Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
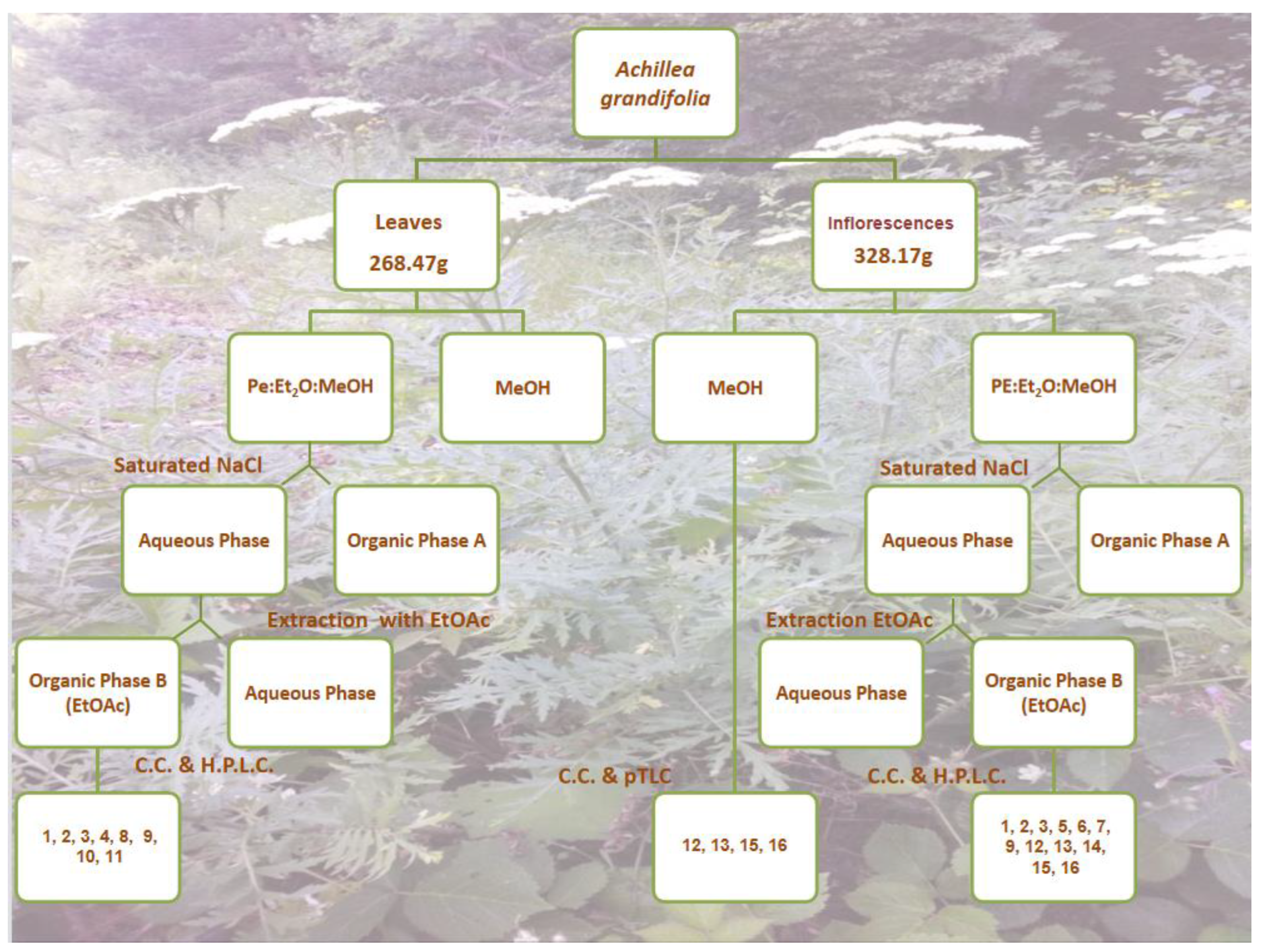
2.2. Extraction and Isolation
2.3. Compound Isolation
2.4. Viability Assay
2.5. Cell Cycle Analysis
3. Results
3.1. Isolated Compounds
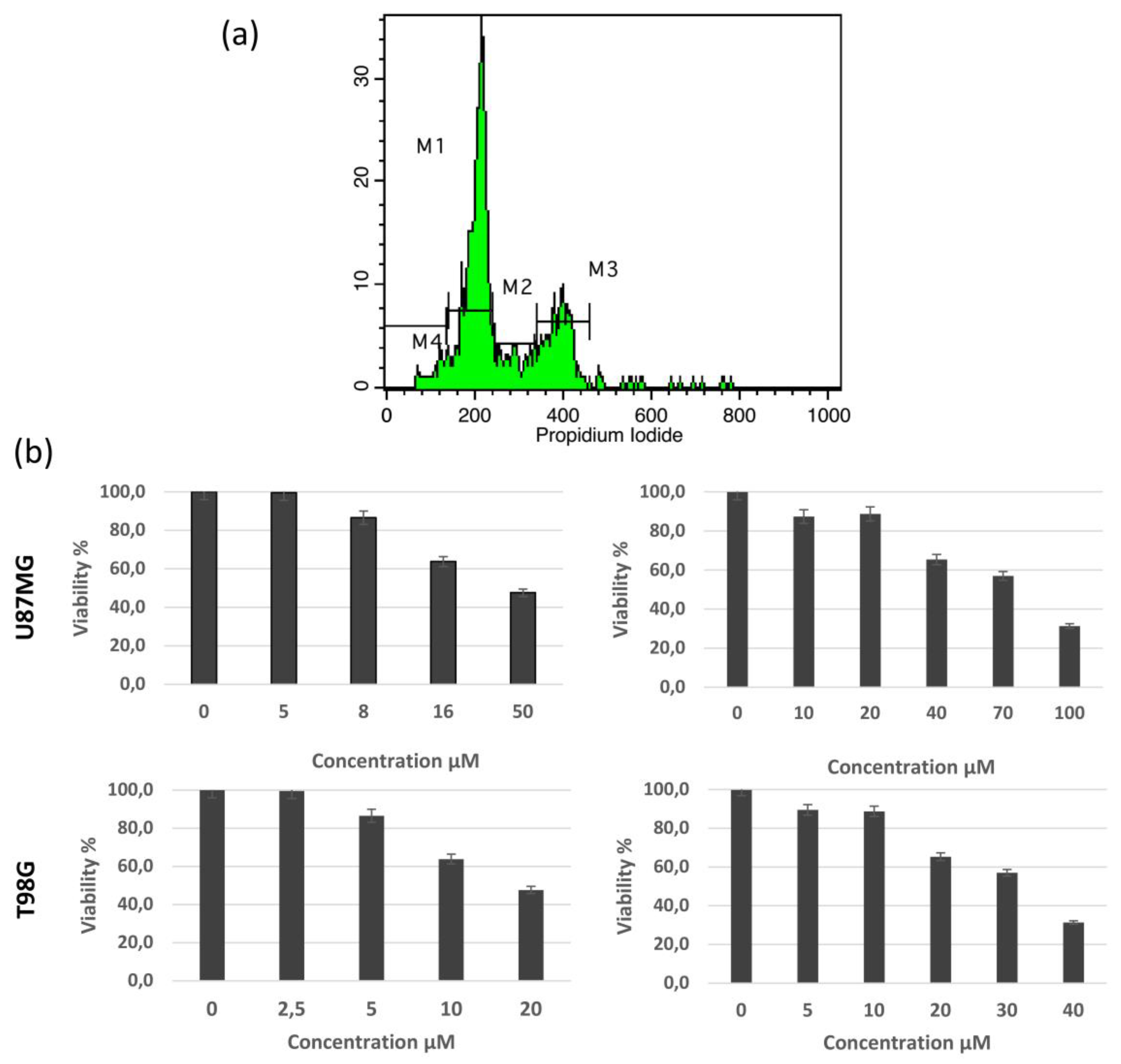
3.2. Biological Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radulović, N.; Zlatković, B.; Palić, R.; Stojanović, G. Chemotaxonomic significance of the Balkan Achillea volatiles. Nat. Prod. Commun. 2007, 2, 453–474. [Google Scholar] [CrossRef]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Čomić, L.; Kocić, B.; Bernstein, N. Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan peninsula. NJAS Wagen. J. Life Sc. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef] [PubMed]
- Bali, E.B.; Açık, L.; Elçi, P.; Sarper, M.; Avcu, F.; Vural, M. In vitro anti-oxidant, cytotoxic and pro-apoptotic effects of Achillea teretifolia Willd. extracts on human prostate cancer cell lines. Pharmacogn. Mag. 2015, 11 (Suppl. S2), S308–S315. [Google Scholar] [CrossRef] [PubMed]
- Choucry, M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharmacogn. Phytother. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Mouhid, L.; Gómez de Cedrón, M.; Vargas, T.; García-Carrascosa, E.; Herranz, N.; García-Risco, M.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Identification of antitumoral agents against human pancreatic cancer cells from Asteraceae and Lamiaceae plant extracts. BMC Complement. Altern. Med. 2018, 18, 254. [Google Scholar] [CrossRef]
- Abou Baker, D.H. Achillea millefolium L. ethyl acetate fraction induces apoptosis and cell cycle arrest in human cervical cancer (HeLa) cells. Ann. Agric. Sci. 2020, 65, 42–48. [Google Scholar] [CrossRef]
- Papakosta, K.; Grafakou, M.E.; Barda, C.; Kostopoulos, I.V.; Tsitsilonis, O.; Skaltsa, H. Cytotoxicity and anti-cancer activity of the genus Achillea L. Curr. Med. Chem. 2020, 27, 6910–6925. [Google Scholar] [CrossRef]
- Taşkın, D.; Alkaya, D.B.; Dölen, E. Evaluation of antioxidant capacity and analysis of major phenolic compounds in Achillea grandifolia by HPLC-DAD with Q-TOF LC/MS Confirmation. Chiang Mai J. Sci. 2018, 45, 287–298. Available online: https://www.thaiscience.info/Journals/Article/CMJS/10989292.pdf (accessed on 29 April 2023).
