Virtual Screening-Based Study of Novel Anti-Cancer Drugs Targeting G-Quadruplex
Abstract
1. Introduction
2. Materials and Methods
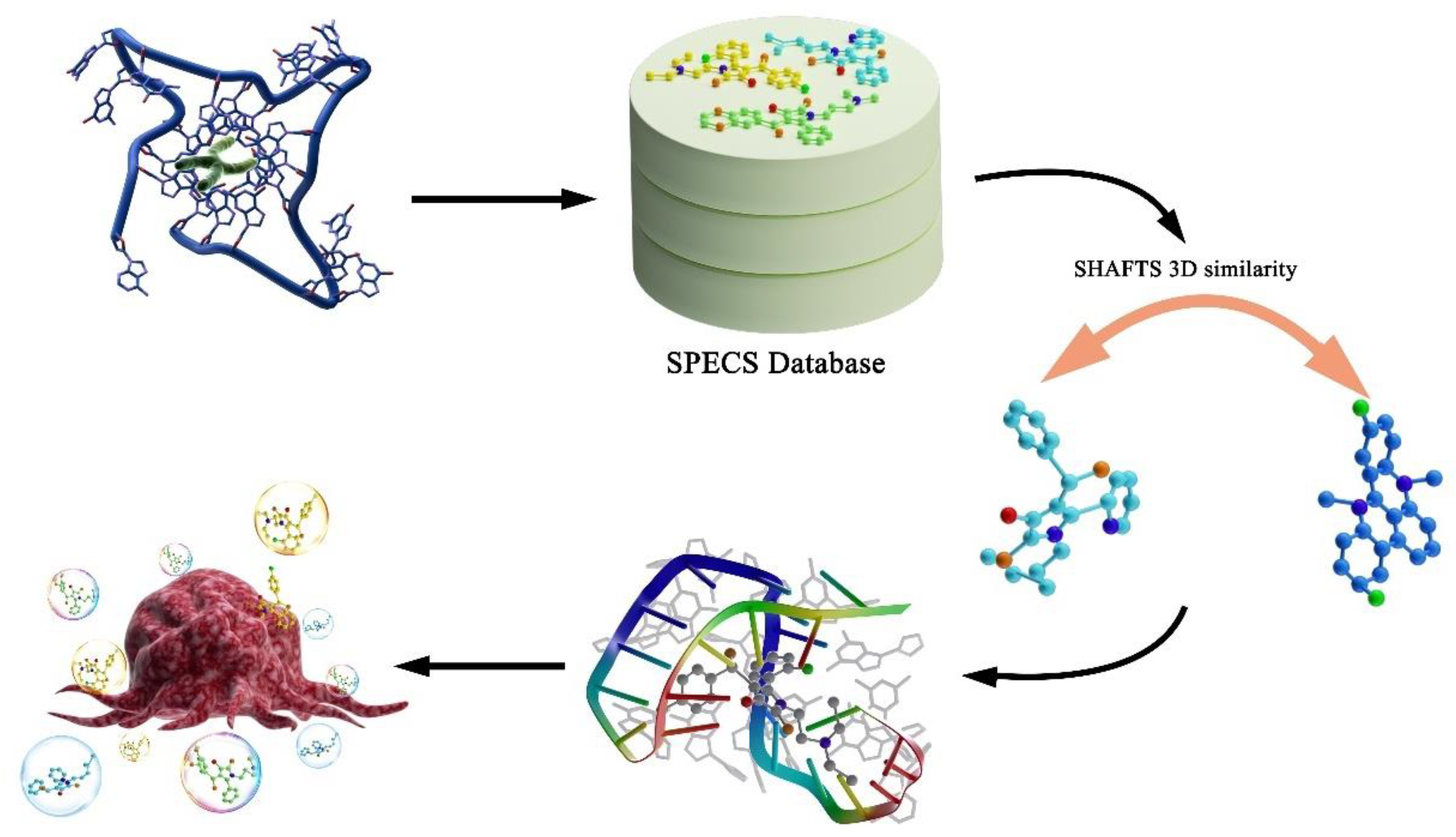
2.1. Database Preparation
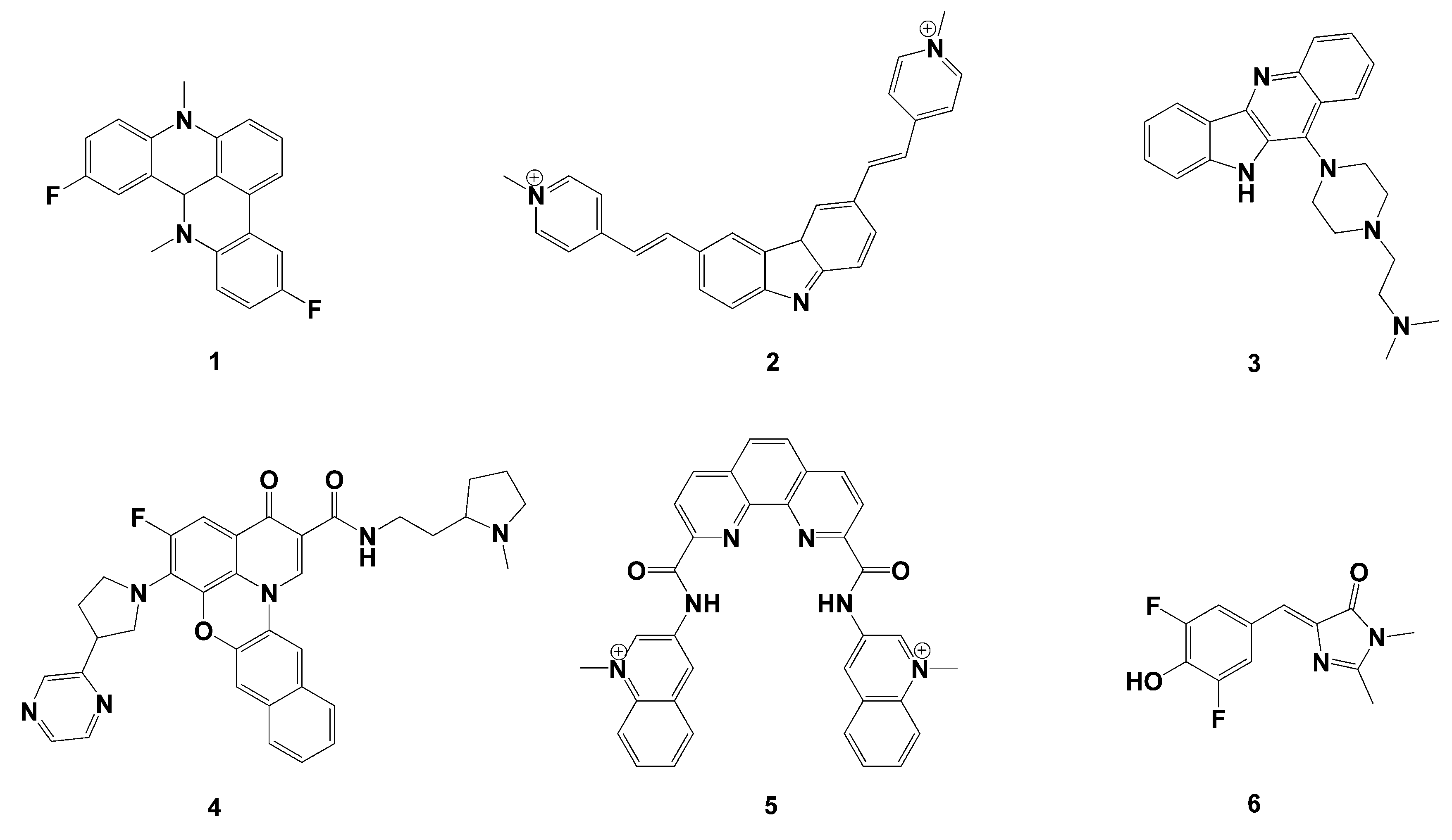
2.2. Query Molecules Selection
2.3. 3D Similarity Calculation
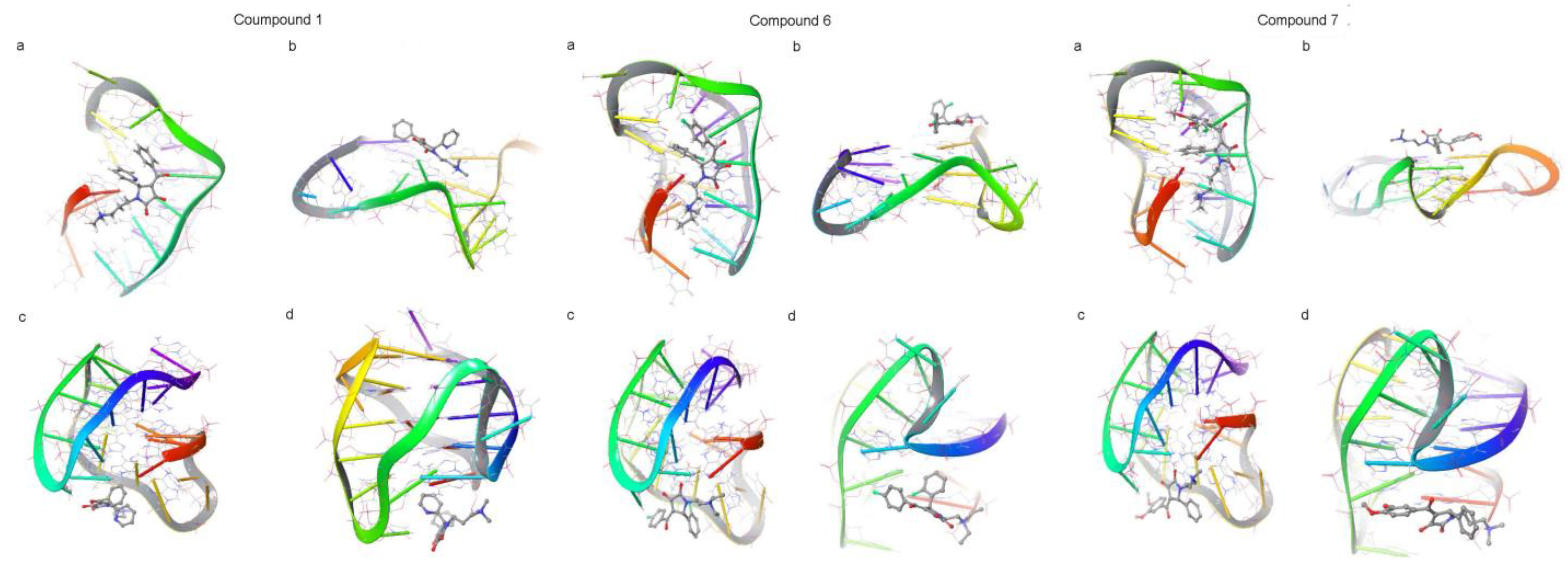
2.4. Molecular Docking
2.5. Cytotoxicity Assay
2.6. In Vitro Inhibition of Compounds 1, 6 and 7
2.7. In Vitro Cell Migration Assays
2.8. Cell Apoptosis Assays
2.9. Flow Cytometry Analysis of Cell Cycle
3. Results and Discussion
3.1. Virtual Screening Results
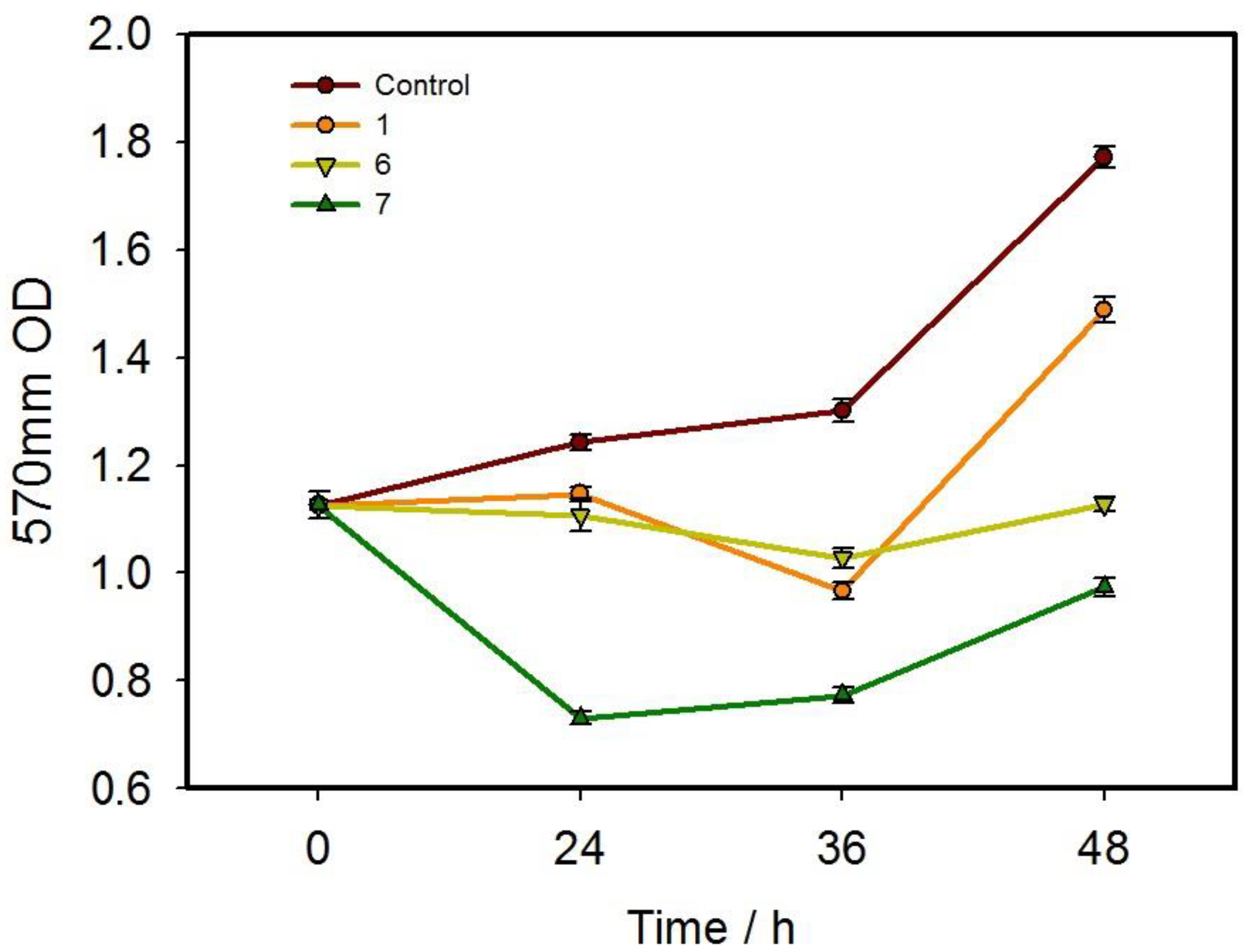
3.2. Cytotoxicity of the Compounds
3.3. In Vitro Inhibition Behavior
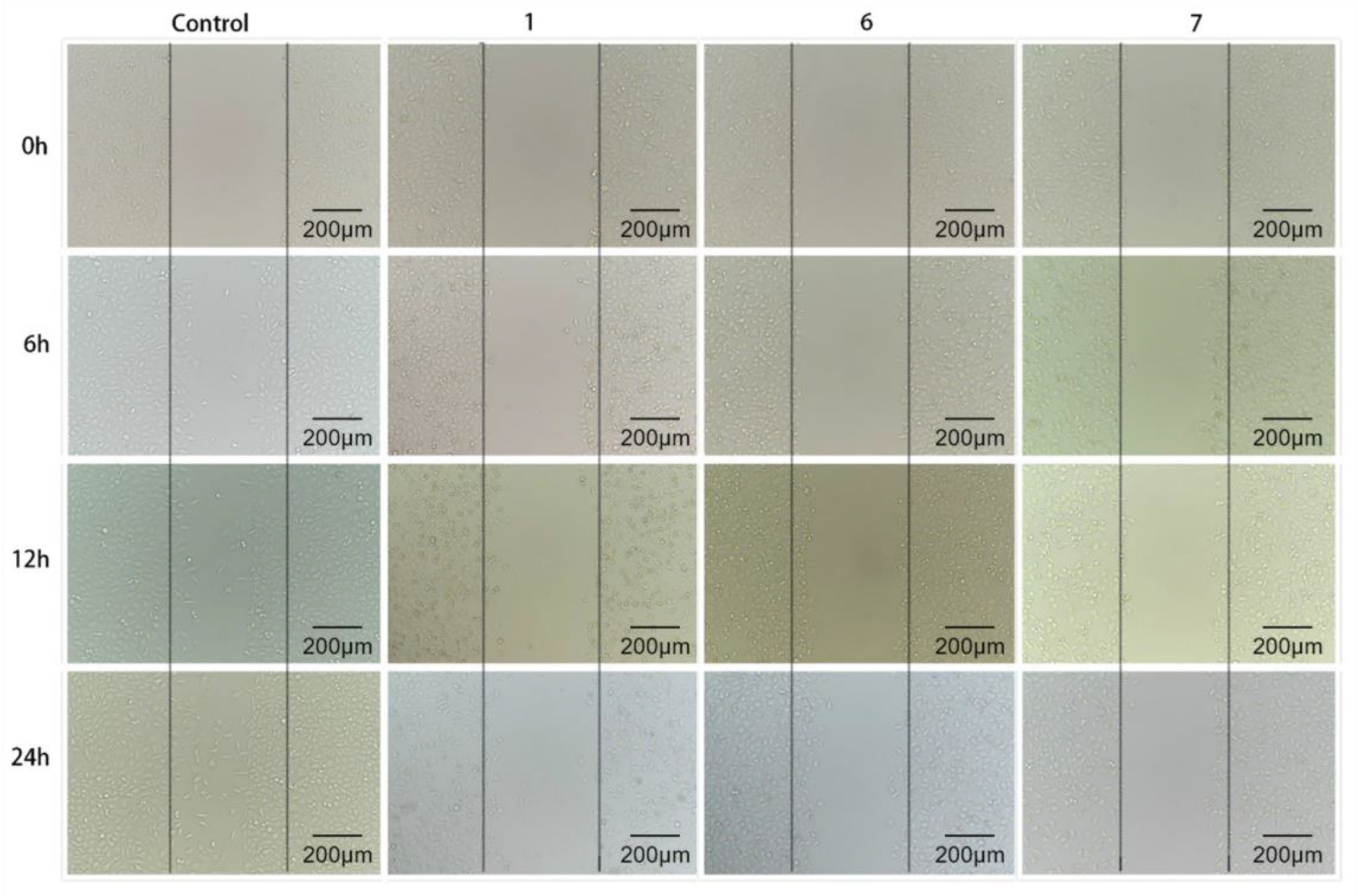
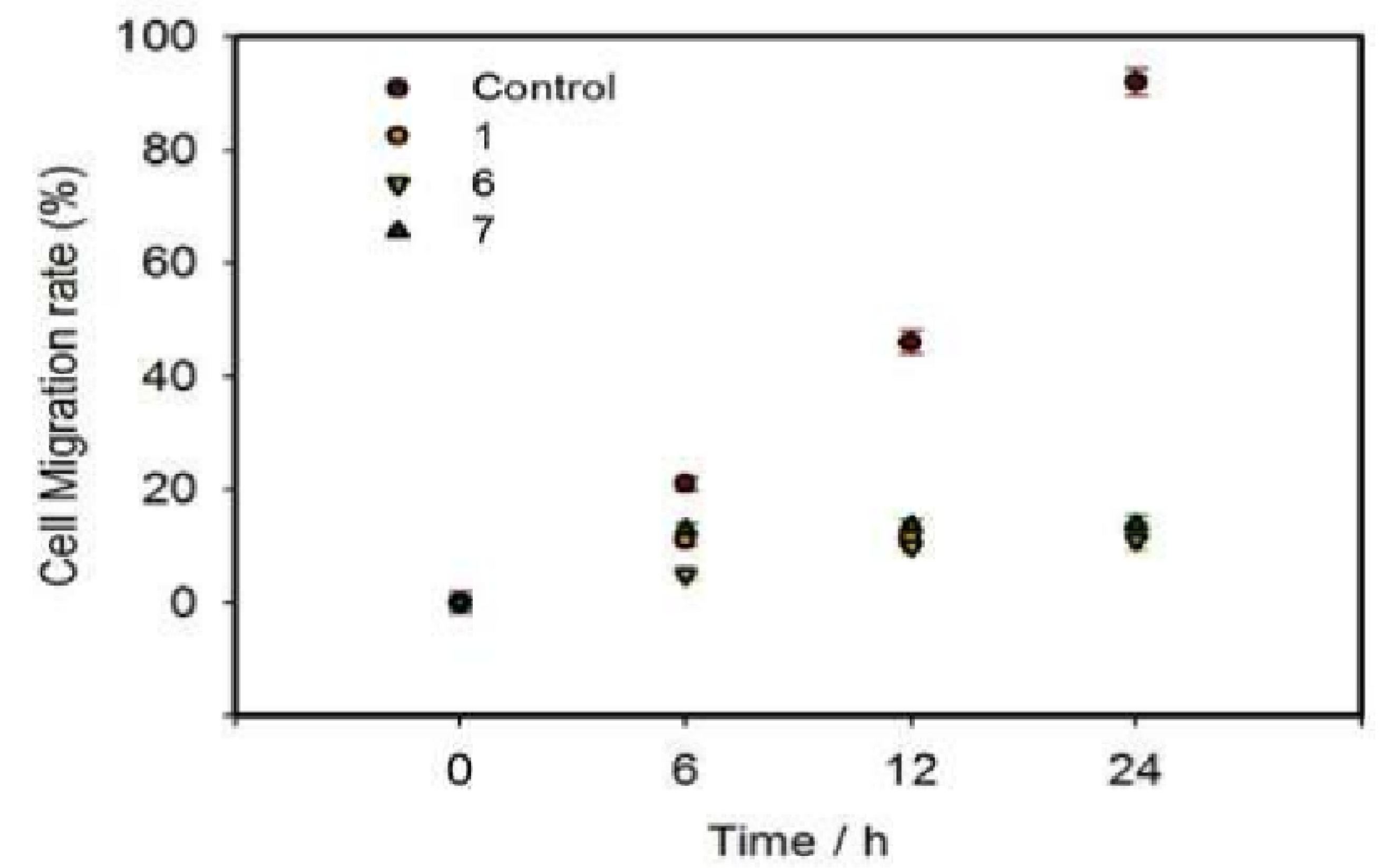
3.4. Investigations of In Vitro Migration
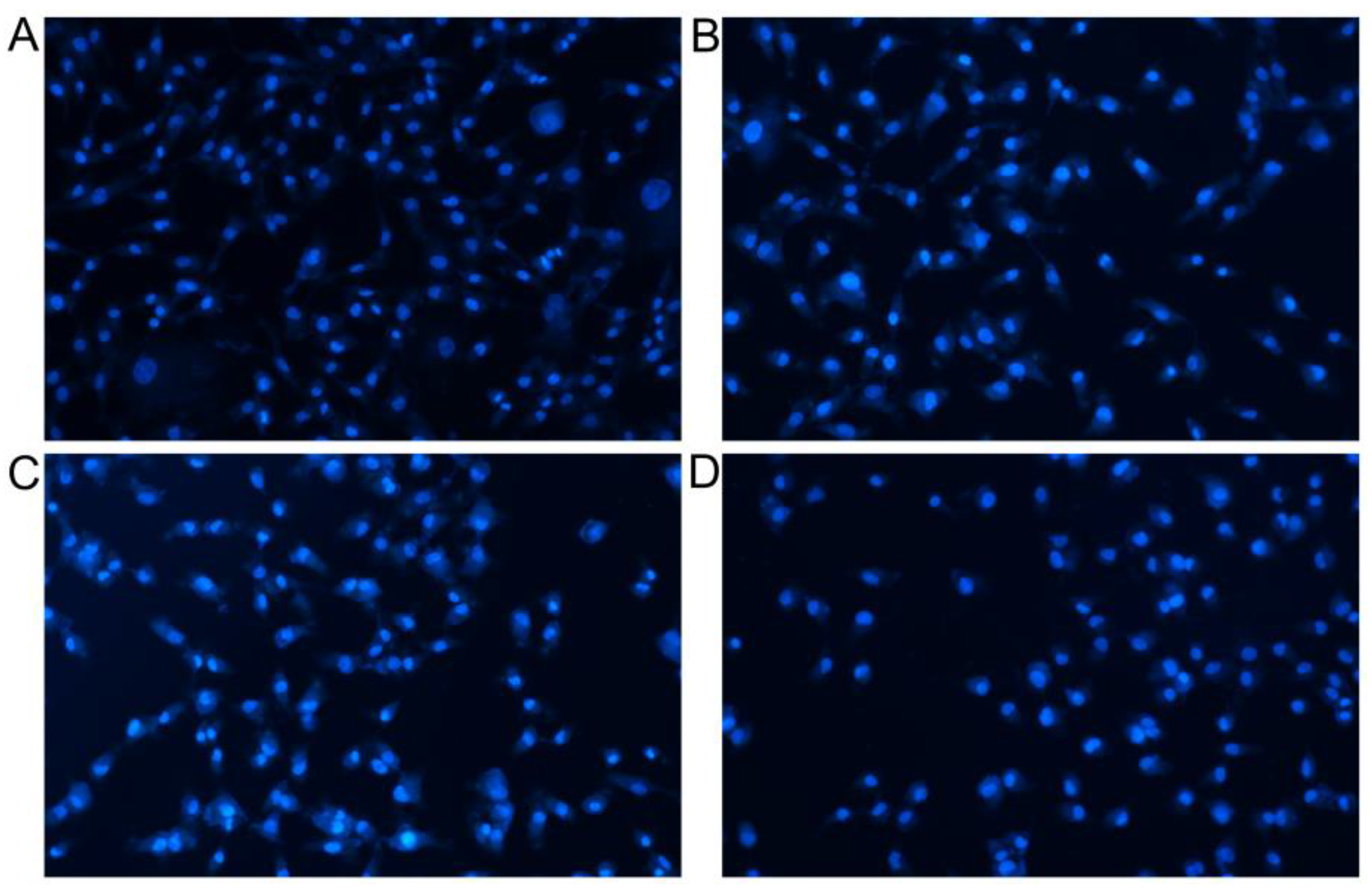
3.5. Analysis of Apoptosis
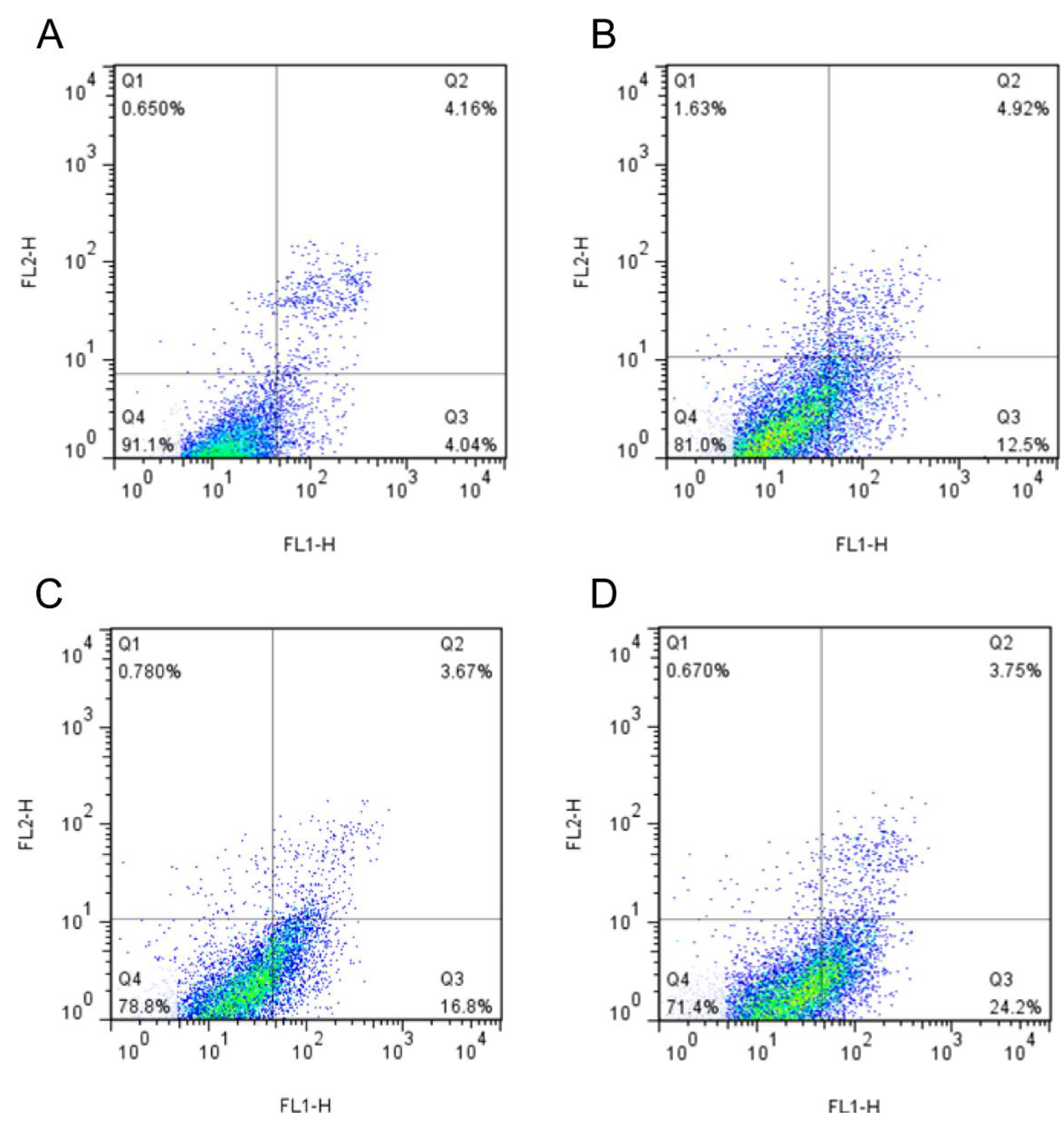
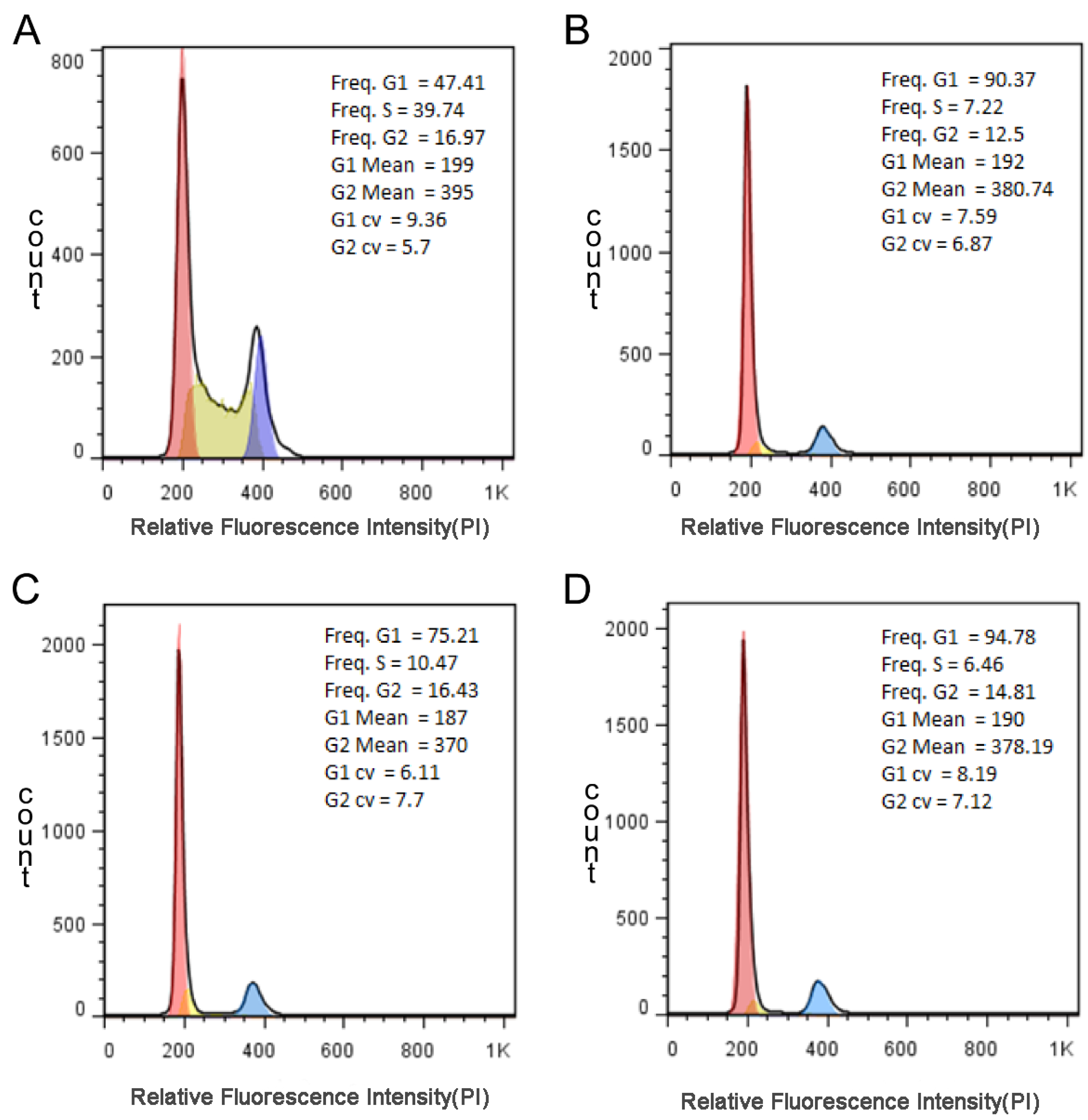
3.6. The Result of Cell Cycle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohn, K.W. Beyond DNA cross-linking: History and prospects of DNA-targeted cancer treatment—Fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1996, 56, 5533–5546. [Google Scholar] [PubMed]
