Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design and Optimization Process
2.2.2. Preparation of Carvedilol-Loaded BSA-Based Nanoparticles
2.2.3. In Vitro Evaluation of Carvedilol-Loaded BSA-Based Nanoparticles
2.2.4. In Vitro Evaluation of Optimized Carvedilol-Loaded BSA-Based Nanoparticle Formulation
2.2.5. In Vivo Evaluation of Optimized Carvedilol-Loaded BSA-Based Nanoparticle Formulation
3. Results and Discussion
3.1. Preparation of Carvedilol-Loaded BSA-Based Nanoparticles
3.2. Experimental Design and Statistical Analysis
3.2.1. Effect of Independent Factors (A and B) on the Particle Size of Carvedilol-Loaded Nanoparticles (Y1 Response)
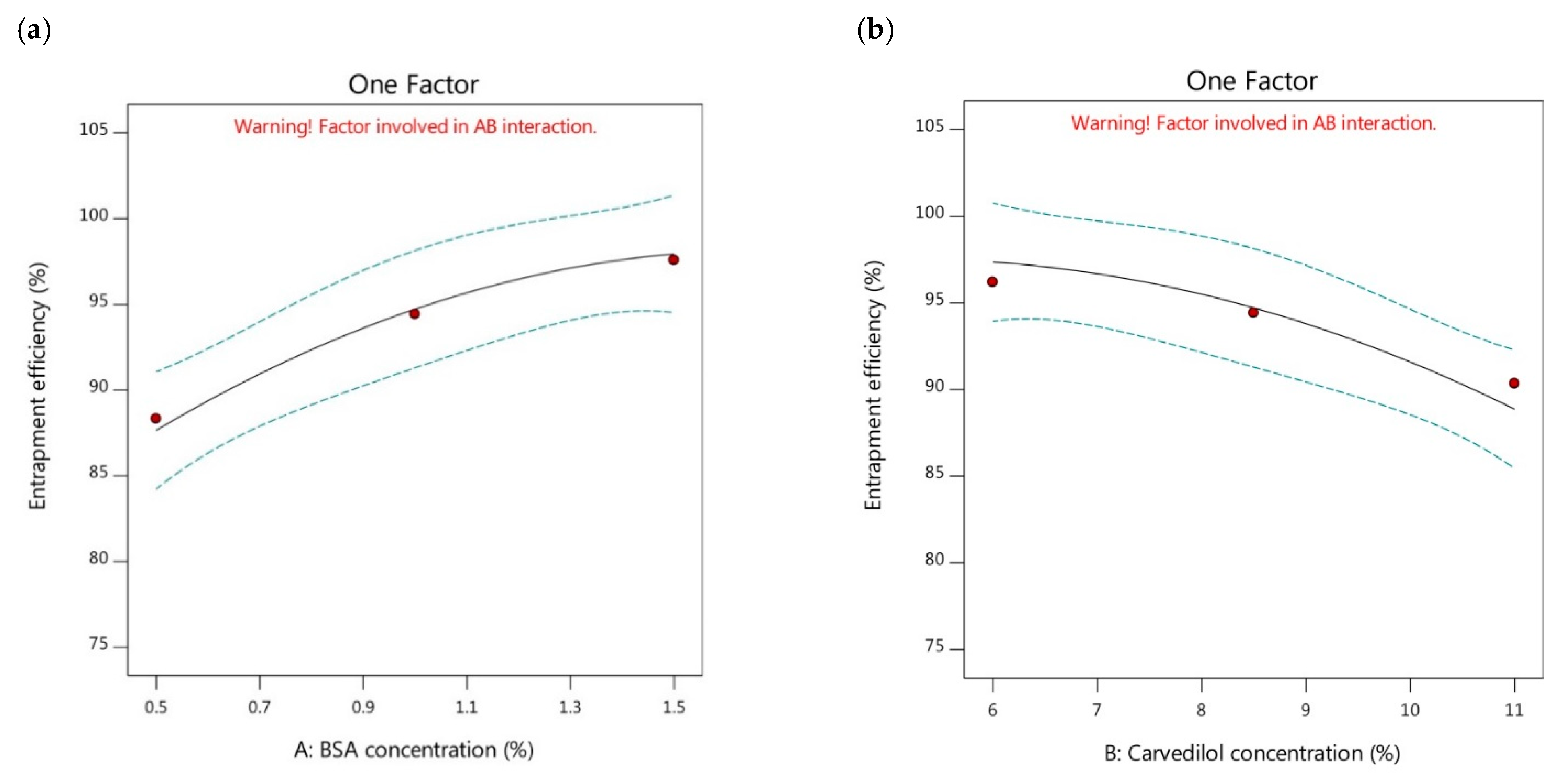
3.2.2. Effect of Independent Factors (A and B) on Entrapment Efficiency of Carvedilol-Loaded Nanoparticles (Y2 Response)
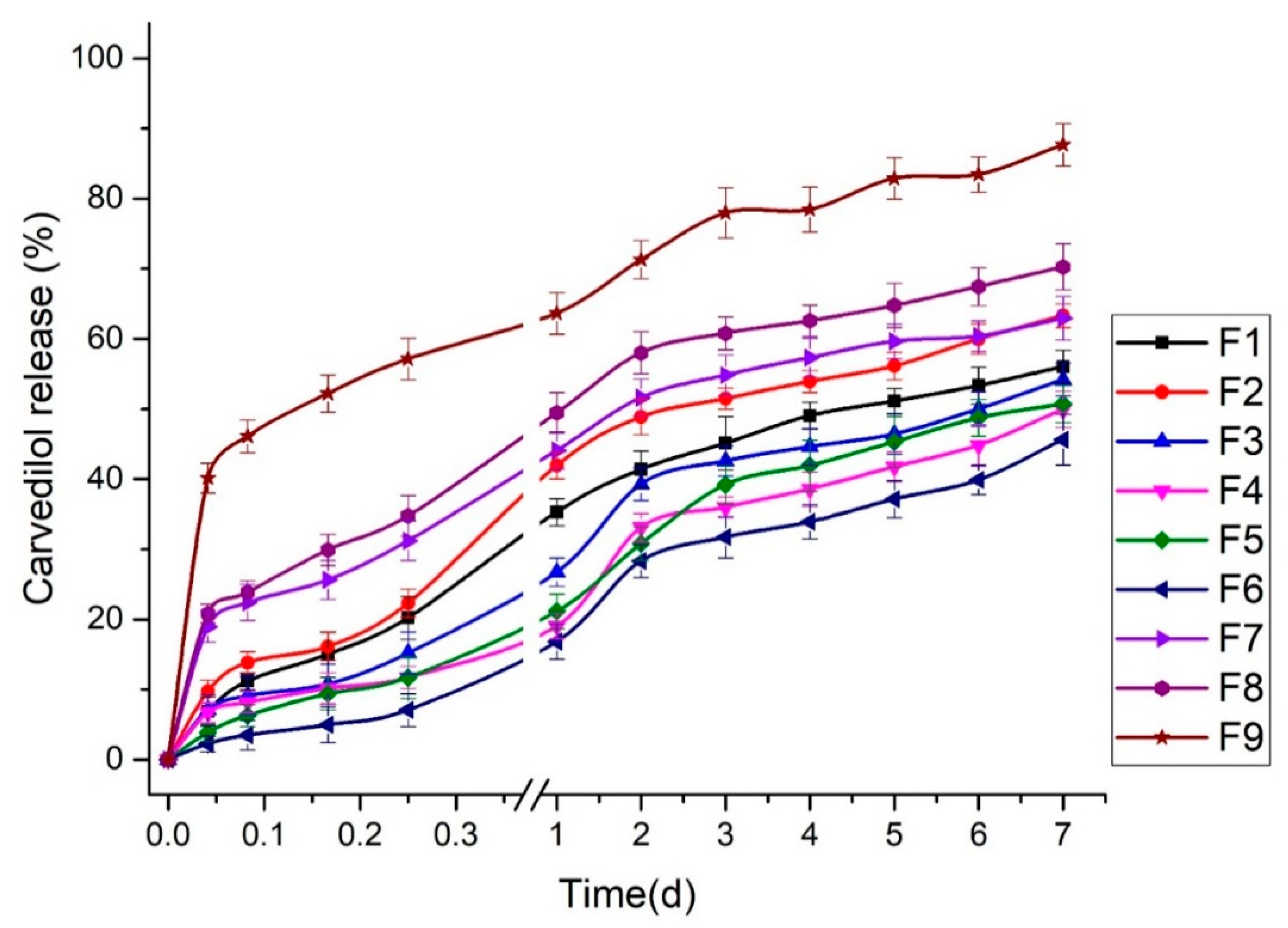
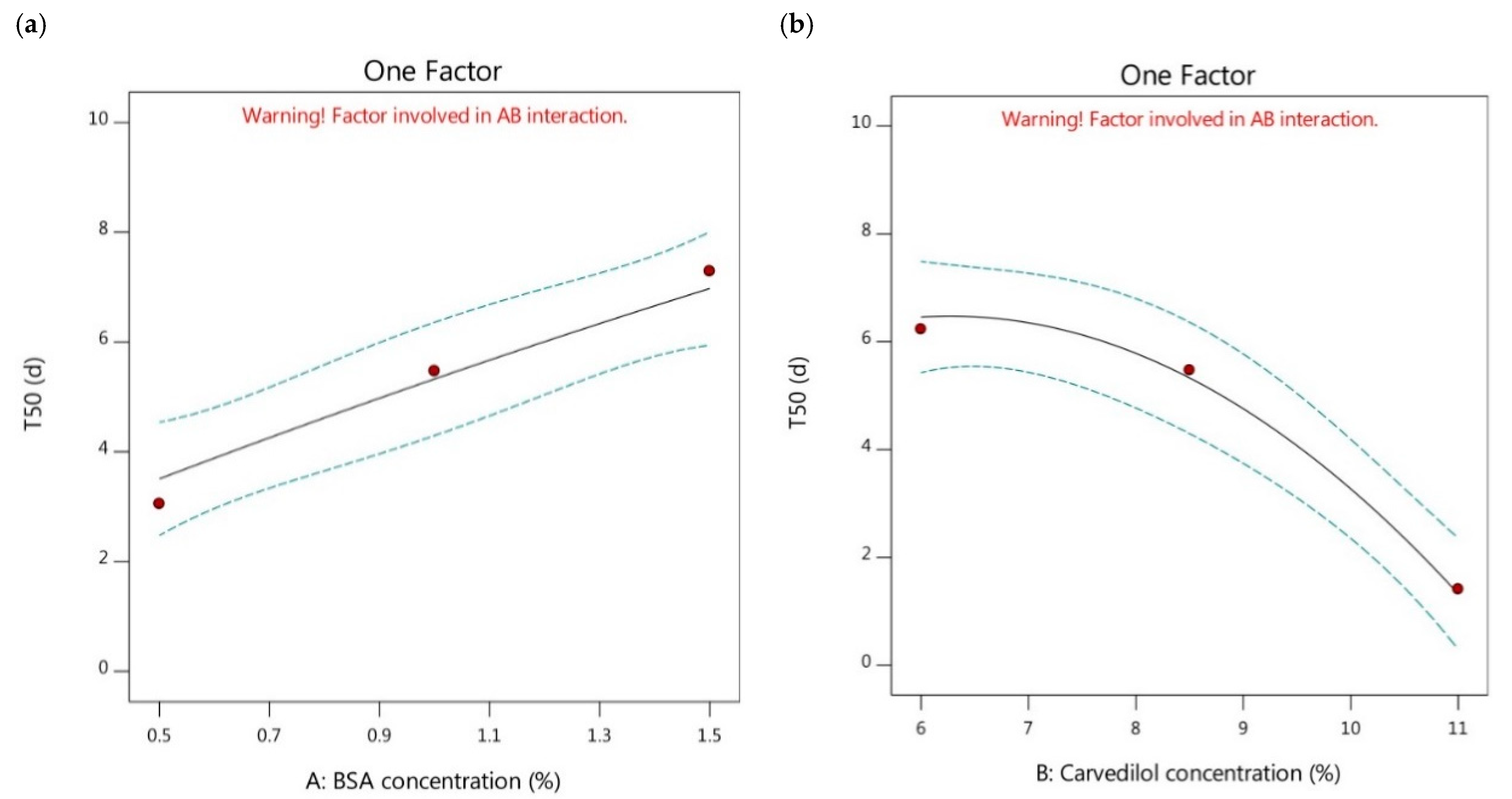
3.2.3. Effect of Independent Factors (A and B) on T50 of Carvedilol-Loaded Nanoparticles (Y3 Response)
3.2.4. Optimization Process
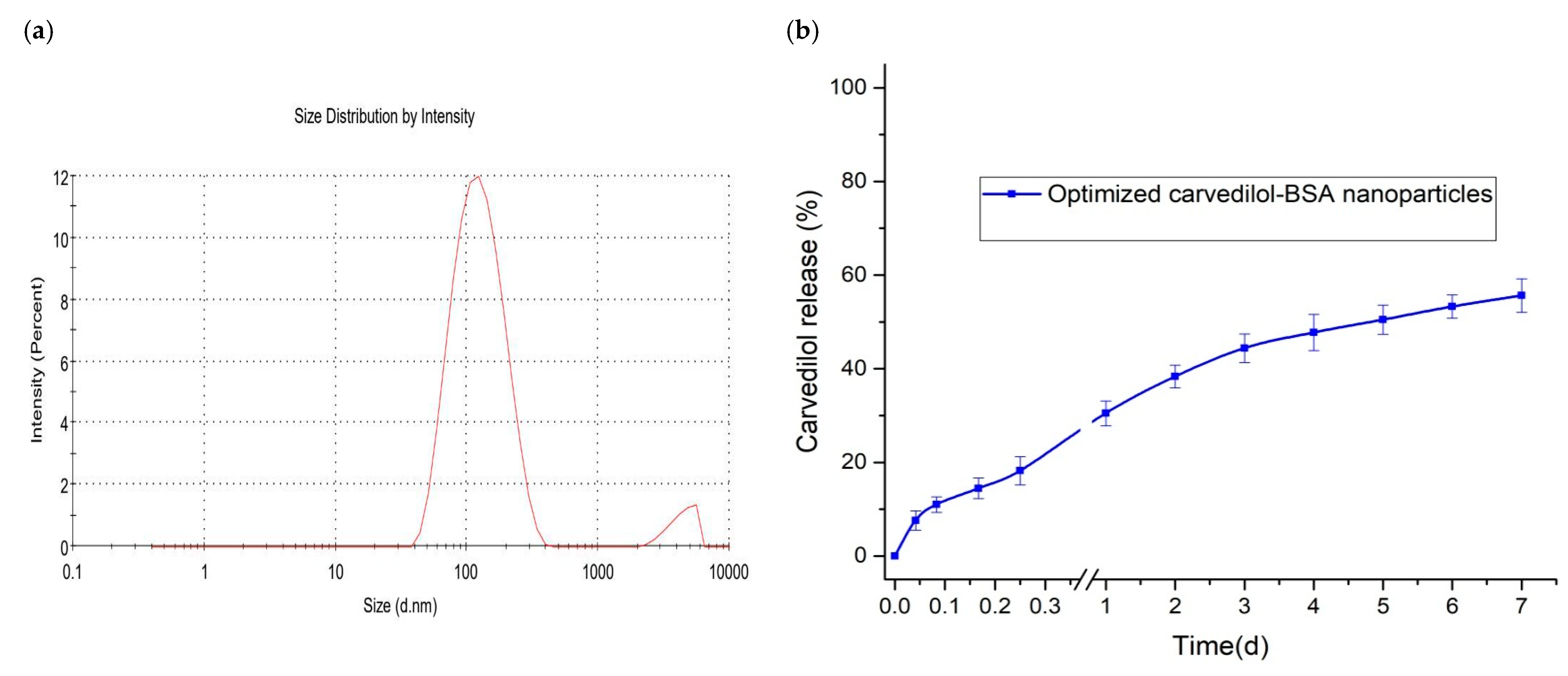
3.3. In Vitro Evaluation of Optimized Carvedilol-Loaded Nanoparticle Formulation
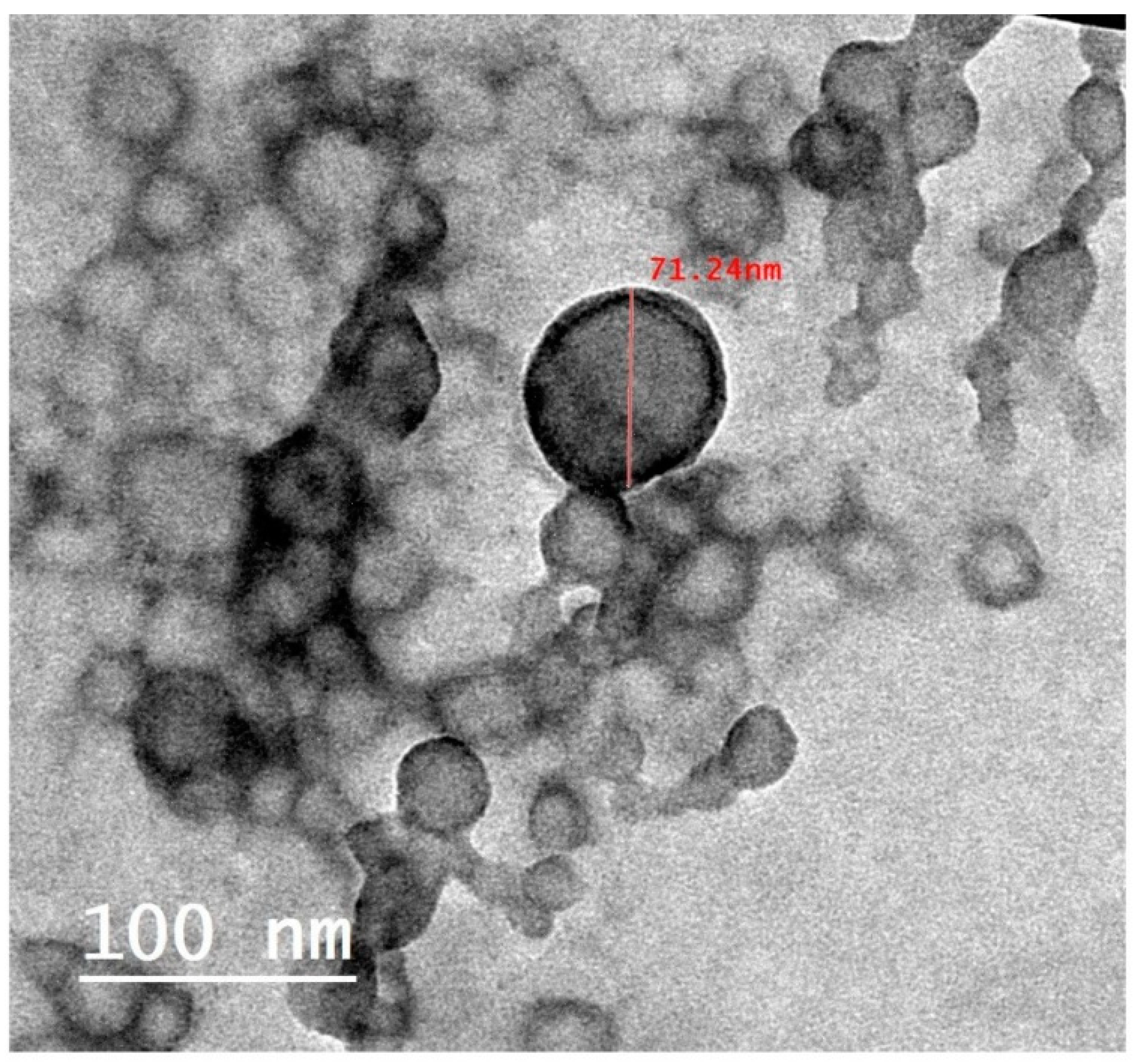
3.3.1. TEM Study
3.3.2. DSC Study
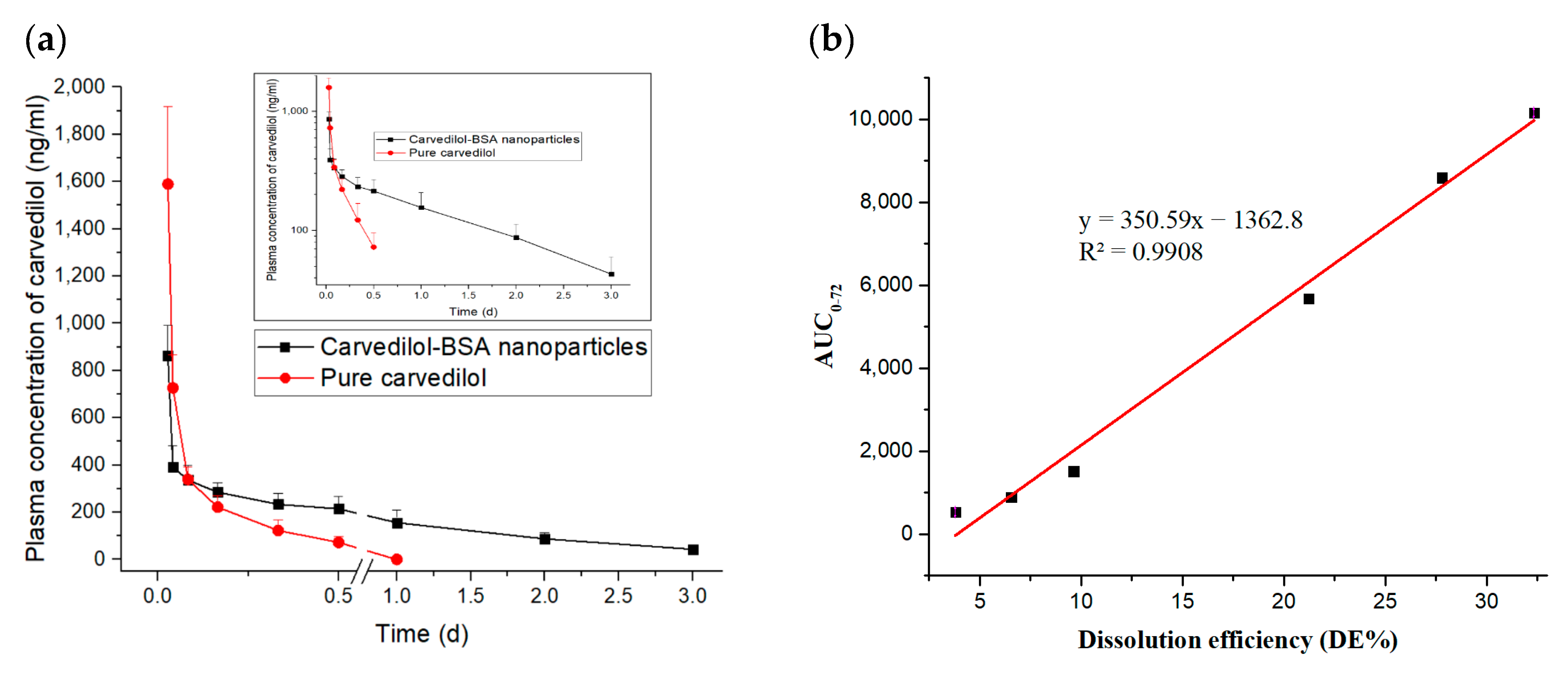
