Biodegradable and Sustainable Synthetic Antibodies—A Perspective
Abstract
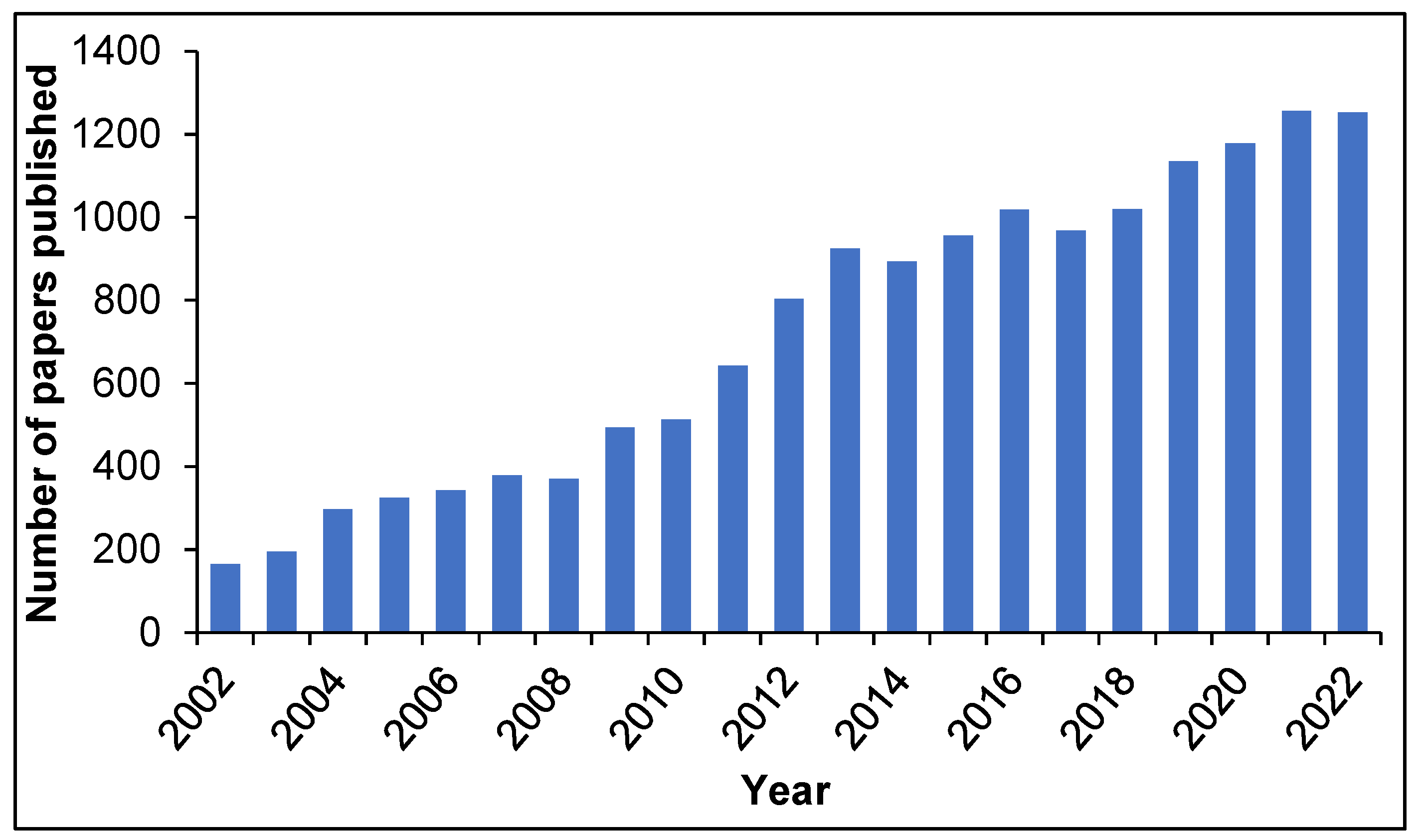
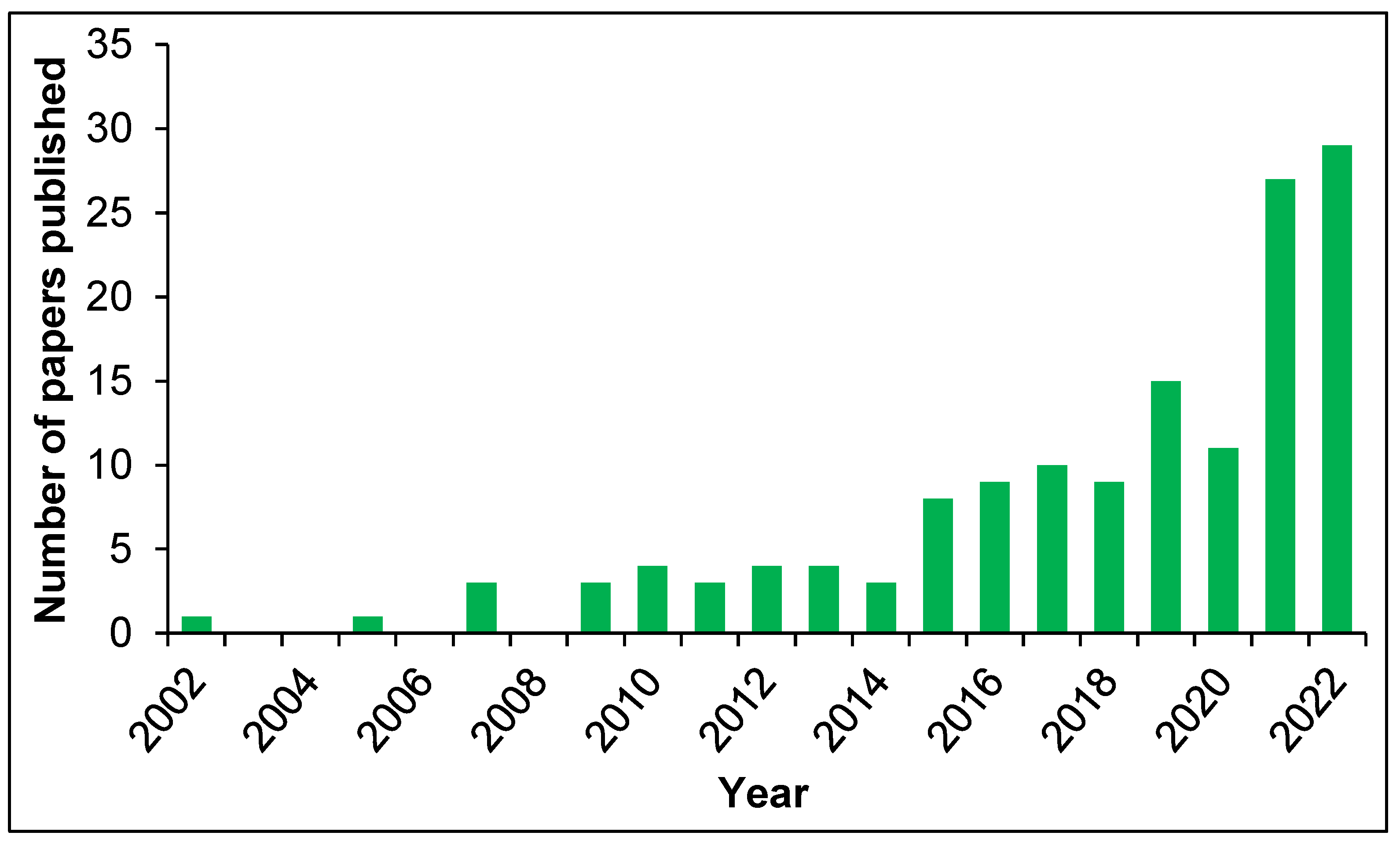
1. Introduction
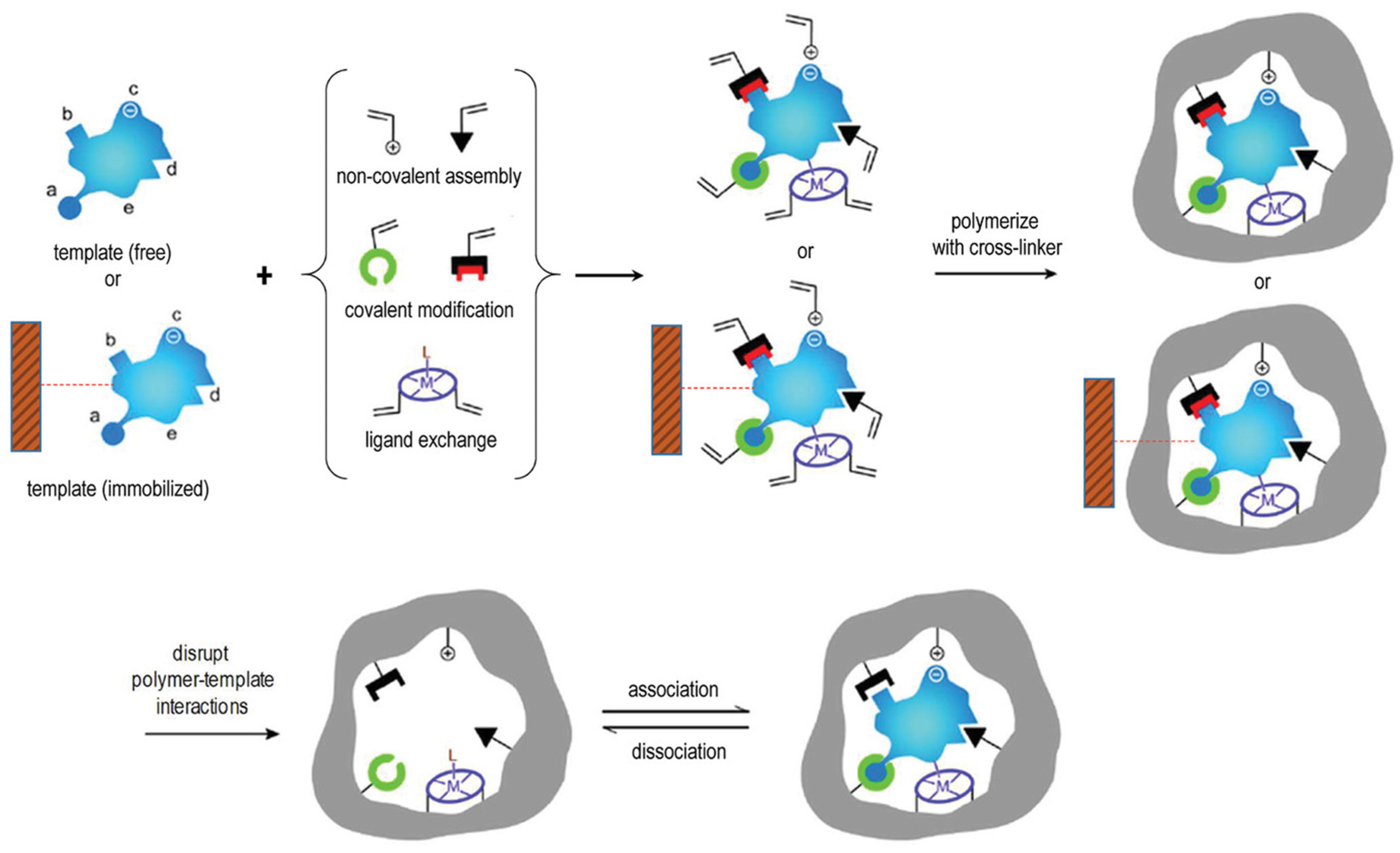
2. MIP NPs Production
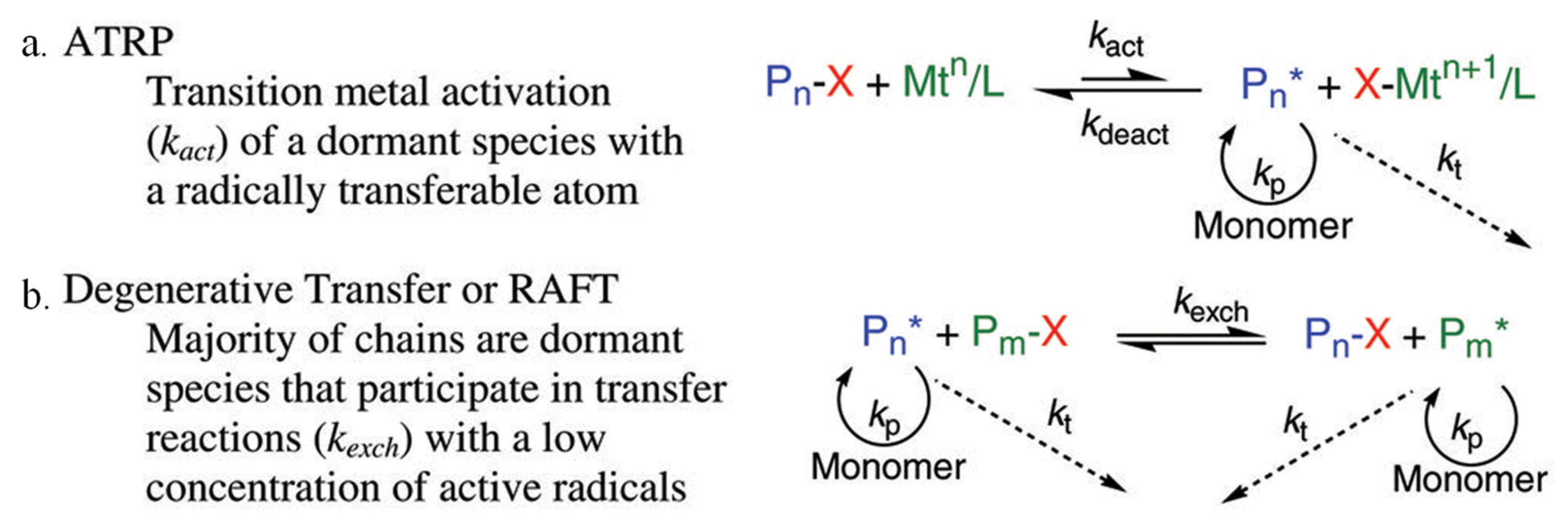
2.1. Polymerization Chemistry
2.2. Manufacturing Methods
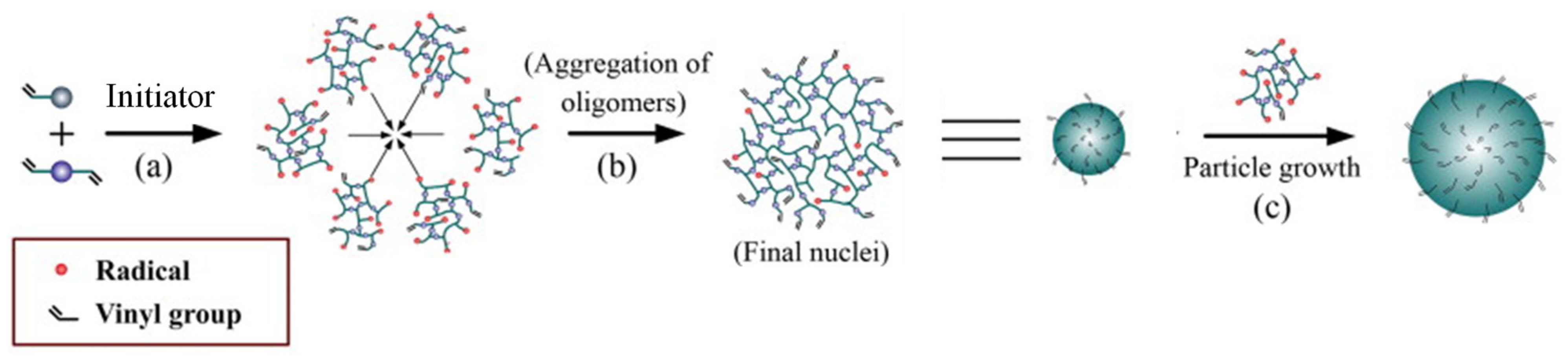
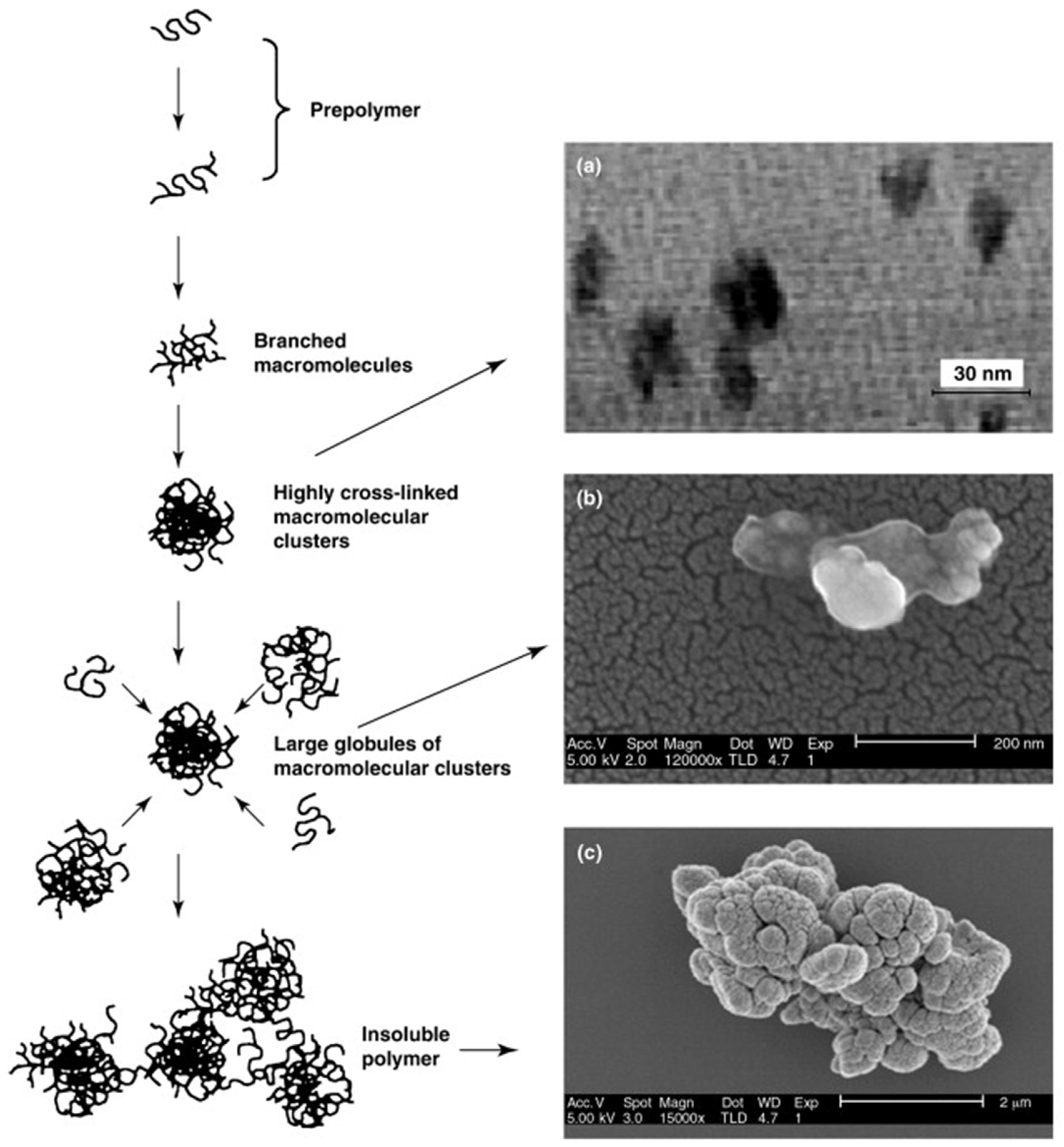
2.2.1. Bulk Polymerization
2.2.2. Precipitation Polymerization
2.2.3. Early-Termination Polymerization
2.2.4. Mini-Emulsion Polymerization
2.2.5. Solid-Phase Synthesis
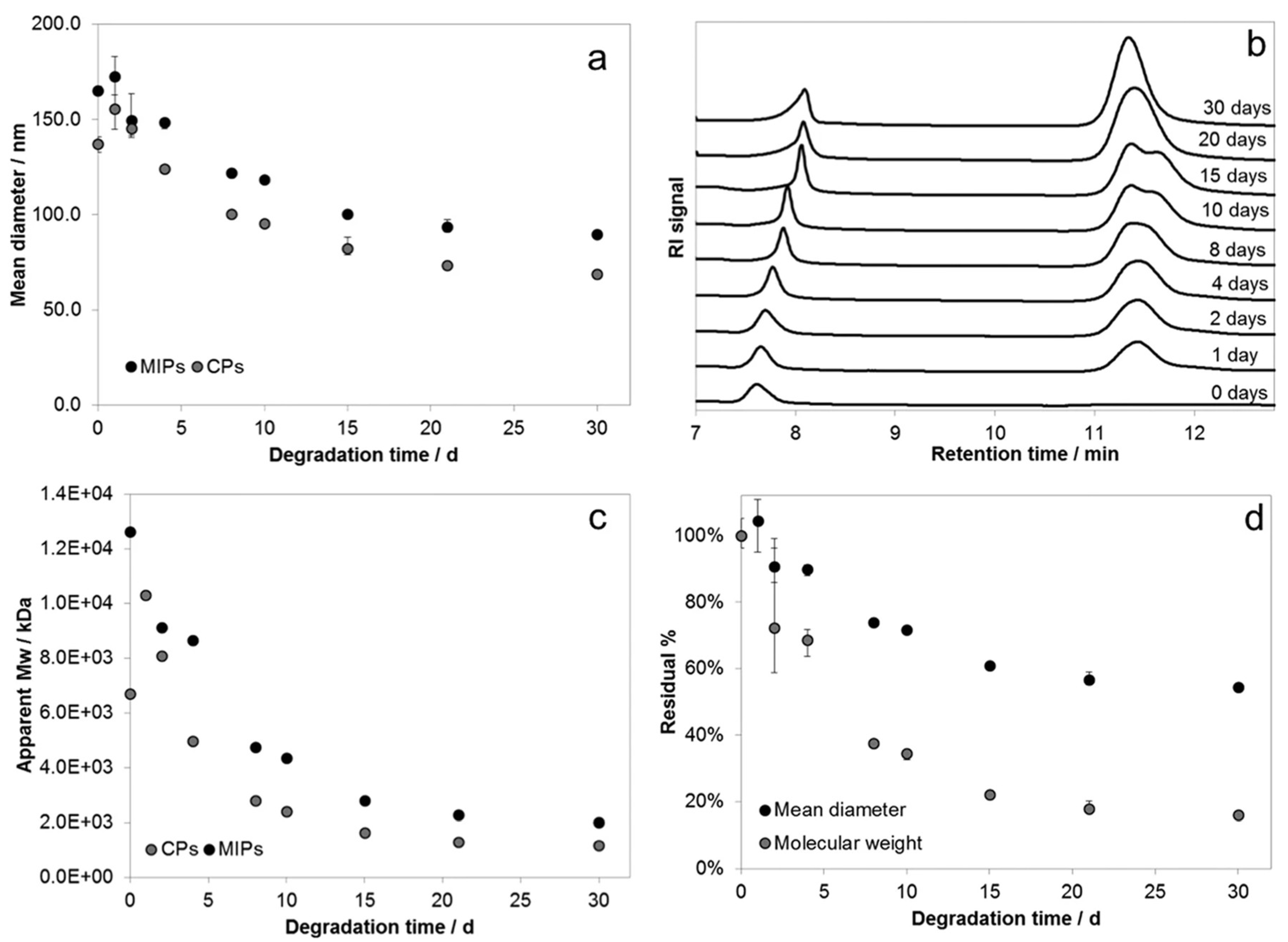
3. Biocompatibility and Biodegradability
4. Waste Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gellman, S.H. Introduction: Molecular Recognition. Chem. Rev. 1997, 97, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arco, L.; Poma, A.; Ruiz-Perez, L.; Scarpa, E.; Ngamkham, K.; Battaglia, G. Molecular bionics–engineering biomaterials at the molecular level using biological principles. Biomaterials 2019, 192, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Kim, D.; Nyberg, S.; Poma, A.; Cecchin, D.; Jain, S.A.; Kim, K.-A.; Shin, Y.-J.; Kim, M.; Baek, S.-H.; et al. LRP-1 functionalized polymersomes enhance the efficacy of carnosine in experimental stroke. Sci. Rep. 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; De Pace, C.; Joseph, A.S.; De Souza, S.C.; Poma, A.; Liatsi-Douvitsa, E.; Contini, C.; De Matteis, V.; Martí, J.S.; Battaglia, G.; et al. Tuning cell behavior with nanoparticle shape. PLoS ONE 2020, 15, e0240197. [Google Scholar] [CrossRef]
- Mercadante, V.; Scarpa, E.; De Matteis, V.; Rizzello, L.; Poma, A. Engineering Polymeric Nanosystems against Oral Diseases. Molecules 2021, 26, 2229. [Google Scholar] [CrossRef]
- Polyakov, M. Adsorption properties and structure of silica gel. Zhur. Fiz. Khim. 1931, 2, 799–805. [Google Scholar]
- Poma, A.; Turner, A.P.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef]
- Poma, A.; Whitcombe, M.; Piletsky, S. Plastic antibodies. In Designing Receptors for the Next Generation of Biosensors; Piletsky, S.A., Whitcombe, M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 105–129. [Google Scholar]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, H.; Knowles, J.C.; Poma, A. Molecularly Imprinted Polymers and Electrospinning: Manufacturing Convergence for Next-Level Applications. Adv. Funct. Mater. 2020, 30, 2001955. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular Imprinting: Green Perspectives and Strategies. Adv. Mater. 2021, 33, e2100543. [Google Scholar] [CrossRef]