- Conklin, K.A. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer 2000, 37, 1–18. [Google Scholar] [CrossRef]
- Schaffitzel, C.; Berger, I.; Postberg, J.; Hanes, J.; Lipps, H.J.; Plükthun, A. In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl. Acad. Sci. USA 2001, 98, 8572–8577. [Google Scholar] [CrossRef]
- Dai, J.; Carver, M.; Yang, D. Polymorphism of human telomeric quadruplex structures. Biochimie 2008, 90, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Haensel-Hertsch, R.; Simeone, A.; Shea, A.; Hui, W.W.I.; Zyner, K.G.; Marsico, G.; Rueda, O.M.; Bruna, A.; Martin, A.; Zhang, X.; et al. Landscape of G-quadruplex DNA structural regions in breast cancer. Nat. Genet. 2020, 52, 878–883. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Di Antonio, M.; Ponjavic, A.; Radzevičius, A.; Ranasinghe, R.T.; Catalano, M.; Zhang, X.; Shen, J.; Needham, L.-M.; Lee, S.F.; Klenerman, D.; et al. Single-molecule visualization of DNA G-quadruplex formation in live cells. Nat. Chem. 2020, 12, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.C.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Marian, C.O.; Cho, S.K.; Mcellin, B.M.; Maher, E.A.; Hatanpaa, K.J.; Madden, C.J.; Mickey, B.E.; Wright, W.E.; Shay, J.W.; Bachoo, R.M. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin. Cancer Res. 2010, 16, 154–163. [Google Scholar] [CrossRef]
- Philippi, C.; Loretz, B.; Schaefer, U.; Lehr, C. Telomerase as an emerging target to fight cancer—Opportunities and challenges for nanomedicine. J. Control. Release 2010, 146, 228–240. [Google Scholar] [CrossRef]
- Musumeci, D.; Amato, J.; Zizza, P.; Platella, C.; Cosconati, S.; Cingolani, C.; Biroccio, A.; Novellino, E.; Randazzo, A.; Giancola, C.; et al. Tandem application of ligand-based virtual screening and G4-OAS assay to identify novel G-quadruplex-targeting chemotypes. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861 Pt B, 1341–1352. [Google Scholar] [CrossRef]
- Ma, Y.; Iida, K.; Nagasawa, K. Topologies of G-quadruplex: Biological functions and regulation by ligands. Biochem. Biophys. Res. Commun. 2020, 531, 3–17. [Google Scholar] [CrossRef] [PubMed]
- De Cian, A.; Lacroix, L.; Douarre, C.; Temime-Smaali, N.; Trentesaux, C.; Riou, J.-F.; Mergny, J.