Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
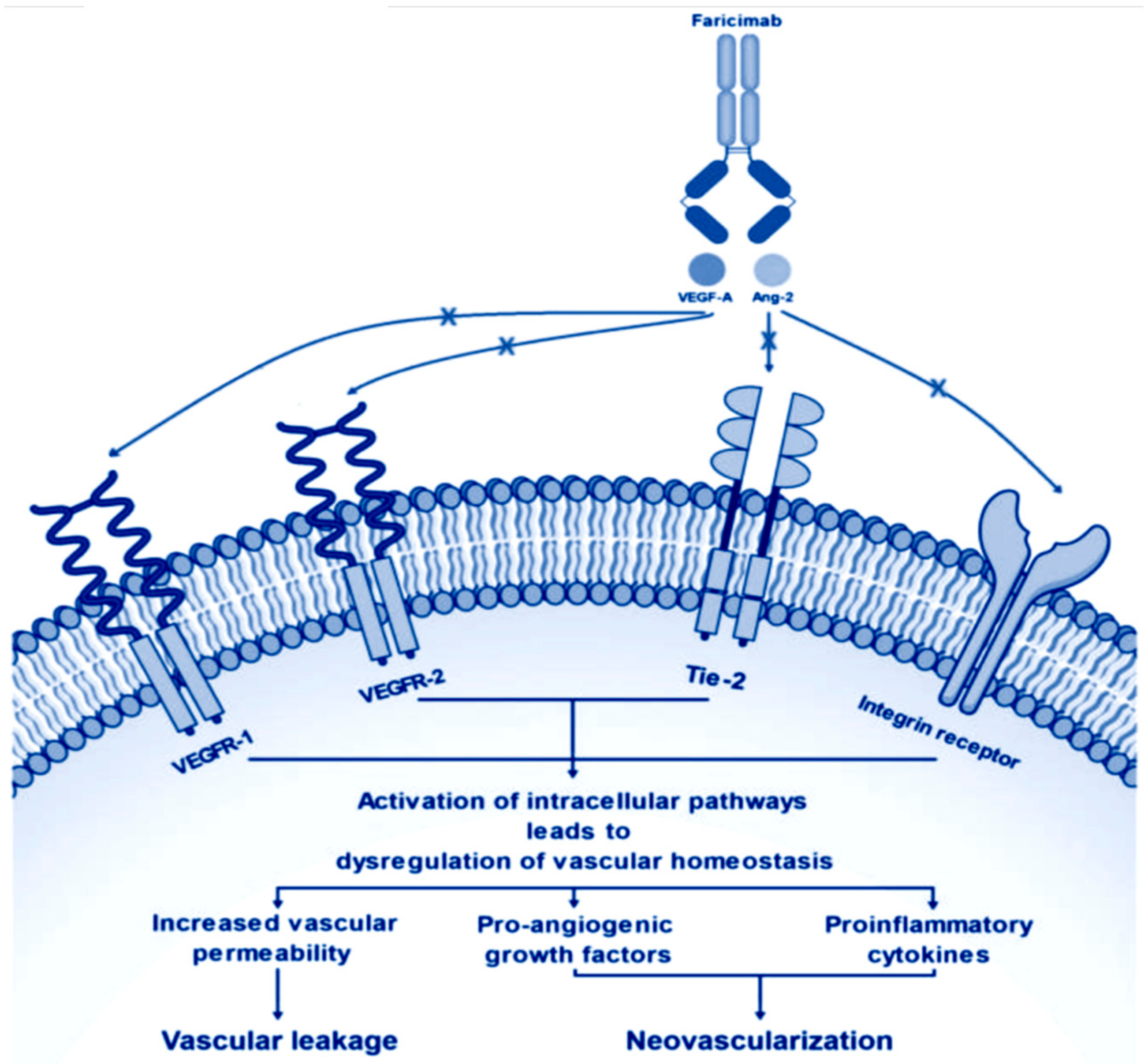
2.1. Preclinical Pharmacology
2.2. Pharmacokinetics Ad Metabolism
2.3. Safety
3. Clinical Trials
3.1. Pase I Studies
3.2. Phase II Studies
3.2.1. Phase II Studies in Patients with w-AMD
3.2.2. Phase II Studies in Patients with DME
3.3. Phase III Studies
3.3.1. Phase III Studies in Patients with w-AMD
3.3.2. Phase III Studies in Patients with DME
3.4. Other Ongoing Phase III and IV Trials
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic macular edema: Evidence-based management. Indian J. Ophthalmol. 2018, 66, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, V.; Dreyfuss, J.L.; Regatieri, C.V. Age-related macular degeneration: A review of current therapies and new treatments. Arq. Bras. Oftalmol. 2020, 83, 552–561. [Google Scholar]
- Keenan, T.D.L.; Cukras, C.A.; Chew, E.Y. Age-Related Macular Degeneration: Epidemiology and Clinical Aspects. Adv. Exp. Med. Biol. 2021, 1256, 1–31. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef]
- Schwarzer, P.; Ebneter, A.; Munk, M.; Wolf, S.; Zinkernagel, M.S. One-Year Results of Using a Treat-and-Extend Regimen without a Loading Phase with Anti-VEGF Agents in Patients with Treatment-Naive Diabetic Macular Edema. Ophthalmologica 2019, 241, 220–225. [Google Scholar] [CrossRef]
- Nicolo, M.; Morlacchi, A.; Cappelli, F.; Ferro Desideri, L.; Colombo, V.; Musetti, D.; Musolino, M.; Saccheggiani, M.; Bonetto, M.; Giacomini, M.; et al. Real-life data in the treatment of neovascular age-related macular degeneration. Results from the I-maculaweb registry evaluated in a single Italian Medical Retina center. Ophthalmologica 2020, 243, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Parodi, M.; Romano, F.; Arrigo, A.; Sacchi, R.; Scanzi, G.; Ferri, C.; Bandello, F. Real-life anti-vascular endothelial growth factor treatment for age-related macular degeneration and diabetic macular edema in an Italian tertiary referral hospital. Eur. J. Ophthalmol. 2020, 30, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Munk, M.R.; Ruckert, R.; Zinkernagel, M.; Ebneter, A.; Wolf, S. The role of anti-VEGF agents in myopic choroidal neovascularization: Current standards and future outlook. Expert. Opin. Biol. Ther. 2016, 16, 477–487. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Traverso, C.E.; Nicolo, M. Abicipar pegol: An investigational anti-VEGF agent for the treatment of wet age-related macular degeneration. Expert. Opin. Investig. Drugs 2020, 29, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Study to Collect Safety and ECG Data on Brolucizumab 6 mg Intravitreal Treatment in Patients With Wet AMD; National Library of Medicine: Bethesda, MD, USA, 2019.