- Tsiftsoglou, O.S.; Atskakani, M.-E.; Krigas, N.; Stefanakis, M.K.; Gounaris, C.; Hadjipavlou-Litina, D.; Lazari, D. Exploring the medicinal potential of Achillea grandifolia in Greek wild-growing populations: Characterization of volatile compounds, anti-inflammatory and antioxidant activities of leaves and inflorescences. Plants 2023, 12, 613. [Google Scholar] [CrossRef]
- Özek, G. Phytochemical characterization of Achillea grandifolia Friv. essential oil and its potent against oxidative damage, acetylcholinesterase and α-amylase. Eskişehir Tech. Univ. J. Sci. Tech. Appl. Sci. Eng. 2018, 19, 671–684. [Google Scholar] [CrossRef]
- Wollenweber, E.; Valant-Vetxhera, K.M.; Ivancheva, S.; Kumanov, B. Flavonoid aglycones from the leaf surfaces of some Achlllea species. Phytochemistry 1987, 26, 181–182. [Google Scholar] [CrossRef]
- Greger, H.; Grenz, M.; Bohlman, F. Piperidides and other amides from Achillea species. Phytochemistry 1982, 21, 1071–1074. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232110 (accessed on 29 April 2023).
- Lee, D.; Kim, K.O.; Lee, D.; Kang, K.S. Anti-Apoptotic and antioxidant effects of 3-Epi-Iso-Seco-Tanapartholide isolated from Artemisia argyi against iodixanol-induced kidney epithelial cell death. Biomolecules 2020, 10, 867. [Google Scholar] [CrossRef]
- Skaltsa, H.; Lazari, D.; Georgiadou, E.; Kakavas, S.; Constantinidis, T. Sesquiterpene lactones from Centaurea Species: C. thessala subsp. drakiensis and C. attica subsp. attica. Planta Med. 1999, 65, 393. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Lazari, D.; Markopoulos, G.; Vartholomatos, E.; Hodaj, E.; Galani, V.; Kyritsis, A.P. Moschamine inhibits proliferation of glioblastoma cells via cell cycle arrest and apoptosis. Tumor Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Alexiou, G.A.; Markopoulos, G.S.; Lazari, D.; Tsiftsoglou, O.; Chousidis, I.; Leonardos, I.; Kyritsis, A.P. Deglucohellebrin: A Potent Agent for Glioblastoma Treatment. Anticancer Agents Med. Chem. 2020, 20, 103–110. [Google Scholar] [CrossRef]
