
| Promoting nerve regeneration | Central nerve injury | Tuning NSCs; improved motor performance; reduced formation of fluid-filled cysts; impeded collapse of musculature and connective tissue | [37,38,39,40,41,42,43] |
| Peripheral nerve injury | Well-organized fibers; unimpaired myelin sheath | [44] | |
| Anti-aging | Skin anti-aging | Reduced trans-epidermal water loss and skin pore number; increased elasticity; enhanced dermal thickness and acoustic density | [45,46,47,48] |
3.2. Collagen for Treating Orthopedic Diseases
3.3. Collagen for Treating Ophthalmic Diseases
3.4. Collagen for Promoting Nerve Regeneration
3.5. Collagen for Anti-Aging
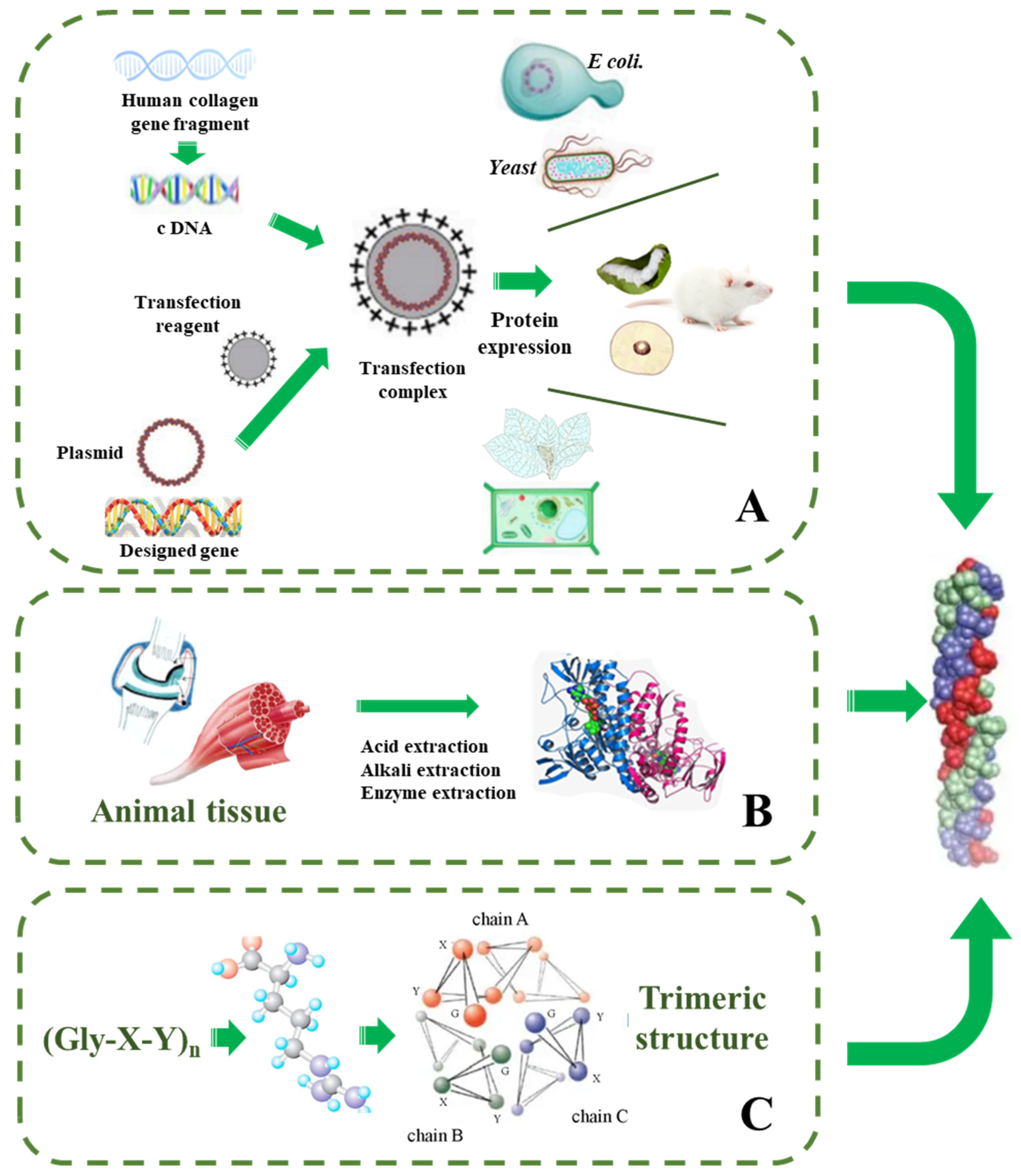
4. Strategies for Synthetic Collagen Production
4.1. Total Synthesis Strategy
4.2. Strategies for Recombinant Collagen Production
4.2.1. Protein Engineering in Animals
4.2.2. Protein Engineering in Escherichia coli
| Expression System | Host for Transfection | Synthetic Conditions | Productivity | Limitations | Ref. |
|---|---|---|---|---|---|
| Transgenic plants | Tobacco, corn, barley, etc. | Transforming collagen gene into plants such as transgenic tobacco and corn for expression | 20 g/L | Its expression quantity and purification steps need to be improved | [79,80,81,82,83] |
| Escherichia coli | E. coli | Designed collagen target genes and vectors in E. coli to induce expression of the target protein | 0.1–0.2 g/L | Affected by pH, temperature, dissolved oxygen, acetic acid concentration, carbon source, nitrogen source, etc. | [77,84] |
| Yeast | Pichia pastoris | Eukaryotic expression system can ensure the post-translational modification of collagen, including glycosylation, etc. | 0.7–1.5 g/L | Affected by pH, temperature, and methanol content | [85,86,87] |
| Transgenic animals | Glands of mice and silkworms | Construction of the vector and animals are transformed with collagen gene fragments | 8–20 g/L | Low level of hydroxyproline and incomplete triple helix structure | [73,74,75,76] |
4.2.3. Protein Engineering in Yeast Expression System
4.2.4. Protein Engineering in Plants
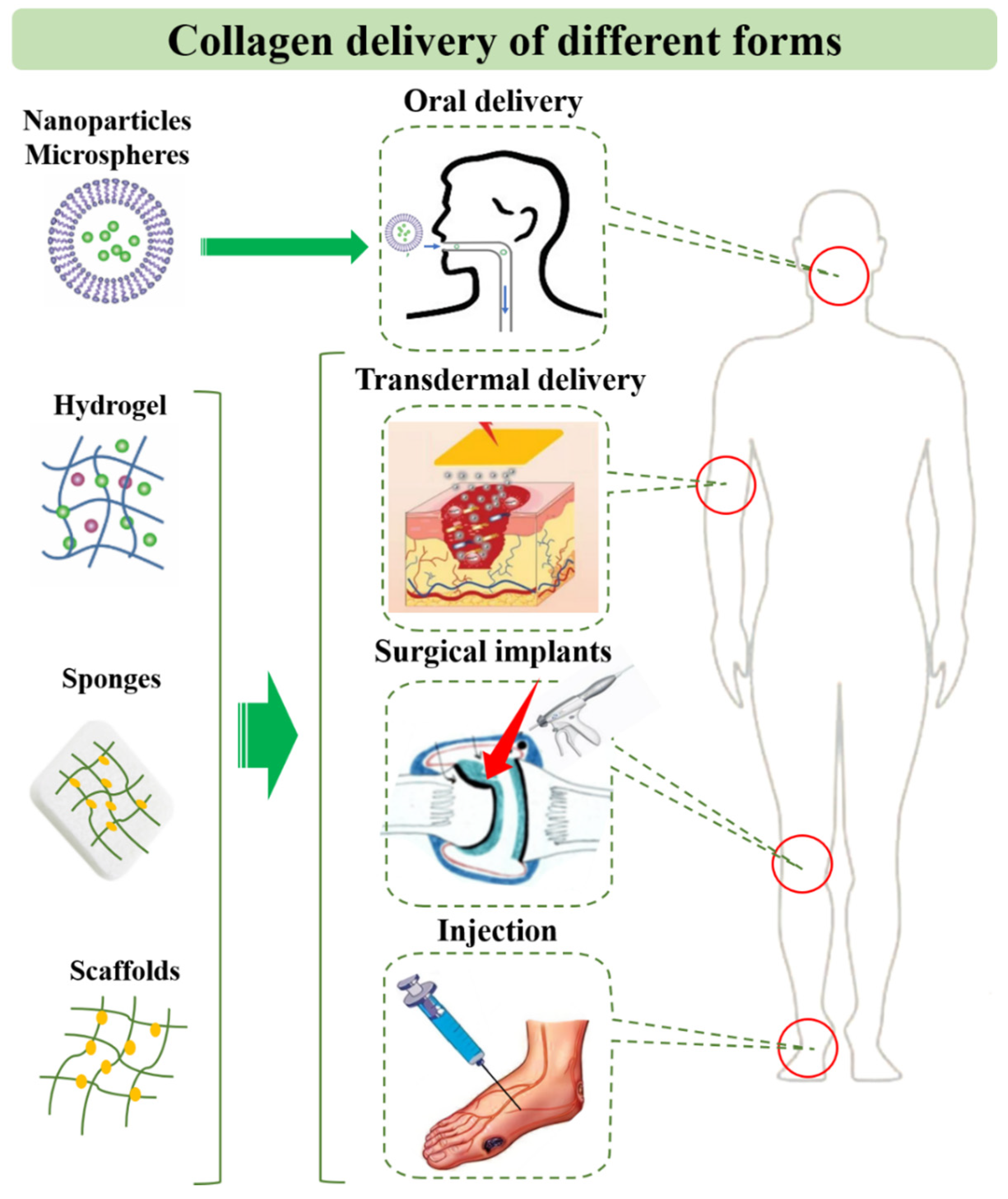
5. Application and Delivery Strategies of Collagen for Diseases
5.1. Collagen Peptide and Solution
5.2. Collagen-Based Drug Delivery Systems
5.3. Collagen-Based Tissue Engineering Systems
6. Discussion and Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ricard-Blum, S.; Ruggiero, F.; van der Rest, M. The collagen superfarmily. In Collagen: Primer in Structure, Processing and Assembly; Brinckmann, J., Notbohm, H., Muller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 35–84. [Google Scholar]
- El Blidi, O.; El Omari, N.; Balahbib, A.; Ghchime, R.; El Menyiy, N.; Ibrahimi, A.; Ben Kaddour, K.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar] [CrossRef]
- Fu, R.; Fan, D.; Yang, W.; Chen, L.; Qu, C.; Yang, S.; Xu, L. Industrial development and biomedical application prospect of recombinant collagen. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2022, 38, 3228–3242. [Google Scholar] [CrossRef]
