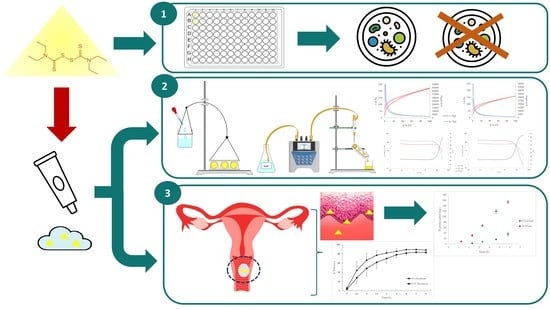
Repurposing Disulfiram as an Antifungal Agent: Development of a New Disulfiram Vaginal Mucoadhesive Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antifungal Susceptibility Testing
2.3. Preparation and Optimization of the Mucoadhesive Gel
2.4. Adhesive Strength of the Gels
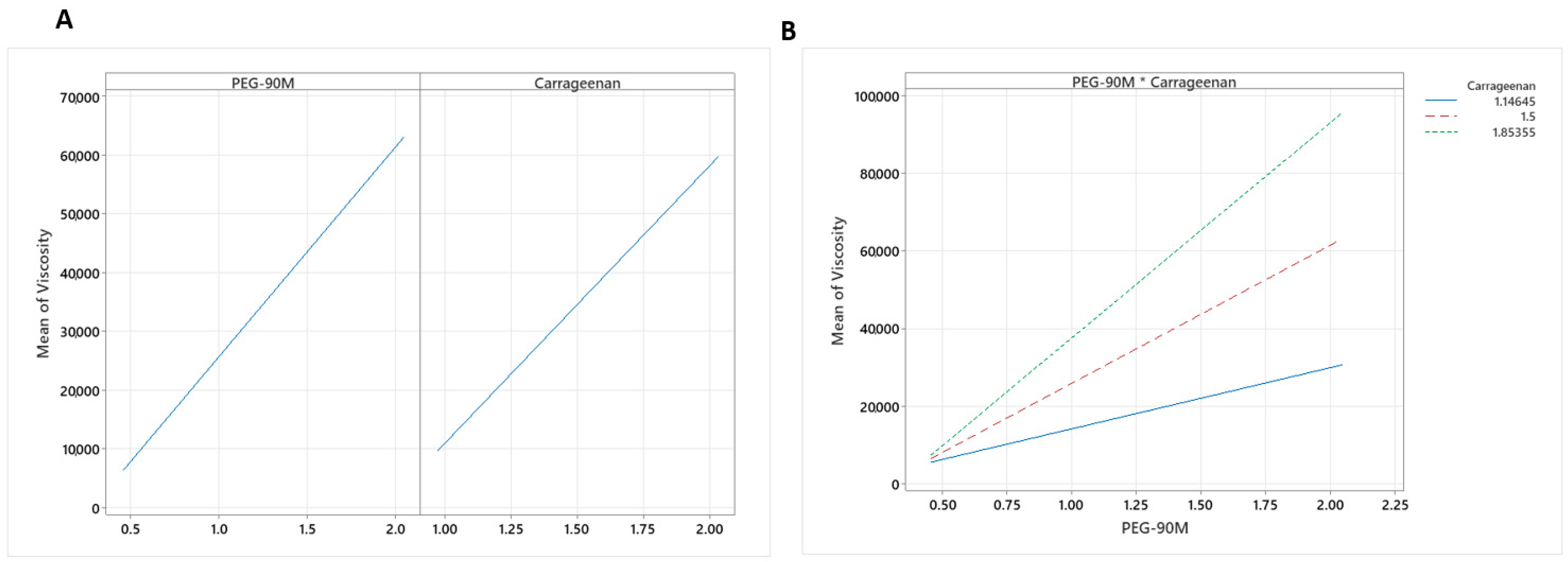
2.5. Viscosity of the Mucoadhesive Gels
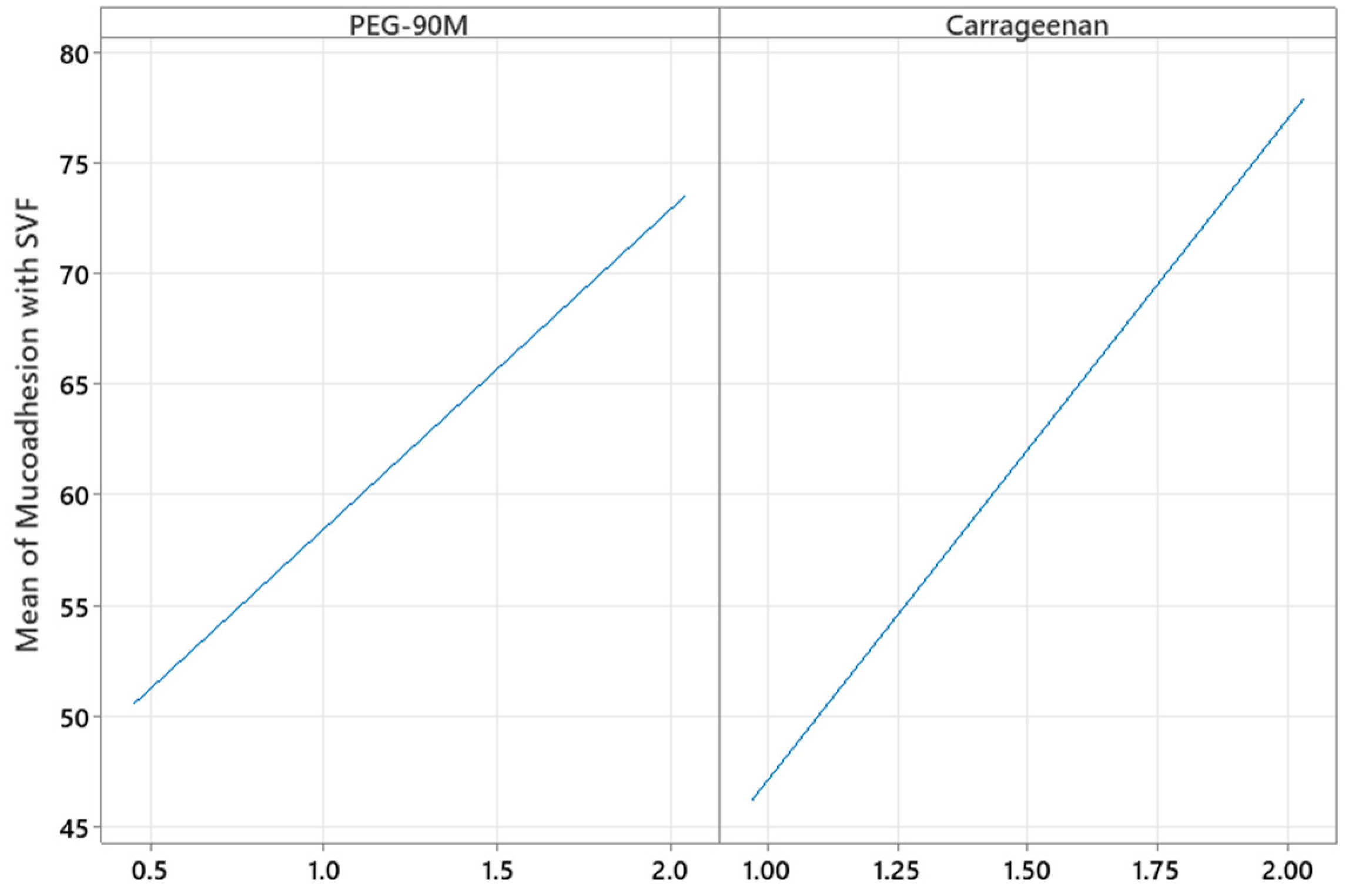
2.6. Mucoadhesive Properties
2.7. Optimization of the Mucoadhesive Gels
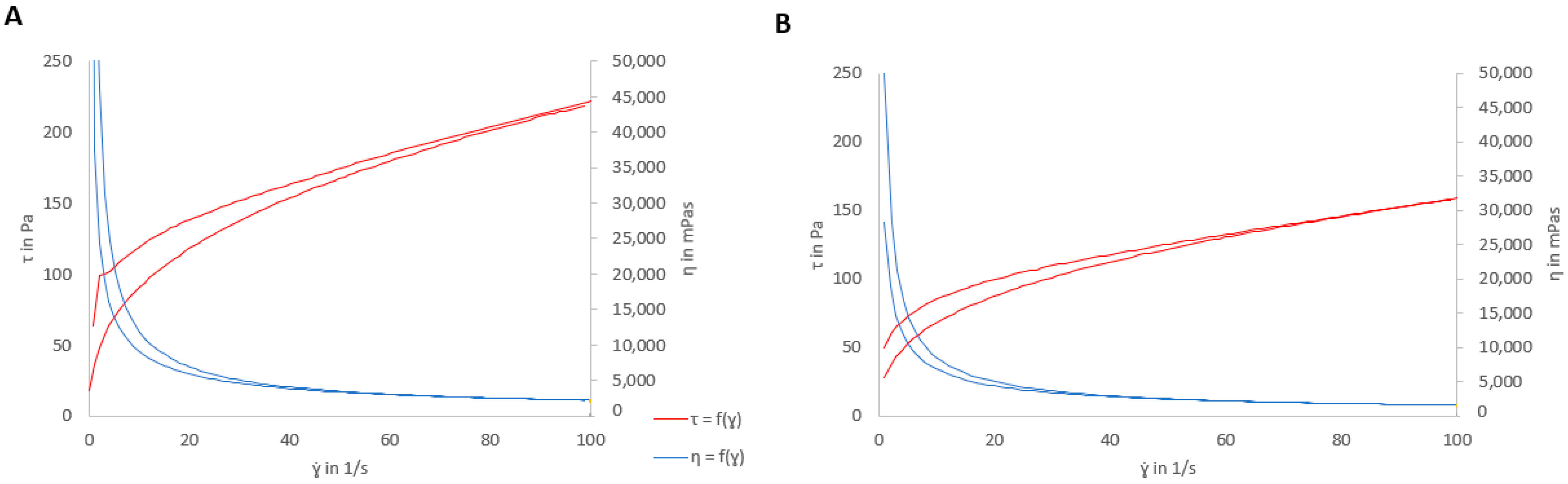
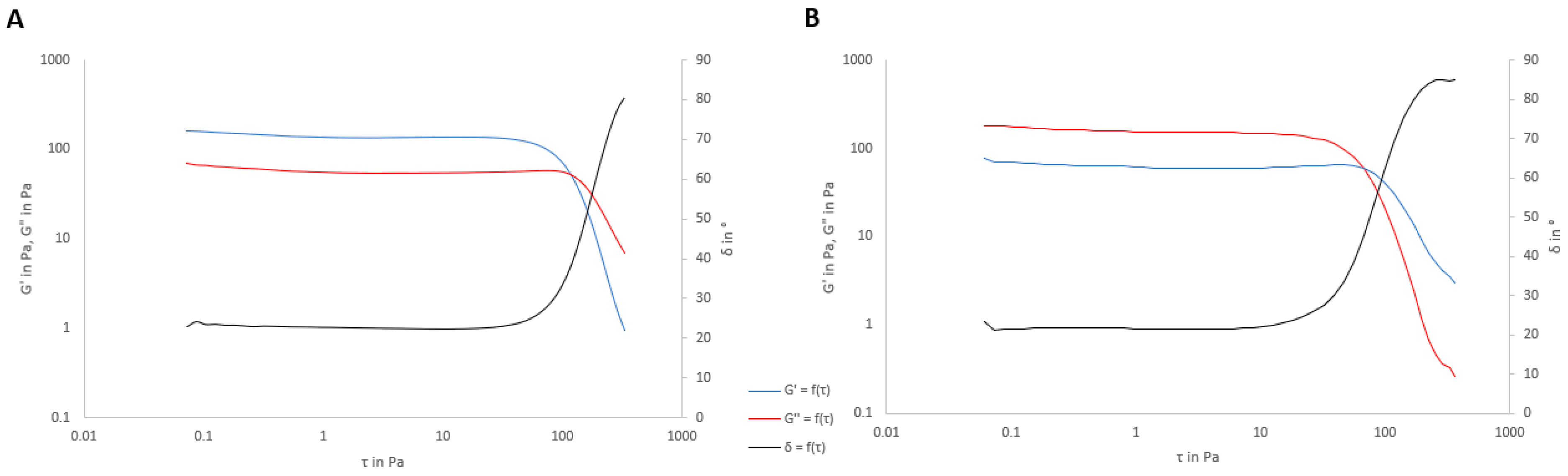
2.8. Final Rheological Properties
2.9. Syringeability of the Formulations
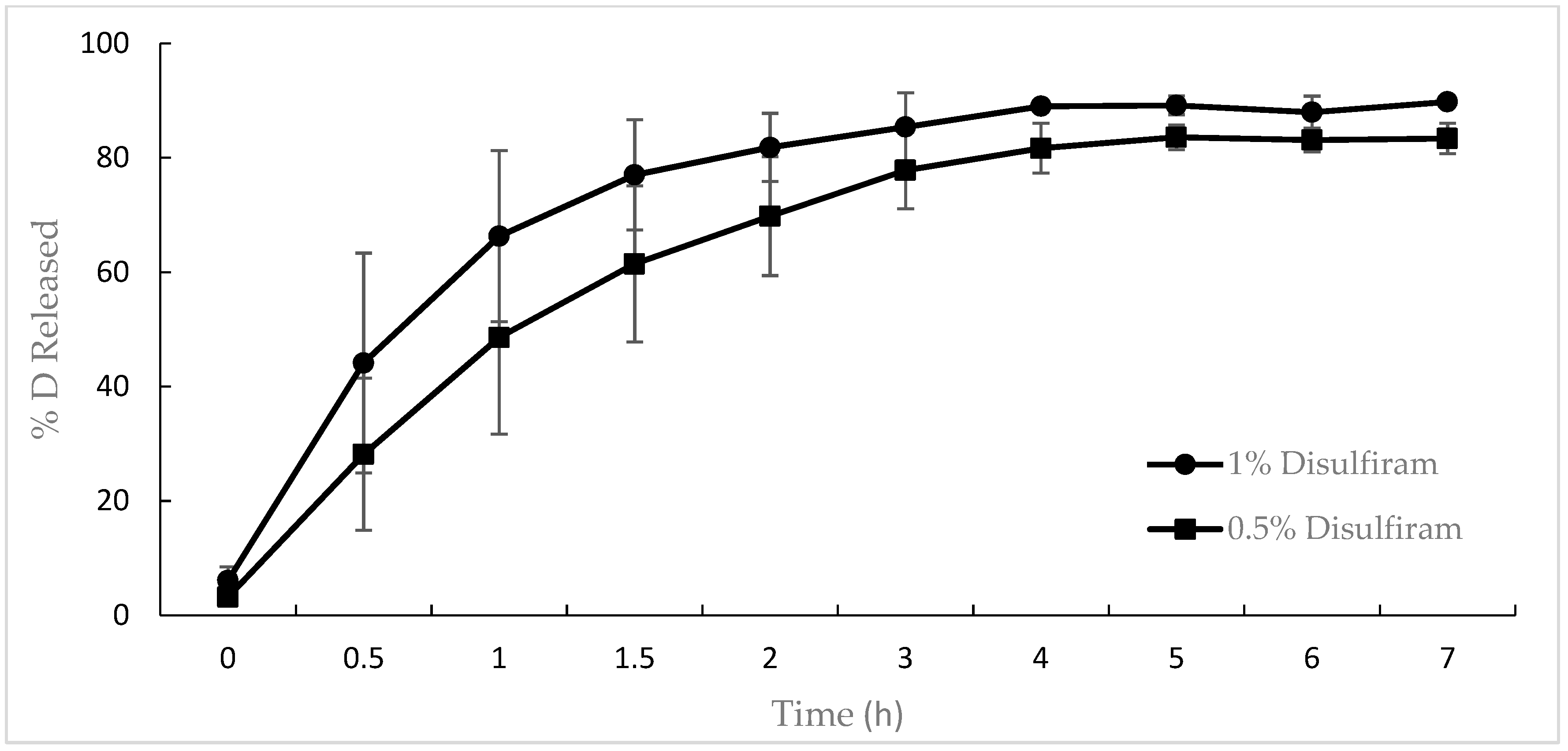
2.10. In Vitro Release Tests of the Disulfiram Gel
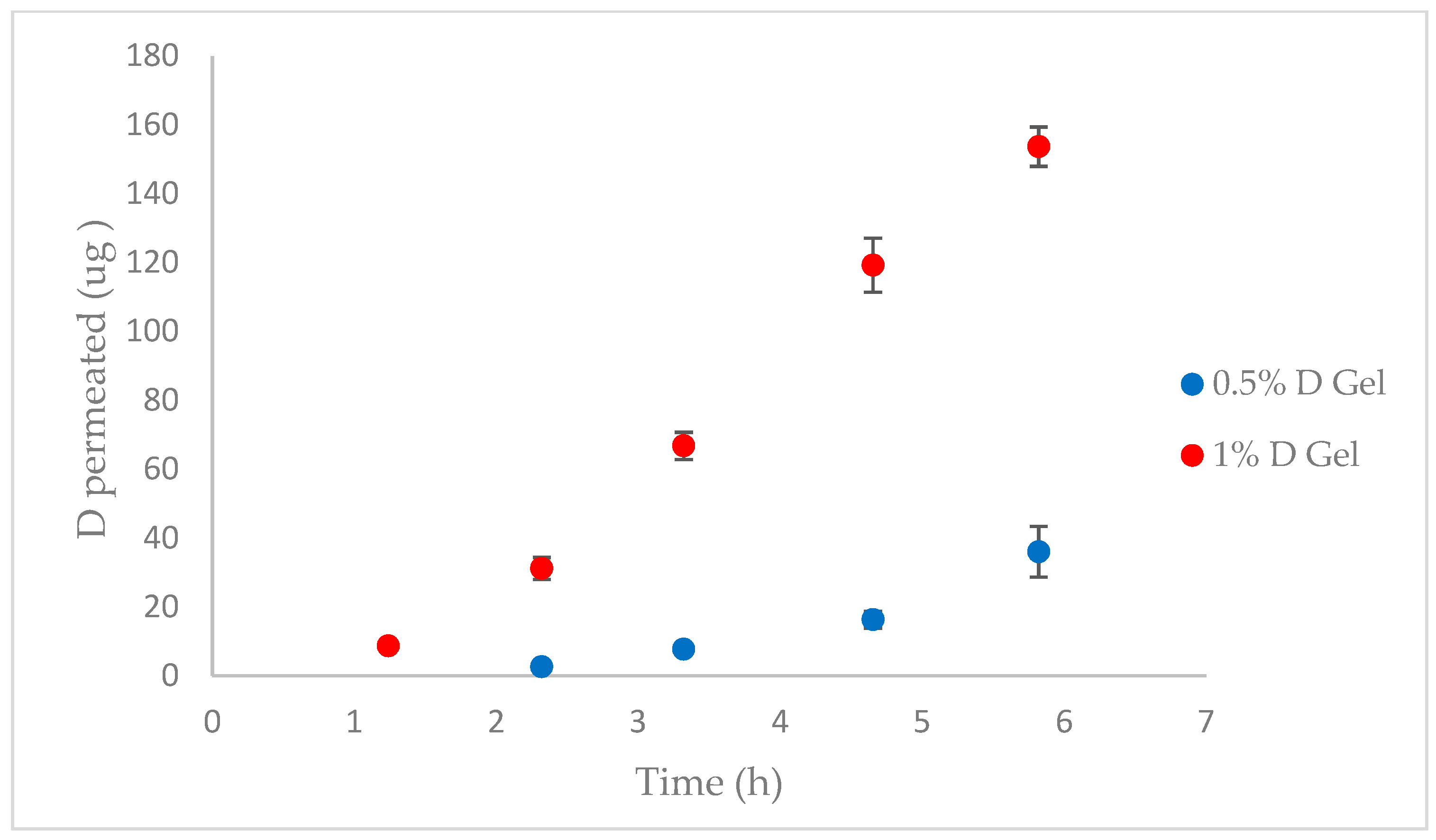
2.11. Pig Vagina Permeation
2.12. Determination of the Concentration Retained in the Pig Vagina
3. Results and Discussion
3.1. Antifungal Susceptibility Testing
3.2. Preparation and Statistical Design to Evaluate the Effect of Experimental Variables on the Mucoadhesive Gel Properties
3.3. Optimization of the Mucoadhesive Gels