- Irwin, M.A.; Geissman, T.A. Ridentin-β: An eudesmanolide from Artemisia tripartita ssp. rupicola. Phytochemistry 1973, 12, 871–873. [Google Scholar] [CrossRef]
- Jakupovic, J.; Klemeyer, H.; Bohlmann, F.; Graven, E.H. Glaucolides and guaianolides from Artemisia afra. Phytochemistry 1988, 27, 1129–1133. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gáti, T.; Taha, A.H.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Tóth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1,10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar] [CrossRef]
- Hajdú, Z.; Hohmann, J.; Forgo, P.; Máthé, I.; Molnár, J.; Zupkó, I. Antiproliferative activity of Artemisia asiatica extract and its constituents on human tumor cell lines. Planta Med. 2014, 80, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Julianti, E.; Jang, K.H.; Lee, S.; Lee, D.; Mar, W.; Oh, K.B.; Shin, J. Sesquiterpenes from the leaves of Laurus nobilis L. Phytochemistry 2012, 80, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Tan, R.X.; Bohlmann, F.; Boldt, P.E.; Jia, Z.J. Sesquiterpene lactones from Artemisia ludoviciana. Phytochemistry 1991, 30, 1573–1577. [Google Scholar] [CrossRef]
- Todorova, M.N.; Vogler, B.; Tsankova, E.T. Acrifolide, a novel sesquiterpene lactone from Achillea crithmifolia. Nat. Prod. Lett. 2000, 14, 463–468. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Z.Y.; Zhu, M.C.; Zhang, K.; Wu, Y.B.; Dong, M.; Shi, Q.W.; Huo, C.H.; Sauriol, F.; Kiyota, H.; et al. New 1, 10-Seco-guaianolides from the flowers of Achillea millefolium. Z. Naturforsch. B 2012, 67, 438–446. [Google Scholar] [CrossRef]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Ersöz, T.; Harput, Ü.Ş.; Saracoğlu, I.; Çaliş, I. Phenolic compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 581–588. Available online: https://journals.tubitak.gov.tr/chem/vol26/iss4/16 (accessed on 29 April 2023).
- Patora, J.; Klimek, B. Flavonoids from lemon balm (Melissa officinalis L., Lamiaceae). Acta Pol. Pharm. 2002, 59, 139–143. Available online: https://europepmc.org/article/med/12365606 (accessed on 29 April 2023).