3.4. In Vivo Evaluation of Optimized Carvedilol-Loaded Nanoparticle Formulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Hypertension. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension. (accessed on 2 January 2023).
- Alam, T.; Khan, S.; Gaba, B.; Haider, M.F.; Baboota, S.; Ali, J. Nanocarriers as Treatment Modalities for Hypertension. Drug Deliv. 2017, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Hypertension. 2022. Available online: https://www.fda.gov/consumers/minority-health-and-health-equity-resources/hypertension (accessed on 2 January 2023).
- Food and Drug Administration (FDA). High Blood Pressure–Understanding the Silent Killer. 2021. Available online: https://www.fda.gov/drugs/special-features/high-blood-pressure-understanding-silent-killer (accessed on 2 January 2023).
- Attia, M.S.; Hasan, A.A.; Ghazy, F.S.; Gomaa, E. Solid Dispersion as a Technical Solution to Boost the Dissolution Rate and Bioavailability of Poorly Water-Soluble Drugs. Indian J. Pharm. Educ. Res. 2021, 55, s327–s339. [Google Scholar] [CrossRef]
- El-Say, K.M.; Hosny, K.M. Optimization of Carvedilol Solid Lipid Nanoparticles: An Approach to Control the Release and Enhance the Oral Bioavailability on Rabbits. PLoS ONE 2018, 13, e0203405. [Google Scholar] [CrossRef] [PubMed]