-L. Targeting telomeres and telomerase. Biochimie 2008, 90, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.N.; Kumar, S.P.; Rawal, R.M.; Pandya, H.A. An integrated computational approach to identify GC minor groove binders using various molecular docking scoring functions, dynamics simulations and binding free energy calculations. J. Biomol. Struct. Dyn. 2020, 38, 3838–3855. [Google Scholar] [CrossRef]
- Holt, P.A.; Chaires, J.; Trent, J.O. Molecular docking of intercalators and groove-binders to nucleic acids using Autodock and Surflex. J. Chem. Inf. Model. 2008, 48, 1602–1615. [Google Scholar] [CrossRef]
- Gong, J.; Cai, C.; Liu, X.; Ku, X.; Jiang, H.; Gao, D.; Li, H. ChemMapper: A versatile web server for exploring pharmacology and chemical structure association based on molecular 3D similarity method. Bioinformatics 2013, 29, 1827–1829. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, H.; Li, H. SHAFTS: A hybrid approach for 3D molecular similarity calculation. 1. Method and assessment of virtual screening. J. Chem. Inf. Model. 2011, 51, 2372–2385. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Van Bay, M.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 Forms Better Binding Affinity. J. Chem. Inf. Model. 2020, 60, 204–211. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Jade, D.D.; Pandey, R.; Kumar, R.; Gupta, D. Ligand-based pharmacophore modeling of TNF-alpha to design novel inhibitors using virtual screening and molecular dynamics. J. Biomol. Struct. Dyn. 2022, 40, 1702–1718. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Liu, X.; Bai, F.; Ouyang, S.; Wang, X.; Li, H.; Jiang, H. Cyndi: A multi-objective evolution algorithm based method for bioactive molecular conformational generation. BMC Bioinform. 2009, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Monchaud, D.; Allain, C.; Bertrand, H.C.; Smargiasso, N.; Rosu, F.; Gabelica, V.; De Cian, A.; Mergny, J.L.; Teulade-Fichou, M.P. Ligands playing musical chairs with G-quadruplex DNA: A rapid and simple displacement assay for identifying selective G-quadruplex binders. Biochimie 2008, 90, 1207–1223. [Google Scholar] [CrossRef]
- Neidle, S. Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer. FEBS J. 2010, 277, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S. A Personal History of Quadruplex-Small Molecule Targeting. Chem. Rec. 2015, 15, 691–710. [Google Scholar] [CrossRef]
- Jahn, A.; Hinselmann, G.; Fechner, N.; Zell, A. Optimal assignment methods for ligand-based virtual screening. J. Cheminform. 2009, 1, 14. [Google Scholar] [CrossRef]
