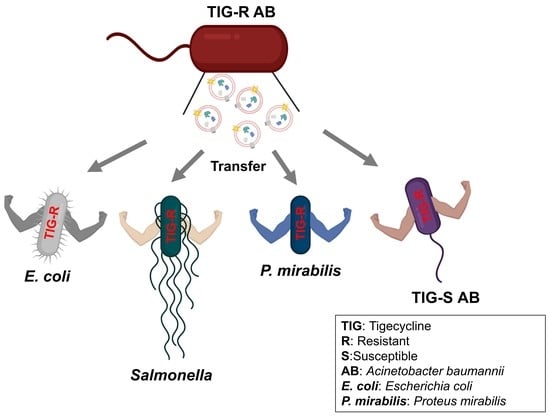
Characterization of Increased Extracellular Vesicle-Mediated Tigecycline Resistance in Acinetobacter baumannii
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth Conditions, and Chemicals
2.2. MIC Determination
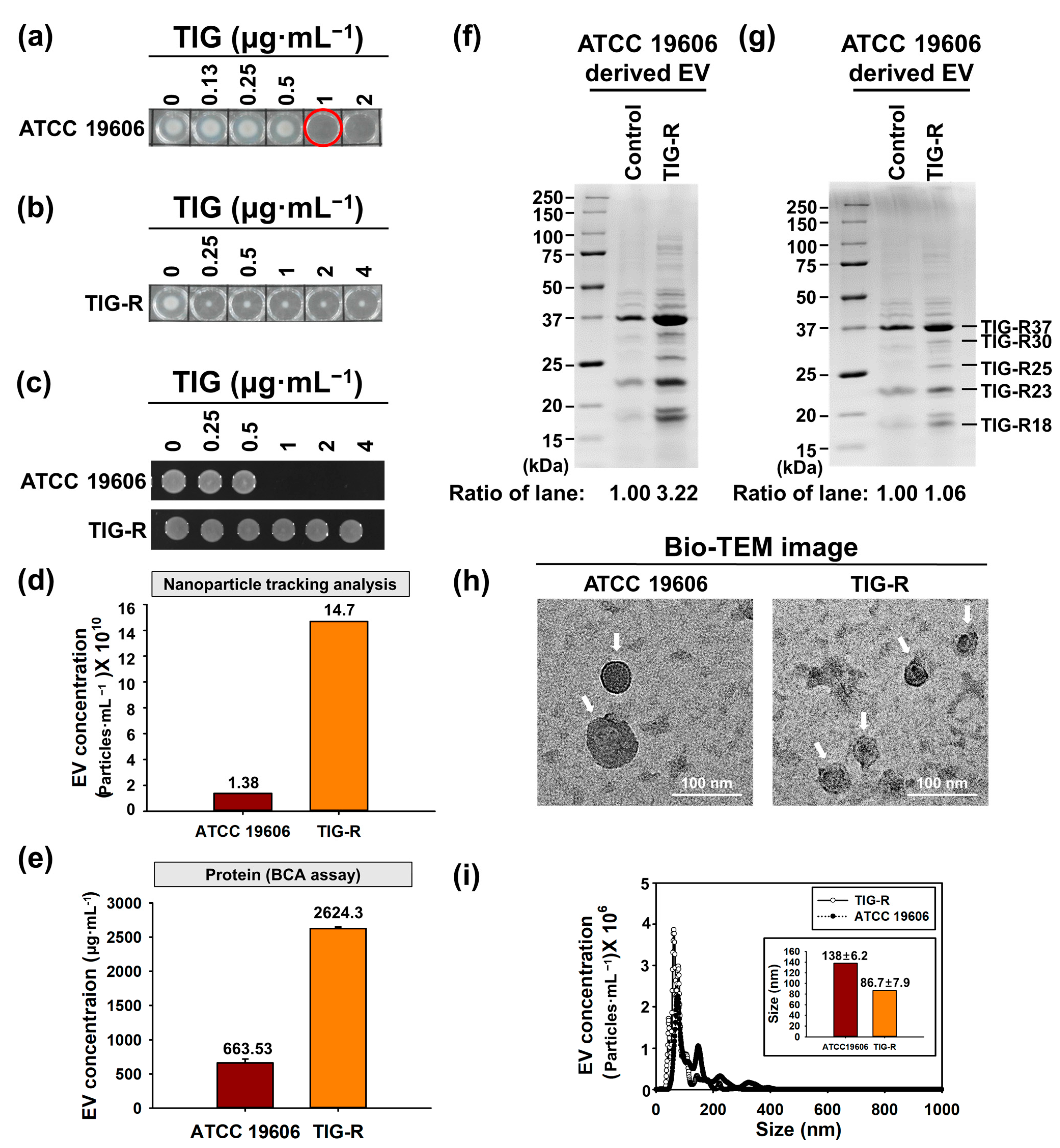
2.3. EVs Isolation
2.4. Characterization of EVs
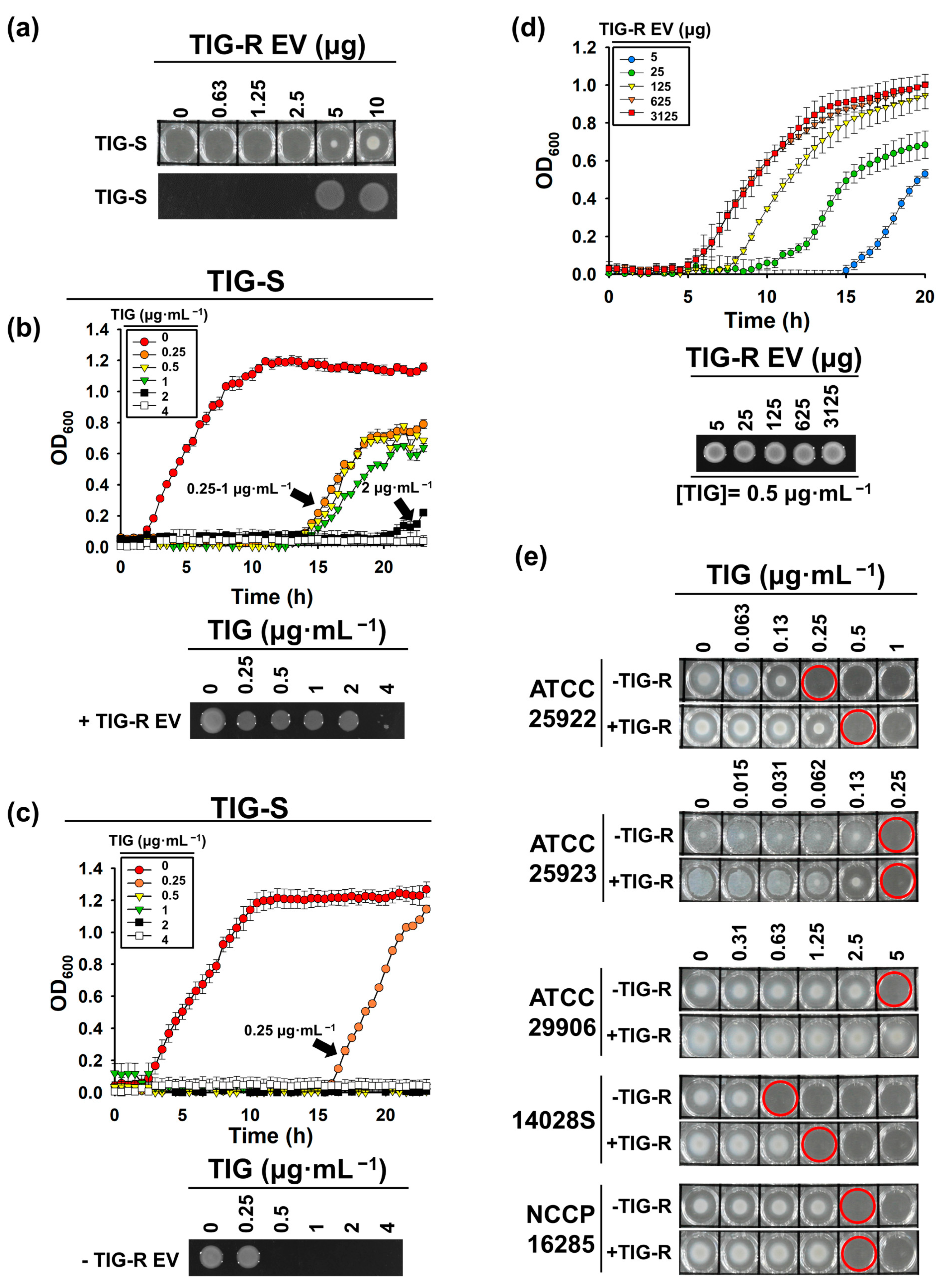
2.5. Evaluation of EV-Mediated TIG-R AB Transfer Activity to TIG-S AB and Other Bacteria
2.6. Identification of Components Involved in EV-Mediated TIG Transfer Activity
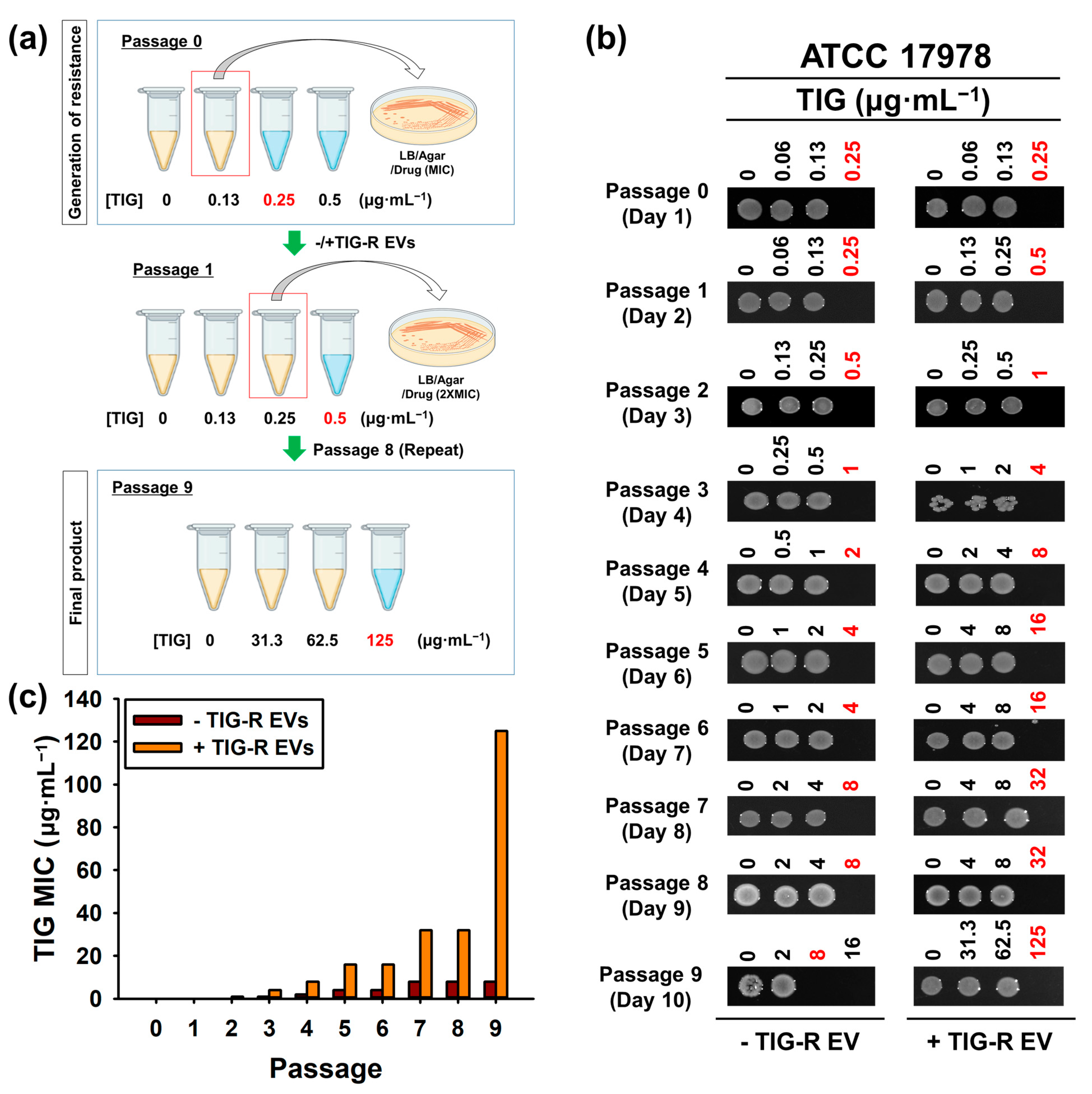
2.7. Evaluation of Recurrence Efficacy of TIG-R by TIG or TIG-R EVs
2.8. Characterization of EV Proteins
| Type of Bacteria | Strain ID | Strains | Feature | Reference |
|---|---|---|---|---|
| Gram- Negative | 17978 | Acinetobacter baumannii (AB) | Acinetobacter baumannii (AB) Bouvet and Grimont | ATCC |
| 19606 | ||||
| TIG-R | Laboratory made TIG-R ATCC 19606 | [27] | ||
| 25922 | Escherichia coli | Smooth LPS (O6 serotype reference strain) | ATCC | |
| 29906 | Proteus mirabilis | Proteus mirabilis Hauser (Type strain) | ATCC | |
| 14028S | Salmonella enterica serovar typhimurium | Wild-type Salmonella enterica serovar typhimurium; a spontaneous mutant resistant to nalidixic acid (NA) | [28] | |
| 16285 | Klebsiella pneumoniae | Klebsiella pneumoniae mcr-1 clinical isolates | NCCP | |
