Abstract
Cancer is one of the leading causes of death, and latest predictions indicate that cancer- related deaths will increase over the next few decades. Despite significant advances in conventional therapies, treatments remain far from ideal due to limitations such as lack of selectivity, non-specific distribution, and multidrug resistance. Current research is focusing on the development of several strategies to improve the efficiency of chemotherapeutic agents and, as a result, overcome the challenges associated with conventional therapies. In this regard, combined therapy with natural compounds and other therapeutic agents, such as chemotherapeutics or nucleic acids, has recently emerged as a new strategy for tackling the drawbacks of conventional therapies. Taking this strategy into consideration, the co-delivery of the above-mentioned agents in lipid-based nanocarriers provides some advantages by improving the potential of the therapeutic agents carried. In this review, we present an analysis of the synergistic anticancer outcomes resulting from the combination of natural compounds and chemotherapeutics or nucleic acids. We also emphasize the importance of these co-delivery strategies when reducing multidrug resistance and adverse toxic effects. Furthermore, the review delves into the challenges and opportunities surrounding the application of these co-delivery strategies towards tangible clinical translation for cancer treatment.
1. Introduction
According to the World Health Organization (WHO), cancer is a serious public health problem around the world, being the leading cause of mortality and causing more than 6 million deaths yearly [1,2]. While the cancer mortality rate has declined in recent years, WHO estimates it will reach 13.1 million cancer-related deaths by 2030 [3,4]. Despite extensive development of cytotoxic agents, current therapy approaches for cancer are still ineffective [5]. There are two major treatment options available: surgical procedures or non-surgical therapy regimens [5]. The surgical intervention is limited by the tumor’s size as well as the stage of metastasis in the tissues and organs from the site of origin. Non-surgical treatment options primarily include chemotherapy and radiotherapy, or a combination of these approaches [6,7]. Even though chemotherapeutic agents have evidenced efficacy in killing cancer cells by interfering with the process of cell division [5], they still face a number of challenges, including low bioavailability and lack of selectivity. Consequently, non-specific body distribution of chemotherapy is a key factor for cancer patient mortality, followed by chemo-resistance of cancer cells, which is another significant barrier that must be overcome in order to provide effective cancer treatment [2,3,7,8,9].
Several strategies have been employed to improve the performance of chemotherapeutic agents and, as a result, overcome the abovementioned challenges. Among these strategies are chemical modification, the development of new chemotherapeutic agents that are not detected by multidrug resistance (MDR) efflux pumps, and the combination of the cytotoxic agent with a chemosensitizer. Moreover, nanocarriers have been proposed to surpass some of the chemotherapy challenges. In this regard, nanocarriers for drug delivery are designed to reach specific organs and act selectively on the target site, providing advantages over conventional chemotherapeutics [1,7,10]. Some of the nanocarriers’ benefits include increased permeability through cell membranes and improved protection of the drugs against physical and chemical degradation. Furthermore, nanocarriers improve the therapeutic potential by optimizing drug properties such as stability, solubility, and bioavailability [1,11].
A common strategy for cancer therapy based on the association of multiple chemotherapeutic agents has been implemented as the standard first-line treatment of various malignancies to improve clinical outcome [2]. This approach has shown great potential, particularly to solve the issue of MDR in cancer cells [12,13] and improve anticancer efficacy [14,15,16]. Nonetheless, the administration of multiple drugs is frequently challenging, as different pharmacological agents have distinct pharmacokinetic profiles, resulting in an uncoordinated uptake by the tumor cell, affecting the expected synergistic effect [17,18]. Since nanocarriers can deliver multiple pharmacological agents to the same tumor cell in a single vehicle, the administration of combined drugs utilizing nanocarriers offers the most recent and most efficient therapy for several cancers. The “same time at same place” strategy is appealing since it may increase therapeutic efficacy while minimizing damage to healthy cells through pharmacological synergism, overcoming MDR, and reducing the effective doses [2,19]. Additionally, due to the importance of minimizing harmful side effects to healthy cells, the pharmaceutical market has been more receptive to lipid-based nanocarriers as they are classified by the FDA as generally recognized as safe (GRAS). Lipid-based nanocarriers are also regarded as safe because they are biodegradable and will not accumulate in the body [20].
There is currently a growing interest in the use of natural products in cancer prevention and therapy. Natural compounds and their derivatives have been clinically researched for their capacity to reverse, inhibit, and prevent cancer progression [21]. Due to their proven efficacy in a wide range of malignant tumors with minimal side effects and toxicity, some authors demonstrated that these agents may be a promising option for combination therapy [22].
For their prospective therapeutic applications, nucleic acids such as plasmid DNA (pDNA), small interfering RNA (siRNA), and micro-RNA (miRNA) have been developed into potent tools. Since nucleic acids are able, among other effects, to modulate the expression of genes responsible for MDR, associating chemotherapeutics with nucleic acids has been suggested as an appropriate strategy to increase the effect of cancer therapy [23,24,25,26]. The combination of natural compounds and nucleic acids is a less well-known strategy that has the potential to be very effective as a therapeutic modality that acts by different mechanisms. This combination can lead to a synergistic improvement of the therapeutic effect, a sensitization of the cancer cells to the anticancer activity of the natural compound, and a synergic effect against MDR that restores the anticancer effect.
This review provides a comprehensive overview of lipid-based nanocarriers used for the co-delivery of natural compounds either with chemotherapeutic drugs or with nucleic acids. The utilization of such co-delivery systems offers several benefits, including synergistic/additive/potentiation effects, sensitization of cancer cells, overcoming of MDR, and reduction in adverse effects. Given their promising features, there is an increasing number of reviews exploring the use of natural compounds in cancer treatment (e.g., [27,28,29,30,31,32,33]). However, to date, there has been no comprehensive investigation into the use of lipid-based nanocarriers for the co-delivery of natural compounds and nucleic acids, nor have there been any examples provided of the use of lyotropic liquid crystalline nanoassemblies (LLCNs). Recently, advanced lipid mesophase delivery systems have emerged as a promising class of nanocarrier system. These systems have the potential to encapsulate various cargos with a wide range of lipophilicity properties, making them one of the most advantageous co-delivery systems for cancer [34]. For these reasons, a thorough and up-to-date overview of the studies currently available in the literature is still lacking. Lastly, the review examines the potential opportunities and challenges associated with the implementation of nanocarriers for co-delivery of natural compounds and/or chemotherapeutic drugs and nucleic acids in a clinical context.
2. Natural Compounds: Advantages of Combination Therapy in Cancer
Conventional therapy has evident benefits in cancer treatment; however, despite the continuous emergence of new anticancer agents, the majority of chemotherapy-based treatment continues to remain ineffective due to an array of factors, which include chemotherapy-induced toxicity and adverse reactions, insufficient target specificity, and, most importantly, drug resistance during cancer progression (Figure 1) [9].