- Ferro Desideri, L.; Traverso, C.E.; Nicolo, M. Brolucizumab: A novel anti-VEGF humanized single-chain antibody fragment for treating w-AMD. Expert. Opin. Biol. Ther. 2021, 21, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug. Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Duran, C.L.; Borriello, L.; Karagiannis, G.S.; Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Targeting Tie2 in the Tumor Microenvironment: From Angiogenesis to Dissemination. Cancers 2021, 13, 5730. [Google Scholar] [CrossRef]
- Thurston, G.; Daly, C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012, 2, a006550. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.M. Angiopoietins and Tie2 in vascular inflammation. Curr. Opin. Hematol. 2017, 24, 432–438. [Google Scholar] [CrossRef]
- Imhof, B.A.; Aurrand-Lions, M. Angiogenesis and inflammation face off. Nat. Med. 2006, 12, 171–172. [Google Scholar] [CrossRef]
- Scholz, A.; Plate, K.H.; Reiss, Y. Angiopoietin-2: A multifaceted cytokine that functions in both angiogenesis and inflammation. Ann. N. Y. Acad. Sci. 2015, 1347, 45–51. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Traverso, C.E.; Nicolo, M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert. Opin. Ther. Targets 2022, 26, 145–154. [Google Scholar] [CrossRef]
- Foxton, R.H.; Uhles, S.; Gruner, S.; Revelant, F.; Ullmer, C. Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol. Med. 2019, 11, e10204. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Faricimab: An investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert. Opin. Investig. Drugs 2021, 30, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Kuppermann, B.D.; Bandello, F.; Loewenstein, A. Faricimab: Expanding horizon beyond VEGF. Eye 2020, 34, 802–804. [Google Scholar] [CrossRef]
- Schaefer, W.; Regula, J.T.; Bahner, M.; Schanzer, J.; Croasdale, R.; Durr, H.; Gassner, C.; Georges, G.; Kettenberger, H.; Imhof-Jung, S.; et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl. Acad. Sci. USA 2011, 108, 11187–11192. [Google Scholar] [CrossRef]
- Surowka, M.; Schaefer, W.; Klein, C. Ten years in the making: Application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins. MAbs 2021, 13, 1967714. [Google Scholar] [CrossRef]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Gemmy Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288, Erratum in EMBO Mol. Med. 2017, 9, 985. [Google Scholar] [CrossRef]
- Oshima, Y.; Oshima, S.; Nambu, H.; Kachi, S.; Takahashi, K.; Umeda, N.; Shen, J.; Dong, A.; Apte, R.S.; Duh, E.; et al. Different effects of angiopoietin-2 in different vascular beds: New vessels are most sensitive. FASEB J. 2005, 19, 963–965. [Google Scholar] [CrossRef]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef]
- Jeansson, M.; Gawlik, A.; Anderson, G.; Li, C.; Kerjaschki, D.; Henkelman, M.; Quaggin, S.E. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Investig. 2011, 121, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Medscape. Faricimab (Rx) Pharmacology. 2022. Available online: https://reference.medscape.com/drug/vabysmo-faricimab-4000244#10 (accessed on 14 April 2023).