- Ostovan, A.; Arabi, M.; Wang, Y.; Li, J.; Li, B.; Wang, X.; Chen, L. Greenificated Molecularly Imprinted Materials for Advanced Applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template–“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Bedwell, T.S.; Anjum, N.; Ma, Y.; Czulak, J.; Poma, A.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. New protocol for optimisation of polymer composition for imprinting of peptides and proteins. RSC Adv. 2019, 9, 27849–27855. [Google Scholar] [CrossRef]
- Liu, R.; Poma, A. Advances in Molecularly Imprinted Polymers as Drug Delivery Systems. Molecules 2021, 26, 3589. [Google Scholar] [CrossRef]
- Marcin, D.; Patrycja, L.; Maciej, C.; Wlodzimierz, K. Nanostructured molecularly imprinted polymers for protein chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. [Google Scholar]
- Zhang, H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef]
- Judith, W.; Romana, S. Applications of Molecularly Imprinted Polymer Nanoparticles and Their Advances toward Industrial Use: A Review. Anal. Chem. 2016, 88, 250–2621. [Google Scholar]
- Xu, S.; Wang, L.; Liu, Z. Molecularly Imprinted Polymer Nanoparticles: An Emerging Versatile Platform for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 60, 3858–3869. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Delahaye, M.; Poma, A.; Du Prez, F.; Battaglia, G. Comparison of metal free polymer–dye conjugation strategies in protic solvents. Polym. Chem. 2016, 7, 3046–3055. [Google Scholar] [CrossRef]
- Poma, A.; Pei, Y.; Ruiz-Perez, L.; Rizzello, L.; Battaglia, G. Polymersomes: Synthesis and applications. In Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2018; pp. 1–43. [Google Scholar]
- Adali-Kaya, Z.; Bui, B.T.S.; Falcimaigne-Cordin, A.; Haupt, K. Molecularly Imprinted Polymer Nanomaterials and Nanocomposites: Atom-Transfer Radical Polymerization with Acidic Monomers. Angew. Chem. Int. Ed. 2015, 54, 5192–5195. [Google Scholar] [CrossRef]
- Martínez, I.V.; Ek, J.I.; Ahn, E.C.; Sustaita, A.O. Molecularly imprinted polymers via reversible addition–fragmentation chain-transfer synthesis in sensing and environmental applications. RSC Adv. 2022, 12, 9186–9201. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Materialstoday 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Pierre, A.C. From random glass networks to random silica gel networks and their use as host for biocatalytic applications. J. Sol-Gel Sci. Technol. 2018, 90, 172–186. [Google Scholar] [CrossRef]