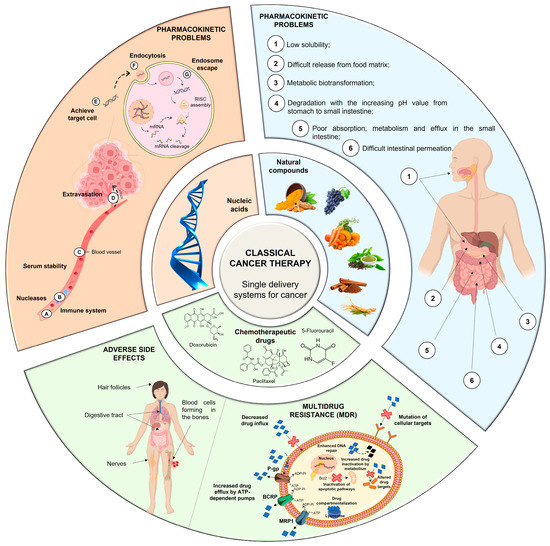
Figure 1.
Problems associated with the classical single-delivery therapy (i.e., administration of each of the therapeutic agents in their free form). Nucleic acids, if delivered in the free form, would face different pharmacokinetic challenges, including inactivation by nucleases (A), lack of serum stability due to the immune system (B) and serum proteins (C), extravasation difficulties (D), non-specific distribution in target cells (E), difficulties entering the cell (F), and degradation if not able to escape endosomes (G). Chemotherapeutic drugs, when delivered in the free form, have a nonspecific distribution in cancer cells and healthy cells causing serious adverse side effects, commonly affecting hair follicles, the digestive tract, blood cells and nerves. Furthermore, several MDR mechanisms, such as drug efflux by multidrug resistance protein 1 (MRP1), P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP), or inactivation of apoptotic pathways by B cell leukemia protein (Bcl2), can impair their efficiency. Natural compounds, when administered in their free form, exhibit a number of pharmacokinetic issues that affect their biodistribution and efficacy (1–6).
In this regard, combination therapy has recently become an emerging strategy for tackling the drawbacks of chemotherapy. Simultaneous delivery of two or more therapeutic agents (chemotherapeutic drugs/natural compounds/nucleic acids) can modify different signaling pathways in cancer cells, providing a synergistic response, improving targeting selectivity, optimizing therapeutic effect, and overcoming MDR (Figure 2) [2,17,35]. Thus, taking benefit of the minimal side effects promoted by natural compounds, there is a tendency to follow the potential strategy of combination therapy [9].
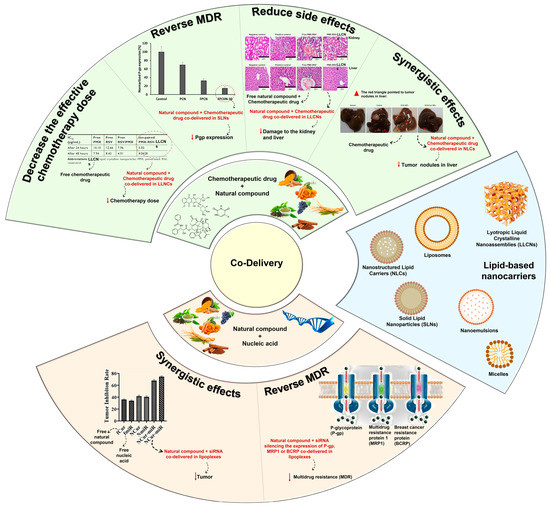
Figure 2.
Schematic illustration of lipid-based nanocarriers and advantages of their use for the co-delivery of natural compounds and chemotherapeutic drugs or nucleic acids. Adapted from [36,37], and from [38,39] with permission from Elsevier.
2.1. Overcoming Multidrug Resistance
MDR is a mechanism that emerges after cells’ exposure to chemotherapeutic agents and refers to the capacity of cancer cells to become resistant to the agents’ effect and can result in the development of malignant cell metastases [40,41]. The cellular mechanisms of MDR can be divided into two general classes: (i) those that block the delivery of chemotherapeutic agents to their target sites, and include the abnormal vasculature which results in low oral chemotherapeutic absorption, early renal clearance, poor bioavailability, and lower tumor site accumulation; or (ii) those that emerge in cancer cells primarily as a result of genetic and epigenetic alterations and directly affect the efficacy of chemotherapeutic agents, and include apoptosis deregulation, increased repair of drug-induced DNA damage, and, enhanced efflux of chemotherapeutic agents [40,41].
Although a wide range of different factors can contribute to MDR, drug efflux changes are considered the major cause of classical MDR [42]. Drug efflux is enhanced by the overexpression of human ATP-binding cassette (ABC) membrane transporters. These transporters are accountable for removing chemotherapeutic agents from cancer cells. Among the ABC transporters, the multidrug resistance protein (MRP) P-glycoprotein (P-gp) is an ATP-dependent drug efflux pump also referred to as multidrug resistance protein 1 (MRP1) (Figure 1). P-gp, the best-studied drug efflux pump, is a significant contributor to chemotherapy failure [42,43]. Furthermore, it has been reported that resistant cells have significantly greater levels of P-gp, and their overexpression is linked to a poor prognosis in a variety of cancers [44].
P-gp-mediated MDR affects several classes of chemotherapeutic agents, such as anthracyclines (e.g., daunorubicin and doxorubicin (DOX)), taxanes (e.g., paclitaxel (PTX) and docetaxel (DTX)), epipodophyllotoxins (e.g., etoposide), and camptothecins (e.g., topotecan and methotrexate (MTX)). As a result, strategies to reverse P-gp-mediated MDR have been extensively researched since the early 1980s, and three generations of P-gp inhibitors are currently classified [40,41,45]. Despite promising in vitro results, there is not, unfortunately, an irrefutable proof of efficacy for the currently available inhibitors, since various clinical trials have been performed to evaluate their anticancer effect, but no significant improvements have been found [21,40]. The development of an ideal inhibitor is commonly associated with the difficulty of finding compounds with high potency and specificity, and with low intrinsic toxicity. Furthermore, it is difficult to achieve specificity of the inhibitors to the ABC transporters, as well as interactions between chemotherapeutic agents and inhibitors [21].
Consequently, in order to overcome such limitations, researchers have shifted their attention to novel approaches for MDR prevention in cancer. In this regard, natural compounds have emerged as an appealing solution, primarily due to their chemosensitizing capacity [46]. Chemosensitizers are small molecules that can increase the sensitivity of cancer cells to chemotherapeutic agents, and those that act as ABC membrane transporter inhibitors are particularly effective. The main example is inhibitors obtained from natural sources, also known as fourth-generation inhibitors, which can interact with ATP binding sites or act directly at MRP binding sites. Natural inhibitors have the potential to be considerably more successful since they offer the most diverse and innovative chemical scaffolds [21]. Moreover, natural compounds with anticancer properties are widely available, as evidenced by the Naturally Occurring Plant-based Anti-Cancer Compound-Activity-Target Database (NPACT) [47]. The main natural compounds evaluated as chemosensitizing agents are highlighted in Figure 3.
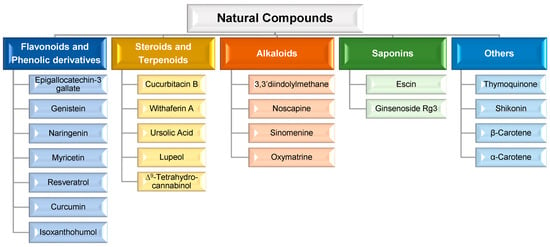
Figure 3.
Main natural compounds considered chemosensitizing agents, according to their chemical family [47].