- Chakravarthy, U.; Bailey, C.; Brown, D.; Campochiaro, P.; Chittum, M.; Csaky, K.; Tufail, A.; Yates, P.; Cech, P.; Giraudon, M.; et al. Phase I Trial of Anti-Vascular Endothelial Growth Factor/Anti-angiopoietin 2 Bispecific Antibody RG7716 for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retina 2017, 1, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Wormald, R.; Evans, J.; Virgili, G.; Keller, P.R.; Lawrenson, J.G.; Li, T. Analysis of a Systematic Review About Blue Light-Filtering Intraocular Lenses for Retinal Protection: Understanding the Limitations of the Evidence. JAMA Ophthalmol. 2019, 137, 694–697. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 964–972. [Google Scholar] [CrossRef]
- Sahni, J.; Patel, S.S.; Dugel, P.U.; Khanani, A.M.; Jhaveri, C.D.; Wykoff, C.C.; Hershberger, V.S.; Pauly-Evers, M.; Sadikhov, S.; Szczesny, P.; et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology 2019, 126, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef]
- A Study to Evaluate the Long-Term Safety and Tolerability of Faricimab in Participants With Diabetic Macular Edema (Rhone-X); National Library of Medicine: Bethesda, MD, USA, 2020.
- Sahni, J.; Dugel, P.U.; Patel, S.S.; Chittum, M.E.; Berger, B.; Del Valle Rubido, M.; Sadikhov, S.; Szczesny, P.; Schwab, D.; Nogoceke, E.; et al. Safety and Efficacy of Different Doses and Regimens of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The AVENUE Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 955–963. [Google Scholar] [CrossRef]
- ClinicalTrial.gov. A Study to Evaluate the Efficacy and Safety of Faricimab in Participants With Macular Edema Secondary to Branch Retinal Vein Occlusion (BALATON). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04740905?term=faricimab&cond=Macular+Degeneration&phase=23&draw=2&rank=8 (accessed on 14 April 2023).
- ClinicalTrial.gov. A Study to Evaluate the Efficacy and Safety of Faricimab in Participants With Macular Edema Secondary to Central Retinal or Hemiretinal Vein Occlusion (COMINO). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04740931?term=faricimab&cond=Macular+Degeneration&phase=23&draw=2&rank=9 (accessed on 14 April 2023).
- ClinicalTrial.gov. A Study to Investigate Faricimab Treatment Response in Treatment-Naive, Underrepresented Patients With Diabetic Macular Edema (ELEVATUM). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05224102?term=faricimab&cond=Macular+Degeneration&phase=3&draw=2&rank=1 (accessed on 14 April 2023).
- ClinicalTrial.gov. A Study to Evaluate the Long-Term Safety and Tolerability of Faricimab in Participants With Neovascular Age-Related Macular Degeneration (AVONELLE-X). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04777201?term=AVONELLE-X&draw=2&rank=1 (accessed on 14 April 2023).