- Nava, G.; Piñón, E.; Mendoza, L.; Mendoza, N.; Quintanar, D.; Ganem, A. Formulation and In Vitro, Ex Vivo and In Vivo Evaluation of Elastic Liposomes for Transdermal Delivery of Ketorolac Tromethamine. Pharmaceutics 2011, 3, 954–970. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Upgrading of Dissolution and Anti-Hypertensive Effect of Carvedilol via Two Combined Approaches: Self-Emulsification and Liquisolid Techniques. Drug Dev. Ind. Pharm. 2018, 44, 873–885. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D. Development and In Vivo Evaluation of an Innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” Formulation with Sustained Release and Enhanced Oral Bioavailability for Potential Hypertension Treatment in Pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Tian, B.; Yuan, K.; Pan, H.; Ma, S.; Yang, X.; Pan, W. Fabrication of Carvedilol Nanosuspensions Through the Anti-Solvent Precipitation–Ultrasonication Method for the Improvement of Dissolution Rate and Oral Bioavailability. AAPS PharmSciTech 2012, 13, 295–304. [Google Scholar] [CrossRef]
- Öztürk, K.; Arslan, F.B.; Öztürk, S.C.; Çalış, S. Mixed Micelles Formulation for Carvedilol Delivery: In-Vitro Characterization and In-Vivo Evaluation. Int. J. Pharm. 2022, 611, 121294. [Google Scholar] [CrossRef]