- Mujahid, A.; Khan, A.I.; Afzal, A.; Hussain, T.; Raza, M.H.; Shah, A.T.; Zaman, W.U. Molecularly imprinted titania nanoparticles for selective recognition and assay of uric acid. Appl. Nanosci. 2015, 5, 527–534. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Michel, A.; Briot, E.; Siaugue, J.M.; Ménager, C.; Wilhelm, C.; Griffete, N. Controlled drug delivery for cancer cell treatment via magnetic doxorubicin imprinted silica nanoparticles. Chem. Commun. 2020, 56, 10255–10258. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Croissant, J.G.; Brinker, C.J. Chapter eight-biodegradable silica-based nanoparticles: Dissolution kinetics and selective bond cleavage. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 181–214. [Google Scholar]
- Vareda, J.P.; García-González, C.A.; Valente, A.J.M.; Simón-Vázquez, R.; Stipetic, M.; Durães, L. Insights on toxicity, safe handling and disposal of silica aerogels and amorphous nanoparticles. Environ. Sci. Nano 2021, 8, 1177–1195. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carrillo, R. The Generally Recognized as Safe (GRAS) Process for Industrial Microbial Enzymes. Ind. Biotechnol. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Wang, Y.; Mei, R.; Fu, L.; Li, J.; Wang, X.; Chen, L. Chiral molecular imprinting-based SERS detection strategy for absolute enantiomeric discrimination. Nat. Commun. 2022, 13, 5757. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ostovan, A.; Zhang, Z.; Wang, Y.; Mei, R.; Fu, L.; Wang, X.; Ma, J.; Chen, L. Label-free SERS detection of Raman-Inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens. Bioelectron. 2020, 174, 112825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Ma, C.; Wang, Y.; Mu, S.; Liu, X.; Zhang, X.; Zhang, H. Molecularly imprinted gelatin nanoparticles for DNA delivery and in-situ fluorescence imaging of telomerase activity. Microchim. Acta 2019, 186, 610. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Guan, G.; Wang, X.; Lou, T. Electrospun molecularly imprinted sodium alginate/polyethylene oxide nanofibrous membranes for selective adsorption of methylene blue. Int. J. Biol. Macromol. 2022, 207, 62–71. [Google Scholar] [CrossRef]
- Zhu, Y.; Poma, A.; Rizzello, L.; Gouveia, V.M.; Ruiz-Perez, L.; Battaglia, G.; Williams, C.K. Metabolically Active, Fully Hydrolysable Polymersomes. Angew. Chem. Int. Ed. 2019, 58, 4581–4586. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Bertero, A.; Bifone, A. Molecularly Imprinted Biodegradable Nanoparticles. Sci. Rep. 2017, 7, 40046. [Google Scholar] [CrossRef]
