Establishment and Validation of a New Co-Culture for the Evaluation of the Permeability through the Blood–Brain Barrier in Patients with Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Products
2.2. Cell Culture and Permeability Studies
2.3. Analysis of the Samples
2.4. Permeability Calculation and Comparison between the Healthy BBB and the BBB with Glioblastoma
2.5. Statistical Analysis
3. Results and Discussion
3.1. Establishment of the New Co-Culture Models
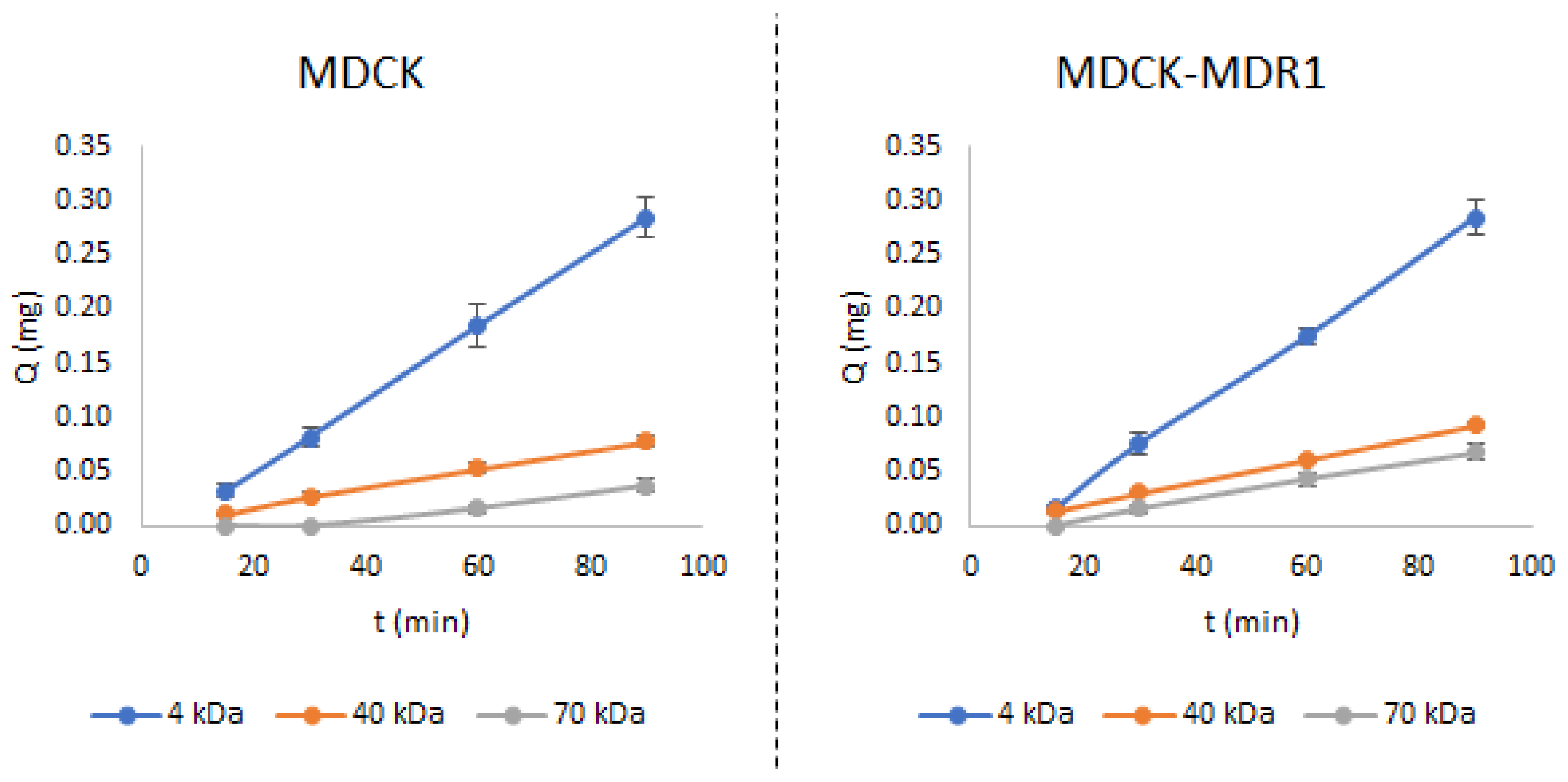
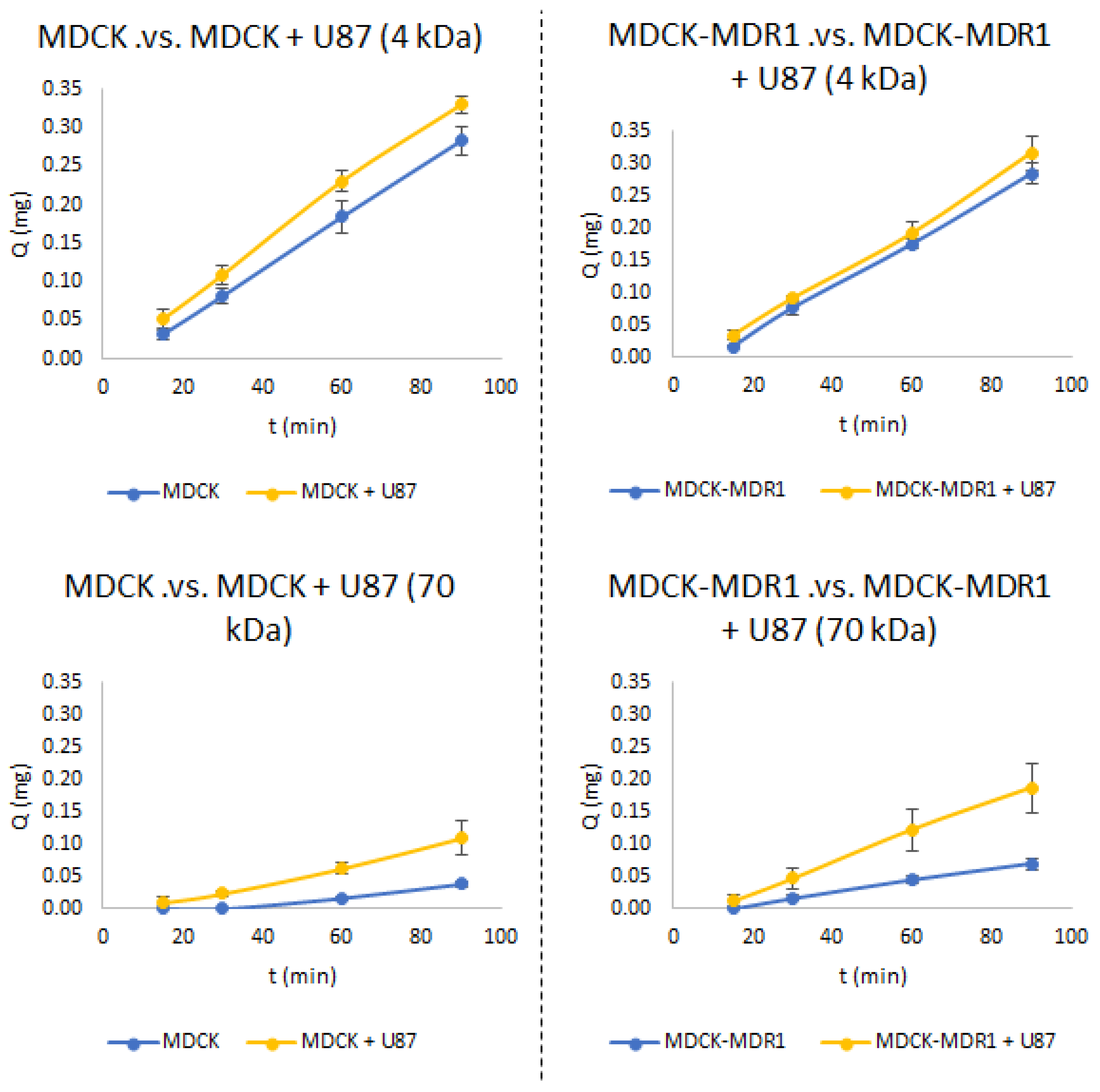
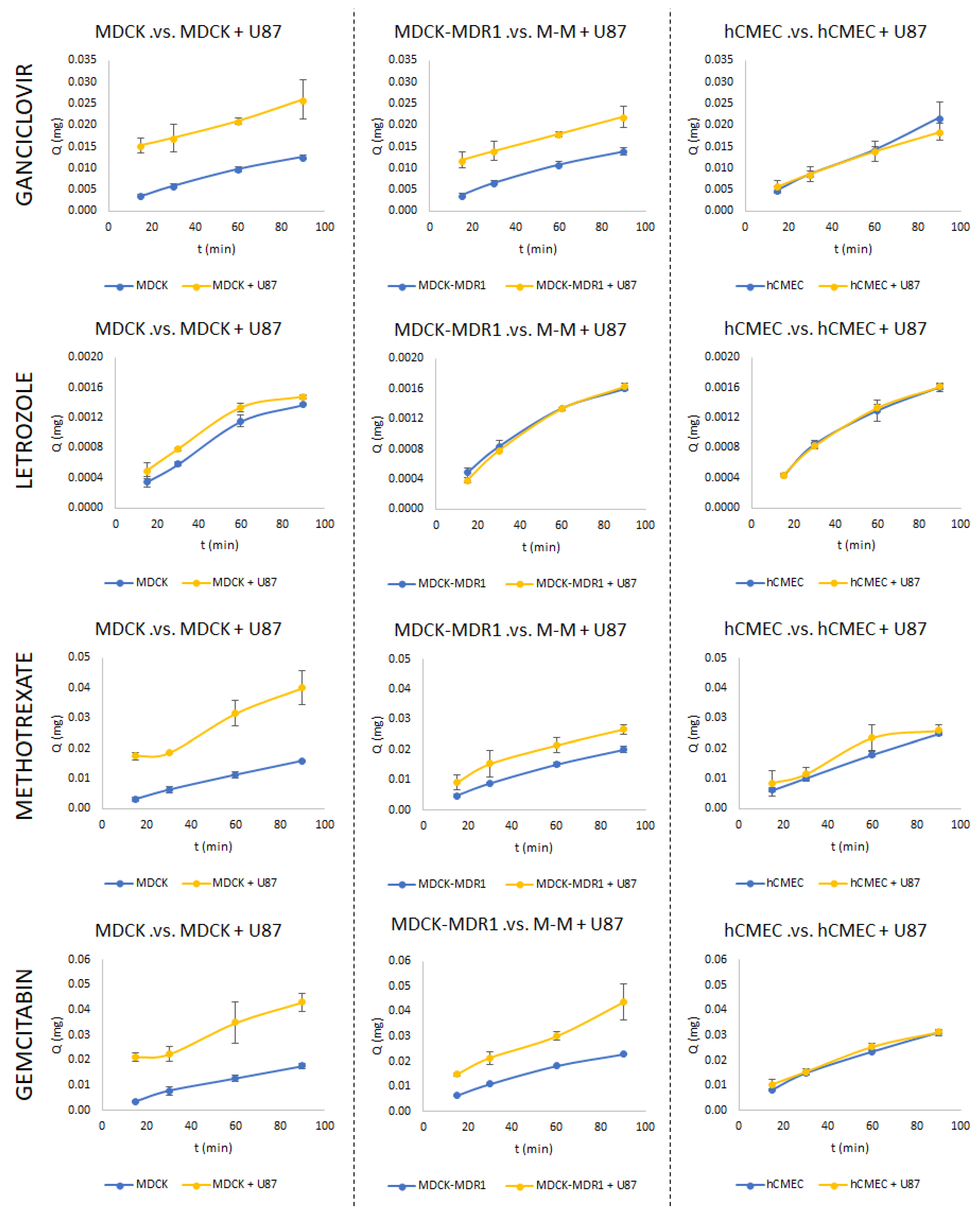
3.2. Permeability Studies
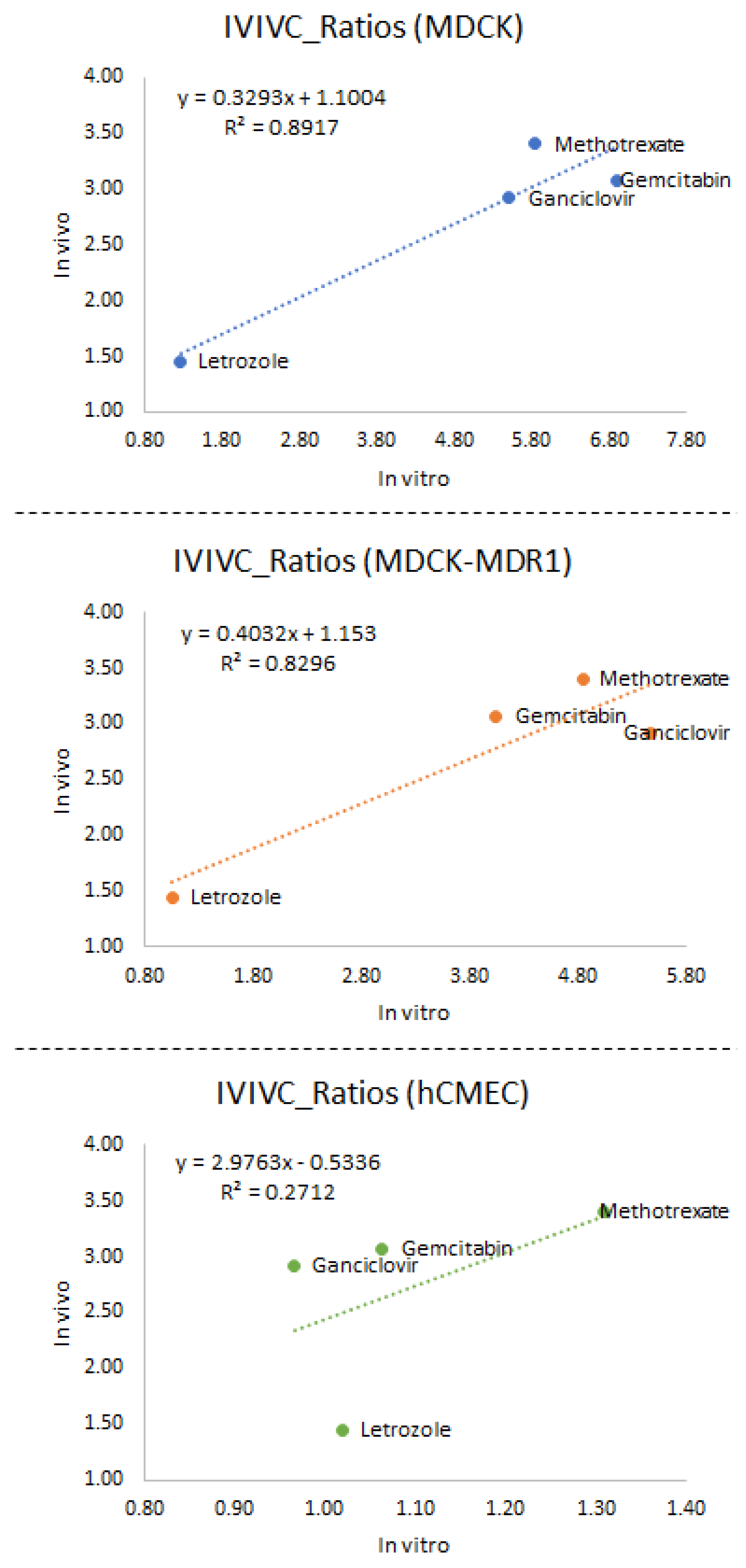
3.3. In Vitro–In Vivo Correlations
4. Conclusions
- (1)
- Checking the access of the molecule in the MDCK-MDR1 BBB model at different concentrations (one of them being the one selected as effective to kill glioma cells) to evaluate if it is or it is not a substrate of the Pgp transporter.