| Gram- Positive | 25923 | Staphylococcus aureus | Staphylococcus aureus subsp. aureus Rosenbach | ATCC |
2.9. Screening of Resistant Antibiotics by TIG-R EVs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical Characterization of EVs Produced by Tigecycline Resistant A. baumannii
3.2. TIG-R EV as a Mediator for TIG Resistance
3.3. Proteins Are Major Determinants for EV-Mediated TIG-R Transfer
3.4. Comparison of EV-Mediated Transfer of Resistance to Antibiotics
3.5. Role of TIG-R EVs on Current Antibiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022.
- World Health Organization; Regional Office for Europe. Central Asian and Eastern European Surveillance of Antimicrobial Resistance: Annual Report 2017; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Curcio, D. Tigecycline in the Treatment of Community-Acquired Pneumonia. Clin. Med. Ther. 2009, 1, CMT.S2351. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Giannella, M.; Fortini, D.; Giordano, A.; Meledandri, M.; Ballardini, M.; Venditti, M.; Bordi, E.; Capozzi, D.; Balice, M.P.; et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 2013, 19, E23–E30. [Google Scholar] [CrossRef]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–236. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Zhou, H.; Jiang, Y.; Chen, Y.; Hua, X.; Yu, Y. Decreased susceptibility to tigecycline in Acinetobacter baumannii mediated by a mutation in trm encoding SAM-dependent methyltransferase. J. Antimicrob. Chemother. 