- Yang, C.L.; Hillas, P.J.; Baez, J.A.; Nokelainen, M.; Balan, J.; Tang, J.; Spiro, R.; Polarek, J.W. The application of recombinant human collagen in tissue engineering. Biodrugs 2004, 18, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Hsiuying, W. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, e2000017. [Google Scholar] [CrossRef]
- Israelowitz, M.; Rizvi, S.W.H.; Kramer, J.; von Schroeder, H.P. Computational modeling of type I collagen fibers to determine the extracellular matrix structure of connective tissues. Protein Eng. Des. Sel. 2005, 18, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef]
- Nishimoto, M.; Sakamoto, R.; Mizuta, S.; Yoshinaka, R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder Paralichthys olivaceus. Food Chem. 2005, 90, 151–156. [Google Scholar] [CrossRef]
- Minor, R.R. Collagen-metabolism—Comparison of diseases of collagen and diseases affecting collagen. Am. J. Pathol. 1980, 98, 225–280. [Google Scholar] [PubMed]
- Asamura, K.; Abe, S.; Imamura, Y.; Aszodi, A.; Suzuki, N.; Hashimoto, S.; Takumi, Y.; Hayashi, T.; Fassler, R.; Nakamura, Y.; et al. Type IX collagen is crucial for normal hearing. Neuroscience 2005, 132, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Goldbloom-Helzner, L.; Hao, D.; Wang, A. Developing Regenerative Treatments for Developmental Defects, Injuries, and Diseases Using Extracellular Matrix Collagen-Targeting Peptides. Int. J. Mol. Sci. 2019, 20, 4072. [Google Scholar] [CrossRef]
- Liu, T.; Dan, W.H.; Dan, N.H.; Liu, X.H.; Liu, X.X.; Peng, X. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Xu, B.; Wang, Y.H.; Li, Y.; Si, H.; Zheng, X.Y.; Chen, Z.Y.; Chen, F.L.; Fan, D.D. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J. Biomater. Sci. Polym. Ed. 2019, 30, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhou, C.C.; Luo, C.; Qian, B.; Liu, S.K.; Zeng, Y.Y.; Hou, J.F.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Han, M.; Sun, Y.S.; Hua, Y.Y.; Chen, G.F.; Zhang, L.F. Oligoarginine mediated collagen/chitosan gel composite for cutaneous wound healing. Int. J. Biol. Macromol. 2019, 122, 1120–1127. [Google Scholar] [CrossRef]
- Masci, V.L.; Taddei, A.R.; Courant, T.; Tezgel, O.; Navarro, F.; Giorgi, F.; Mariolle, D.; Fausto, A.M.; Texier, I. Characterization of Collagen/Lipid Nanoparticle-Curcumin Cryostructurates for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800446. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing following cesarean section in rats. Food Nutr. Res. 2015, 59, 26411. [Google Scholar] [CrossRef]