- (2)
- If the new molecule is a substrate of Pgp, then the access in glioblastoma illness should be evaluated by combining MDCK-MDR1 and U87-MG cells. If the molecule does not bind the Pgp, the permeability should be tested with the MDCK and U87-MG cell lines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbot, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Passeleu-Le Bourdonnec, C.; Carrupt, P.-A.; Scherrmann, J.M.; Martel, S. Methodologies to Assess Drug Permeation Through the Blood–Brain Barrier for Pharmaceutical Research. Pharm. Res. 2013, 30, 2729–2756. [Google Scholar] [CrossRef]
- Morofuji, Y.; Nakagawa, S. Drug Development for Central Nervous System Diseases Using In Vitro Blood-Brain Barrier Models and Drug Repositioning. Curr. Pharm. Des. 2020, 26, 1466–1485. [Google Scholar] [CrossRef]
- Bowman, P.D.; Ennis, S.R.; Rarey, K.E.; Lorris Betz, A.; Goldstein, G.W. Brain Microvessel Endothelial Cells in Tissue Culture: A Model for Study of Blood-Brain Barrier Permeability. Ann. Neurol. 1983, 14, 396–402. [Google Scholar] [CrossRef]
- Zenker, D.; Begley, D.; Bratzke, H.; Rubsamen-Waigmann, H.; von Briesen, H. Human Blood-Derived Macrophages Enhance Barrier Function of Cultured Primary Bovine and Human Brain Capillary Endothelial Cells. J. Physiol. 2003, 551, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Gutmann, H.; Krähenbühl, S.; Weksler, B.; Romero, I.; Couraud, P.-O.; Tuffin, G.; Drewe, J.; Huwyler, J. The Human Brain Endothelial Cell Line HCMEC/D3 as a Human Blood-Brain Barrier Model for Drug Transport Studies. J. Neurochem. 2008, 107, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain Barrier-specific Properties of a Human Adult Brain Endothelial Cell Line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In Vitro Models for the Blood-Brain Barrier. Toxicol. In Vitro 2005, 19, 299–334. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, J.J.; Jalkanen, A.J.; Kääriäinen, T.M.; Keski-Rahkonen, P.; Venäläinen, T.; Hokkanen, J.; Mönkkönen, J.; Suhonen, M.; Forsberg, M.M. Comparison of In Vitro Cell Models in Predicting In Vivo Brain Entry of Drugs. Int. J. Pharm. 2010, 402, 27–36. [Google Scholar] [CrossRef]
- Megard, I.; Garrigues, A.; Orlowski, S.; Jorajuria, S.; Clayette, P.; Ezan, E.; Mabondzo, A. A Co-Culture-Based Model of Human Blood–Brain Barrier: Application to Active Transport of Indinavir and In Vivo–In Vitro Correlation. Brain Res. 2002, 927, 153–167. [Google Scholar] [CrossRef]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. In Vitro and In Vivo Models of BBB to Evaluate Brain Targeting Drug Delivery. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–101. [Google Scholar]
- Walimbe, T.; Panitch, A.; Sivasankar, M.P. An In Vitro Scaffold-Free Epithelial-Fibroblast Coculture Model for the Larynx. Laryngoscope 2017, 127, E185–E192. [Google Scholar] [CrossRef] [PubMed]
- Kaeokhamloed, N.; Roger, E.; Béjaud, J.; Lautram, N.; Manero, F.; Perrot, R.; Briet, M.; Abbara, C.; Legeay, S. New In Vitro Coculture Model for Evaluating Intestinal Absorption of Different Lipid Nanocapsules. Pharmaceutics 2021, 13, 595. [Google Scholar] [CrossRef]