3.4. Rheological Properties
3.5. Syringeability of the Formulations
3.6. In Vitro Release of Disulfiram from the Gel
3.7. Pig Vagina Permeation
3.8. Disulfiram Concentration Retained in the Pig Vagina
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, N.; Febrer, N.B.-D.; Calpena-Campmany, A.C.; Nardi-Ricart, A.; Rodríguez-Lagunas, M.J.; Morales-Molina, J.A.; Soriano-Ruiz, J.L.; Fernández-Campos, F.; Clares-Naveros, B. New formulations loading caspofungin for topical therapy of vulvovaginal candidiasis. Gels 2021, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef]
- Felix, T.C.; de Brito Röder, D.V.D.; dos Santos Pedroso, R. Alternative and complementary therapies for vulvovaginal candidiasis. Folia Microbiol. 2018, 64, 133–141. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2015, 42, 905–927. [Google Scholar] [CrossRef]
- Mtibaa, L.; Fakhfakh, N.; Kallel, A.; Belhadj, S.; Salah, N.B.; Bada, N.; Kallel, K. Les candidoses vulvovaginales: Étiologies, symptômes et facteurs de risque. J. Mycol. Med. 2017, 27, 153–158. [Google Scholar] [CrossRef]
- Ravani, L.; Esposito, E.; Bories, C.; Moal, V.L.-L.; Loiseau, P.M.; Djabourov, M.; Cortesi, R.; Bouchemal, K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013, 454, 695–702. [Google Scholar] [CrossRef]
- Sobel, J. Current treatment options for vulvovaginal candidiasis. Women’s Health 2005, 1, 253–261. [Google Scholar] [CrossRef]
- Azie, N.; Angulo, D.; Dehn, B.; Sobel, J.D. Oral Ibrexafungerp: An investigational agent for the treatment of vulvovaginal candidiasis. Expert. Opin. Investig. Drugs 2020, 29, 893–900. [Google Scholar] [CrossRef]
- Mei, L.; Chen, J.; Yu, S.; Huang, Y.; Xie, Y.; Wang, H.; Pan, X.; Wu, C. Expansible thermal gelling foam aerosol for vaginal drug delivery. Drug Deliv. 2017, 24, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Sauna, Z.E.; Shukla, S.; Ambudkar, S.V. Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Mol. Biosyst. 2005, 1, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018, 19, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Nair, R.; Banerjee, A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet. Biol. 2019, 132, 103252. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Shukla, S.; Sauna, Z.E.; Prasad, R.; Ambudkar, S.V. Disulfiram is a potent modulator of multidrug transporter Cdr1p of Candida albicans. Biochem. Biophys. Res. Commun. 2004, 322, 520–525. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Monod, M.; Bille, J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 1996, 40, 2300–2305. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Ren, Y.; Zhang, X. Disulfiram: A Novel Repurposed Drug for Cancer Therapy. Cancer Chemother. Pharmacol. 2021, 87, 159–172. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Disulfiram: Chemical and Physical Properties. PubChem Compound Summary CID 3117. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Disulfiram#section=Chemical-and-Physical-Properties (accessed on 20 March 2023).
- Lajarin-Reinares, M.; Martinez-Esteve, E.; Pena-Rodr, E.; Cañellas-Santos, M.; Fernandez-Campos, F. The Efficacy and Biopharmaceutical Properties of a Fixed-Dose Combination of Disulfiram and Benzyl Benzoate. Int. J. Mol. Sci. 2022, 23, 10969. [Google Scholar] [CrossRef]
- Lajarin-Reinares, M.; Pena-Rodríguez, E.; Cañellas-Santos, M.; Rosell-Vives, E.; Cortés, P.; Casas, M.L.; Calvo, M.À.; Fernandez-Campos, F. Repurposing Disulfiram as an Antimicrobial Agent in Topical Infections. Antibiotics 2022, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Wu, C.; Wang, L.; Chen, Z.S.; Cui, W. The combination of disulfiram and copper for cancer treatment. Drug Discov. Today 2020, 25, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Garg, T.; Chopra, S.; Dasgupta, A. Repurposing disulfiram to target infections caused by non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2019, 74, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Sauna, Z.E.; Peng, X.H.; Nandigama, K.; Tekle, S.; Ambudkar, S.V. The Molecular Basis of the Action of Disulfiram as a Modulator of the Multidrug Resistance-Linked ATP Binding Cassette Transporters MDR1 (ABCB1) and MRP1 (ABCC1). Mol. Pharmacol. 2004, 65, 675–684. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- M27-A3 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition. Published online 2008. Available online: www.clsi.org (accessed on 7 September 2022).