- Shamma, R.N.; Basha, M. Soluplus®: A Novel Polymeric Solubilizer for Optimization of Carvedilol Solid Dispersions: Formulation Design and Effect of Method of Preparation. Powder Technol. 2013, 237, 406–414. [Google Scholar] [CrossRef]
- Tazhbayev, Y.; Galiyeva, A.; Zhumagaliyeva, T.; Burkeyev, M.; Karimova, B. Isoniazid—Loaded Albumin Nanoparticles: Taguchi Optimization Method. Polymers 2021, 13, 3808. [Google Scholar] [CrossRef]
- Hobson, J.; Savage, A.; Dwyer, A.; Unsworth, C.; Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.; Neary, M.; et al. Scalable Nanoprecipitation of Niclosamide and In Vivo Demonstration of Long-Acting Delivery After Intramuscular Injection. Nanoscale 2021, 13, 6410–6416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-Based Nanoparticles: From Preparation to Encapsulation of Active Molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A Review on Blending of Corn Starch with Natural and Synthetic Polymers, and Inorganic Nanoparticles with Mathematical Modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Kandav, G.; Bhatt, D.C.; Jindal, D.K.; Singh, S.K. Formulation, Optimization, and Evaluation of Allopurinol-Loaded Bovine Serum Albumin Nanoparticles for Targeting Kidney in Management of