- Yoosefi, S.; Esfandyari-Manesh, M.; Ghorbani-Bidkorpeh, F.; Ahmadi, M.; Moraffah, F.; Dinarvand, R. Novel biodegradable molecularly imprinted polymer nanoparticles for drug delivery of methotrexate anti-cancer; synthesis, characterization and cellular studies. DARU J. Pharm. Sci. 2022, 30, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Endo, T. General Mechanisms in ring-opening polymerization. In Handbook of Ring-Opening Polymerization; Wiley: New York, NY, USA, 2009; pp. 53–63. [Google Scholar]
- Harrier, D.D.; Guironnet, D. Design rules for performing water-sensitive ring-opening polymerizations in an aqueous dispersion. Polym. Chem. 2022, 13, 2459–2468. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Z.-Y.; Xiong, W.; Wang, D.; Zhao, R.; Xie, Y.; Song, Y.-Q.; Zhu, J.; Lu, H. Water-assisted and protein-initiated fast and controlled ring-opening polymerization of proline N-carboxyanhydride. Natl. Sci. Rev. 2022, 9, nwac033. [Google Scholar] [CrossRef]
- Yuan, P.; Sun, Y.; Xu, X.; Luo, Y.; Hong, M. Towards high-performance sustainable polymers via isomerization-driven irreversible ring-opening polymerization of five-membered thionolactones. Nat. Chem. 2022, 14, 294–303. [Google Scholar] [CrossRef]
- Abbasi, S.; Haeri, S.A.; Naghipour, A.; Sajjadifar, S. Enrichment of cardiovascular drugs using rhamnolipid bioaggregates after dispersive solid phase extraction based water compatible magnetic molecularly imprinted biopolymers. Microchem. J. 2020, 157, 104874. [Google Scholar] [CrossRef]
- Dhanashree, S.; Priyanka, M.; Manisha, K.; Vilasrao, K. Molecularly Imprinted Polymers: Novel Discovery for Drug Delivery. Curr. Drug Deliv. 2016, 13, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Rizk, M.S.; El-Beshlawy, M.M. Molecularly-imprinted polymer-base bulk optode for the determination of ivabradine hydrochloride in Procoralan®. RSC Adv. 2022, 12, 17645–17654. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Rong, F.; Yuan, C. Morphologies and binding characteristics of molecularly imprinted polymers prepared by precipitation polymerization. Polym. Int. 2003, 52, 1799–1806. [Google Scholar] [CrossRef]
- Curcio, P.; Zandanel, C.; Wagner, A.; Mioskowski, C.; Baati, R. Semi-Covalent Surface Molecular Imprinting of Polymers by One-Stage Mini-emulsion Polymerization: Glucopyranoside as a Model Analyte. Macromol. Biosci. 2009, 9, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Matsuda, K.; Nakazawa, T.; Mori, S.; Yoshida, M.; Shimizu, R.; Tatsumi, H.; Jin, J. Synthesis and characterization of molecularly imprinted polymers for detection of the local anesthetic lidocaine in urine. Sep. Sci. Plus 2022, 6, 2200081. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Piletska, E.V.; Sergeyeva, T.A.; Nicholls, I.A.; Weston, D.; Turner, A.P.F. Synthesis of biologically active molecules by imprinting polymerisation. Biopolym. Cell 2006, 22, 63–67. [Google Scholar] [CrossRef]
