1. Introduction
Age-related macular degeneration (AMD) is a common disease that may cause blindness in people over the age of 50 [
1]. The incidence of this disease has been increasing due to the high percentage of middle-aged and older adults among the population, and it affects about 50 million people around the world [
2]. The pathophysiology of AMD is not fully known. In the early stages of the disease, the accumulation of granules called drusen, which consist of a mixture of lipids, proteins, and minerals including cholesterol, phospholipids, triglycerides, complement factors, immunoglobulins, apolipoproteins, calcium, zinc, iron, glycosaminoglycans, extracellular matrix proteins, and inflammatory molecules, is observed in the Bruch’s membrane, which is considered residue from retinal pigment epithelium (RPE) cells. Since these accumulations rarely affect the photoreceptor layer of the retina, they usually do not cause any symptoms. However, the accumulation of drusen thickens the Bruch’s membrane, which causes ischemia in RPE cells. Ischemia instigates increased vascular endothelial growth factor (VEGF) secretion from the basal parts of RPE cells, and increased VEGF secretion triggers pathological angiogenesis in the choroid layer. Leakage from the new vessels in the choroid layer causes damage at the cellular level, and vision loss occurs [
3,
4]. Many studies have shown that choroid angiogenesis regresses with anti-VEGF agents [
3]. Ranibizumab is the first anti-VEGF antibody approved for use in ophthalmology by the American Food and Drug Administration in 2006 [
5]. Today, anti-VEGF antibodies are used in many eye diseases (diabetic retinopathy, premature retinopathy, etc.) in the pathophysiology of which VEGF plays a role [
6,
7]. Ranibizumab is a humanized monoclonal antibody fragment created using standard recombinant DNA technology and designed to fight against VEGF [
5]. This fragment is one-third the size of the antibody and can easily pass through all layers of the retina when injected into the eye, and it binds to VEGF with high affinity [
5]. Clinical studies have shown that the intravitreal use of ranibizumab is very effective and reliable in treating age-related macular degeneration [
5,
8]. The clinical protocol for using the drug is three doses of intravitreal injections that are one month apart [
9,
10]. The continuation of monthly injections after 3 months is decided according to the patient’s clinical, angiographic, and optical coherence tomography (OCT) findings. However, intravitreal drugs cannot stay in the vitreous for a long time due to the natural physiology of the eye, and they are eliminated quickly [
6]. The available literature suggests that ranibizumab is eliminated from the body at a relatively slow rate, with a half-life ranging from 2.88 to 10.3 days, depending on the specific compartment measured. In addition, recurrent injections at short intervals may cause severe intraocular infections, especially endophthalmitis, and complications that can reduce the level of vision, such as retinal detachment, cataracts, and intraocular hemorrhage [
3,
4].
Currently, there are four different drug delivery techniques used for the posterior segment of the eye: topical, periocular, intraocular, and systemic [
11]. In topical drug applications, sufficient drug concentrations could not be attained since the drug cannot freely pass into the intraocular tissues [
12]. Similarly, in systemic applications, due to the existing blood–retinal barrier, there is not sufficient drug transition, and systemic side effects occur when the drug dose is increased. Intraocular (intravitreal) injection stands out among these drug administration techniques, since the drug is applied at the desired concentration, and treatment can be performed effectively; however, this method may cause various complications. Several studies have been carried out in recent years on the development of enhanced drug delivery systems using microspheres [
13,
14,
15], polymeric materials [
16,
17,
18], micelles [
19,
20,
21,
22], liposome systems [
23,
24], and hydrogels [
24,
25,
26] in order to achieve the most effective concentration and the desired duration with the least invasive intervention. These systems aim to increase the half-life of ophthalmic drugs by preventing them from being easily removed. Delivery systems are able to maintain the drug concentration at a constant level and prevent the side effects that accompany the drug being administered with a high-dose single injection [
27]. The drug’s molecular weight and lipophilic properties affect its half-life in the eye. Small-molecule drugs can be removed more easily from the vitreous part of the eye than larger ones. Therefore, when small molecular-weight anti-angiogenic drugs such as pegaptanib were coated with polyethylene glycol material, their intraocular half-life was prolonged [
28]. Although such modifications increase the drug’s half-life, the length of the duration achieved through this method is insufficient considering the treatment duration of the disease. Therefore, intraocular transport and release systems that provide a longer drug half-life are needed. Non-degradable implants, which allow the drug to be released for an extended period, consist of a core section encapsulating the drug, a permeable membrane surrounding the drug (e.g., polyvinyl alcohol), and a semi-permeable membrane surrounding the whole system (e.g., silicone), and they are capable of constant drug release for at least one year [
28]. Moreover, recently, an ocular implant (Susvimo) from Genentech acquired approval from the FDA, and it offers two treatments per year [
29].
Biodegradable release systems can provide a more modular design with different shapes and sizes using different biocompatible materials that decompose into non-toxic substances when broken down by the body. In these systems, the drug release regime depends on the type of the polymer used, the morphological state in which it is injected, the amount of drug loaded, and the pore size of the system. Ozurdex
TM (formerly Posurdex
TM; Allergan, Inc., Irvine, CA, USA), a carrier system used to treat retinal vascular blockages, has been approved by the American Food and Drug Administration and enables the long-term release of dexamethasone from polylactic glycolic acid [
27]. Micro/nano-spheres for drug encapsulation have also been investigated as an alternative treatment method. In these systems, homogeneous distribution of the drug is achieved inside the microcapsules. Polylactic acid microspheres have been shown to enable a constant release for 1.5 months in a rabbit eye model [
15]. Despite being injectable, these systems cause short-term temporary vision impairment following injection. Vision impairment passes when microspheres collapse into the lower part of the vitreous over time.
Although drug release systems ensure that the drug is protected from intraocular enzymes and released for an extended duration inside the eye, some transport systems have disadvantages such as loss of the structure and function of the drug when integrated with the carrier [
30]. For antiangiogenic drugs that are made of proteins, integration into a carrier material is especially critical, since the protein structure is vital for its function and integration should not disrupt its structure.