Although a wide range of natural compounds, such as terpenoids, alkaloids, steroids, and saponins (Figure 3), have recently been employed to overcome MDR [46,47], phenolic derivatives and flavonoids have been the most cited and studied. According to in vitro biochemical and pharmacological studies, the majority of flavonoids could modulate ABC transporters by competitively binding to the substrate-binding sites and, as a result, delaying cellular efflux [21]. From these chemical families of natural compounds, resveratrol (RSV), curcumin (CUR), and epigallocatechin-3-gallate (EGCG) are the most promising as they can also directly interact with MDR genes [47]. For example, CUR, a polyphenol found in plants of the genus Curcuma, modulates cancer signaling pathways, primarily by inhibiting the nuclear factor kappa B (NF-kB) pathway, as shown in Figure 4. In more detail, CUR modifies signaling pathways of the apoptosis process, by interfering with X-linked inhibitor of apoptosis protein (XIAP), cell proliferation (cyclin D1, ciclo-oxigenase-2 (COX-2), C-myc), cellular inhibitor of apoptosis protein-1 (CIAP-1), cell metastasis (C-X-C chemokine receptor type 4 (CXCR4), ICAM-I), cell invasion (matrix metallopeptidase 9 (MMP-9)), and angiogenesis (vascular endothelial growth factor (VEGF)) [47]. This natural compound also displays P-gp inhibitory activity by downregulating the phosphoinositide 3-kinases (PI3K)/protein kinase B (Akt) [36].
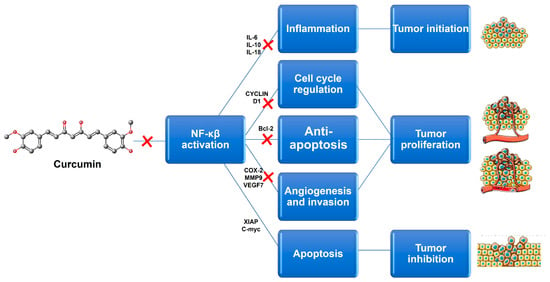
Figure 4.
Potential targets associated with Curcumin anticancer activity. This natural compound induces a reduction in its target genes by inhibiting NF-kβ signaling. Bcl-2-B-cell limphoma-2; COX-2-ciclo-oxigenase-2; IL-6-interleukin 6; IL-10-interleukin 10; IL-18-interleukin 18; MMP9-matrix metallopeptidase 9; NF-kβ-nuclear factor kappa B; VEGF-vascular endothelial growth factor; XIAP-X-linked inhibitor of apoptosis protein.
2.2. Synergistic, Additive, and Potentiation Effects
The combination of therapeutic agents can result in the following complementary effects [35,48]: (i) synergistic, when the final effect is greater than the sum of individual agents’ effects, resulting in cooperative targeting of activity regulation but with each agent targeting different sites; (ii) additive, that promotes greater or equal effect to the sum of individual agents’ effect; however, both agents act on the same target or pathway; and, (iii) potentiation, in which one agent can enhance the effect of the other or minimize its side effects by regulating pharmacokinetics and/or pharmacodynamics. Furthermore, when both agents in a combination therapy act on the same pathway or target, an undesirable antagonist effect may occur (i.e., when the resultant therapeutic effect is less than the sum of effects of each agent delivered).
2.3. Reducing the Side Effects
Combination therapy may also avoid the toxic side effects that normally affect healthy cells. This could happen if one of the co-delivered agents is antagonistic to the other in terms of cytotoxicity. For example, antioxidant supplementation during anticancer treatment may decrease adverse reactions, primarily due to the prevention of reactive oxygen species (ROS)-mediated injury, without compromising anticancer activity [47].
2.4. Decreasing the Effective Chemotherapy Dose
One significant drawback of chemotherapy is the high dose of cytotoxic drugs required to achieve a therapeutic effect, which causes serious side effects. In this context, combination therapy appears to be a promising alternative, since the combination of a natural compound and a chemotherapeutic drug may promote an increase in the cytotoxic effect (due to previously described synergistic, additive, or potentiation effects), improve chemotherapeutic performance, and reduce the effective dose required to achieve the necessary therapeutic outcomes [47].
3. Lipid-Based Nanocarriers for the Co-Delivery of Natural Compounds and Other Therapeutic Agents
Classic single-delivery therapy (i.e., single administration of the therapeutic agents in their free form) can be challenging due to several drawbacks, including the presence of highly organized physical, physiological, and enzymatic barriers, which make targeting cancer cells with minimal side effects particularly difficult (Figure 1). Furthermore, the varying physiochemical and pharmacodynamic properties of different agents can limit their successful co-delivery [49]. Thus, as previously stated, nanocarriers are an advantageous option for overcoming these challenges, since they are designed to reach specific organs and act selectively on the target site [1,7,10]. In this regard, several nanocarriers have been widely explored for the delivery of anticancer drugs [5]. Lipid-based nanocarriers present some attractive features such as: non-toxic degradation products, biodegradable matrix, low toxicity, high capacity to incorporate lipophilic and/or hydrophilic compounds, and ability to achieve controlled release of encapsulated therapeutic agent [50,51].
3.1. Co-Delivery of Natural Compounds and Chemotherapeutics
Table 1 provides several examples of lipid-based nanocarriers co-encapsulating a chemotherapeutic agent and a natural compound for cancer treatment.

Table 1.
Examples of lipid-based nanocarriers co-encapsulating a chemotherapeutic agent and a natural compound for cancer treatment.
Co-delivery of natural compounds acting as chemosensitizers and chemotherapeutic agents with different or comparable mechanisms of action has been identified as the most promising strategy for overcoming undesirable toxicity and other side effects while improving therapeutic effect [5]. However, this co-delivery is also being investigated as a strategy to treat drug-resistant cancers, because of its ability to interfere with a number of signaling pathways in cancer cells [77]. In this regard, the co-encapsulation of different chemotherapeutic agents, particularly DOX, PTX, and 5-fluorouracil (5-FU), with diverse natural compounds using different lipid-based nanocarriers has been reported (Table 1).