- Ge, B.S.; Wang, H.N.; Li, J.; Liu, H.H.; Yin, Y.H.; Zhang, N.L.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, C.H.; Fan, D.D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Rahman, M.S.; Ullah, M.A.; Siddika, A.; Hossain, M.L.; Akhter, M.S.; Hasan, M.Z.; Asaduzzaman, S.M. Amnion and collagen-based blended hydrogel improves burn healing efficacy on a rat skin wound model in the presence of wound dressing biomembrane. Bio-Med. Mater. Eng. 2020, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Ying, H.Y.; Zhou, J.; Wang, M.Y.; Su, D.D.; Ma, Q.Q.; Lv, G.Z.; Chen, J.H. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Song, H.; Li, B. Effect of Collagen Hydrolysates from Silver Carp Skin (Hypophthalmichthys molitrix) on Osteoporosis in Chronologically Aged Mice: Increasing Bone Remodeling. Nutrients 2018, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Guo, J.L.; Zhang, Q.; Li, J.; Llu, Y.S.; Hou, Z.Y.; Chen, W.; Jin, L.; Tian, Y.; Ju, L.L.; Liu, B.; et al. Local application of an ibandronate/collagen sponge improves femoral fracture healing in ovariectomized rats. PLoS ONE 2017, 12, e0187683. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; HosseinKhani, H.; Heirani-Tabasi, A.; Havakhah, S.; Nekooei, S.; Jafarian, A.H.; Rezaie, F.; Peivandi, M.T.; et al. Bone defect healing is induced by collagen sponge/polyglycolic acid. J. Mater. Sci. Mater. Med. 2019, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Nabavi, M.A.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef]
- Calderon-Colon, X.; Xia, Z.Y.; Breidenich, J.L.; Mulreany, D.G.; Guo, Q.Y.; Uy, O.M.; Tiffany, J.E.; Freund, D.E.; McCally, R.L.; Schein, O.D.; et al. Structure and properties of collagen vitrigel membranes for ocular repair and regeneration applications. Biomaterials 2012, 33, 8286–8295. [Google Scholar] [CrossRef]
- Connon, C.J.; Meek, K.M.; Newton, R.H.; Kenney, M.C.; Alba, S.A.; Karageozian, H. Hyaluronidase treatment, collagen fibril packing, and normal transparency in rabbit corneas. J. Refract. Surg. 2000, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G. Crosslinking treatment of progressive keratoconus: New hope. Curr. Opin. Ophthalmol. 2006, 17, 356–360. [Google Scholar] [CrossRef]
- Dias, J.; Diakonis, V.F.; Kankariya, V.P.; Yoo, S.H.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp. Eye Res. 2013, 116, 58–62. [Google Scholar] [CrossRef]
- Li, N.; Peng, X.J.; Fan, Z.J.; Xia, Y.; Wu, T.F. New techniques to improve classical corneal collagen cross-linking treatment. Chin. Med. J. 2014, 127, 1558–1565. [Google Scholar]
- Han, Q.; Sun, W.; Lin, H.; Zhao, W.; Gao, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Hu, W.; Li, Y.; et al. Linear Ordered Collagen Scaffolds Loaded with Collagen-Binding Brain-Derived Neurotrophic Factor Improve the Recovery of Spinal Cord Injury in Rats. Tissue Eng. Part A 2009, 15, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xiao, Z.; Zhang, H.; Chen, B.; Tang, G.; Hou, X.; Ding, W.; Wang, B.; Zhang, P.; Dai, J.; et al. Linear Ordered Collagen Scaffolds Loaded with Collagen-Binding Neurotrophin-3 Promote Axonal Regeneration and Partial Functional Recovery after Complete Spinal Cord Transection. J. Neurotrauma 2010, 27, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.H.; Hsu, H.-P.; Spector, M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012, 33, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.-Y.; Knowles, J.C.; Lee, G.-S.; Kim, J.-W.; Kim, H.-W.; Son, Y.-J.; Hyun, J.K. Effects of phosphate glass fiber-collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012, 8, 1802–1812. [Google Scholar] [CrossRef]