Due to their low toxicity, excellent biocompatibility, and capacity to target small therapeutic drug molecules and proteins, supramolecular self-assembled peptide amphiphile nanostructures are a promising alternative to conventional delivery vehicles. Self-assembled peptide amphiphile molecules are a bottom-up way of creating scalable nanosized delivery systems. Molecular self-assembly is a viable method for spontaneously organizing molecules into ordered structures using free-energy processes such as van der Waals, electrostatic, hydrogen bonding, and π-π stacking interactions [
31]. Peptide hydrogels also offer advantageous mechanical features, such as shear-thinning for easy distribution and extended-release patterns to maximize drug uptake while reducing overall drug dose. When employed to deliver therapeutics to the brain [
32], eye [
33,
34], cardiovascular system [
35], bone tissue [
36], or cancer cells [
37,
38], self-assembled peptide nanostructures have exhibited promising results. Several studies have demonstrated the efficacy of PA-based delivery systems for anti-VEGF therapy in animal models of ocular diseases. For example, one study reported on the use of a PA-based hydrogel to deliver anti-VEGF proteins to the retina of rats with laser-induced choroidal neovascularization. The hydrogel was able to sustain the release of the anti-VEGF protein for up to 21 days and significantly reduced the size of the neovascular lesions compared to control groups. Another study reported the use of a PA-based nanofiber system to deliver anti-VEGF proteins to the vitreous of rabbits with experimental retinal neovascularization. The nanofiber system was able to sustain the release of the anti-VEGF protein for up to 28 days and significantly reduced the area of neovascularization compared to control groups [
11].
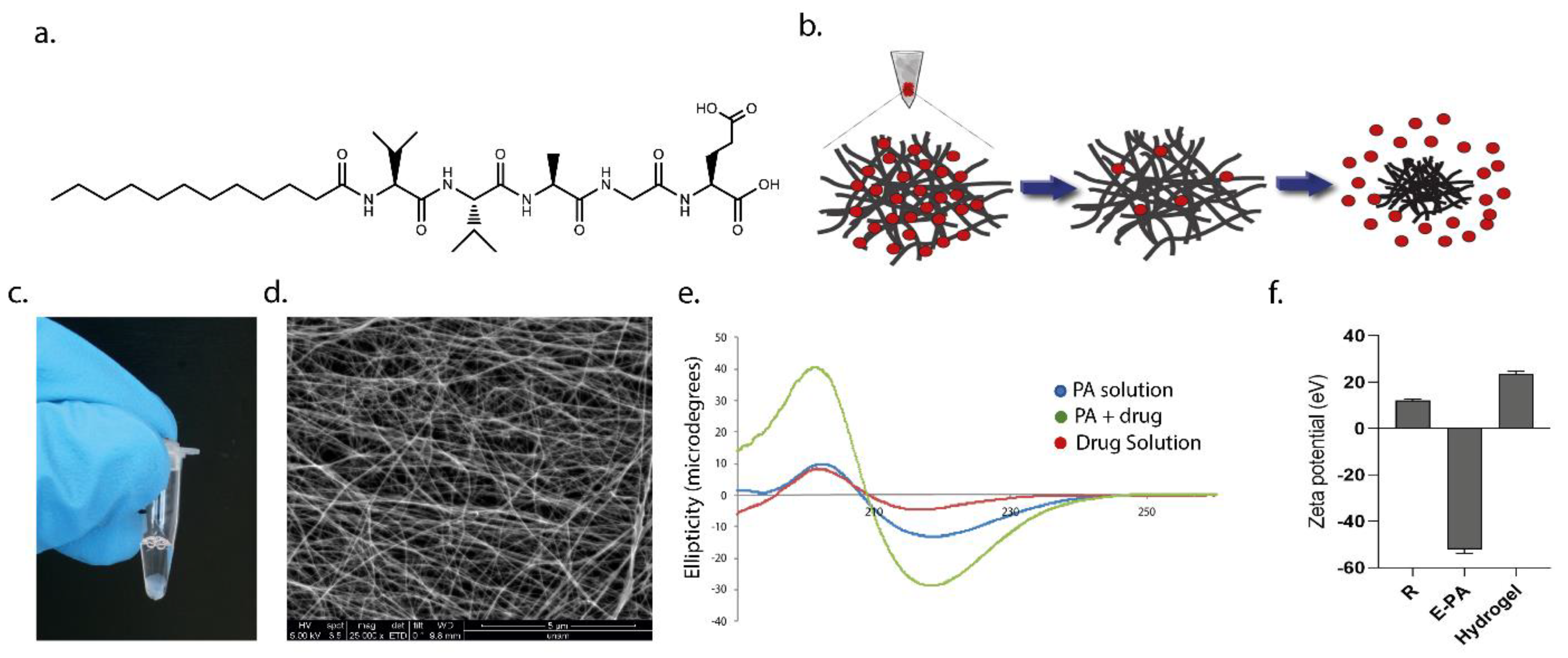
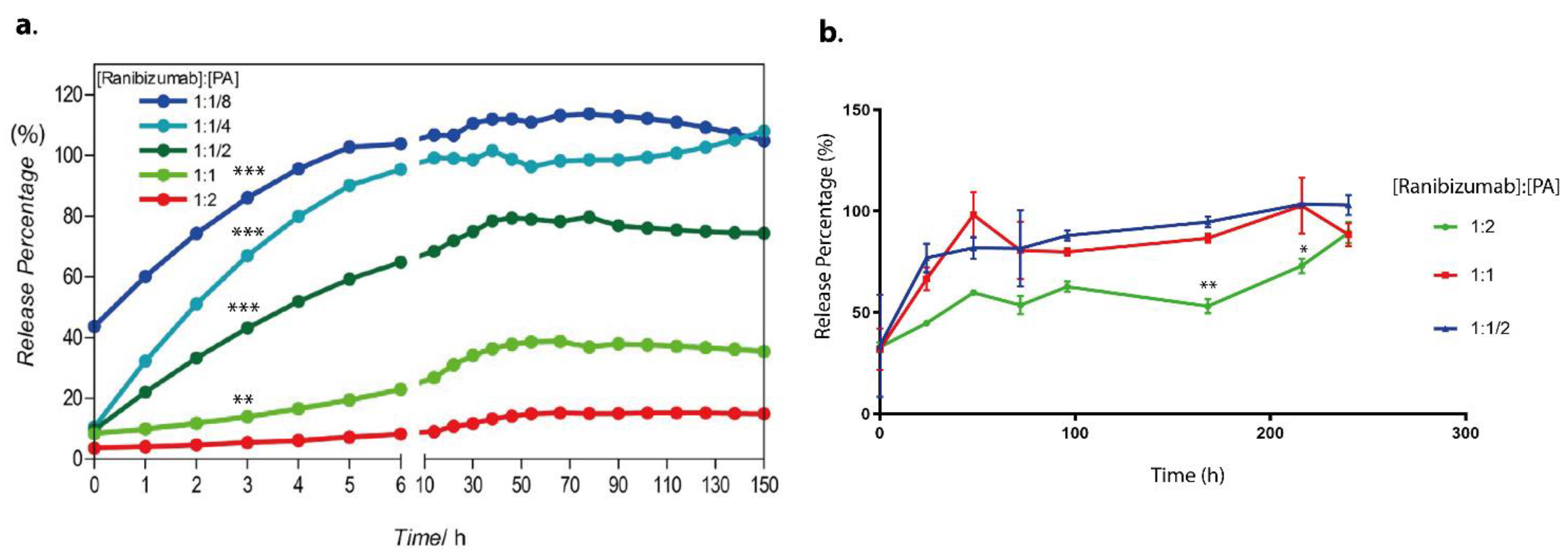
In this present study, we utilized a biodegradable self-assembled peptide nanofiber hydrogel system for the sustained release of an anti-VEGF drug, ranibizumab. The negatively charged peptide amphiphile molecule (E-PA) spontaneously formed an injectable supramolecular hydrogel nanofiber with the ranibizumab drug solution. The resulting hydrogel nanofiber encapsulating ranibizumab provided extended-release with a single dose of the drug in vitro with no dose dumping or fluctuation over time. The drug level can be sustained and controlled within the specific dosage window over a significant period (between 10–150 h). Moreover, depending on the concentration of the peptide amphiphile molecules used in the formation of hydrogel nanofiber, the amount of encapsulated ranibizumab can be modulated easily. When a peptide solution with a higher molar concentration is used, the drug is entrapped in a tighter, more compact, and less porous hydrogel, thereby reducing the diffusion rate. On the other hand, when a peptide solution with a lower molar concentration is used, the drug diffuses from a highly porous hydrogel faster. In addition, drugs released from hydrogel nanofibers are fully functional as assessed by their effects on human umbilical vein endothelial cells (HUVECs) in vitro. In the in vivo study performed in the rabbit eye, the drug concentration remained higher for 7 days compared to the control group.