- Luyts, K.; Napierska, D.; Dinsdale, D.; Klein, S.G.; Serchi, T.; Hoet, P.H.M. A Coculture Model of the Lung–Blood Barrier: The Role of Activated Phagocytic Cells. Toxicol. In Vitro 2015, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Dingezweni, S. The Blood–Brain Barrier. S. Afr. J. Anaesth. Analg. 2020, 26, S32–S34. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-Brain Barrier Permeability and Physical Exercise. J. Neuroinflamm. 2019, 16, 15. [Google Scholar] [CrossRef]
- Mendes, B.; Marques, C.; Carvalho, I.; Costa, P.; Martins, S.; Ferreira, D.; Sarmento, B. Influence of Glioma Cells on a New Co-Culture In Vitro Blood–Brain Barrier Model for Characterization and Validation of Permeability. Int. J. Pharm. 2015, 490, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gynther, M.; Kääriäinen, T.M.; Hakkarainen, J.J.; Jalkanen, A.J.; Petsalo, A.; Lehtonen, M.; Peura, L.; Kurkipuro, J.; Samaranayake, H.; Ylä-Herttuala, S.; et al. Brain Pharmacokinetics of Ganciclovir in Rats with Orthotopic BT4C Glioma. Drug Metab. Dispos. 2015, 43, 140–146. [Google Scholar] [CrossRef]
- Dave, N.; Gudelsky, G.A.; Desai, P.B. The Pharmacokinetics of Letrozole in Brain and Brain Tumor in Rats with Orthotopically Implanted C6 Glioma, Assessed Using Intracerebral Microdialysis. Cancer Chemother. Pharmacol. 2013, 72, 349–357. [Google Scholar] [CrossRef]
- Devineni, D.; Klein-Szanto, A.; Gallo, J.M. In Vivo Microdialysis to Characterize Drug Transport in Brain Tumors: Analysis of Methotrexate Uptake in Rat Glioma-2 (RG-2)-Bearing Rats. Cancer Chemother. Pharmacol. 1996, 38, 499–507. [Google Scholar] [CrossRef]
- Apparaju, S.K.; Gudelsky, G.A.; Desai, P.B. Pharmacokinetics of Gemcitabine in Tumor and Non-Tumor Extracellular Fluid of Brain: An In Vivo Assessment in Rats Employing Intracerebral Microdialysis. Cancer Chemother. Pharmacol. 2007, 61, 223–229. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Sousa, F.; Bermejo, M.; González-Álvarez, M.; Sarmento, B. In Vitro Model for Predicting the Access and Distribution of Drugs in the Brain Using HCMEC/D3 Cells. Eur. J. Pharm. Biopharm. 2021, 163, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Modified Nonsink Equation for Permeability Estimation in Cell Monolayers: Comparison with Standard Methods. Mol. Pharm. 2014, 11, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Hebda, J.K.; Le Guelte, A.; Galan-Moya, E.-M.; Smith, S.S.; Azzi, S.; Bidere, N.; Gavard, J. Glioblastoma Cell-Secreted Interleukin-8 Induces Brain Endothelial Cell Permeability via CXCR2. PLoS ONE 2012, 7, e45562. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I.; Mészáros, M.; Bocsik, A.; Hellinger, É.; Vastag, M.; Rákhely, G.; et al. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model with Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front. Mol. Neurosci. 2018, 11, 166. [Google Scholar] [CrossRef]
- Mangas-Sanjuan, V.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Bermejo, M. In Vitro Methods for Assessing Drug Access to the Brain. In Advances in Non-Invasive Drug Delivery to the Brain; Future Science Ltd.: Huntington, WV, USA, 2015; pp. 44–61. ISBN 9781909453937. [Google Scholar]