- Hussien, T.A.; El-Toumy, S.A.; Hassan, H.M.; Hetta, M.H. Cytotoxic and antioxidant activities of secondary metabolites from Pulicaria undulata. Int. J. Pharm. Pharm. Sci. 2016, 8, 150–155. [Google Scholar] [CrossRef]
- Benetis, R.; Radušienė, J.; Januli, V. Variability of phenolic compounds in flowers of Achillea millefolium wild populations in Lithuania. Medicina 2008, 44, 775–781. [Google Scholar] [CrossRef]
- Karlová, K. Accumulation of flavonoid compounds in flowering shoots of Achillea collina Becker ex. Rchb. alba during flower development. Hortic. Sci. 2006, 33, 158–162. [Google Scholar] [CrossRef]
- Haliloglu, Y.; Ozek, T.; Tekin, M.; Goger, F.; Baser, K.H.C.; Ozek, G. Phytochemicals, antioxidant, and antityrosinase activities of Achillea sivasica Çelik and Akpulat. Int. J. Food Prop. 2017, 20, S693–S706. [Google Scholar] [CrossRef]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- Yusupov, M.I.; Kasymov, S.Z.; Abdullaev, N.D.; Sidyakin, G.P.; Yagudaev, M.R. A new lactone, isoridentin, from Achillea biebersteinii. Chem. Nat. Compd. 1977, 13, 674–676. [Google Scholar] [CrossRef]
- Mohamed, A.E.H.H.; Mohamed, N.S.; Hamed, A.R.; Hegazy, M.E.F. Anti-inflammatory activity of highly oxygenated terpenoids from Achillea biebersteinii Afan. Z. Naturforsch. 2016, 71, 429–432. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Jurenitsch, J.; Korhammer, S.; Haslinger, E.; Sosa, S.; Della Loggia, R.; Kubelka, W.; Franz, C. Sesquiterpene lactones of Achillea setacea with antiphlogistic activity. Planta Med. 1991, 57, 444–446. [Google Scholar] [CrossRef]
- Milosavljević, S.; Aljančić, I.; Macura, S.; Milinković, D.; Stefanović, M. Sesquiterpene lactones from Achillea crithmifolia. Phytochemistry 1991, 30, 3464–3466. [Google Scholar] [CrossRef]
- Todorova, M.; Krasteva, M.; Markova, M.; Tsankova, E.; Taskova, R.; Peev, D.R. Terpenoids from Achillea clypeolata. Phytochemistry 1998, 49, 2371–2374. [Google Scholar] [CrossRef]
- Todorova, M.; Tsankova, E. Sesquiterpene lactones from Achillea chrysocoma and Achillea coarctata. Z. Naturforsch. C 2001, 56, 957–960. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Abdel-Lateff, A.; Gamal-Eldeen, A.M.; Turky, F.; Hirata, T.; Paré, P.W.; Karchesy, J.; Kamel, M.S.; Ahmed, A.A. Anti-inflammatory activity of new guaiane acid derivatives from Achillea coarctata. Nat. Prod. Commun. 2008, 3, 851–856. [Google Scholar] [CrossRef]