2. Materials and Methods
2.1. Materials
Wang resin, 9-Fluorenylmethoxycarbonyl (Fmoc) and tert-butoxycarbonyl (Boc)-protected amino acids, and 2-(1H-benzotriazol-1-yl)-1,1,3,3 tetramethyluronium hexaf luoro-phosphate (HBTU) for peptide amphiphile synthesis were purchased from NovaBiochem (Alexandria, NSW, Australia). Lauric acid and N,N- diisopropylethylamine (DIEA) were purchased from Merck (Rahway, NJ, USA). Other chemicals were purchased from Alfa Aesar (Haverhill, MA, USA) or Sigma-Aldrich (St. Louis, MI, USA) and used without any purification. We ensured that all chemicals were of analytical grade and were used without further purification.
Millipore Milli-Q deionized water were used during the experiments with a resistance of 18 MΩ·cm. Vascular endothelial growth factor (VEGF) was purchased from R&D Systems (293-VE-050/CF). The cell proliferation kit was purchased from Sigma (Cat. No. 11 465 007 001), and the goat-anti-human IgG/F (ab)2 antibody labeled with horseradish peroxidase was purchased from Thermo Fisher (Catalog #31122), Waltham, MA, USA.
All cell culture chemicals were purchased from Biological Industries (Kibbutz Beit Haemek, Israel), and cell culture consumables were purchased from Nest Scientific USA Inc. (Woodbridg, NJ, USA). Finally, the Lucentis used in our study was donated by Gazi University, Department of Ophthalmology.
2.2. Synthesis, Purification, and Characterization of Peptide Amphiphile Molecules
The peptide amphiphile molecule, E-PA, was synthesized using the Fmoc-protected solid-phase peptide synthesis method. E-PA was composed of an alkyl group, a β-sheet-forming peptide sequence, the VVAG, and a negatively charged amino acid, E. For synthesis, Rink amide resin was preloaded with 1.1 mmol/g Fmoc-Glu (OtBu)-OH, with a resultant concentration of 0.72 mmol/g. All amino acid couplings were performed with two equivalents of Fmoc-protected amino acid, 1.95 equivalents of hexafluorophosphate benzotriazole tetramethyl uronium (HBTU), and three equivalents of N,N-diisopropylethylamine (DIEA) in dimethylformamide (DMF) for 3 h. Fmoc deprotection was performed with 20% piperidine/DMF solution for 20 min. The cleavage of the peptides from the resin and deprotection of acid-labile protected amino acids was carried out through treatment with a mixture of trifluoroacetic acid (TFA):triisoproplysilane (TIS):water in the ratio of 95:2.5:2.5 for 2.5 h. Excess TFA was removed by rotary evaporation. The remaining residue was triturated with ice-cold diethyl ether, and the resulting white pellet was freeze-dried. The purity and molecular mass of the synthesized peptides were determined using the Agilent 6530–1200 QTOF LC-MS system with the addition of ESI-MS. Zorbax Extend-C18 2.1 × 50 mm columns were used for basic conditions. As a gradient, 0.1% TFA/water and 0.1% TFA/acetonitrile solutions were used. The purification was performed on a Zorbax Extend C18 prep-HT with a water/acetonitrile (0.1% NH4OH) gradient. Due to the acidic character of the E-PA, an Agilent 6530 accurate-Mass Q-TOF LC/MS was operated by eluting it from an Agilent Zorbax Extend-C18 (50 × 2.1 mm) column with a water/acetonitrile mixture (0.1% NH4OH) for the elucidation of the molecule.