- Altinova, H.; Moellers, S.; Fuehrmann, T.; Deumens, R.; Bozkurt, A.; Heschel, I.; Damink, L.H.H.O.; Schuegner, F.; Weis, J.; Brook, G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014, 1585, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, J. Bridging the gap with functional collagen scaffolds: Tuning endogenous neural stem cells for severe spinal cord injury repair. Biomater. Sci. 2018, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Samadian, H.; Vaez, A.; Ehterami, A.; Salehi, M.; Farzamfar, S.; Sahrapeyma, H.; Norouzi, P. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J. Mater. Sci. Mater. Med. 2019, 30, 107. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 LevelsA Randomized, Double-Blind, Placebo-Controlled Trial. Mar. Drugs 2018, 16, 482. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Coll. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tang, T.; Qi, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liang, C.; Luo, P.; Huang, J.; He, J.; Wang, Z.; Cao, X.; Peng, C.; Wu, S. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res. Ther. 2018, 9, 174. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International Cartilage Repair Society (ICRS) Recommended Guidelines for Histological Endpoints for Cartilage Repair Studies in Animal Models and Clinical Trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Lindert, U.; Gnoli, M.; Maioli, M.; Bedeschi, M.F.; Sangiorgi, L.; Rohrbach, M.; Giunta, C. Insight into the Pathology of a COL1A1 Signal Peptide Heterozygous Mutation Leading to Severe Osteogenesis Imperfecta. Calcif. Tissue Int. 2018, 102, 373–379. [Google Scholar] [CrossRef]
- Tabeta, K.; Du, X.; Arimatsu, K.; Yokoji, M.; Takahashi, N.; Amizuka, N.; Hasegawa, T.; Crozat, K.; Maekawa, T.; Miyauchi, S.; et al. An ENU-induced splice site mutation of mouse Col1a1 causing recessive osteogenesis imperfecta and revealing a novel splicing rescue. Sci. Rep. 2017, 7, 11717. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Valle, D. Cornea, Fundamentals, Diagnosis and Management. Arch. De La Soc. Española De Oftalmol. 2005, 80, 373–374. [Google Scholar]
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional differences between infant and adult human corneal basement membranes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999. [Google Scholar] [CrossRef] [PubMed]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef]