- Zhang, H. Controlled/“living” radical precipitation polymerization: A versatile polymerization technique for advanced functional polymers. Eur. Polym. J. 2013, 49, 579–600. [Google Scholar] [CrossRef]
- Yang, K.; Berg, M.M.; Zhao, C.; Ye, L. One-Pot Synthesis of Hydrophilic Molecularly Imprinted Nanoparticles. Macromolecules 2009, 42, 8739–8746. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yang, P.; Jin, S.; Yu, M.; Guo, J.; Wang, C. Fabrication of polymeric microgels using reflux-precipitation polymerization and its application for phosphoprotein enrichment. J. Mater. Chem. B 2013, 2, 2575–2582. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Reimhult, K.; Krozer, A.; Mosbach, K.; Sode, K.; Ye, L. Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal. Chim. Acta 2007, 584, 112–121. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kodama, T.; Okahata, Y.; Shea, K.J. Peptide Imprinted Polymer Nanoparticles: A Plastic Antibody. J. Am. Chem. Soc. 2008, 130, 15242–15243. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, P.; Attieh, M.D.; Gonzato, C.; Haupt, K. A New Versatile Water-Soluble Iniferter Platform for the Preparation of Molecularly Imprinted Nanoparticles by Photopolymerisation in Aqueous Media. Chem. A Eur. J. 2016, 22, 10150–10154. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ye, L.; Haupt, K.; Mosbach, K. Formation of a class of enzyme inhibitors (drugs), including a chiral compound, by using imprinted polymers or biomolecules as molecular-scale reaction vessels. Angew. Chem. Int. Ed. 2002, 41, 4459–4463. [Google Scholar] [CrossRef]
- Attieh, M.D.; Zhao, Y.; Elkak, A.; Falcimaigne-Cordin, A.; Haupt, K. Enzyme-Initiated Free-Radical Polymerization of Molecularly Imprinted Polymer Nanogels on a Solid Phase with an Immobilized Radical Source. Angew. Chem. Int. Ed. 2017, 56, 3339–3343. [Google Scholar] [CrossRef] [PubMed]
- Cutivet, A.; Schembri, C.; Kovensky, J.; Haupt, K. Molecularly Imprinted Microgels as Enzyme Inhibitors. J. Am. Chem. Soc. 2009, 131, 14699–14702. [Google Scholar] [CrossRef]
- Hoshino, Y.; Koide, H.; Furuya, K.; Haberaecker, W.W.; Lee, S.-H.; Kodama, T.; Kanazawa, H.; Oku, N.; Shea, K.J. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. Proc. Natl. Acad. Sci. USA 2011, 109, 33–38. [Google Scholar] [CrossRef]
- Wulff, G.; Chong, B.-O.; Kolb, U. Soluble Single-Molecule Nanogels of Controlled Structure as a Matrix for Efficient Artificial Enzymes. Angew. Chem. Int. Ed. 2006, 45, 2955–2958. [Google Scholar] [CrossRef]