DOX, a potent anthracycline, exhibits a broad-spectrum of anticancer activity [61,78]. In brief, DOX’s anticancer mechanism involves two primary possible pathways: (i) producing ROS that cause DNA damage [79,80] and (ii) intercalating into DNA strands and inhibiting topoisomerase II [81,82]. Despite its high efficacy in cancer treatment, DOX’s clinical application is hampered by severe side effects, the majority of which are caused by non-selective DOX-induced apoptosis in tissues and organs [83,84,85], as well as the development of MDR during chemotherapy [86,87]. One co-delivery approach focuses on the combination of DOX with natural compounds, such as CUR [9,39,56], palmitoyl ascorbate (PA) [59], and oleanolic acid (OA) [58], using different lipid-based nanocarriers in order to obtain a synergistic effect. For example, Barui et al. [56] and Tefas et al. [9] developed liposomes co-encapsulating CUR and DOX, and demonstrated their synergism in inhibiting the proliferation, invasion, and migration of tumor cells [9,40]. Zhao et al. [39] studied the cell proliferation inhibition effect of lipid nanoparticles co-loaded with DOX and CUR. The results confirmed the synergistic effect on apoptosis, proliferation, and angiogenesis of hepatocellular carcinoma (HCC), by the increase in Caspase-3 and Bax/Bcl-2 ratio and the decrease in C-myc and VEGF. In addition to CUR, the co-encapsulation in liposomes of DOX with PA, a lipophilic derivative of ascorbic acid, caused an anticancer synergistic effect [59]. Furthermore, the addition of PA not only improved DOX’s anticancer effects [80], but it also demonstrated that this natural compound can mitigate the tissue toxicity of DOX resulting from oxidation [59], as previously described by Shimpo et al. [88]. Sarfraz et al. [58] explored the effect of a liposomal formulation that co-encapsulated DOX and OA, a natural pentacyclic triterpenoid, in a HepG2 mouse model of HCC. This combination had an anticancer synergistic effect, as well as an antagonistic oxidative effect at the cardiomyocytes level, which reduced DOX cardiotoxicity [58]. Overcoming MDR with flavonoids, such as quercetin (QUER) and baicalein (BCL), is the most widely discussed strategy for increasing anticancer effect in several drug-resistant cell lines [55,89,90]. As an example of this strategy, Liu et al. [55] developed hyaluronic acid (HA)-decorated nanostructured lipid carriers (NLCs) to co-deliver DOX and BCL, and reported a synergistic cytotoxic effect in DOX-resistant MCF-7 breast cancer cells. DOX-QUER co-loaded in a lipid-based nanocarrier was also developed as a promising approach for active targeting with the goal of increasing cellular uptake and toxicity against cancer cells [43,91]. Zhang et al. [43] confirmed that QUER can avoid the MDR effect and that biotin (BIO) enhances P-gp inhibition synergistically, resulting in improved antitumor activity. Furthermore, to overcome MDR, the use of DOX combined with other natural compounds, such as docosahexaenoic acid (DHA) [57], α-tocopherol succinate (TS) [61], and Brucea javanica oil (BJO) [62], has been reported in the literature. Mussi et al. [57] proposed NLCs co-loaded with DOX and DHA that increased cytotoxicity activity and penetration of DOX, inferring a bypassing of P-gp bomb efflux. The potential of solid lipid nanoparticles (SLNs) co-loaded with DOX and TS—a vitamin E analogue—to overcome MDR and to increase DOX cytotoxicity have been confirmed by two independent studies [61,92]. Li et al. [62] developed lyotropic liquid crystalline nanoassemblies (LLCNs) co-loaded with DOX and BJO in human breast carcinoma cell lines (MCF-7) that have shown an improved anti-tumor effect [62].
PTX is an antimicrotubule chemotherapeutic agent widely used in cancer treatment [70,74]. In cancer cells, PTX induces apoptosis and, as a mitotic inhibitor of cell replication, interferes with microtubule breakdown, which leads to cell cycle arrest [72]. Nevertheless, due to the development of MDR, the potential application of PTX in several cancers is severely limited [36,93]. PTX is a substrate for MDR1 (i.e., for the P-gp channel) [94], and thus the primary strategy for reversing MDR using combined therapy is the co-delivery of PTX with potential P-gp modulators. Several studies were developed in order to reverse MDR, using different natural compounds, such as borneol (BOR) [54], CUR [55,56], cyclosporine A (CycA) [57] and parthenolide (PTN) [74]. Abouzeid et al. [55] and Ganta et al. [56] demonstrated a synergistic effect in MDR cells of CUR and PTX co-loaded in PEG-PE/vitamin E micelles [55] and nanoemulsions [56]. Indeed, CUR enhanced PTX cytotoxicity by down-regulation of the NF-kB and Akt pathways [55,56] (Figure 4). Tang et al. [69] studied lipid–albumin nanoassemblies (LANs) co-loaded with BOR and PTX to achieve greater cellular uptake and improved anti-tumor efficacy. BOR/PTX LANs significantly increased cytotoxicity and drug accumulation in cancer cells, corroborating its potential to enhance the efficacy of chemotherapy. Sarisozen et al. [72] developed actively targeted PEG-PE-based micelles co-encapsulating PTX and CycA, a first-generation P-gp inhibitor, to reverse PTX resistance in P-gp-expressing cells. The authors concluded that the formulation showed a significant increase in cytotoxicity, specifically in drug-resistant cells [72]. Gill et al. [74] studied PTX and PTN co-loaded in micelles that significantly improved anticancer activity against PTX-resistant cell lines. Moreover, the anti-proliferative and pro-apoptotic activity of RSV against MDR tumor cells has been reported [95]. Meng et al. [2] demonstrated that co-encapsulating RSV and PTX in PEGylated liposomes had the potential to reverse PTX-resistance of MCF-7/Adr tumors and improve the efficacy of RSV and PTX, implying their promising use in the treatment of drug-resistant malignancies. The BCL oxidative stress-inducing potential was also considered by Meng et al. [68], who proposed co-loading PTX and BCL in nanoemulsions to enhance antitumor effect and suppress MDR in breast cancer. The antioxidant activity of PA has also improved PTX anticancer activity when they were co-encapsulated in liposomes [73].
5-FU, an antimetabolite, is commonly used to treat colorectal, breast, head, and neck cancers. The anticancer effect of 5-FU is due to the inhibition of thymidylate synthase and the incorporation of its metabolites into DNA and RNA, thereby inhibiting their production [96]. Previous research has shown that RSV synergistically promotes 5-FU-mediated cancer cell apoptosis [52,53]. Mohan et al. [53] investigated the influence of RSV and 5-FU co-loaded in PEGylated liposomes on a head and neck squamous cancer cell line, reporting differential combination effects on gene expression that resulted in cancer cell apoptosis. Furthermore, Cosco et al. [52] evaluated the efficacy of ultradeformable liposomes co-loaded with both agents against squamous cell carcinoma-related lesions. The authors reported that liposomes improved RSV and 5-FU permeation into deeper skin strata, where antioxidant and antiproliferative effects of RSV are essential [52].
3.2. Co-Delivery of Natural Compounds and Nucleic Acids
Currently, the efforts to overcome the drawbacks of traditional cancer treatment are mostly focused on strategies that can block the efflux pump effects generated by long-term pharmacological therapy [97]. The combination of natural compounds with nucleic acids is another promising option for a co-delivery system [17]. Furthermore, this is a desirable method for cancer treatment able to overcome MDR and generate synergistic apoptotic effects while reducing toxicity and other side effects [98]. Given their multifunctionality and ability to encapsulate drugs, nanocarriers are the most widely used drug delivery systems [99]. However, many of these delivery systems suffer from non-degradability, complexity, and insufficient biological activity [100]. Lipid-based nanocarriers developed for the co-delivery of nucleic acids and natural compounds (Table 2) are promising due to their low toxicity, biocompatibility, and ease of scaling up [101].

Table 2.
Examples of lipid-based nanocarriers co-encapsulating a nucleic acid and a natural compound for cancer treatment.
Co-delivery of natural compounds and pDNA [103], siRNA [102,104], or miRNA [38], has been reported and the expression of specific genes can be restored, upregulated, downregulated, or even silenced depending on the type of nucleic acid used [109].
pDNA are small DNA molecules that can carry a gene that will be transcribed into a specific protein of interest, thus improve or restore its function and consequently, different cellular pathways [110]. For example, Xu et al. [103] studied the potential of lipoplexes (i.e., nucleic acids condensed by liposomes) for the co-delivery of p53 pDNA and RSV. The developed formulations were able to up-regulate p53, and the combination of two therapeutic agents demonstrated an anticancer synergistic effect by cell growth inhibition [103].