Hyperuricemic Nephrolithiasis. AAPS PharmSciTech 2020, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Zhang, X. Strategies for Preparing Albumin-Based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Karami, E.; Behdani, M.; Kazemi-Lomedasht, F. Albumin Nanoparticles as Nanocarriers for Drug Delivery: Focusing on Antibody and Nanobody Delivery and Albumin-Based Drugs. J. Drug Deliv. Sci. Technol. 2020, 55, 101471. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-Based Nanoparticles: A Promising Strategy to Overcome Cancer Drug Resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Ayoub, M.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Gomaa, E. Investigation of Alogliptin-Loaded In Situ Gel Implants by 23 Factorial Design with Glycemic Assessment in Rats. Pharmaceutics 2022, 14, 1867. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhattacharya, S.; Pal, T.K. Application of Statistical Design to Evaluate Critical Process Parameters and Optimize Formulation Technique of Polymeric Nanoparticles. R. Soc. Open Sci. 2019, 6, 190896. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Von Storp, B.; Engel, A.; Boeker, A.; Ploeger, M.; Langer, K. Albumin Nanoparticles with Predictable Size by Desolvation Procedure. J. Microencapsul. 2012, 29, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hong, G.; Liu, Z.; Yang, D.; Kankala, R.K.; Wu, W. Synergistic Antitumor Efficacy of Doxorubicin and Gambogic Acid-Encapsulated Albumin Nanocomposites. Colloids Surf. B Biointerfaces 2020, 196, 111286. [Google Scholar] [CrossRef]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of Some Formulation Parameters on Flurbiprofen Encapsulation and Release Rates of Niosomes Prepared from Proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef]
- Gao, Y.; Nai, J.; Yang, Z.; Zhang, J.; Ma, S.; Zhao, Y.; Li, H.; Li, J.; Yang, Y.; Yang, M.; et al. A Novel Preparative Method for Nanoparticle Albumin-Bound Paclitaxel with High Drug Loading and its Evaluation Both In Vitro and In Vivo. PLoS ONE 2021, 16, e0250670. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, J.; Zhao, W.; Shen, Y.; Song, Q.; Shum, H.C.; Wang, Y.; Rong, J. Biodegradable Celastrol-Loaded Albumin Nanoparticles Ameliorate Inflammation and Lipid Accumulation in Diet-Induced Obese Mice. Biomater. Sci. 2022, 10, 984–996. [Google Scholar] [CrossRef]
- International Council on Harmonization (ICH). ICH Guideline Q3C (R6) on Impurities: Guideline for Residual Solvents. 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-33.pdf (accessed on 19 March 2023).