- Wykoff, C.C.; Clark, W.L.; Nielsen, J.S.; Brill, J.V.; Greene, L.S.; Heggen, C.L. Optimizing Anti-VEGF Treatment Outcomes for Patients with Neovascular Age-Related Macular Degeneration. J. Manag. Care Spec. Pharm. 2018, 24, S3–S15. [Google Scholar] [CrossRef]
- Munk, M.R.; Kiss, C.; Huf, W.; Sulzbacher, F.; Roberts, P.; Mittermuller, T.J.; Sacu, S.; Simader, C.; Schmidt-Erfurth, U. One year follow-up of functional recovery in neovascular AMD during monthly anti-VEGF treatment. Am. J. Ophthalmol. 2013, 156, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and Future Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. J. Exp. Pharmacol. 2021, 13, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mantel, I. Optimizing the Anti-VEGF Treatment Strategy for Neovascular Age-Related Macular Degeneration: From Clinical Trials to Real-Life Requirements. Transl. Vis. Sci. Technol. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Munk, M.R.; Kurmann, T.; De Zanet, S.; Mosinska, A.; Karagoz, I.K.; Zinkernagel, M.S.; Wolf, S.; Sznitman, R. Machine Learning Can Predict Anti-VEGF Treatment Demand in a Treat-and-Extend Regimen for Patients with Neovascular AMD, DME, and RVO Associated Macular Edema. Ophthalmol. Retin. 2021, 5, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Mahr, M.A.; Hodge, D.O.; Erie, J.C. Racial Differences in Age-Related Macular Degeneration and Associated Anti-Vascular Endothelial Growth Factor Intravitreal Injections among Medicare Beneficiaries. Ophthalmol. Retin. 2018, 2, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Boyer, D.; Brown, D.M.; Chaudhry, N.; Elman, M.; Liang, C.; O’Shaughnessy, D.; Parsons, E.C.; Patel, S.; Slakter, J.S.; et al. Oral Tyrosine Kinase Inhibitor for Neovascular Age-Related Macular Degeneration: A Phase 1 Dose-Escalation Study. JAMA Ophthalmol. 2017, 135, 761–767. [Google Scholar] [CrossRef]
- Ciucci, F.; Ioele, G.; Bardocci, A.; Lofoco, G.; Antonelli, B.; Gaetano, C.; Polimanti, G.; Luca, M.; Ragno, G.; Gattegna, R. Central retinal thickness fluctuations in patients treated with anti-VEGF for neovascular age related macular degeneration. Eur. J. Ophthalmol. 2022, 32, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Stanga, P.E.; Valentín-Bravo, F.J.; Stanga, S.E.F.; Reinstein, U.I.; Pastor-Idoate, S.; Downes, S.M. Faricimab in neovascular AMD: First report of real-world outcomes in an independent retina clinic. Eye 2023. [Google Scholar] [CrossRef]
- Rush, R.B.; Rush, S.W. Faricimab for Treatment-Resistant Diabetic Macular Edema. Clin. Ophthalmol. 2022, 16, 2797–2801. [Google Scholar] [CrossRef]
- Brown, D.M.; Boyer, D.S.; Csaky, K.; Vitti, R.; Perlee, L.; Chu, K.W.; Asmus, F.; Leal, S.; Zeitz, O.; Cheng, Y.; et al. Intravitreal Nesvacumab (Antiangiopoietin 2) plus Aflibercept in Diabetic Macular Edema: Phase 2 RUBY Randomized Trial. Retina 2022, 42, 1111–1120. [Google Scholar] [CrossRef]
- Khanani, A.M.; Zarbin, M.A.; Barakat, M.R.; Albini, T.A.; Kaiser, P.K.; Guruprasad, B.; Agashivala, N.; Yu, J.S.; Wykoff, C.C.; MacCumber, M.W. Safety Outcomes of Brolucizumab in Neovascular Age-Related Macular Degeneration: Results From the IRIS Registry and Komodo Healthcare Map. JAMA Ophthalmol. 2022, 140, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Iida, T.; Maruko, I.; Sadda, S.R. A consensus on risk mitigation for brolucizumab in neovascular age-related macular degeneration: Patient Selection, Evaluation, and Treatment. Retina 2022, 42, 1629–1637. [Google Scholar] [CrossRef]