- Danysh, B.P.; Duncan, M.K. The lens capsule. Exp. Eye Res. 2009, 88, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Alavi, M.V.; Labelle-Dumais, C.; Gould, D.B. Type IV Collagens and Basement Membrane Diseases: Cell Biology and Pathogenic Mechanisms. Curr. Top. Membr. 2015, 76, 61–116. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Jerdon, J.A.; Glaser, B.M. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1615–1621. [Google Scholar]
- Sun, J.H.; Huang, M.; Fang, Z.; Li, T.X.; Wu, T.T.; Chen, Y.; Quan, D.P.; Xu, Y.Y.; Wang, Y.M.; Yang, Y.; et al. Nerve bundle formation during the promotion of peripheral nerve regeneration: Collagen VI-neural cell adhesion molecule 1 interaction. Neural Regen. Res. 2022, 17, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Zuccolotto, G.; Nobbio, L.; Colombelli, C.; Filaferro, M.; Vitale, G.; Feltri, M.L.; Bonaldo, P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015, 129, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhou, L.; Zheng, X.; Hu, Y. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phenotype. Eur. J. Neurosci. 2017, 45, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, H.; Wang, S.; Zhao, X.; Li, B. Effects of early enteral nutrition supplemented with collagen peptides on post-burn inflammatory responses in a mouse model. Food Funct. 2017, 8, 1933–1941. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Yoon, H.I.; Choi, Y.C.; Cho, Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012, 28, 973–980. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perälä, M.; Hämäläinen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- John, D.C.; Watson, R.; Kind, A.J.; Scott, A.R.; Kadler, K.E.; Bulleid, N.J. Expression of an engineered form of recombinant procollagen in mouse milk. Nat. Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Toman, P.D.; Pieper, F.; Sakai, N.; Karatzas, C.; Platenburg, E.; de Wit, I.; Samuel, C.; Dekker, A.; Daniels, G.A.; Berg, R.A.; et al. Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res. 1999, 8, 415–427. [Google Scholar] [CrossRef]
- Tomita, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef]
- Adachi, T.; Tomita, M.; Shimizu, K.; Ogawa, S.; Yoshizato, K. Generation of hybrid transgenic silkworms that express Bombyx mori prolyl-hydroxylase alpha-subunits and human collagens in posterior silk glands: Production of cocoons that contained collagens with hydroxylated proline residues. J. Biotechnol. 2006, 126, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef]
- Liao, H.M.; Fang, J.S.; Chen, Y.J.; Wu, K.L.; Lee, K.F.; Chen, C.H. Clinical and molecular characterization of a transmitted reciprocal translocation t(1;12)(p32.1;q21.3) in a family co-segregating with mental retardation, language delay, and microcephaly. BMC Med. Genet. 2011, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Baez, J.; Pappu, K.M.; Glatz, C.E. Purification and characterization of a transgenic corn grain-derived recombinant collagen type I alpha 1. Biotechnol. Prog. 2009, 25, 1660–1668. [Google Scholar] [CrossRef]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Ritala, A.; Suntio, T.; Blumer, S.; Holkeri, H.; Wahlström, E.H.; Baez, J.; Mäkinen, K.; Maria, N.A. Production of a recombinant full-length collagen type I alpha-1 and of a 45-kDa collagen type I alpha-1 fragment in barley seeds. Plant Biotechnol. J. 2009, 7, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Perret, S.; Lacour, T.; Jonval, V.; Hudaverdian, S.; Garrone, R.; Ruggiero, F.; Theisen, M. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett. 2002, 515, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Rezaei, N.; Chan, C.K.; Xu, C.; Panwar, P.; Brömme, D.; Merschrod, S.E.F. Development and characterization of a eukaryotic expression system for human type II procollagen. BMC Biotechnol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Xi, C.; Liu, N.; Liang, F.; Zhao, X.; Long, J.; Yuan, F.; Yun, S.; Sun, Y.; Xi, Y. Molecular assembly of recombinant chicken type II collagen in the yeast Pichia pastoris. Sci. China Life Sci. 2018, 61, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Levin, R.; Landau, S.; Kaduri, M.; Adir, O.; Ianovici, I.; Krinsky, N.; Doppelt-Flikshtain, O.; Shklover, J.; Shainsky-Roitman, J.; et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc. Natl. Acad. Sci. USA 2022, 119, e2207525119. [Google Scholar] [CrossRef]
- Jianjun, Y.; Hua, Z. Progress of Domestic Recombinant Human-Like Collagen Research and Application. J. China Deterg. Cosmet. 2022, 7, 58–62. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I. COLLAGENS: Molecular biology, diseases, and potentials for therapy. In Annual Review of Biochemistry; Richardson, C.C., Ed.; Annual Reviews: San Mateo, CA, USA, 1995; Volume 64, pp. 403–434. [Google Scholar]
- Twyman, R.M.; Stoger, E.; Schillberg, S.; Christou, P.; Fischer, R. Molecular farming in plants: Host systems and expression technology. Trends Biotechnol. 2003, 21, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Gu, T.W.; Xu, Y.; Dad, H.A.; Liu, J.X.; Lian, J.Z.; Huang, L.Q. Gene delivery strategies for therapeutic proteins production in plants: Emerging opportunities and challenges. Biotechnol. Adv. 2022, 54, 107845. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. JDD 2019, 18, 9–16. [Google Scholar]
- Gu, L.S.; Shan, T.T.; Ma, Y.X.; Tay, F.R.; Niu, L.N. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J Cosmet Derm. 2020, 19, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L.; Corbo, A.; Sibilla, S. An Insight into the Changes in Skin Texture and Properties following Dietary Intervention with a Nutricosmeceutical Containing a Blend of Collagen Bioactive Peptides and Antioxidants. Ski. Pharmacol. Physiol. 2017, 30, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the chondroprotective effects of glucosamine and fish collagen peptide on a rabbit ACLT model using serum biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429. [Google Scholar] [CrossRef]