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; Von Briesen, H.; Schubert, D. Optimization of the Preparation Process for Human Serum Albumin (HSA) Nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Kufleitner, J.; Worek, F.; Kreuter, J. Incorporation of Obidoxime into Human Serum Albumin Nanoparticles: Optimisation of Preparation Parameters for the Development of a Stable Formulation. J. Microencapsul. 2010, 27, 594–601. [Google Scholar] [CrossRef]
- Aniesrani Delfiya, D.S.; Thangavel, K.; Amirtham, D. Preparation of Curcumin Loaded Egg Albumin Nanoparticles Using Acetone and Optimization of Desolvation Process. Protein J. 2016, 35, 124–135. [Google Scholar] [CrossRef]
- Esim, O.; Gedik, M.E.; Dogan, A.L.; Gunaydin, G.; Hascicek, C. Development of Carboplatin Loaded Bovine Serum Albumin Nanoparticles and Evaluation of its Effect on an Ovarian Cancer Cell Line. J. Drug Deliv. Sci. Technol. 2021, 64, 102655. [Google Scholar] [CrossRef]
- İnan, B.; Özçimen, D. Microalgal Bioprocess Toward the Production of Microalgal Oil Loaded Bovine Serum Albumin Nanoparticles. Protein J. 2021, 40, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Choi, G.; Piao, H.; Choy, J. Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine Against COVID-19. Materials 2021, 14, 3792. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Selvam, J.; Mukundan, G.K.; Premakumari, K.B.; Jenita, J.L. Albumin Nanoparticles Coated with Polysorbate 80 for the Targeted Delivery of Antiepileptic Drug Levetiracetam into the Brain. Drug Deliv. Transl. Res. 2020, 10, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; et al. Development of Meloxicam-Human Serum Albumin Nanoparticles for Nose-to-Brain Delivery via Application of a Quality by Design Approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Huang, X.; Wu, H. Development of a Novel Intraarticular Injection of Diclofenac for the Treatment of Arthritis: A Preclinical Study in the Rabbit Model. Acta Biochim. Pol. 2021, 68, 71–76. [Google Scholar] [CrossRef]
- Şenol, Y.; Kaplan, O.; Varan, C.; Demirtürk, N.; Öncül, S.; Fidan, B.B.; Ercan, A.; Bilensoy, E.; Çelebier, M. Pharmacometabolomic Assessment of Vitamin E Loaded Human Serum Albumin Nanoparticles on HepG2 Cancer Cell Lines. J. Drug Deliv. Sci. Technol. 2023, 79, 104017. [Google Scholar] [CrossRef]
- Kimura, K.; Yamasaki, K.; Nakamura, H.; Haratake, M.; Taguchi, K.; Otagiri, M. Preparation and In Vitro Analysis of Human Serum Albumin Nanoparticles Loaded with Anthracycline Derivatives. Chem. Pharm. Bull. 2018, 66, 382–390. [Google Scholar] [CrossRef]
- Abolhassani, H.; Shojaosadati, S.A. A Comparative and Systematic Approach to Desolvation and Self-Assembly Methods for Synthesis of Piperine-Loaded Human Serum Albumin Nanoparticles. Colloids Surf. B Biointerfaces 2019, 184, 110534. [Google Scholar] [CrossRef]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, Characterization and Biodistribution of the Lactone form of 10-Hydroxycamptothecin (HCPT)-Loaded Bovine Serum Albumin (BSA) Nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef]
- Jose, P.; Sundar, K.; Anjali, C.H.; Ravindran, A. Metformin-Loaded BSA Nanoparticles in Cancer Therapy: A New Perspective for an Old Antidiabetic Drug. Cell Biochem. Biophys. 2014, 71, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Llabot, J.M.; Redin, I.L.; Agüeros, M.; Caballero, M.J.D.; Boiero, C.; Irache, J.M.; Allemandi, D. In Vitro Characterization of New Stabilizing Albumin Nanoparticles as a Potential Topical Drug Delivery System in the Treatment of Corneal Neovascularization (CNV). J. Drug Deliv. Sci. Technol. 2019, 52, 379–385. [Google Scholar] [CrossRef]
- Gomaa, E.; Eissa, N.G.; Ibrahim, T.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Ayoub, M.M. Development of Depot PLGA-Based In-Situ Implant of Linagliptin: Sustained Release and Glycemic Control. Saudi Pharm. J. 2023, 31, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Farahnaky, A.; Badii, F.; Farhat, I.A.; Mitchell, J.R.; Hill, S.E. Enthalpy Relaxation of Bovine Serum Albumin and Implications for its Storage in the Glassy State. Biopolymers 2005, 78, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; El-Kamel, A.H.; Hammad, G.O.; Heikal, L.A. Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal. Pharmaceutics 2022, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.K.; Ahmad, E.; Khan, J.M.; Alam, P.; Ishtikhar, M.; Khan, R.H. Elucidating the interaction of limonene with bovine serum albumin: A multi-technique approach. Mol. BioSyst. 2015, 11, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, M.; Matsuki, H.; Kaneshina, S.; Ogli, K. Study on the Interaction between Bovine Serum Albumin and Inhalation Anesthetic Halothane by Differential Scanning Calorimetry; Elsevier: Amsterdam, The Netherlands, 2005; pp. 326–327. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Lei, L.; Xia, X.; Wang, X.; Tong, F.; Li, Y.; Gao, H. Co-Delivery of Ibrutinib and Hydroxychloroquine by Albumin Nanoparticles for Enhanced Chemotherapy of Glioma. Int. J. Pharm. 2023, 630, 122436. [Google Scholar] [CrossRef]