- Wulff, G.; Liu, J. Design of Biomimetic Catalysts by Molecular Imprinting in Synthetic Polymers: The Role of Transition State Stabilization. Acc. Chem. Res. 2012, 45, 239–247. [Google Scholar] [CrossRef]
- Guerreiro, A.R.; Chianella, I.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. Selection of imprinted nanoparticles by affinity chromatography. Biosens. Bioelectron. 2009, 24, 2740–2743. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, J.; Zhang, Z.; Pan, X.; Zhang, W.; Cheng, Z.; Zhu, X. New selenium-based iniferter agent for living free radical polymerization of styrene under UV irradiation. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2211–2218. [Google Scholar] [CrossRef]
- Otsu, T. Iniferter concept and living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2121–2136. [Google Scholar] [CrossRef]
- Lehnen, A.-C.; Kurki, J.A.M.; Hartlieb, M. The difference between photo-iniferter and conventional RAFT polymerization: High livingness enables the straightforward synthesis of multiblock copolymers. Polym. Chem. 2022, 13, 1537–1546. [Google Scholar] [CrossRef]
- Van Herk, A.M.; Monteiro, M. Heterogeneous systems. In Handbook of Radical Polymerization; Matyjaszewski, K., Davis, T.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 301–331. [Google Scholar] [CrossRef]
- Vaihinger, D.; Landfester, K.; Kräuter, I.; Brunner, H.; Tovar, G.E.M. Molecularly imprinted polymer nanospheres as synthetic affinity receptors obtained by miniemulsion polymerisation. Macromol. Chem. Phys. 2002, 203, 1965–1973. [Google Scholar] [CrossRef]
- Decompte, E.; Lobaz, V.; Monperrus, M.; Deniau, E.; Save, M. Molecularly Imprinted Polymer Colloids Synthesized by Miniemulsion Polymerization for Recognition and Separation of Nonylphenol. ACS Appl. Polym. Mater. 2020, 2, 3543–3556. [Google Scholar] [CrossRef]
- Priego-Capote, F.; Ye, L.; Shakil, S.; Shamsi, S.A.; Nilsson, S. Monoclonal Behavior of Molecularly Imprinted Polymer Nanoparticles in Capillary Electrochromatography. Anal. Chem. 2008, 80, 2881–2887. [Google Scholar] [CrossRef]
- Zeng, Z.; Hoshino, Y.; Rodriguez, A.; Yoo, H.; Shea, K.J. Synthetic Polymer Nanoparticles with Antibody-like Affinity for a Hydrophilic Peptide. ACS Nano 2010, 4, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.J.; Tong, Y.W. The Effect of Protein Structural Conformation on Nanoparticle Molecular Imprinting of Ribonuclease A Using Miniemulsion Polymerization. Langmuir 2007, 23, 2722–2730. [Google Scholar] [CrossRef]