2013, 69, 72–76. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Govinden, U.; Bester, L.A.; Essack, S.Y. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: Emerging resistance mechanisms and detection methods. J. Appl. Microbiol. 2016, 121, 601–617. [Google Scholar] [CrossRef]
- Hua, X.; He, J.; Wang, J.; Zhang, L.; Zhang, L.; Xu, Q.; Shi, K.; Leptihn, S.; Shi, Y.; Fu, X.; et al. Novel tigecycline resistance mechanisms in Acinetobacter baumannii mediated by mutations in adeS, rpoB and rrf. Emerg. Microbes Infect. 2021, 10, 1404–1417. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.; Li, H.; Wang, Z.; Yin, Y.; Wang, S.; Chen, H.; Wang, Q.; Wang, H. Genomic and Phenotypic Evolution of Tigecycline-Resistant Acinetobacter baumannii in Critically Ill Patients. Microbiol. Spectr. 2022, 10, e0159321. [Google Scholar] [CrossRef]
- Kuehn, M.J.; Kesty, N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wang, S.; Du, Y.; Wei, B.; Wu, Q.; Wang, H. Inhibitors of Bacterial Extracellular Vesicles. Front. Microbiol. 2022, 13, 835058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, J.; Wang, S.; Zhou, J.; Qin, J.; Jia, Z.; Wang, Y.; Wang, Z.; Zhang, Y.; Hao, H. Research Progress on Bacterial Membrane Vesicles and Antibiotic Resistance. Int. J. Mol. Sci. 2022, 23, 11553. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Fernández-Moreira, E.; Merino, M.; Poza, M.; Mendez, J.A.; Soares, N.C.; Mosquera, A.; Chaves, F.; Bou, G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 3084–3090. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Shin, B.; Kang, M.; Yang, J.; Lee, T.K.; Park, W. A novel decoy strategy for polymyxin resistance in Acinetobacter baumannii. eLife 2021, 10, e66988. [Google Scholar] [CrossRef]
- Kim, T.; Bak, G.; Lee, J.; Kim, K.S. Systematic analysis of the role of bacterial Hfq-interacting sRNAs in the response to antibiotics. J. Antimicrob. Chemother. 2015, 70, 1659–1668. [Google Scholar] [CrossRef]
- Lee, W.-H.; Choi, H.-I.; Hong, S.-W.; Kim, K.-S.; Gho, Y.S.; Jeon, S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015, 47, e183. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, J.S.; Park, S.B.; Lee, A.R.; Lee, J.S.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Kim, J.H.; et al. Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Sci. Rep. 2020, 10, 21066. [Google Scholar] [CrossRef]
- Choi, H.; Kim, M.; Jeon, J.; Han, J.K.; Kim, K.-S. Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem. Biophys. Res. Commun. 2017, 490, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Celia, C.; Mincione, G.; Stringaro, A.; Di Marzio, L.; Colone, M.; Di Marcantonio, M.C.; Savino, L.; Puca, V.; Santoliquido, R.; et al. Detection and Physicochemical Characterization of Membrane Vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 2017, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Chebotar, I.V.; Konchakova, E.D.; Maianskii, A.N. Vesicle formation as a result of interaction between polymorphonuclear neutrophils and Staphylococcus aureus biofilm. J. Med. Microbiol. 