- Nicholls, A.; Grant, J.A. Molecular shape and electrostatics in the encoding of relevant chemical information. J. Comput.-Aided Mol. Des. 2005, 19, 661–686. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson-Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Xin, X.; Niu, X.; Liu, W.; Wang, D. Hybrid Solvation Model with First Solvation Shell for Calculation of Solvation Free Energy. Chemphyschem 2020, 21, 762–769. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Sugiyama, A.; Purqon, A.; Nagao, H.; Nishikawa, K. Binding Free Energy Calculation and Structural Analysis for Antigen-Antibody Complex. AIP Conf. Proc. 2006, 832, 566–569. [Google Scholar]
- Mobley, D.L.; Gilson, M.K. Predicting Binding Free Energies: Frontiers and Benchmarks. Annu. Rev. Biophys. 2017, 46, 531–558. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Weynand, J.; Bonnet, H.; Loiseau, F.; Decottignies, A.; Dejeu, J.; Defrancq, E.; Elias, B. Flexible RuII Schiff Base Complexes: G-Quadruplex DNA Binding and Photo-Induced Cancer Cell Death. Chem. Eur. J. 2020, 26, 13849–13860. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- International, B.R. Retracted: Apoptosis and Molecular Targeting Therapy in Cancer. BioMed Res. Int. 2020, 2020, 2451249. [Google Scholar]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Hustedt, N.; Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell. Biol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
| Number | Docking Score | |||
|---|---|---|---|---|
| 143D | 1KF1 | 2HY9 | 2JPZ | |
| 1 | −6.36921 | −3.30817 | −5.4937 | −3.87285 |
| 6 | −5.01954 | −2.71853 | −5.00908 | −4.88085 |
| 7 | −5.01123 | −3.61745 | −4.81507 | −4.20793 |
| Number | Template | Feature Score | Shape Score | Hybrid Score |
|---|---|---|---|---|
| 1 | 1 | 0.44 | 0.785 | 1.227 |
| 6 | 1 | 0.521 | 0.729 | 1.25 |
| 7 | 3 | 0.273 | 0.676 | 0.95 |
| Compounds | 1 | 2 | 3 | 4 | 5 |
| IC50 ± SEM (μΜ/L) | 201.35 ± 1.2 | 259.64 ± 2.6 | 345.75 ± 3.8 | 318.64 ± 4.1 | 298.27 ± 1.8 |
| Compounds | 6 | 7 | 8 | 9 | 10 |
| IC50 ± SEM (μΜ/L) | 198.38 ± 2.3 | 185.89 ± 1.7 | 314.19 ± 3.1 | 245.74 ± 0.87 | 349.87 ± 5.6 |
| Compounds | 11 | 12 | 13 | 14 | 15 |
| IC50 ± SEM (μΜ/L) | 326.48 ± 4.6 | >400 | >400 | 396.54 ± 4.8 | >400 |
| Compounds | 16 | 17 | 18 | 19 | 20 |
| IC50 ± SEM (μΜ/L) | 390.23 ± 2.8 | >400 | >400 | 374.56 ± 5.2 | >400 |