- Shen, X.; Ye, L. Interfacial Molecular Imprinting in Nanoparticle-Stabilized Emulsions. Macromolecules 2011, 44, 5631–5637. [Google Scholar] [CrossRef]
- Ayari, M.G.; Kadhirvel, P.; Favetta, P.; Plano, B.; Dejous, C.; Carbonnier, B.; Agrofoglio, L.A. Synthesis of imprinted hydrogel microbeads by inverse Pickering emulsion to controlled release of adenosine 5′-monophosphate. Mater. Sci. Eng. C 2019, 101, 254–263. [Google Scholar] [CrossRef]
- Ou, H.; Chen, Q.; Pan, J.; Zhang, Y.; Huang, Y.; Qi, X. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef]
- Mahajan, R.; Rouhi, M.; Shinde, S.; Bedwell, T.; Incel, A.; Mavliutova, L.; Piletsky, S.; Nicholls, I.A.; Sellergren, B.K.-E. Highly Efficient Synthesis and Assay of Protein-Imprinted Nanogels by Using Magnetic Templates. Angew. Chem. Int. Ed. 2019, 58, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Guerreiro, A.; Caygill, S.; Moczko, E.; Piletsky, S. Automatic reactor for solid-phase synthesis of molecularly imprinted polymeric nanoparticles (MIP NPs) in water. RSC Adv. 2013, 4, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Granek, E.F.; Brander, S.M.; Holland, E.B. Microplastics in aquatic organisms: Improving understanding and identifying research directions for the next decade. Limnol. Oceanogr. Lett. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Qin, Y.-T.; Feng, Y.-S.; Ma, Y.-J.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Tumor-Sensitive Biodegradable Nanoparticles of Molecularly Imprinted Polymer-Stabilized Fluorescent Zeolitic Imidazolate Framework-8 for Targeted Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 24585–24598. [Google Scholar] [CrossRef]
- Liu, T.; Qiao, Z.; Wang, J.; Zhang, P.; Zhang, Z.; Guo, D.-S.; Yang, X. Molecular imprinted S-nitrosothiols nanoparticles for nitric oxide control release as cancer target chemotherapy. Colloids Surfaces B Biointerfaces 2019, 173, 356–365. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Zhang, Y.; Ma, Z.-B.; Xiao, N.-N.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Preparation of Dual-Template Epitope Imprinted Polymers for Targeted Fluorescence Imaging and Targeted Drug Delivery to Pancreatic Cancer BxPC-3 Cells. ACS Appl. Mater. Interfaces 2019, 11, 32431–32440. [Google Scholar] [CrossRef]
- Qin, Y.-T.; Peng, H.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Highly Effective Drug Delivery and Cell Imaging Using Fluorescent Double-Imprinted Nanoparticles by Targeting Recognition of the Epitope of Membrane Protein. Anal. Chem. 2019, 91, 12696–12703. [Google Scholar] [CrossRef]