- Tolentino, M.J.; Tolentino, A.J. Investigational drugs in clinical trials for macular degeneration. Expert. Opin. Investig. Drugs 2022, 31, 1067–1085. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Somfai, G.M.; de Smet, M.D.; Donati, G.; Menke, M.N.; Garweg, J.G.; Ceklic, L. The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers. Int. J. Mol. Sci. 2022, 23, 7585. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, L.; Ferro Desideri, L.; Vagge, A.; Nicolo, M.; Traverso, C.E. KSI-301. Antibody–biopolymer conjugate targeting VEGF, Treatment of wet age-related macular degeneration, Treatment of diabetic macular edema, Treatment of retinal vein occlusion. Drugs Future 2021, 46, 865. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody biopolymer conjugate in retinal disorders. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027708. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.V.; Wieland, M.R.; Tam, T.; Rea, J.C.; Horvath, J.; Hieb, A.R.; Jia, W.; Grace, L.; Barteselli, G.; Stewart, J.M. The Port Delivery System with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug. Deliv. 2022, 29, 1326–1334. [Google Scholar] [CrossRef]
- Pieramici, D.J.; Wieland, M.R.; Stewart, J.M.; Chang, R.T.; Gune, S.A.; Malhotra, V.K.; Barteselli, G.; Awh, C.C. Implant Insertion Procedure of the Port Delivery System With Ranibizumab: Overview and Clinical Pearls. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, T.A.C.; Georgiou, M.; Bainbridge, J.W.B.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157. [Google Scholar] [CrossRef]
- Tosi, G.M.; Orlandini, M.; Galvagni, F. The Controversial Role of TGF-beta in Neovascular Age-Related Macular Degeneration Pathogenesis. Int. J. Mol. Sci. 2018, 19, 3363. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Grierson, P.M.; Picus, J.; Lockhart, A.C.; Roth, B.J.; Liu, J.; Morton, A.; Chan, E.; Huffman, J.; Liang, C.; et al. Vorolanib (X-82), an oral anti-VEGFR/PDGFR/CSF1R tyrosine kinase inhibitor, with everolimus in solid tumors: Results of a phase I study. Investig. New Drugs 2021, 39, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
| Trial Identifier | Phase | Indication | Number of Patients and Interventions | Results in BCVA | Status |
|---|---|---|---|---|---|
| AVENUE NCT02484690 [40] | II | w-AMD | 263 patients; monthly ranibizumab 0.5 mg, monthly faricimab 1.5 mg, monthly faricimab 6 mg, bimonthly faricimab 6 mg, and ranibizumab 0.5 mg/faricimab 6 mg sequential therapy | At week 36, adjusted mean change in BCVA vs. ranibizumab was 1.6 (80% CI, −1.6 to 4.7) letters for faricimab 1.5 mg every 4 weeks (p = 0.52), −1.6 (80% CI, −4.9 to 1.7) letters for faricimab 6.0 mg every 4 weeks (p = 0.53), and −1.5 (80% CI, −4.6 to 1.6) letters for faricimab 6.0 mg every 8 weeks (p = 0.53). For faricimab 6.0 mg every 4 weeks adjusted mean change was −1.7 (80% CI, −3.8 to 0.4) letters (p = 0.30) | Completed |
| STAIRWAY NCT03038880 [35] | II | w-AMD | 76 patients; monthly ranibizumab 0.5 mg, faricimab, 6.0 mg, every 12 or 16 weeks | At week 40, adjusted mean BCVA gains from baseline were +11.4 (80% CI, 7.8–15.0), +9.3 (80% CI, 6.4–12.3), and +12.5 (80% CI, 9.9–15.1) for the ranibizumab every 4 weeks, faricimab every 12 weeks, and faricimab every 16 weeks arms, respectively | Completed |
| LUCERNE NCT03823300 [37] | III | w-AMD | 1012 patients; faricimab 6.0 mg up to every 16 weeks vs. bimonthly aflibercept 2.0 mg | Mean BCVA gain +6.6 letters [5.3 to 7.8] with faricimab 6.0 mg up to every 16 weeks and +6.6 letters with aflibercept 2.0 mg every 8 weeks [5.3 to 7.8]; treatment difference 0·0 letters [−1.7 to 1.8] | Completed |