2.2.1. Hydrogel Preparation
To obtain supramolecular hydrogels, E-PA dissolved in HEPES buffer (10 mM) was mixed with ranibizumab drug solution at the same volume at a specified ratio between 1:8 to 1:1. Since E-PA exhibited a negative charge at physiological pH, three-dimensional networks were formed when E-PA and the drug were mixed due to hydrogen bonding, van der Waals, and electrostatic forces.
2.2.2. Circular Dichroism (CD)
A Jasco J-815 CD spectrophotometer was used for CD analysis. Nanofiber formation was induced by mixing the drug and E-PA solutions, and the secondary structure of the mixture was spectrophotometrically monitored with a data range of 0.1 nm and a scan rate of 500 nm/min from 260 to 190 nm. The resulting raw data were converted to molar ellipticity.
2.2.3. Zeta Potential Analysis
Zeta potential measurements of E-PA, ranibizumab and hydrogel nanofibers were performed using a Malvern Zeta-ZS Zetasizer with 5 × 10−5 M at the indicated volume ratios.
2.2.4. Rheology Measurements
The mechanical properties of the drug and E-PA mixture were examined by oscillatory rheology. Oscillatory rheology measurements were performed with an Anton Paar Physica MCR301. The viscoelastic behavior of the drug encapsulating hydrogel was determined by recording the storage (G′) and loss (G′′) moduli of the hydrogels for an hour at constant shear strain and angular frequency. A gap distance of 0.5 mm was used with an angular frequency of 10 rad/s and shear strain of 0.1%. All time-sweep experiments were carried out at room temperature. Measurements were performed with three replicates.
2.2.5. Scanning Electron Microscopy
The morphological properties of nanofiber networks were examined through scanning electron microscopy with an FEI Quanta 200 FEG, using the GSED detector at ESEM mode with 3−10 keV beam energy. After 10 min of gelation on glass surfaces, the nanofibers were treated with gradually increasing ethanol concentrations and then dried using the Tousimis Autosamdri®-815b critical point dryer. The dried samples were coated with 3 nm Au/Pd and imaged with FEI Quanta 200 FEG SEM under a high vacuum.
2.3. Drug Encapsulation and Release Experiments
In the study, the preparation of hydrogels and drug-loaded hydrogels was carried out using serial dilutions of peptide solutions to obtain various hydrogel concentrations. The drug used in the experiments was ranibizumab, and its amount was kept constant at 10 mg/mL for all formulations. The 4% (w/v) E-PA solution was prepared in HEPES buffer at pH 7.4. To ensure homogeneity of the peptide solution, it was sonicated for 5 min. Then, serial dilutions of the stock peptide solution (4% (w/v) E-PA) were prepared to obtain different concentrations of 2%, 1%, 0.5%, 0.25%, 0.125%, and 0.0675% (w/v) peptide solutions.
To form the drug-loaded hydrogels, 10 µL of the E-PA solution, which was diluted from the stock solution (4% (w/v)), at different weight/volume ratios, was mixed with 10 µL of the ranibizumab drug solution. The resulting hydrogels were at weight ratios of 1:2, 1:1, 1:1/2, 1:1/4, and 1:1/8 of [Ranibizumab]: [PA]. After mixing, the hydrogels were incubated at 37 °C for 1 h to ensure hydrogel stability. Then, 50 µL of water was slowly added on top of each hydrogel, and the initial measurement was taken at t = 0.
In order to generate a standard curve, various ranibizumab concentrations were prepared, and the A280 protocol on the NanoDrop 2000c Spectrophotometer [
39] was used to assess them (
Figure S4). This approach is preferred, as it produces results in samples with a small volume (2 µL). Following the initial measurement at time zero, additional measurements were taken every hour for the first 10 h, and then measurements were performed every 8 h for three days. Three replicates of the measurements were made at room temperature.