- Rex, J.H.; Cooper, C.R.; Merz, W.G.; Galgiani, J.N.; Anaissie, E.J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 1995, 39, 906–909. [Google Scholar] [CrossRef]
- Villegas, I.; Rosillo, M.; Alarcón-De-La-Lastra, C.; Vázquez-Román, V.; Llorente, M.; Sánchez, S.; Gil, A.G.; Alcalde, P.; González, E.; Rosell, E.; et al. Amoxicillin and clarithromycin mucoadhesive delivery system for helicobacter pylori infection in a mouse model: Characterization, pharmacokinetics, and efficacy. Pharmaceutics 2021, 13, 153. [Google Scholar] [CrossRef]
- Geonnotti, A.R.; Katz, D.F. Compartmental transport model of microbicide delivery by an intravaginal ring. J. Pharm. Sci. 2010, 99, 3514. [Google Scholar] [CrossRef] [PubMed]
- Friedl, H.E.; Dünnhaupt, S.; Waldner, C.; Bernkop-Schnürch, A. Preactivated thiomers for vaginal drug delivery vehicles. Biomaterials 2013, 34, 7811–7818. [Google Scholar] [CrossRef]
- Park, E.K.; Song, K.W. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations: Steady shear flow behavior. Arch. Pharmacal. Res. 2010, 33, 141–150. [Google Scholar] [CrossRef]
- Venkatesh, M.P.; Kumar, T.P.; Pai, D.R. Targeted drug delivery of Methotrexate in situ gels for the treatment of Rheumatoid Arthritis. Saudi Pharm. J. 2020, 28, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Monica, L.L.; Jordi, G.; Francisco, F.C. In situ bioadhesive film-forming system for topical delivery of mometasone furoate: Characterization and biopharmaceutical properties. J. Drug Deliv. Sci. Technol. 2020, 59, 101852. [Google Scholar] [CrossRef]
- Pena-Rodríguez, E.; Lajarin-Reinares, M.; Mata-Ventosa, A.; Pérez-Torras, S.; Fernández-Campos, F. Dexamethasone-loaded lipomers: Development, characterization, and skin biodistribution studies. Pharmaceutics 2021, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Amores, S.; Domenech, J.; Colom, H.; Calpena, A.C.; Clares, B.; Gimeno, Á.; Lauroba, J. An improved cryopreservation method for porcine buccal mucosa in ex vivo drug permeation studies using Franz diffusion cells. Eur. J. Pharm. Sci. 2014, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kaomongkolgit, R.; Jamdee, K.; Wongnoi, S.; Chimnoi, N.; Techasakul, S. Antifungal activity of coronarin D against Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 61–66. [Google Scholar] [CrossRef]
- Radetsky, M.; Wheeler, R.C.; Roe, M.H.; Todd, J.K. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. J. Clin. Microbiol. 1986, 24, 600–606. [Google Scholar] [CrossRef]
- Khan, S.; Singhal, S.; Mathur, T.; Upadhyay, D.J.; Rattan, A. Antifungal potential of disulfiram. Jpn. J. Med. Mycol. 2007, 48, 109–113. [Google Scholar] [CrossRef]
- Hao, W.; Qiao, D.; Han, Y.; Du, N.; Li, X.; Fan, Y.; Ge, X.; Zhang, H. Identification of disulfiram as a potential antifungal drug by screening small molecular libraries. J. Infect. Chemother. 2020, 27, 696–701. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.; Garland, M.; Woolfson, A.; Donnelly, R. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Ahuja, A.; Khar, R.K.; Ali, J. Mucoadhesive Drug Delivery Systems. Drug. Dev. Ind. Pharm. 1997, 23, 489–515. [Google Scholar] [CrossRef]
- De Souza Ferreira, S.B.; Da Silva, J.B.; Volpato Junqueira, M.; Belincanta Borghi-Pangoni, F.; Guttierres Gomes, R.; Luciano Bruschi, M. The importance of the relationship between mechanical analyses and rheometry of mucoadhesive thermoresponsive polymeric materials for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 74, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Resins, W. POLYOX® Water-Soluble Resins. Chem. Eng. News Arch. 1995, 73, 40. [Google Scholar] [CrossRef]
- Da Silva, J.B.; de Ferreira, S.B.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Gentile, M.; Sandri, G.; Bonferoni, M.C.; Cavalloro, V.; Martino, E.; Collina, S.; Ferrari, F. Development of a Mucoadhesive and an in Situ Gelling Formulation Based on κ-Carrageenan for Application on Oral Mucosa and Esophagus Walls. I.I. Loading of a Bioactive Hydroalcoholic Extract. Mar. Drugs 2019, 17, 153. [Google Scholar] [CrossRef]