- Li, D.X.; Fan, H.S.; Zhu, X.D.; Tan, Y.F.; Xiao, W.Q.; Lu, J.; Xiao, Y.M.; Chen, J.Y.; Zhang, X.D. Controllable release of salmon-calcitonin in injectable calcium phosphate cement modified by chitosan oligosaccharide and collagen polypeptide. J. Mater. Sci. Mater. Med. 2007, 18, 2225–2231. [Google Scholar] [CrossRef]
- Vardar, E.; Larsson, H.M.; Allazetta, S.; Engelhardt, E.M.; Pinnagoda, K.; Vythilingam, G.; Hubbell, J.A.; Lutolf, M.P.; Frey, P. Microfluidic production of bioactive fibrin micro-beads embedded in crosslinked collagen used as an injectable bulking agent for urinary incontinence treatment. Acta Biomater. 2018, 67, 156–166. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef]
- Ahn, S.; Yoon, H.; Kim, G.; Kim, Y.; Lee, S.; Chun, W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng. Part C Methods 2010, 16, 813–820. [Google Scholar] [CrossRef]
- Vaneerdeweg, W.; Bresseleers, T.; Du Jardin, P.; Lauwers, P.; Pauli, S.; Thyssens, K.; Van Marck, E.; Elseviers, M.; Eyskens, E. Comparison between plain and gentamicin containing collagen sponges in infected peritoneal cavity in rats. Eur. J. Surg. = Acta Chir. 1998, 164, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Stemberger, A.; Grimm, H.; Bader, F.; Rahn, H.D.; Ascherl, R. Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur. J. Surg. Suppl. Acta Chir. Suppl. 1997, 578, 17–26. [Google Scholar]
- Barrientos, I.J.H.; Paladino, E.; Szabo, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Nash, J.R.; Kingsnorth, A.N. Effect of transforming growth factor beta and basic fibroblast growth factor on steroid-impaired healing intestinal wounds. Br. J. Surg. 1992, 79, 69–72. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Parkhurst, M.R.; Parsons-Wingerter, P.; Zhu, W.H. Three-dimensional cell cultures mimic tissues. Ann. N. Y. Acad. Sci. 1992, 665, 259–273. [Google Scholar] [CrossRef]
- Cascone, M.G.; Sim, B.; Downes, S. Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials 1995, 16, 569–574. [Google Scholar] [CrossRef]
- Uchio, Y.; Ochi, M.; Matsusaki, M.; Kurioka, H.; Katsube, K. Human chondrocyte proliferation and matrix synthesis cultured in Atelocollagen gel. J. Biomed. Mater. Res. 2000, 50, 138–143. [Google Scholar] [CrossRef]
- Rubin, A.L.; Stenzel, K.H.; Miyata, T.; White, M.J.; Dunn, M. Collagen as a vehicle for drug delivery. Preliminary report. J. Clin. Pharmacol. 1973, 13, 309–312. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. A Rev. J. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Jin, X.; Liu, W.; Wang, J.; Xiao, Z.; Niu, Y.; Chen, B.; Zhao, Y.; Dai, J. Clinical study of injectable collagen scaffold with autologous fat cells for repair of severe vocal fold injury. Biomed. Mater. 2022, 17, 035004. [Google Scholar] [CrossRef]
- Panayi, A.C.; Haug, V.; Liu, Q.; Wu, M.; Karvar, M.; Aoki, S.; Ma, C.; Hamaguchi, R.; Endo, Y.; Orgill, D.P. Novel application of autologous micrografts in a collagen-glycosaminoglycan scaffold for diabetic wound healing. Biomed. Mater. 2021, 16, 035032. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kadota, K.; Kajihara, M.; Sano, A.; Fujioka, K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J. Control. Release Off. J. Control. Release Soc. 2001, 77, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.J. Biological dressings in burns--a review. Ann. Plast. Surg. 1980, 4, 133–137. [Google Scholar] [CrossRef]