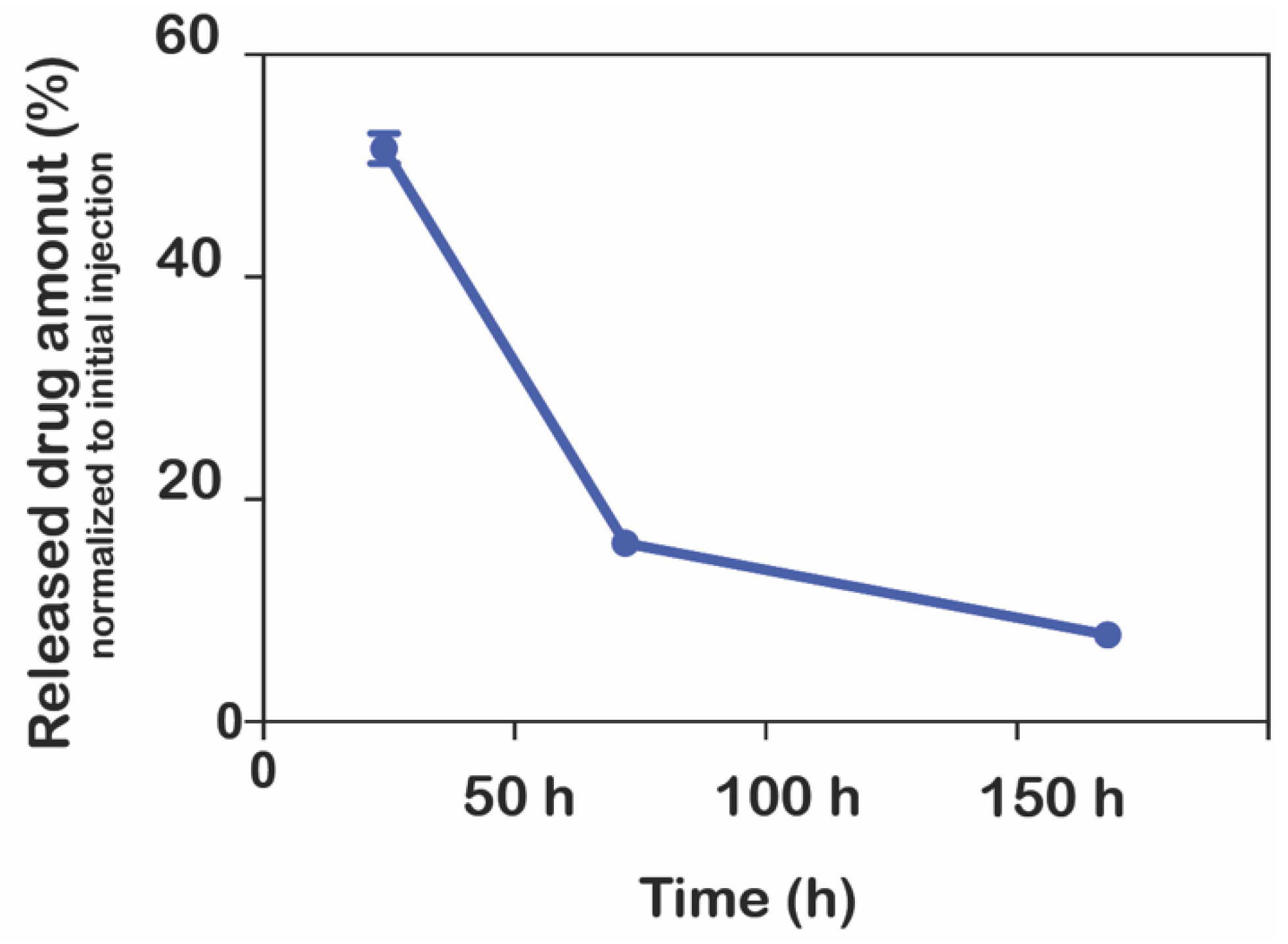
Moreover, the release of ranibizumab from hydrogels was also achieved into the vitrein. Instead of water, 50 µL of vitrein was added onto the hydrogels containing the drug prepared in the ratios of 1:2, 1:1, and 1:1/2 as mentioned above, and samples were taken to measure the release of ranibizumab for 150 h in the same way. Measurements were performed as described in
Section 2.7 using the ELISA protocol, unlike the other release study. Three replicates of the measurements were made at room temperature.
2.4. Enzymatic Degradation of Hydrogel Nanofiber
The enzymatic degradation of the peptide-based hydrogel nanofiber structure was also analyzed. E-PA was dissolved in 100 µL of double-distilled water to form a peptide solution at 10 µM concentration. To form nanofibers, nanofibers were formed by mixing drug solution (ranibizumab, 10 mg/mL) and E-PA (10 µM). A total of 750 µM rhodamine B (20 µL) was added to the E-PA solution to monitor the enzyme degradation while the PA molecules were mixed. After the nanofibers were prepared in quartz cuvettes, they were left to incubate for 3 h to equilibrate the formed nanofibers. Afterwards, 2.2 mL of proteinase K solution (1 mg/mL) or Tris buffer solution (10 mM) as a control was added onto the nanofibers (n = 3). The absorbances of the solutions were measured at room temperature between 400 and 600 nm with a Varian Cary 100 UV-Vis spectrophotometer at certain time intervals, including 0 h, 6 h, 12 h, 24 h, 48 h, 72 h, and 96 h.