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Innovative In Vitro Method to Predict Rate and Extent of Drug Delivery to the Brain across the Blood-Brain Barrier. Mol. Pharm. 2013, 10, 3822–3831. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; Gonzalez-Alvarez, I.; Bermejo, M.; Gonzalez-Alvarez, M. Physiologically Based Pharmacokinetic (PBPK) Modeling for Predicting Brain Levels of Drug in Rat. Pharmaceutics 2021, 13, 1402. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; González-Álvarez, M.; Bermejo, M. New In Vitro Methodology for Kinetics Distribution Prediction in the Brain. An Additional Step towards an Animal-Free Approach. Animals 2021, 11, 3521. [Google Scholar] [CrossRef]

| MW (g/mol) | Solubility logS (pH 7) | logP | Strongest acidic pKa | Strongest Basic pKa | Charge (pH 7.4) | |
|---|---|---|---|---|---|---|
| Ganciclovir | 255.23 | −1.4 | −1.66 | 10.16 | 0.58 | 0 |
| Letrozole | 285.30 | −3.6 | 2.50 | 1.89 | 0 | |
| Methotrexate | 454.43 | −3.7 | −1.85 | 2.95 | 14.55 | - |
| Gemcitabin | 263.19 | −1.1 | −1.40 | 11.52 | 3.65 | 0 |
| Drug | Transporters |
|---|---|
| Ganciclovir | - |
| Letrozole | ABCB1 (P-gp1) * |
| Methotrexate | ABCC3 *, ABCC4 *, ABCC1 *, ABCC2 *, ABCB1 (P-gp1) *, ABCG2 * SLCO1C1 **, SLC16A1 **, SLC15A1 ** |
| Gemcitabin | ABCB1 (P-gp1) * |
| MDCK | MDCK-MDR1 | hCMEC/D3 | Rat * | |
|---|---|---|---|---|
| Ganciclovir | 5.51 | 5.47 | 0.97 | 2.92 |
| Letrozole | 1.28 | 1.06 | 1.02 | 1.45 |
| Methotrexate | 5.83 | 4.85 | 1.31 | 3.40 |
| Gemcitabin | 6.91 | 4.05 | 1.06 | 3.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Dengra, B.; García-Montoya, E.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Establishment and Validation of a New Co-Culture for the Evaluation of the Permeability through the Blood–Brain Barrier in Patients with Glioblastoma. Pharmaceutics 2023, 15, 1431. https://doi.org/10.3390/pharmaceutics15051431
Sánchez-Dengra B, García-Montoya E, González-Álvarez I, Bermejo M, González-Álvarez M. Establishment and Validation of a New Co-Culture for the Evaluation of the Permeability through the Blood–Brain Barrier in Patients with Glioblastoma. Pharmaceutics. 2023; 15(5):1431. https://doi.org/10.3390/pharmaceutics15051431
Chicago/Turabian StyleSánchez-Dengra, Bárbara, Elena García-Montoya, Isabel González-Álvarez, Marival Bermejo, and Marta González-Álvarez. 2023. "Establishment and Validation of a New Co-Culture for the Evaluation of the Permeability through the Blood–Brain Barrier in Patients with Glioblastoma" Pharmaceutics 15, no. 5: 1431. https://doi.org/10.3390/pharmaceutics15051431
APA StyleSánchez-Dengra, B., García-Montoya, E., González-Álvarez, I., Bermejo, M., & González-Álvarez, M. (2023). Establishment and Validation of a New Co-Culture for the Evaluation of the Permeability through the Blood–Brain Barrier in Patients with Glioblastoma. Pharmaceutics, 15(5), 1431. https://doi.org/10.3390/pharmaceutics15051431