In comparison to pDNA, miRNA and siRNA act via RNA interference mechanisms.
miRNA can direct and regulate the expression of multiple genes encoding proteins involved in different cellular pathways, both at the transcriptional and translational levels [111]. Thus, Abtahi et al. [38] studied nioplexes (i.e., nucleic acids condensed by niosomes) for co-delivery of CUR and miR-34a, one of the p53 network members. The results showed that combining miR-34a and CUR enabled a synergistic effect, allowing for a reduction in NF-kB expression (Figure 4) and a consequent increase in p53 expression [38].
siRNA has been widely used to selectively down-regulate abnormal protein expression in tumor cells, which is a promising strategy for preventing disease progression [102,106]. The combination of siRNA with different natural compounds, such as gambogenic acid (GNA) [102], CUR [38,104,105,106,107], and gedunin (GED) [108], using lipid-based nanosystems has also been reported. GNA, isolated from Gamboge, is considered a potential anticancer compound as it regulates the expression of cyclin D1 and COX-2 [112,113,114]. Yu et al. [102] studied lipoplexes for co-delivery of VEGF-siRNA and GNA to improve anticancer efficiency in HepG2 cells. According to this study, VEGF-siRNA seemed to mediate VEGF silencing, and the combination with GNA enhanced cell sensitivity and promoted apoptosis [102]. GED, a tetranortriterpenoid isolated from the Indian neem tree, is a Hsp90 inhibitor that demonstrated anti-proliferative effects in several cancers [115,116]. Rana et al. [108] developed lipoplexes for the co-delivery of GED and P-gp siRNA, to enhance the inhibition of breast cancer stem cell proliferation by modulating P-gp and cyclin D1 as well as apoptosis-related genes [108]. CUR has received considerable attention in cancer treatment as the bioactive compound most co-loaded with siRNA. Anup et al. [104,105] developed lipoplexes co-loaded with CUR and STAT3 siRNA. The authors demonstrated that the lipoplexes administrated iontophoretically showed similar efficiency in inhibiting tumor progression and STAT3 protein suppression as intratumorally administration. The authors also reported a synergistic effect of CUR and STAT3 siRNA in cancer cell inhibition [104,105]. Muddineti et al. [106] studied micelleplexes (i.e., nucleic acids condensed by micelles) for co-delivery of CUR and siRNA in a time-dependent manner via a clathrin-dependent endocytosis mechanism [106]. Jia et al. [107] developed CUR and siCCAT1 co-delivered in lipopolyplexes (i.e., nucleic acids condensed by liposomes containing polymers) for colorectal cancer therapy. The results confirmed the ability of the lipopolyplexes to perform endosomal/lysosomal escape efficiently due to the proton sponge effect of the polymer component. Furthermore, the co-delivery of CUR and siCCAT1 could effectively silence CCAT1 and achieve a synergistic effect, thereby enhancing B-cell limphoma-2-mediated apoptosis in human colon cancer cells [107].
3.3. Challenges and Opportunities of Co-Delivery Strategies
Although co-delivery of natural compounds and other therapeutic agents in a single nanocarrier is a promising strategy for cancer treatment, its clinical achievement is restricted due to challenges occurring at different stages of the nanocarrier’s development including loading capacity, stability, pharmacokinetics, tumor targeting efficiency, pharmacodynamics, and toxicity [32,117,118,119,120,121]. The first challenge is to choose an appropriate nanocarrier composition capable of simultaneously loading natural compounds and other therapeutics with different physicochemical properties and able to establish distinct chemical interactions. In particular, the co-delivered therapeutics’ association with the nanocarrier should be strong enough to ensure their in vivo stability, avoiding interaction with serum proteins and, as a result, an early release and poor tissue distribution. In this regard, the most striking finding is that LLCNs have not been chosen more often for combination therapy in cancer and co-delivery purposes of therapeutic agents and natural compounds. Indeed, LLCNs merit further investigation and represent a future opportunity for combination cancer therapy purposes, as evidenced by previous studies [37,62,122], because their periodic lipid membranes and networks of aqueous channels present in the inner liquid crystalline organization provide benefits for the entrapment, solubilization, and protection of one or more therapeutics. The internal structure of these nanocarriers, which is formed of liquid crystalline lipid bilayers stacked in precise lattice layouts to form complicated three-dimensional networks of aqueous channels, is what makes LLCNs topology unique. Therefore, LLCNs exhibit a substantially greater surface area when compared to other lipid-based nanocarriers and can encapsulate higher quantities of hydrophobic and hydrophilic therapeutics at sizes equivalent to those of other lipid-based nanocarriers.
Another critical challenge is combining distinct pharmacokinetic behaviors of individual therapeutics, which can result in inconsistent in vivo biodistribution after their co-delivery. As a result, a strict modulation of the nanocarrier is required for precise control of the dose and chronological sequence of each co-delivered therapeutic release. Furthermore, one of the primary goals of co-delivery is to increase the therapeutics’ potency through synergistic or additive effects. However, this goal can be jeopardized by suboptimal therapeutic doses at tumor tissues, so active targeting strategies are required but difficult to implement in order to simultaneously fulfill the different targeting sites of the combined therapeutics. At a pharmacodynamic level it is also challenging to define concentration-dependent effects of natural compounds, and their co-delivery with other therapeutic agents may also produce antagonistic effects. Finally, a major challenge to therapeutic efficacy and clinical translation is that co-delivered therapeutics may cause synergistic systemic toxicity and, as a result, unexpected adverse effects when combined. Therefore, hypersensitivity reactions, long-term toxicity evaluation, and biosafety of nanocarriers and their cargo need to be considered, and only after these issues are fully addressed will co-delivery therapy be widely available in the clinic.
Despite all the above challenges, most co-delivery-based nanotherapeutics have been developed empirically by trial-and-error strategies instead of rational development programs and quality-by-design approaches [120,123]. Few research studies have attempted to comprehensively examine co-delivery combinations using proper characterization and analytical techniques throughout the development process. The characterization of co-delivery-based nanotherapeutics is required from a physicochemical point of view to comprehend its corresponding biological behavior (e.g., knowing how nanocarrier size and shape can be tailored for improved hemodynamics or how to modulate the innate immunity to reduce nanocarrier clearance), and to provide guidance for the process control and safety assessment. In terms of the quantity of parameters necessary for an accurate and thorough characterization, this categorization does not meet consensus. The adoption of reference nanomaterials and internationally recognized procedures is suggested as the solution to unifying the many viewpoints on this subject [117,118]. A characterization of co-delivery-based nanotherapeutics should ideally take place at several points, from the design stage to the assessment of its in vitro and in vivo performance. Timing, chemotherapeutic and/or natural compound metabolic processes, and biological interaction patterns explored in preclinical models will all have an impact on the final clinical outcome of combined treatments. Therefore, a robust biocompatibility testing program, which typically consists of in vivo studies reinforced with chosen in vitro assays to assure safety, is required for the pre-clinical evaluation of co-delivery based nanotherapeutics.