2.5. Cellular Viability and Proliferation
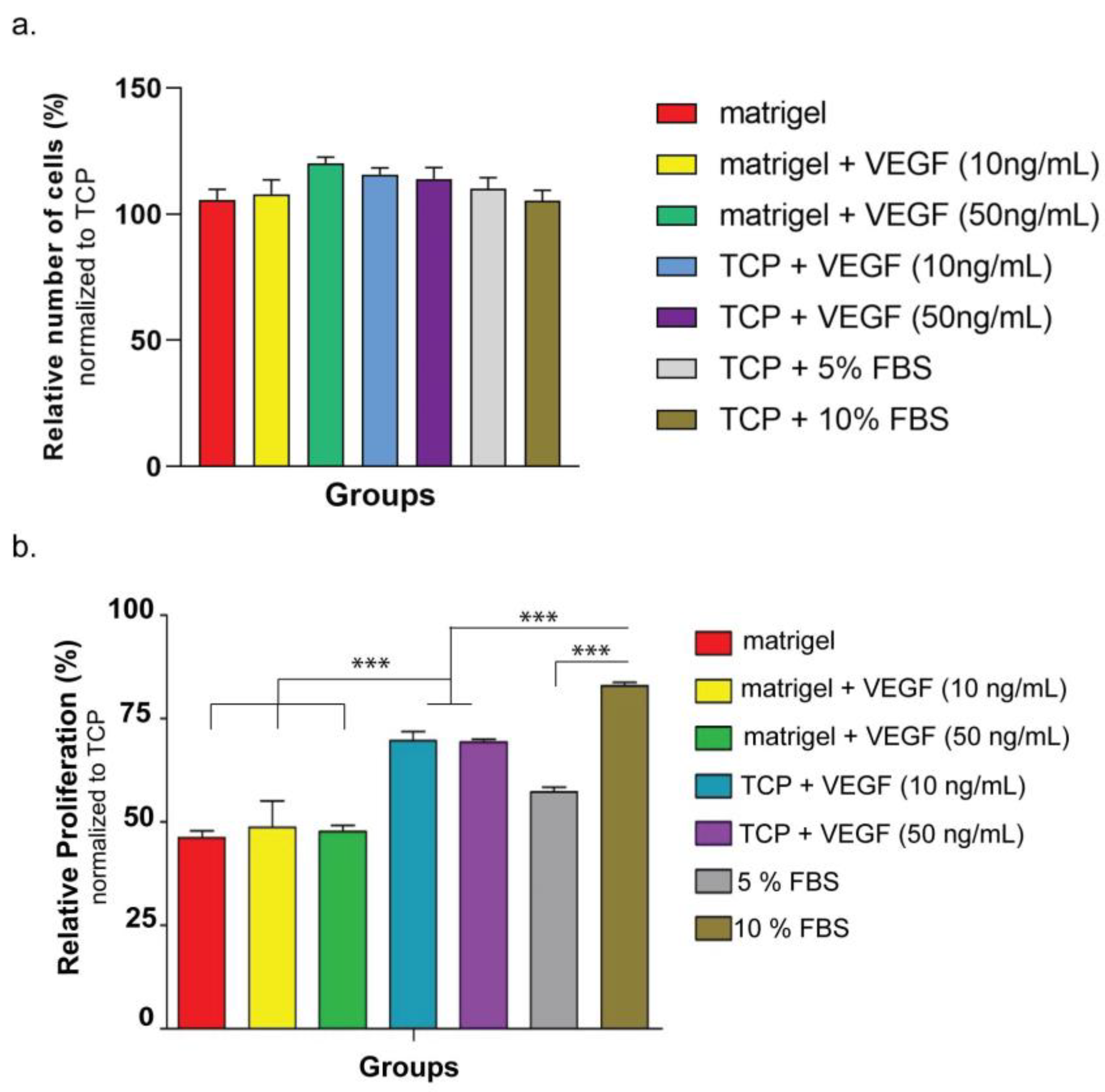
The biocompatibility of the hydrogel nanofiber was monitored on two different cell lines: HUVECSs (ATCC: CRL-1730) and ARPE-19 (ATCC: CRL-2302™). The first set of experiments used ARPE-10 cells and aimed to understand the biocompatibility of hydrogel nanofiber. For the coating of culture plates, E-PA was dissolved in HEPES buffer (1 mM) and mixed with ranibizumab drug solution (10 mg/mL) at the same volume of E-PA to a total volume of 150 µL/cm2, and the gels were allowed to dry overnight under the hood. The coated cell culture plates were then sterilized by keeping them under UV for 30 min. For the ranibizumab group, 20 µL of ranibizumab (10 mg/mL) was added to growth medium of cells. At 24 h, 48 h, and 72 h after seeding, the cellular viability in all groups was determined according to the manufacturer’s protocol for the MTT (Sigma Aldrich, St. Louis, MI, USA) kit by normalizing spectrophotometric reads to ARPE-19 cultured on bare tissue culture plate (TCP) in 10% FBS. The cells used in the second series of cell culture studies were HUVECs. Prior to conducting an in vitro angiogenesis study using HUVECs, the purpose of this study was to assess the cell survival and proliferation variables, with the parameters of VEGF, FBS, and Matrigel. In this investigation, HUVECs cells were therefore cultivated in both Matrigel and non-Matrigel culture dishes. In order to understand the effect of VEGF on HUVECs’ cellular proliferation, cells were cultured with VEGF at varying concentrations. The fetal bovine serum (FBS) ratio was also changed so as to see its effect on HUVECs’ viability in other experiments. At 24 h after seeding, cellular proliferation in all groups was determined according to the manufacturer’s protocol for the MTT kit by normalizing spectrophotometric reads to HUVECs cultured on a bare tissue culture plate (TCP) in %15 FBS. Cellular viability was determined by staining HUVECs with Calcein AM (Invitrogen, Waltham, MA, USA). Prior to staining, 1250 cells/cm2 of HUVECs were seeded on Matrigel or TCP in similar mediums used for the proliferation experiment. At the 4th hour, the viable cells were imaged and counted using Image J software, and the results were normalized to HUVECs cultured on TCP in %15 FBS.
2.6. In Vitro Angiogenesis Model Analysis
HUVECs were cultured in standard cell culture conditions (5% CO2 and 37 °C) in DMEM containing 10% FBS and 1% penicillin–streptomycin. The vascular formation of HUVECs in the presence of VEGF was studied using the Matrigel system.