- Gogia, P.P.; Marquez, R.R. Effects of helium-neon laser on wound healing. Ostomy/Wound Manag. 1992, 38, 33, 36, 38–41. [Google Scholar]
- Boyce, S.T. Skin substitutes from cultured cells and collagen-GAG polymers. Med. Biol. Eng. Comput. 1998, 36, 791–800. [Google Scholar] [CrossRef]
- Mi, S.; Connon, C.J. The formation of a tissue-engineered cornea using plastically compressed collagen scaffolds and limbal stem cells. In Corneal Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2013; Volume 1014, pp. 143–155. [Google Scholar] [CrossRef]
- Takezawa, T.; Ozaki, K.; Nitani, A.; Takabayashi, C.; Shimo-Oka, T. Collagen vitrigel: A novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004, 13, 463–473. [Google Scholar] [CrossRef]
- King, V.R.; Alovskaya, A.; Wei, D.Y.T.; Brown, R.A.; Priestley, J.V. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials 2010, 31, 4447–4456. [Google Scholar] [CrossRef]
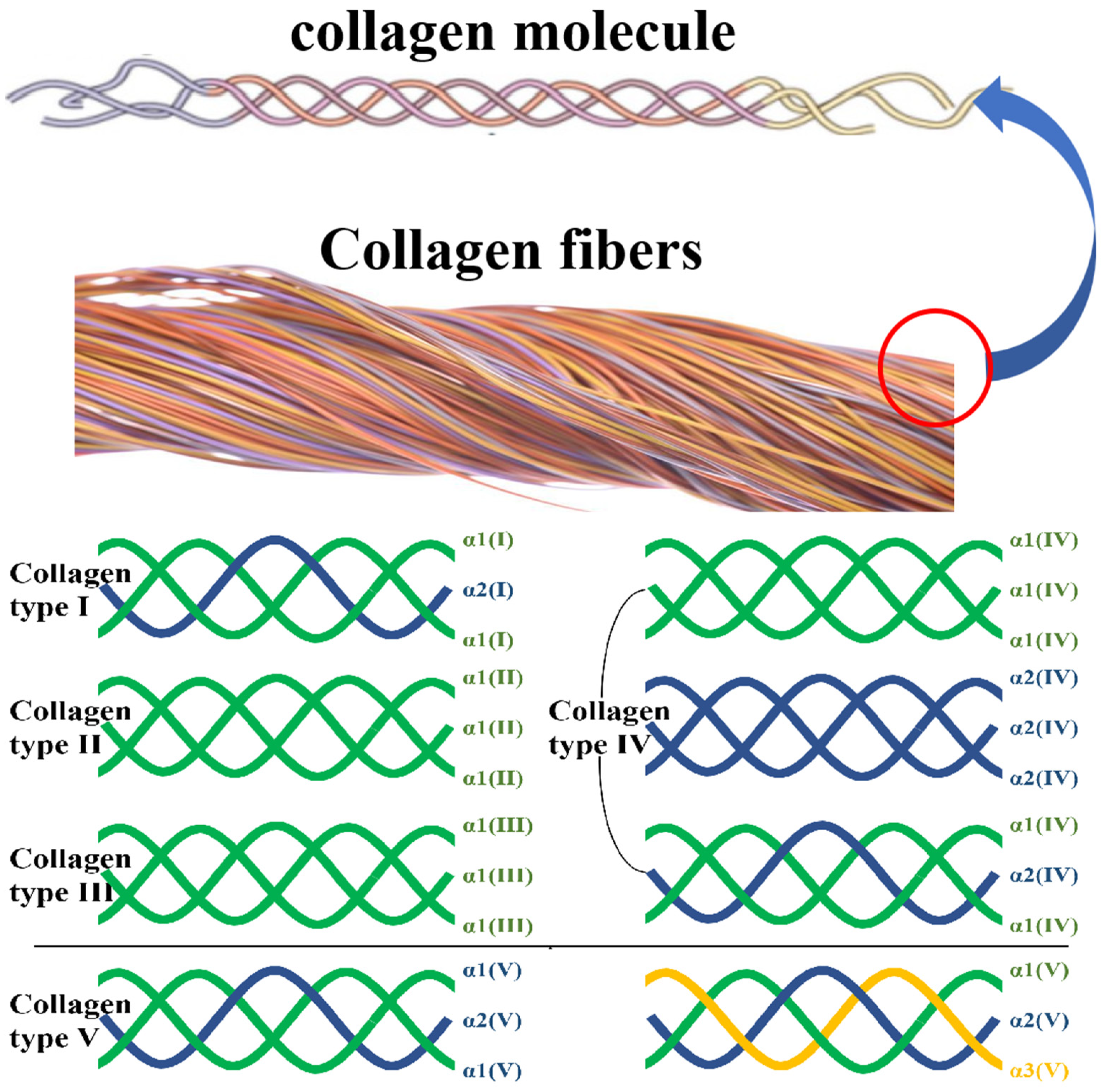
| Types | Subunits and Composition | Composition of Molecular Aggregates | Tissue Distribution | Functions |
|---|---|---|---|---|
| Ⅰ | α1(I) × 2, α2(I) | Large-diameter cross strip fiber | Bone, cornea, skin, tendon, ligament, tumor | Support fiber |
| II | α1(II) × 3 | Small-diameter cross strip fiber | Hyaline cartilage, vitreous body, intervertebral disc | Support fiber |
| III | α1(III) × 3 | Small-diameter cross strip fiber | Skin, blood vessels, muscles, internal organs | Support fiber |
| IV | α1(IV) × 3; α2(IV) × 3; α1(IV) × 2, α2(IV) | Non-fibrous reticular structure | Basement membrane | Reticular scaffold, control of multifunctional cells, site binding |
| V | α1(V) × 2, α2(V); α1(V), α2(V), α3(V) | Small-diameter cross fibers, or forming molecules with type VI chains | Smooth muscle, cultured cells, embryonic tissue, peritoneum, placenta, skin, bone | Small fibers around the supporting cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Liu, Y.-D.; Zhang, Y.; Gu, T.-W.; Peng, L.-H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics 2023, 15, 1443. https://doi.org/10.3390/pharmaceutics15051443
Zhou N, Liu Y-D, Zhang Y, Gu T-W, Peng L-H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics. 2023; 15(5):1443. https://doi.org/10.3390/pharmaceutics15051443
Chicago/Turabian StyleZhou, Nan, Yu-Da Liu, Yue Zhang, Ting-Wei Gu, and Li-Hua Peng. 2023. "Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial" Pharmaceutics 15, no. 5: 1443. https://doi.org/10.3390/pharmaceutics15051443
APA StyleZhou, N., Liu, Y.-D., Zhang, Y., Gu, T.-W., & Peng, L.-H. (2023). Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics, 15(5), 1443. https://doi.org/10.3390/pharmaceutics15051443