| Independent Factors | Unit | Symbol | Actual Levels (Coded) | ||
|---|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |||
| BSA concentration | % | A | 0.5 | 1 | 1.5 |
| Carvedilol percentage in BSA nanoparticles | % | B | 6 | 8.5 | 11 |
| Dependent responses | Unit | Symbol | Goal | ||
| Particle size | nm | Y1 | Minimize | ||
| Entrapment efficiency | % | Y2 | Maximize | ||
| T50 | d | Y3 | Target to 3.5 d | ||
| Formulation | Actual Levels | Responses | |||
|---|---|---|---|---|---|
| A (%) | B (%) | Y1 (nm) | Y2 (%) | Y3 (d) | |
| F1 | 0.5 | 6 | 123.33 ± 7.83 | 93.14 ± 0.97 | 4.46 ± 0.62 |
| F2 | 0.5 | 8.5 | 147.84 ± 22.84 | 88.32 ± 1.03 | 3.05 ± 0.38 |
| F3 | 1 | 8.5 | 177.56 ± 13.21 | 94.41 ± 0.77 | 5.47 ± 0.71 |
| F4 | 1.5 | 8.5 | 196.66 ± 16.11 | 97.57 ± 0.91 | 7.29 ± 0.79 |
| F5 | 1 | 6 | 148.60 ± 22.16 | 96.19 ± 1.31 | 6.23 ± 0.78 |
| F6 | 1.5 | 6 | 167.83 ± 7.08 | 98.89 ± 1.02 | 8.51 ± 0.79 |
| F7 | 1.5 | 11 | 243.96 ± 13.26 | 94.13 ± 0.98 | 2.25 ± 0.55 |
| F8 | 1 | 11 | 210.36 ± 13.39 | 90.34 ± 0.42 | 1.41 ± 0.33 |
| F9 | 0.5 | 11 | 186.30 ± 18.41 | 78.28 ± 1.25 | 0.14 ± 0.05 |
| Source | Y1 Response | Y2 Response | Y3 Response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | Significance | F-Value | p-Value | Significance | F-Value | p-Value | Significance | |
| Model | 164.01 | 0.0007 | Significant | 29.31 | 0.0095 | Significant | 66.20 | 0.0029 | Significant |
| A | 292.75 | 0.0004 | Significant | 76.26 | 0.0032 | Significant | 95.27 | 0.0023 | Significant |
| B | 518.14 | 0.0002 | Significant | 51.98 | 0.0055 | Significant | 208.89 | 0.0007 | Significant |
| AB | 3.34 | 0.1652 | Non-significant | 12.26 | 0.0394 | Significant | 4.97 | 0.1120 | Non-significant |
| A2 | 0.217 | 0.6731 | Non-significant | 3.56 | 0.1555 | Non-significant | 0.0794 | 0.7964 | Non-significant |
| B2 | 5.63 | 0.0983 | Non-significant | 2.48 | 0.2136 | Non-significant | 21.82 | 0.0185 | Significant |
| Formulation | Zero Order Model | First Order Model | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | ||
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | T50 | |
| F1 | 0.4897 | 0.6793 | 0.9095 | 0.6239 | 0.9854 | 0.327 | 4.46 |
| F2 | 0.4309 | 0.6665 | 0.8847 | 0.5990 | 0.9789 | 0.313 | 3.05 |
| F3 | 0.6357 | 0.7785 | 0.9559 | 0.7370 | 0.9880 | 0.374 | 5.47 |
| F4 | 0.7322 | 0.8309 | 0.9781 | 0.8023 | 0.9910 | 0.412 | 7.29 |
| F5 | 0.7837 | 0.8824 | 0.9909 | 0.8547 | 0.9951 | 0.447 | 6.23 |
| F6 | 0.8429 | 0.9075 | 0.9882 | 0.8887 | 0.9882 | 0.503 | 8.51 |
| F7 | −0.0716 | 0.2968 | 0.6720 | 0.1896 | 0.9929 | 0.226 | 2.25 |
| F8 | −0.0883 | 0.3443 | 0.6621 | 0.2227 | 0.9915 | 0.224 | 1.41 |
| F9 | −1.1107 | 0.3320 | 0.0792 | −0.2575 | 0.9962 | 0.140 | 0.14 |
| Optimized | 0.5762 | 0.7423 | 0.9436 | 0.6948 | 0.9959 | 0.348 | 4.77 |
| Parameters | Pure Carvedilol Suspension | Optimized Carvedilol-BSA Nanoparticles |
|---|---|---|
| t1/2 (h) | 4.34 ± 0.77 | 23.56 ± 1.92 |
| AUC0–72 (ng/mL.h) | 3115.09 ± 784.53 | 10,154.75 ± 2652.49 |
| AUC0–∞ (ng/mL.h) | 3671.54 ± 1041.31 | 11,804.37 ± 3425.80 |
| MRT (h) | 4.98 ± 0.77 | 32.21 ± 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, M.S.; Radwan, M.F.; Ibrahim, T.S.; Ibrahim, T.M. Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance. Pharmaceutics 2023, 15, 1425. https://doi.org/10.3390/pharmaceutics15051425
Attia MS, Radwan MF, Ibrahim TS, Ibrahim TM. Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance. Pharmaceutics. 2023; 15(5):1425. https://doi.org/10.3390/pharmaceutics15051425
Chicago/Turabian StyleAttia, Mohamed S., Mohamed F. Radwan, Tarek S. Ibrahim, and Tarek M. Ibrahim. 2023. "Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance" Pharmaceutics 15, no. 5: 1425. https://doi.org/10.3390/pharmaceutics15051425
APA StyleAttia, M. S., Radwan, M. F., Ibrahim, T. S., & Ibrahim, T. M. (2023). Development of Carvedilol-Loaded Albumin-Based Nanoparticles with Factorial Design to Optimize In Vitro and In Vivo Performance. Pharmaceutics, 15(5), 1425. https://doi.org/10.3390/pharmaceutics15051425