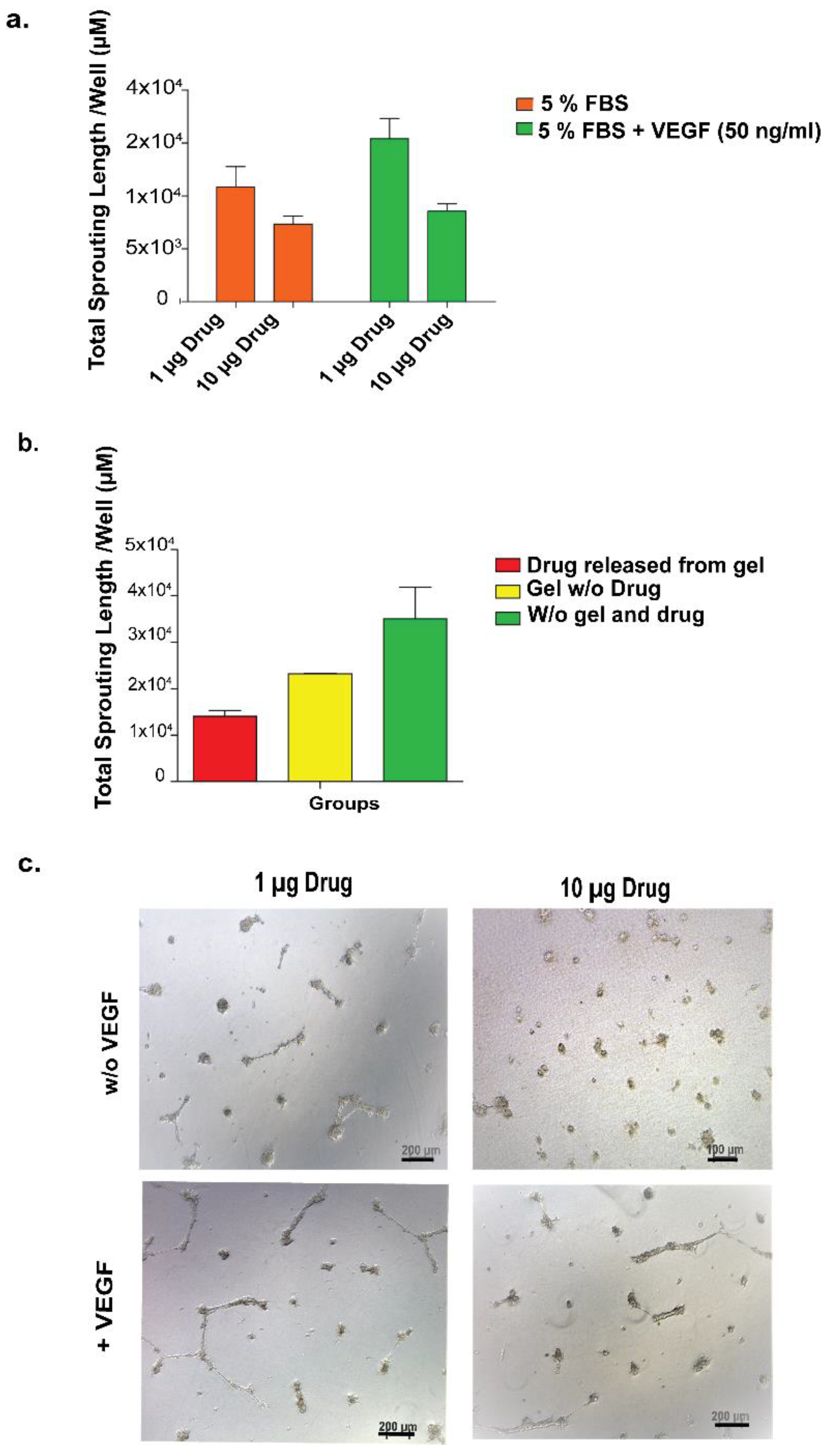
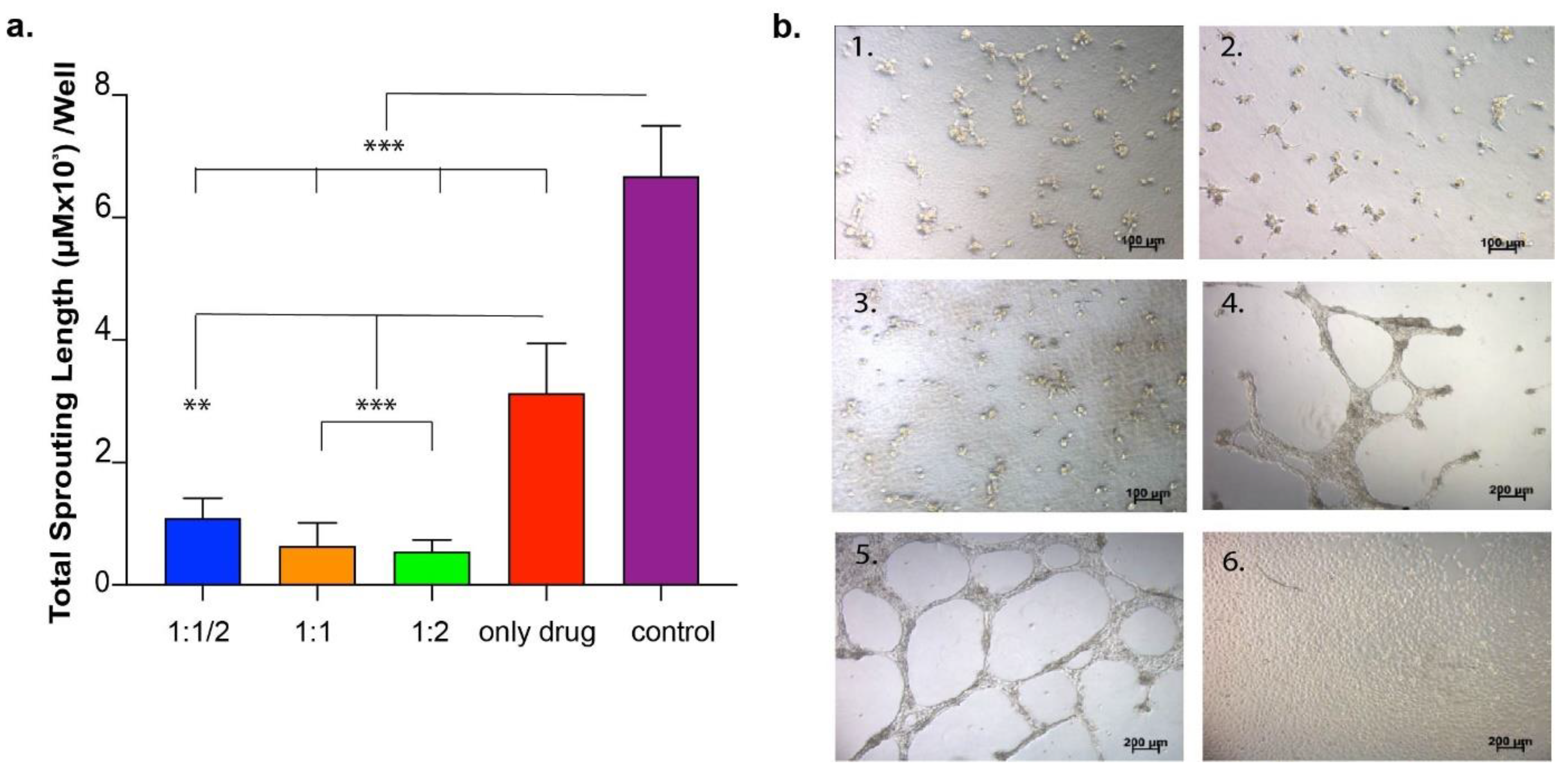
First, the concentration-dependent effect of VEGF on in vitro angiogenesis model was studied. HUVECs seeded on Matrigel or TCP (negative control) were treated with VEGF at two different concentrations (10 ng/mL and 50 ng/mL). At 24 h after cell seeding, the number of sprouting formations was calculated from images taken from each well. In the second set of experiments, the drug functionality was studied, during which the drug was directly applied to HUVECs seeded on Matrigel or TCP at two different concentrations (1 ng/well and 10 ng/well) so as to observe the inhibition of the sprouting formation. The number of sproutings in each well was calculated as above. In the third set of experiments, the drug was encapsulated into the peptide amphiphile hydrogel system and applied to HUVECs cultured on Matrigel or TCP in order to observe the functionality of the released drug.