- Boitard, C.; Curcio, A.; Rollet, A.-L.; Wilhelm, C.; Ménager, C.; Griffete, N. Biological Fate of Magnetic Protein-Specific Molecularly Imprinted Polymers: Toxicity and Degradation. ACS Appl. Mater. Interfaces 2019, 11, 35556–35565. [Google Scholar] [CrossRef]
- Kassem, S.; Piletsky, S.S.; Yesilkaya, H.; Gazioglu, O.; Habtom, M.; Canfarotta, F.; Piletska, E.; Spivey, A.C.; Aboagye, E.O.; Piletsky, S.A. Assessing the In Vivo Biocompatibility of Molecularly Imprinted Polymer Nanoparticles. Polymers 2022, 14, 4582. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Bărăian, A.-I.; Iacob, B.-C.; Bodoki, A.E.; Bodoki, E. In Vivo Applications of Molecularly Imprinted Polymers for Drug Delivery: A Pharmaceutical Perspective. Int. J. Mol. Sci. 2022, 23, 14071. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Bhakta, S.; Reza, K.K.; Kaushik, A. Exploring molecularly imprinted polymers as artificial antibodies for efficient diagnostics and commercialization: A critical overview. Hybrid Adv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Chianella, I.; Karim, K.; Piletska, E.V.; Preston, C.; Piletsky, S.A. Computational design and synthesis of molecularly imprinted polymers with high binding capacity for pharmaceutical applications-model case: Adsorbent for abacavir. Anal. Chim. Acta 2006, 559, 73–78. [Google Scholar] [CrossRef]
- Bates, F.; Busato, M.; Piletska, E.; Whitcombe, M.J.; Karim, K.; Guerreiro, A.; del Valle, M.; Giorgetti, A.; Piletsky, S. Computational design of molecularly imprinted polymer for direct detection of melamine in milk. Sep. Sci. Technol. 2017, 52, 1441–1453. [Google Scholar] [CrossRef]
- Busato, M.; Distefano, R.; Bates, F.; Karim, K.; Bossi, A.M.; Vilariño, J.M.L.; Piletsky, S.; Bombieri, N.; Giorgetti, A. MIRATE: MIps RATional dEsign Science Gateway. J. Integr. Bioinform. 2018, 15, 20170075. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Knowles, J.C.; Poma, A. Biodegradable and Sustainable Synthetic Antibodies—A Perspective. Pharmaceutics 2023, 15, 1440. https://doi.org/10.3390/pharmaceutics15051440
Ma X, Knowles JC, Poma A. Biodegradable and Sustainable Synthetic Antibodies—A Perspective. Pharmaceutics. 2023; 15(5):1440. https://doi.org/10.3390/pharmaceutics15051440
Chicago/Turabian StyleMa, Xiaohan, Jonathan C. Knowles, and Alessandro Poma. 2023. "Biodegradable and Sustainable Synthetic Antibodies—A Perspective" Pharmaceutics 15, no. 5: 1440. https://doi.org/10.3390/pharmaceutics15051440
APA StyleMa, X., Knowles, J. C., & Poma, A. (2023). Biodegradable and Sustainable Synthetic Antibodies—A Perspective. Pharmaceutics, 15(5), 1440. https://doi.org/10.3390/pharmaceutics15051440