| TENAYA NCT03823287 [37] | III | w-AMD | 989 patients; faricimab 6.0 mg PTI up to every 16 weeks vs. bimonthly aflibercept 2.0 mg | Adjusted mean change + 5.8 letters [95% CI 4·6 to 7·1] with faricimab 6.0 mg up to every 16 weeks and + 5.1 letters [3.9 to 6.4] with aflibercept 2.0 mg every 8 weeks; treatment difference 0.7 letters [−1.1 to 2.5] | Completed |
| AVONELLE-X NCT04777201 [44] | III | w-AMD | 1036 patients; faricimab 6.0 mg PTI up to 16 weeks | Awaiting results | Active, not recruiting |
| BOULEVARD NCT02699450 [36] | II | DME | 229 patients; 6.0 mg faricimab, 1.5 mg faricimab, or 0.3 mg ranibizumab with monthly regimen | In 6.0 mg faricimab, 1.5 mg faricimab, and 0.3 mg ranibizumab mean improvements of +13.9, +11.7, and +10.3 ETDRS letters from baseline, respectively. The 6.0-mg faricimab dose showed statistically significant gain of +3.6 letters over ranibizumab (p = 0.03) | Completed |
| RHINE NCT03622593 [38] | III | DME | 1715 patients; faricimab 6.0 mg every 8 weeks, faricimab 6.0 mg per PTI, or aflibercept 2.0 mg every 8 weeks | Faricimab every 8 weeks +11.8 ETDRS letters [10.6 to 13.0] vs. +10.3 ETDRS aflibercept 2.0 mg every 8 weeks letters [9.1 to 11.4] letters, difference +1.5 ETDRS letters [−0.1 to 3.2] Faricimab PTI +10.8 ETDRS letters [9.6 to 11.9], difference +0.5 ETDRS letters [−1.1 to 2.1] | Completed |
| YOSEMITE NCT03622580 [38] | III | DME | 1532 patients; faricimab 6.0 mg every 8 weeks, faricimab 6.0 mg per PTI, or aflibercept 2.0 mg every 8 weeks | Faricimab every 8 weeks + 10.7 ETDRS letters [97·52% CI 9.4 to 12.0] vs. +10.9 ETDRS letters with aflibercept 2.0 mg every 8 weeks letters [9.6 to 12.2], difference −0.2 ETDRS letters [−2.0 to 1.6] Faricimab PTI + 11.6 ETDRS letters [10.3 to 12.9], difference +0.7 ETDRS letters with 8-week aflibercept 2.0 mg [−1.1 to 2.5]; | Completed |
| ELEVATUM NCT05224102 [43] | IV | DME | 120 patients; faricimab 6.0 mg every 4 weeks up to week 20, followed by faricimab 6.0 mg once every 8 weeks up to week 52 | Awaiting results | Recruiting |
| RHONE-X NCT04432831 [39] | III | DME | 1479 patients; faricimab 6.0 mg PTI up to 16 weeks | Awaiting results | Active, not recruiting |
| BALATON NCT04740905 [41] | III | BRVO | 553 patients; faricimab 6.0 mg Q4W (Part 1) followed by faricimab PTI (Part 2) vs. aflibercept 2.0 mg Q4W (Part 1) followed by faricimab PTI (Part 2) | Awaiting results | Active, not recruiting |
| COMINO NCT04740931 [42] | III | CRVO/HRVO | 730 patients, faricimab 6.0 mg Q4W (Part 1) followed by faricimab PTI (Part 2) vs. aflibercept 2.0 mg Q4W (Part 1) followed by faricimab PTI (Part 2) | Awaiting results | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro Desideri, L.; Traverso, C.E.; Nicolò, M.; Munk, M.R. Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration. Pharmaceutics 2023, 15, 1413. https://doi.org/10.3390/pharmaceutics15051413
Ferro Desideri L, Traverso CE, Nicolò M, Munk MR. Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration. Pharmaceutics. 2023; 15(5):1413. https://doi.org/10.3390/pharmaceutics15051413
Chicago/Turabian StyleFerro Desideri, Lorenzo, Carlo Enrico Traverso, Massimo Nicolò, and Marion R. Munk. 2023. "Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration" Pharmaceutics 15, no. 5: 1413. https://doi.org/10.3390/pharmaceutics15051413
APA StyleFerro Desideri, L., Traverso, C. E., Nicolò, M., & Munk, M. R. (2023). Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration. Pharmaceutics, 15(5), 1413. https://doi.org/10.3390/pharmaceutics15051413