- Khazaeli, P.; Mehrabani, M.; Mosadegh, A.; Bios, S.; Zareshahi, R.; Hasan Moshafi, M. Formulation, Physiochemical, and Microbial Assay of Henna Oil Vaginal Suppository Formulated with Polyethylene Glycol Bases. Iran. J. Med. Sci. 2020, 45, 207. [Google Scholar] [CrossRef]
- Kaewnopparat, S.; Kaewnopparat, N. Formulation and Evaluation of Vaginal Suppositories Containing Lactobacillus. World Acad. Sci. Eng. Technol. 2009, 55, 117–120. [Google Scholar]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.D. Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Mar. Drugs 2020, 18, 249. [Google Scholar] [CrossRef]
- Elias, C.J.; Coggins, C.; Alvarez, F.; Brache, V.; Fraser, I.S.; Lacarra, M.; Lähteenmäkl, P.; Massai, R.; Mishell, D.R.; Phillips, D.M.; et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception 1997, 56, 387–389. [Google Scholar] [CrossRef]
- Valenta, C.; Schultz, K. Influence of carrageenan on the rheology and skin permeation of microemulsion formulations. J. Control. Release 2004, 95, 257–265. [Google Scholar] [CrossRef]
- Cha, D.S.; Choi, J.H.; Chinnan, M.S.; Park, H.J. Antimicrobial Films Based on Na-alginate and κ-carrageenan. LWT 2002, 35, 715–719. [Google Scholar] [CrossRef]
- Seol, K.H.; Lim, D.G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 °C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, C.; Cho, S.; Kim, S.B. Effects of thermal treatment on the physical properties of edible calcium alginate gel beads: Response surface methodological approach. Foods 2019, 8, 578. [Google Scholar] [CrossRef]
- Osswald, T.A.; Book, N.R. Polymer Rheology Fundamentals and Applications; Hanser Publications: Munich, Germany, 2015. [Google Scholar]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control Release. 1998, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Xie, R.; Zhang, L.; Gui, H.; Huang, M. Use of Rubber Process Analyzer for Characterizing the Molecular Weight Parameters of Natural Rubber. Int. J. Polym. Sci. 2015, 2015, 517260. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.; et al. Advances and future perspectives in epithelial drug delivery. Adv. Drug Deliv. Rev. 2022, 186, 1–38. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina Ex Vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef]
- Van Eyk, A.D.; Van Der Bijl, P. Porcine vaginal mucosa as an in vitro permeability model for human vaginal mucosa. Int. J. Pharm. 2005, 305, 105–111. [Google Scholar] [CrossRef]




| Factor | Lower Level | Higher Level |
|---|---|---|
| % PEG-90M (w/w) | 0.5 | 1.5 |
| % Carrageenan (w/w) | 1.0 | 2.0 |
| Rheological Model | Equation |
|---|---|
| Newton | |
| Bingham | |
| Ostwald–de Waele | |
| Herschel–Bulkley | |
| Casson | |
| Cross |
| Kinetic Model | Equation | Parameter(s) |
|---|---|---|
| First-Order | , K1 | |
| Higuchi | KH | |
| Korsmeyer–Peppas | F = KKP × tn | KKP, n |
| Weibull | , α, β |
| MIC (µg/mL) | ||
|---|---|---|
| Test Media | ||
| Candida spp. | RPMI 1640 | Sabouraud Dextrose |
| C. albicans | 2 | 2 |
| C. glabrata | 4 | 2 |
| C. parapsilosis | 8 | 8 |
| Batch | PEG-90M (% w/w) | Carrageenan (% w/w) | Viscosity (cP) | Viscosity with SVF (cP) | Mucoadhesion (mN/cm2) | Mucoadhesion with SVF (mN/cm2) | Adhered Fluorescein (%) |
|---|---|---|---|---|---|---|---|
| LP-83 | 0.50 | 1.50 | 19,910 | 16,170 | 50.6 | 60.5 | 91.40 |
| LP-84 | 2.00 | 1.50 | 61,660 | 39,350 | 65.3 | 79.3 | 99.64 |
| LP-86 | 1.25 | 2.00 | 52,840 | 31,360 | 71.9 | 80.9 | 99.50 |
| LP-87 | 1.25 | 1.50 | 38,000 | 27,070 | 67.3 | 70.8 | 99.52 |
| LP-88 | 1.25 | 1.50 | 33,170 | 22,210 | 57.4 | 58.1 | 99.60 |
| LP-89 | 1.25 | 1.50 | 27,890 | 21,730 | 59.4 | 61.8 | 99.59 |
| LP-90 | 1.25 | 1.50 | 33,370 | 25,070 | 65.5 | 68.2 | 99.43 |
| LP-91 | 1.25 | 1.50 | 32,260 | 23,250 | 53.6 | 63.3 | 99.60 |
| LP-92 | 1.78 | 1.14 | 29,750 | 26,180 | 47.9 | 50.3 | 97.33 |
| LP-93 | 0.72 | 1.85 | 20,100 | 16,790 | 48.3 | 51.9 | 98.10 |
| LP-94 | 0.72 | 1.14 | 4720 | 10,490 | 34.9 | 38.8 | 90.28 |