Another issue in the translation of co-delivery-based nanotherapeutics to market and clinical practice is controlling the production process by identifying the critical factors and technology required for a reproducible and economically viable scale-up. The impact of siRNA nanotechnology on pandemic prevention has been demonstrated by COVID-19 vaccines [124]. However, the clinical applications of gene therapy for cancer treatment are currently limited due to the growing stage of the technology, potential unknown risks, higher costs, and selectivity in treating certain types of cancer [97]. Therefore, if this technology is not mature enough in a single delivery context, it is even further away from clinical translation when nucleic acid and natural compound co-delivery is considered. Moreover, based on current understanding, there is an academic interest in co-delivery of natural compounds with chemotherapeutics and/or nucleic acids for cancer treatment that does not mirror the lack of ongoing clinical trials pertaining to the subject matter. This may be attributed to the emergence of new challenges as well as the expenses involved in the design and development of such intricate formulations. The bench-to-market translation of co-delivery-based nanotherapeutics is also severely constrained by the regulatory framework surrounding clinical application. Therefore, it is imperative to address the regulatory and scientific gaps to facilitate the advancement of nanomedicine as a driving force for future biomedical innovation.
4. Conclusions
Conventional cancer therapies are still unable to achieve the desired outcomes due to current limitations related with inefficiency and selectivity. As a result, the development of novel therapeutic strategies to overcome these limitations has become critical. Combination therapy has been extensively explored in this context, since co-delivery of natural compounds and chemotherapeutic agents or nucleic acids can achieve stronger anticancer effects through synergistic/additive/potentiation mechanisms, or by improving selectivity, and overcoming MDR either by inhibition of ABC membrane transporters or interaction with MDR genes.
However, while this strategy provides new therapeutic results, it also introduces several new challenges, such as the need to clearly identify the mechanism behind the enhanced anticancer activity by comparing the co-delivery effect with co-administration effect. It is also required to better define the concentration-dependent effect of natural compounds, as well as to evaluate the improvement of their pharmacokinetic parameters when delivered by lipid-based nanocarriers. Moreover, despite lipid-based nanocarriers being considered biocompatible, the safety of the loaded cargo has also to be addressed, namely by long-term toxicity assessment and by studying the immunogenicity issues that may arise from the nucleic acid conjugation. Furthermore, as far as we know, no clinical trials with nanocarriers co-delivering natural compounds or other therapeutic agents have been conducted, which may be due to these challenges as well as the costs of designing and developing such complex formulations.
Despite the critical points that remain unresolved, the co-delivery strategy of natural compounds and chemotherapeutic agents/nucleic acids is undeniably very promising, especially by further exploring versatile nanocarriers such as LLCNs. We strongly believe that this approach, allied with thorough characterization, rational development, and pre-clinical studies, will fulfill the translation of lipid-based nanocarriers into clinical applications.
Author Contributions
Conceptualization C.M.L., M.L. and F.A.; writing—original draft preparation, P.V.T., E.F. and T.B.S.; writing—review and editing, P.V.T., C.M.L., M.L. and F.A.; visualization, M.L. and P.V.T.; supervision, C.M.L., M.L. and F.A.; funding acquisition, M.L. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding UIDB/04650/2020 and UIDB/04050/2020 (CF-UM-UP and CBMA), UIDB/04046/2020 and UIDP/04046/2020 (BioISI), UIDP/04378/2020 and UIDB/04378/2020 (UCIBIO), and LA/P/0140/2020 (i4HB). E.F. thanks FCT for the PhD grant (SFRH/BD/147938/2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Pardakhty, A.; Azarmi, S.; Jazayeri, J.A.; Nokhodchi, A.; Omri, A. Role of nanocarrier systems in cancer nanotherapy. J. Liposome Res. 2009, 19, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Levin, B. World Cancer Report 2008; IARC Press, International Agency for Research on Cancer: Lyon, France, 2008. [Google Scholar]
- Qi, S.S.; Sun, J.H.; Yu, H.H.; Yu, S.Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef]
- Leng, F.; Liu, F.; Yang, Y.; Wu, Y.; Tian, W. Strategies on Nanodiagnostics and Nanotherapies of the Three Common Cancers. Nanomaterials 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal. Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Hofmann, M.; Guschel, M.; Bernd, A.; Bereiter-Hahn, J.; Kaufmann, R.; Tandi, C.; Wiig, H.; Kippenberger, S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia 2006, 8, 89–95. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Devel. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm. 2005, 2, 194–205. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Noguchi, K.; Katayama, K.; Mitsuhashi, J.; Sugimoto, Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 2009, 61, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.C.R.; Xu, P. Multicompartment intracellular self-expanding nanogel for targeted delivery of drug cocktail. Adv. Mater. 2012, 24, 6479–6483. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Young, R.C.; Canellos, G.P. Combination versus single agent chemotherapy: A review of the basis for selection of drug treatment of cancer. Cancer 1975, 35, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.; Zhang, L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharm. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Chen, C.; Fan, A.; Kong, D.; Wang, Z.; Zhao, Y. On-demand combinational delivery of curcumin and doxorubicin via a pH-labile micellar nanocarrier. Int. J. Pharm. 2015, 495, 572–578. [Google Scholar] [CrossRef]
- Qi, J.; Zhuang, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. In vivo fate of lipid-based nanoparticles. Drug Discov. Today 2017, 22, 166–172. [Google Scholar] [CrossRef]
- Wu, C.-P.; Ohnuma, S.; Ambudkar, S.V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharm. Pharmacol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Creixell, M.; Peppas, N.A. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today 2012, 7, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef]