- Todorova, M.N.; Markova, M.; Tsankova, E.T. Crithmifolide: A sesquiterpene lactone from Achillea crithmifolia. Phytochemistry 1998, 49, 2429–2432. [Google Scholar] [CrossRef]
- Trifunović, S.; Aljančić, Ι.; Vajs, V.; Macura, S.; Milosavljević, S. Sesquiterpene lactones and flavonoids of Achillea depressa. Biochem. Syst. Ecol. 2005, 33, 317–322. [Google Scholar] [CrossRef]
- Zhang, S.J.; Ma, Y.L.; Wang, J.L.; Li, J.; Zhao, M.; Bai, L.M. Chemical constituents of Artemisia argyi. Zhong Cao Yao 2019, 50, 1906–1914. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-851198 (accessed on 29 April 2023).
- Zhang, L.B.; Lv, J.L. Sesquiterpenoids from Artemisia argyi and their COXs inhibitory activities. Fitoterapia 2019, 139, 104372. [Google Scholar] [CrossRef]
- Tan, R.X.; Jia, Z.J.; Jakupovic, J.; Bohlmann, F.; Huneck, S. Sesquiterpene lactones from Artemisia rutifolia. Phytochemistry 1991, 30, 3033–3035. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Shafizadeh, F. Sesquiterpene lactones and systematics of the genus Artemisia. Phytochemistry 1979, 18, 1591–1611. [Google Scholar] [CrossRef]
- Geissman, T.A.; Griffin, T.S.; Irwin, M.A. Sesquiterpene lactones of Artemisia. Artecalin from A. californica and A. tripartita ssp. rupicola. Phytochemistry 1969, 8, 1297–1300. [Google Scholar] [CrossRef]
- Abduazimov, B.K.; Yunusov, A.I.; Sidyakin, G.P. Sesquiterpene lactones of Tanacetum santolina. Chem. Nat. Compd. 1980, 16, 452–454. [Google Scholar] [CrossRef]
- Fernández, I.; Pedro, J.R.; Vidal, R. Norisoprenoids from Centaurea aspera and C. salmantica. Phytochemistry 1993, 34, 733–736. [Google Scholar] [CrossRef]
- Wang, J.; Yi, Z.; Ma, Y.; Zhao, M.; Li, J.; Zhang, S. Study on chemical constituents of Artemisia integrifolia. Zhong Cao Yao 2016, 47, 2241–2245. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Adams, A.; Li, W.; Wang, Z.; De Kimpe, N. Chemical constituents from the aerial parts of Gynura bicolor. Nat. Prod. Commun. 2012, 7, 1563–1564. Available online: https://journals.sagepub.com/doi/pdf/10.1177/1934578X1200701203 (accessed on 29 April 2023). [CrossRef]