| LP-95 | 1.78 | 1.85 | 87,280 | 40,590 | 64.8 | 74.9 | 99.54 |
| LP-96 | 1.25 | 1.00 | 10,000 | 13,690 | 38.4 | 47.8 | 92.42 |
| Quadratic Polynomial Model Equation | R2 | Adj R2 |
|---|---|---|
| Y1 = −0.8 + 14.40 X1 + 29.88 X2 | 0.702 | 0.642 |
| Y2 = 24,704 − 48,649 X1 − 23,052 X2 + 56,200 X1 X2 | 0.945 | 0.927 |
| Y3 = 28.85 + 32.60 X1 + 56.06 X2 − 6.652 X12 − 13.21 X22 − 7.48 X1X2 | 0.987 | 0.978 |
| PEG-90M | Carrageenan | Fluorescein Adhesion Fit | Mucoadhesion with SVF Fit | Viscosity Fit | Composite Desirability |
|---|---|---|---|---|---|
| 0.866 | 2 | 98.42 | 71.46 | 33,824 | 0.76 |
| Property | Theoretical Value (95% CI) | Placebo Gel | 0.5% D Gel | 1% D Gel | Bias (%) |
|---|---|---|---|---|---|
| Mucoadhesion (mN/cm2) | 71.5 (60.85; 82.07) | 72.9 | 68.2 | 69.4 | 1.86 |
| Viscosity (cP) | 33,824 (22,885; 44,763) | 29,835 | 30,400 | 32,780 | 8.30 |
| Fluorescein adhered (%) | 98.42 (97.23; 99.62) | 98.67 | 98.58 | 98.81 | 0.27 |
| Rheological Model | Placebo Gel | Placebo Gel with SVF | ||
|---|---|---|---|---|
| Chi² | r | Chi² | r | |
| Newton | 1.773 × 105 | 0.326 | 1.004 × 105 | −0.137 |
| Bingham | 1.147 × 104 | 0.970 | 6127 | 0.968 |
| Ostwald–de Waele | 8.997 | 1.000 | 13.18 | 0.999 |
| Herschel–Bulkley | 7.020 | 1.000 | 12.900 | 0.999 |
| Casson | 9.220 | 0.988 | 1791 | 0.991 |
| Cross | 1.164 | 1.000 | 1.359 | 1.000 |
| Cross Equation Parameter | Placebo Gel | Placebo Gel with SVF |
|---|---|---|
| 318.7 | 141.9 | |
| 0.1517 | 0.1600 | |
| 0.0422 | 0.1235 | |
| N | 0.6488 | 0.6853 |
| Parameter | Formulation | Mean ± SD |
|---|---|---|
| G′ | Placebo gel | 129.92 ± 25.20 Pa |
| Placebo gel with SVF | 149.66 ± 25.33 Pa | |
| G″ | Placebo gel | 57.90 ± 4.60 Pa |
| Placebo gel with SVF | 63.67 ± 4.01 Pa | |
| η* | Placebo gel | 22,709 ± 3657 mPa |
| Placebo gel with SVF | 25,947 ± 3649 mPa | |
| δ | Placebo gel | 24.20 ± 4.46° |
| Placebo gel with SVF | 23.67 ± 5.07° | |
| tan(δ) | Placebo gel | 0.45 ± 0.11 |
| Placebo gel with SVF | 0.44 ± 0.08 |
| Formulation | Model | Parameters | Value |
|---|---|---|---|
| 0.5% D gel | First-Order | K1 (h−1) Fmax (%) | 0.95 ± 0.48 85.79 ± 2.13 |
| 1% D gel | Weibull | α β Fmax (%) | 0.88 ± 0.43 0.73 ± 0.23 93.42 ± 3.21 |
| Formulation | Parameter | Mean | SD |
|---|---|---|---|
| 0.5% D gel | Jsup (µg/h·cm2) | 9.0106 | 0.6562 |
| R2 | 0.9113 | 0.0445 | |
| Kp (cm/h) | 0.2481 | 0.0181 | |
| Tlag (h) | 2.2834 | 0.6721 | |
| P (cm/h2) | 3.4376 | 1.2485 | |
| Dif (1/h) | 0.0763 | 0.0225 | |
| 1% D gel | Jsup (µg/h·cm2) | 32.9769 | 1.1737 |
| R2 | 0.9913 | 0.0001 | |
| Kp (cm/h) | 0.4542 | 0.0162 | |
| Tlag (h) | 1.1698 | 0.0514 | |
| P (cm/h2) | 3.1851 | 0.0265 | |
| Dif (1/h) | 0.1426 | 0.0063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajarin-Reinares, M.; Naveira-Souto, I.; Mallandrich, M.; Suñer-Carbó, J.; Llagostera Casas, M.; Calvo, M.A.; Fernandez-Campos, F. Repurposing Disulfiram as an Antifungal Agent: Development of a New Disulfiram Vaginal Mucoadhesive Gel. Pharmaceutics 2023, 15, 1436. https://doi.org/10.3390/pharmaceutics15051436
Lajarin-Reinares M, Naveira-Souto I, Mallandrich M, Suñer-Carbó J, Llagostera Casas M, Calvo MA, Fernandez-Campos F. Repurposing Disulfiram as an Antifungal Agent: Development of a New Disulfiram Vaginal Mucoadhesive Gel. Pharmaceutics. 2023; 15(5):1436. https://doi.org/10.3390/pharmaceutics15051436
Chicago/Turabian StyleLajarin-Reinares, Maria, Iria Naveira-Souto, Mireia Mallandrich, Joaquim Suñer-Carbó, Montserrat Llagostera Casas, Maria Angels Calvo, and Francisco Fernandez-Campos. 2023. "Repurposing Disulfiram as an Antifungal Agent: Development of a New Disulfiram Vaginal Mucoadhesive Gel" Pharmaceutics 15, no. 5: 1436. https://doi.org/10.3390/pharmaceutics15051436
APA StyleLajarin-Reinares, M., Naveira-Souto, I., Mallandrich, M., Suñer-Carbó, J., Llagostera Casas, M., Calvo, M. A., & Fernandez-Campos, F. (2023). Repurposing Disulfiram as an Antifungal Agent: Development of a New Disulfiram Vaginal Mucoadhesive Gel. Pharmaceutics, 15(5), 1436. https://doi.org/10.3390/pharmaceutics15051436