- Amani, A.; Alizadeh, M.R.; Yaghoubi, H.; Nohtani, M. Novel multi-targeted nanoparticles for targeted co-delivery of nucleic acid and chemotherapeutic agents to breast cancer tissues. Mater. Sci. Eng. C 2021, 118, 111494. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Lin, S.-R.; Chang, C.-H.; Hsu, C.-F.; Tsai, M.-J.; Cheng, H.; Leong, M.K.; Sung, P.-J.; Chen, J.-C.; Weng, C.-F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Cho, Y.; Park, M.N.; Noh, S.; Kang, S.Y.; Kim, B. Review of Natural Compounds for the Management and Prevention of Lymphoma. Processes 2020, 8, 1164. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert. Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Rahmani Moghadam, E.; Raei, M.; Kalantari, M.; Tavakol, S.; Mohammadinejad, R.; Najafi, M.; et al. Progress in Natural Compounds/siRNA Co-delivery Employing Nanovehicles for Cancer Therapy. ACS Comb. Sci. 2020, 22, 669–700. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.V.; Adega, F.; Martins-Lopes, P.; Machado, R.; Lopes, C.M.; Lúcio, M. pH-Responsive Hybrid Nanoassemblies for Cancer Treatment: Formulation Development, Optimization, and In Vitro Therapeutic Performance. Pharmaceutics 2023, 15, 326. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells. Oncotarget 2017, 8, 30369–30382. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Samaha, M.W.; Fang, J.Y.; Freag, M.S. Liquid crystalline assembly for potential combinatorial chemo-herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019, 14, 499–517. [Google Scholar] [CrossRef]
- Abtahi, N.A.; Naghib, S.M.; Ghalekohneh, S.J.; Mohammadpour, Z.; Nazari, H.; Mosavi, S.M.; Gheibihayat, S.M.; Haghiralsadat, F.; Reza, J.Z.; Doulabi, B.Z. Multifunctional stimuli-responsive niosomal nanoparticles for co-delivery and co-administration of gene and bioactive compound: In vitro and in vivo studies. Chem. Eng. J. 2022, 429, 132090. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559–84571. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Liu, K.; Chen, W.; Yang, T.; Wen, B.; Ding, D.; Keidar, M.; Tang, J.; Zhang, W. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int. J. Nanomed. 2017, 12, 8239–8255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, Y.; Zhao, X.; Li, X.; Li, K.; Chen, D.; Qiao, M.; Hu, H.; Zhao, X. Co-delivery of doxorubicin and the traditional Chinese medicine quercetin using biotin–PEG2000–DSPE modified liposomes for the treatment of multidrug resistant breast cancer. RSC Adv. 2016, 6, 113173–113184. [Google Scholar] [CrossRef]
- Israel, B.B.; Tilghman, S.L.; Parker-Lemieux, K.; Payton-Stewart, F. Phytochemicals: Current strategies for treating breast cancer. Oncol. Lett. 2018, 15, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- de Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, F.; Ma, X.; Cao, Z.; Cao, Z.W.; Li, Y.; Li, Y.X.; Chen, Y.Z. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Rev. Drug Discov. 2009, 8, 111–128. [Google Scholar] [CrossRef]
- Xu, M.; Li, G.; Zhang, H.; Chen, X.; Li, Y.; Yao, Q.; Xie, M. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020, 27, 983–995. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Marzio, L.D.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed. Res. Int. 2014, 2014, 424239. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.; Surapaneni, S.K.; Tikoo, K.; Singh, C.; Burman, R.; Gill, M.S.; Suresh, S. Folic acid functionalized long-circulating co-encapsulated docetaxel and curcumin solid lipid nanoparticles: In vitro evaluation, pharmacokinetic and biodistribution in rats. Drug Deliv. 2016, 23, 1453–1468. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016, 23, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Saha, S.; Mondal, G.; Haseena, S.; Chaudhuri, A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials 2014, 35, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.; Torchilin, V.P. Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Raza, S.M.; Bashir, S.; Madni, A.; Khan, M.W.; Ma, X.; Xiang, G. Liposomal co-delivered oleanolic acid attenuates doxorubicin-induced multi-organ toxicity in hepatocellular carcinoma. Oncotarget 2017, 8, 47136–47153. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Liu, Q.; Dai, Y.; Zhu, X.; Wen, Y.; Xu, J.; Lu, Y.; Zhao, D.; Chen, X.; et al. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur. J. Pharm. Sci. 2017, 105, 219–229. [Google Scholar] [CrossRef]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Aryasomayajula, B.; Pattni, B.; Mussi, S.V.; Ferreira, L.A.M.; Torchilin, V.P. Solid lipid nanoparticles co-loaded with doxorubicin and alpha-tocopherol succinate are effective against drug-resistant cancer cells in monolayer and 3-D spheroid cancer cell models. Int. J. Pharm. 2016, 512, 292–300. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Jiang, H.; Geng, D.; Liu, H.; Li, Z.; Cao, J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug Deliv. 2016, 23, 3665–3673. [Google Scholar] [CrossRef]
- Shukla, P.; Mathur, V.; Kumar, A.; Khedgikar, V.; Teja, V.B.; Chaudhary, D.; Kushwaha, P.; Bora, H.K.; Konwar, R.; Trivedi, R.; et al. Nanoemulsion based concomitant delivery of curcumin and etoposide: Impact on cross talk between prostate cancer cells and osteoblast during metastasis. J. Biomed. Nanotechnol. 2014, 10, 3381–3391. [Google Scholar] [CrossRef]
- Lu, Z.; Su, J.; Li, Z.; Zhan, Y.; Ye, D. Hyaluronic acid-coated, prodrug-based nanostructured lipid carriers for enhanced pancreatic cancer therapy. Drug Dev. Ind. Pharm. 2017, 43, 160–170. [Google Scholar] [CrossRef]
- Soe, Z.C.; Thapa, R.K.; Ou, W.; Gautam, M.; Nguyen, H.T.; Jin, S.G.; Ku, S.K.; Oh, K.T.; Choi, H.G.; Yong, C.S.; et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B. Biointerfaces 2018, 170, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Kushwah, V.; Garg, N.K.; Kesharwani, P.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: A preclinical study for breast cancer. Nanomedicine 2017, 12, 1851–1872. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Wang, L.; Cheng, G. Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to C6 glioma cells with P-gp inhibition and its tumor targeting. Asian J. Pharm. Sci. 2015, 10, 363–371. [Google Scholar] [CrossRef]
- Abouzeid, A.H.; Patel, N.R.; Torchilin, V.P. Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer. Int. J. Pharm. 2014, 464, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef]
- Sarisozen, C.; Vural, I.; Levchenko, T.; Hincal, A.A.; Torchilin, V.P. PEG-PE-based micelles co-loaded with paclitaxel and cyclosporine A or loaded with paclitaxel and targeted by anticancer antibody overcome drug resistance in cancer cells. Drug Deliv. 2012, 19, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Vaze, O.S.; Rockwell, K.; Torchilin, V.P. Palmitoyl ascorbate-modified liposomes as nanoparticle platform for ascorbate-mediated cytotoxicity and paclitaxel co-delivery. Eur. J. Pharm. Biopharm. 2010, 75, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: Enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012, 46, 64–71. [Google Scholar]
- Kabary, D.M.; Helmy, M.W.; Elkhodairy, K.A.; Fang, J.Y.; Elzoghby, A.O. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf. B Biointerfaces 2018, 169, 183–194. [Google Scholar] [CrossRef]
- Webb, M.S.; Johnstone, S.; Morris, T.J.; Kennedy, A.; Gallagher, R.; Harasym, N.; Harasym, T.; Shew, C.R.; Tardi, P.; Dragowska, W.H.; et al. In vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserine. Eur. J. Pharm. Biopharm. 2007, 65, 289–299. [Google Scholar] [CrossRef]
- Lúcio, M.; Lopes, C.M.; Oliveira, M.E.C.R. Functional Lipid Nanosystems in Cancer; Jenny Stanford Publishing: New York, NY, USA, 2021. [Google Scholar]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Štěrba, M.; Popelová, O.; Vávrová, A.; Jirkovský, E.; Kovaříková, P.; Geršl, V.; Šimůnek, T. Oxidative Stress, Redox Signaling, and Metal Chelation in Anthracycline Cardiotoxicity and Pharmacological Cardioprotection. Antioxid. Redox Signal. 2012, 18, 899–929. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338. [Google Scholar] [CrossRef]