As the last set of experiments for this section, the effect of the drug released from various hydrogel systems on HUVECs was investigated in an in vitro angiogenesis model. HUVECs were cultured in 24-well plates coated with Matrigel. In order to analyze the effect of released drug from peptide hydrogels, hydrogel was formed in an insert system so as to expose cells to drug-loaded hydrogels during culturing. Peptide solutions were prepared and sterilized under UV for 30 min. There were three experimental samples and four control samples. Experimental samples were the same except for their peptide concentrations in the gel in the insert system. E1: 10 µL drug + 10 µL peptide solution 1 (2%) (1:2); E2: 10 µL drug + 10 µL peptide solution 2 (1%) (1:1); E3: 10 µL drug + 10 µL peptide solution 3 (0.5%) (1:1/2). At the end of 24 h of culture, vascular tube formation was imaged under the microscope and quantified using Image J software on eight images taken from each culture plate well.
2.7. In Vivo Analysis
The Animal Ethics Committee of Gazi University approved all animal procedures. For in vivo tests, six white female/male New Zealand rabbits (mean weight, 2500 g; age, 12 weeks) were employed. In order to analyze the in vivo release kinetics of the delivery system, a nanofiber delivery system was applied to the vitreous chamber of New Zealand rabbits. Pupil dilation was achieved topically with one drop of phenylephrine (2.5%) and one drop of tropicamide (0.5%) just before the injection. General anesthesia was administered with ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (5 mg/kg), and topical anesthesia was administered with proparacaine (0.5%). After cleaning the eyelid and its surroundings with povidone–iodine, a sterile perforated cover was applied, and the lid was excluded with a sterile blepharostat immediately before Intravitreal injection. For 1 min, 10% povidine was placed on the cornea–conjunctiva surface, and povidine was cleaned off with 0.09% NaCl. All procedures were performed under sterile conditions. Then, the ranibizumab hydrogel system was applied intravitreally with a 30-gauge tip and insulin injector 3 mm behind the limbus in the upper temporal quadrant. Thirty microliters of physiological saline were mixed with 30 µL of ranibizumab (10 µg/µL) and injected into the right eyes of 6 rabbits; 30 µL of 4% (
w/
v) E-PA was mixed with 30 µL of ranibizumab (10 µg/µL) (
Table 1) and injected into the left eyes of 6 rabbits. After this procedure, moxifloxacin (0.5%) was dripped 3 × 1 drops daily for one week, and the eyes of the animals were monitored daily in terms of inflammation and infection. The animals were sacrificed at the end of the experiment via intravenous injection of 100 mg/kg sodium pentobarbital. Both eyeballs were promptly enucleated and placed in a −80 °C freezer. On the day of the analysis, the vitreous was eviscerated from the eye (
Figure S10). Before analysis, the frozen vitreous was weighed and then defrosted and solubilized in 1.0 mL of phosphate-buffered saline (PBS) on a rotator overnight at 4 °C in 1.0 mL of 1% bovine serum albumin in PBS. The samples were centrifuged at 2000 rpm for 10 min the next day. After centrifugation, the volume of the sample was measured. Before the immunoassay, all samples were diluted. The 165-amino acid variant of human recombinant VEGF (R&D Systems, 293-VE-010/CF) was immobilized on high-binding plates suitable for luminescent detection. The VEGF was put onto each plate as 100 µL/well for overnight incubation at 4 °C. Then, plates were washed three times with PBS and blocked for 4 h at 4 °C with 1% bovine serum albumin in PBS. A standard curve was generated using ranibizumab of known concentrations for each assay (
Figure S9). A goat-anti-human IgG/F (ab)2 antibody (Thermo Fisher, Catalog #31122) conjugated with horseradish peroxidase was used to identify the bound ranibizumab. The tagged anti-humanized monoclonal IgG/F (ab)2 was diluted in the buffer at 1:20,000. The diluted secondary antibody was aliquoted at 100 L/well onto the VEGF plate and incubated for 45 min at room temperature with agitation. The plate was rinsed three times with 0.05% Tween 20/PBS after this incubation. According to the manufacturer’s instructions, the chemiluminescent signal was triggered using the luminol-based SuperSignal ELISA Pico Chemiluminescent Substrate (Pierce Biotechnology). The chemiluminescent signal was detected on a microplate reader (Molecular Devices, SpectraMax M Series) and acquired over a time frame of 0.1 s/well [
39].