- Ni, Z.; Wu, Y.; Zhang, K.; Dong, M.; Sauriol, F.; Huo, C.; Gu, Y.; Shi, Q. A monoterpene and two sesquiterpenoids from the flowers of Achillea millefolium. Chem. Nat. Compd. 2013, 49, 450–453. [Google Scholar] [CrossRef]
- Akkol, E.; Küpeli Koca, U.; Pesin, I.; Yilmazer, D. Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by in vivo excision and incision Models. Evid. Based Complementary Altern. Med. 2011, 2011, 474026. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid lactones as potential anti-cancer agents: An update on molecular mechanisms and recent studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Mantziou, S.; Alexiou, G.A.; Lazari, D.; Sioka, C.; Kyritsis, A.; Markopoulos, G.S. An NF-κB- and Therapy-Related Regulatory Network in Glioma: A Potential Mechanism of Action for Natural Antiglioma Agents. Biomedicines 2022, 10, 935. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Z.; Zhu, M.; Dong, M.; Wang, S.; Shi, Q.; Zhang, M.; Wang, Y.; Huo, C.; Kiyota, H.; et al. Antitumour activities of sesquiterpene lactones from Inula helenium and Inula japonica. Z. Naturforsch. C. 2012, 67, 375–380. [Google Scholar] [CrossRef]
- Surowiak, A.K.; Balcerzak, L.; Lochyński, S.; Strub, D.J. Biological activity of selected natural and synthetic terpenoid lactones. Int. J. Mol. Sci. 2021, 22, 5036. [Google Scholar] [CrossRef]
- Tohme, R.; Al Aaraj, L.; Ghaddar, T.; Gali-Muhtasib, H.; Saliba, N.A.; Darwiche, N. Differential growth inhibitory effects of highly oxygenated guaianolides isolated from the Middle Eastern indigenous plant Achillea falcata in HCT-116 colorectal cancer cells. Molecules 2013, 18, 8275–8288. [Google Scholar] [CrossRef]
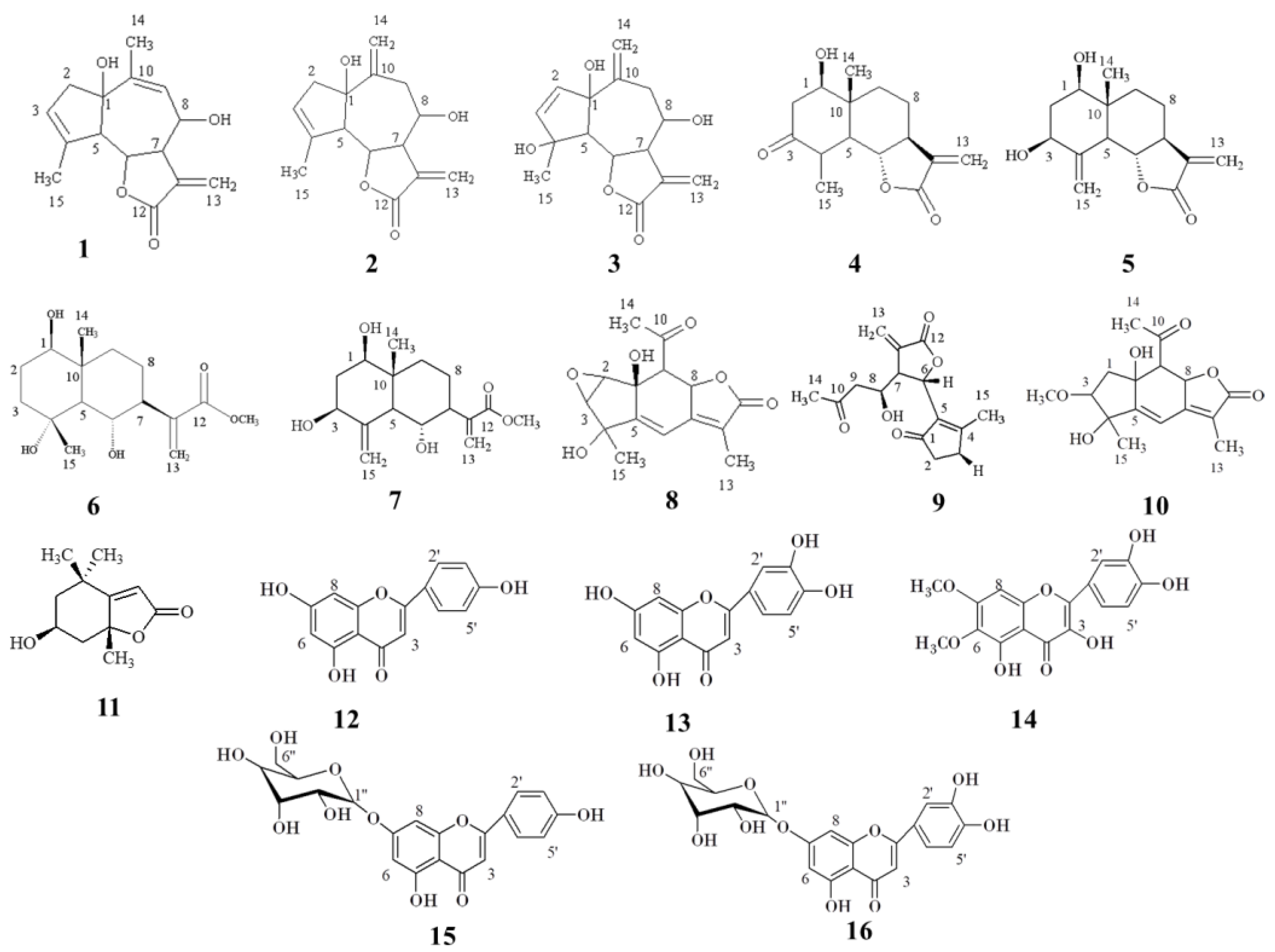
| Isolated in Fractions | Compound Number | Compound Name |
|---|---|---|
| DI(L), EG(F), EO(I), FF(I), FG(I) | 1 | Rupicolin A |
| DI(L), EG(I), EO(L), FL(L), FG(I) | 2 | Rupicolin B |
| DI(L), GG(I), GQ(L) | 3 | 4S,6aS,9R,9aS,9bS)-4,6a,9-Trihydroxy-9-methyl-3,6-dimethylene-3a,4,5,6,6a,9,9a,9b-octahydro-3H-azuleno[4,5-b]furan-2-one) |
| EL(L) | 4 | Artecalin |
| FH(I) | 5 | Ridentin B |