- Sun, J.; Song, Y.; Lu, M.; Lin, X.; Liu, Y.; Zhou, S.; Su, Y.; Deng, Y. Evaluation of the antitumor effect of dexamethasone palmitate and doxorubicin co-loaded liposomes modified with a sialic acid-octadecylamine conjugate. Eur. J. Pharm. Sci. 2016, 93, 177–183. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Mady, M.M. Ehrlich tumor inhibition using doxorubicin containing liposomes. Saudi Pharm. J. 2015, 23, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Sahoo, S.K. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. Eur. J. Pharm. Sci. 2010, 39, 152–163. [Google Scholar] [CrossRef]
- Qiu, L.; Qiao, M.; Chen, Q.; Tian, C.; Long, M.; Wang, M.; Li, Z.; Hu, W.; Li, G.; Cheng, L.; et al. Enhanced effect of pH-sensitive mixed copolymer micelles for overcoming multidrug resistance of doxorubicin. Biomaterials 2014, 35, 9877–9887. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, G.; Mathur, A.; Mallade, P.; Gerlach, S.; Willis, J.; Datta, A.; Srivastav, S.; Abdel-Mageed, A.B.; Mondal, D. Nelfinavir targets multiple drug resistance mechanisms to increase the efficacy of doxorubicin in MCF-7/Dox breast cancer cells. Biochimie 2016, 124, 53–64. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–69. [Google Scholar] [CrossRef]
- Shimpo, K.; Nagatsu, T.; Yamada, K.; Sato, T.; Niimi, H.; Shamoto, M.; Takeuchi, T.; Umezawa, H.; Fujita, K. Ascorbic acid and adriamycin toxicity. Am. J. Clin. Nutr. 1991, 54, 1298s–1301s. [Google Scholar] [CrossRef] [PubMed]
- Borska, S.; Chmielewska, M.; Wysocka, T.; Drag-Zalesinska, M.; Zabel, M.; Dziegiel, P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem. Toxicol. 2012, 50, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, K.S.; Choo, H.; Chong, Y. Quercetin-POM (pivaloxymethyl) conjugates: Modulatory activity for P-glycoprotein-based multidrug resistance. Phytomedicine 2015, 22, 778–785. [Google Scholar] [CrossRef]
- Kim, J.H.; Li, Y.; Kim, M.S.; Kang, S.W.; Jeong, J.H.; Lee, D.S. Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers. Int. J. Pharm. 2012, 427, 435–442. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Mussi, S.V.; Gomes, D.A.; Yoshida, M.I.; Frezard, F.; Carregal, V.M.; Ferreira, L.A.M. α-Tocopherol succinate improves encapsulation and anticancer activity of doxorubicin loaded in solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 140, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, Y.; Qiu, L. Enhanced combination therapy effect on paclitaxel-resistant carcinoma by chloroquine co-delivery via liposomes. Int. J. Nanomed. 2015, 10, 6615–6632. [Google Scholar] [CrossRef]
- Jang, S.H.; Wientjes, M.G.; Au, J.L. Kinetics of P-glycoprotein-mediated efflux of paclitaxel. J. Pharm. Pharmacol. Exp. Ther. 2001, 298, 1236–1242. [Google Scholar]
- Quan, F.; Pan, C.; Ma, Q.; Zhang, S.; Yan, L. Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed. Pharm. Pharmacother. 2008, 62, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, D.; Cao, Z.; Zhang, C.; Cheng, D.; Liu, J.; Shuai, X. Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 2015, 3, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, M.; Gong, S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater. Today 2014, 17, 298–306. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Jogdeo, C.M.; Panja, S.; Kanvinde, S.; Kapoor, E.; Siddhanta, K.; Oupický, D. Advances in Lipid-Based Codelivery Systems for Cancer and Inflammatory Diseases. Adv. Healthc. Mater. 2023, 12, 2202400. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Zhou, Y.; Ge, Q.; Chang, J.; Chen, Y.; Zhang, K.; Peng, D.; Chen, W. Co-delivery of gambogenic acid and VEGF-siRNA with anionic liposome and polyethylenimine complexes to HepG2 cells. J. Liposome Res. 2019, 29, 322–331. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Bai, Y.; Li, Y.; Zhang, C.; Cui, S.; Piao, Y.; Zhang, S. Co-delivery of resveratrol and p53 gene via peptide cationic liposomal nanocarrier for the synergistic treatment of cervical cancer and breast cancer cells. J. Drug Deliv. Sci. Technol. 2019, 51, 746–753. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Li, Y.; Deng, X.; Wang, X.; Cui, X.; Lu, J.; Pan, Z.; Wu, Y. Self-assembled fluorescent hybrid nanoparticles-mediated collaborative lncRNA CCAT1 silencing and curcumin delivery for synchronous colorectal cancer theranostics. J. Nanobiotechnol. 2021, 19, 238. [Google Scholar] [CrossRef]
- Rana, M.S.; Ediriweera, M.K.; Rajagopalan, U.; Karunaratne, D.N.; Tennekoon, K.H.; Samarakoon, S.R. A new liposomal nanocarrier for co-delivery of gedunin and p-glycoprotein siRNA to target breast cancer stem cells. Nat. Prod. Prod. Res. 2022, 36, 6389–6392. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, R.B.; Maghsoudnia, N.; Samimi, S.; Zamzami, A.; Dorkoosh, F.A. Co-Delivery Nanosystems for Cancer Treatment: A Review. Pharm. Nanotechnol. 2019, 7, 90–112. [Google Scholar] [CrossRef]
- Oliveto, S.; Mancino, M.; Manfrini, N.; Biffo, S. Role of microRNAs in translation regulation and cancer. World J. Biol. Chem. 2017, 8, 45–56. [Google Scholar] [CrossRef]
- Yu, X.J.; Han, Q.B.; Wen, Z.S.; Ma, L.; Gao, J.; Zhou, G.B. Gambogenic acid induces G1 arrest via GSK3β-dependent cyclin D1 degradation and triggers autophagy in lung cancer cells. Cancer Lett. 2012, 322, 185–194. [Google Scholar] [CrossRef]
- Su, J.; Cheng, H.; Zhang, D.; Wang, M.; Xie, C.; Hu, Y.; Chang, H.C.; Li, Q. Synergistic effects of 5-fluorouracil and gambogenic acid on A549 cells: Activation of cell death caused by apoptotic and necroptotic mechanisms via the ROS-mitochondria pathway. Biol. Pharm. Bull. 2014, 37, 1259–1268. [Google Scholar] [CrossRef]
- Tang, X.; Sun, J.; Ge, T.; Zhang, K.; Gui, Q.; Zhang, S.; Chen, W. PEGylated liposomes as delivery systems for Gambogenic acid: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 172, 26–36. [Google Scholar] [CrossRef]
- Patwardhan, C.A.; Fauq, A.; Peterson, L.B.; Miller, C.; Blagg, B.S.J.; Chadli, A. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J. Biol. Chem. 2013, 288, 7313–7325. [Google Scholar] [CrossRef] [PubMed]
- Nwokwu, C.D.U.; Samarakoon, S.R.; Karunaratne, D.N.; Katuvawila, N.P.; Pamunuwa, G.K.; Ediriweera, M.K.; Tennekoon, K.H. Induction of apoptosis in response to improved gedunin by liposomal nano-encapsulation in human non-small-cell lung cancer (NCI-H292) cell line. Trop. J. Pharm. Res. 2017, 16, 2079–2087. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Vitorino, C.; Lopes, C.M.; Lúcio, M. Nanomedicine and Drug Delivery Approaches. In Organelle and Molecular Targeting; CRC Press: Boca Raton, FL, USA, 2021; pp. 23–112. [Google Scholar]
- Maniam, G.; Mai, C.-W.; Zulkefeli, M.; Dufès, C.; Tan, D.M.-Y.; Fu, J.-Y. Challenges and Opportunities of Nanotechnology as Delivery Platform for Tocotrienols in Cancer Therapy. Front. Pharmacol. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Fernandes, E.; Soares, T.B.; Gonçalves, H.; Lúcio, M. Spectroscopic Studies as a Toolbox for Biophysical and Chemical Characterization of Lipid-Based Nanotherapeutics. Front. Chem. 2018, 6, 323. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).