| Compounds | 21 | 22 | 23 | ||
| IC50 ± SEM (μΜ/L) | >400 | 389.65 ± 3.5 | >400 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, R.; Liu, J.; Wang, S.; Zhang, W.; Feng, K.; Liu, C.; Liu, B.; Miao, Y.; Zhou, S. Virtual Screening-Based Study of Novel Anti-Cancer Drugs Targeting G-Quadruplex. Pharmaceutics 2023, 15, 1414. https://doi.org/10.3390/pharmaceutics15051414
Ouyang R, Liu J, Wang S, Zhang W, Feng K, Liu C, Liu B, Miao Y, Zhou S. Virtual Screening-Based Study of Novel Anti-Cancer Drugs Targeting G-Quadruplex. Pharmaceutics. 2023; 15(5):1414. https://doi.org/10.3390/pharmaceutics15051414
Chicago/Turabian StyleOuyang, Ruizhuo, Jinyao Liu, Shen Wang, Weilun Zhang, Kai Feng, Conghao Liu, Baolin Liu, Yuqing Miao, and Shuang Zhou. 2023. "Virtual Screening-Based Study of Novel Anti-Cancer Drugs Targeting G-Quadruplex" Pharmaceutics 15, no. 5: 1414. https://doi.org/10.3390/pharmaceutics15051414
APA StyleOuyang, R., Liu, J., Wang, S., Zhang, W., Feng, K., Liu, C., Liu, B., Miao, Y., & Zhou, S. (2023). Virtual Screening-Based Study of Novel Anti-Cancer Drugs Targeting G-Quadruplex. Pharmaceutics, 15(5), 1414. https://doi.org/10.3390/pharmaceutics15051414