| GD(I) | 6 | (1S,2S,4αR,5R,8R,8αS)-decahydro-1,5,8-trihydroxy-4α,8-dimethyl–methylene-2-naphthaleneacetic acid methyl ester |
| GG(I) | 7 | 1β,3β,6α-Trihydroxycostic acid methyl ester |
| FH(L) | 8 | Acrifolide |
| GH(L) | 9 | Arteludovicinolide A |
| FF(L) | 10 | Lingustolide A |
| EI(L) | 11 | Loliolide |
| DLB(I), EG(I), EH(L) | 12 | Apigenin |
| EG(I), EL(L), FIH(I) | 13 | Luteolin |
| EFK(F) | 14 | Eupatolitin |
| EL(L) | 15 | Apigenin 7-O-glucopyranoside |
| EFF(L) | 16 | Luteolin 7-O-glucopyranoside |
| Cell Line | U87MG | T98G | ||||
|---|---|---|---|---|---|---|
| Cell Cycle Phases | Control | Rupicolin A | Rupicolin B | Control | Rupicolin A | Rupicolin B |
| G1 | 64.73 | 15.87 | 23.60 | 58.27 | 19.27 | 32.60 |
| S | 9.20 | 24.27 | 37.60 | 13.67 | 24.13 | 17.20 |
| G2/M | 20.87 | 54.40 | 37.00 | 25.73 | 50.67 | 42.93 |
| subG1 | 3.13 | 3.20 | 3.40 | 0.80 | 2.40 | 4.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiftsoglou, O.S.; Krigas, N.; Gounaris, C.; Papitsa, C.; Nanouli, M.; Vartholomatos, E.; Markopoulos, G.S.; Isyhou, R.; Alexiou, G.; Lazari, D. Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model. Pharmaceutics 2023, 15, 1383. https://doi.org/10.3390/pharmaceutics15051383
Tsiftsoglou OS, Krigas N, Gounaris C, Papitsa C, Nanouli M, Vartholomatos E, Markopoulos GS, Isyhou R, Alexiou G, Lazari D. Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model. Pharmaceutics. 2023; 15(5):1383. https://doi.org/10.3390/pharmaceutics15051383
Chicago/Turabian StyleTsiftsoglou, Olga S., Nikos Krigas, Christos Gounaris, Christina Papitsa, Maria Nanouli, Evrysthenis Vartholomatos, Georgios S. Markopoulos, Rafaela Isyhou, George Alexiou, and Diamanto Lazari. 2023. "Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model" Pharmaceutics 15, no. 5: 1383. https://doi.org/10.3390/pharmaceutics15051383
APA StyleTsiftsoglou, O. S., Krigas, N., Gounaris, C., Papitsa, C., Nanouli, M., Vartholomatos, E., Markopoulos, G. S., Isyhou, R., Alexiou, G., & Lazari, D. (2023). Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds’ Effects on a Glioblastoma Cellular Model. Pharmaceutics, 15(5), 1383. https://doi.org/10.3390/pharmaceutics15051383