2013, 62, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.-s. Repurposing of Ciclopirox to Overcome the Limitations of Zidovudine (Azidothymidine) against Multidrug-Resistant Gram-Negative Bacteria. Pharmaceutics 2022, 14, 552. [Google Scholar] [CrossRef]
- Jarvik, T.; Smillie, C.; Groisman, E.A.; Ochman, H. Short-term signatures of evolutionary change in the Salmonella enterica serovar Typhimurium 14028 genome. J. Bacteriol. 2010, 192, 560. [Google Scholar] [CrossRef]
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [Google Scholar] [CrossRef]
- Ho, M.-H.; Chen, C.-H.; Goodwin, J.S.; Wang, B.-Y.; Xie, H. Functional Advantages of Porphyromonas gingivalis Vesicles. PLoS ONE 2015, 10, e0123448. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Daniel, O.; Karch, H.; Mellmann, A. Dissemination of the blaCTX-M-15 gene among Enterobacteriaceae via outer membrane vesicles. J. Antimicrob. Chemother. 2020, 75, 2442–2451. [Google Scholar] [CrossRef]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance from Salmonella enterica sv. Typhi ΔtolR and ΔdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef]
- Lee, A.R.; Park, S.B.; Kim, S.W.; Jung, J.W.; Chun, J.H.; Kim, J.; Kim, Y.R.; Lazarte, J.M.S.; Jang, H.B.; Thompson, K.D.; et al. Membrane vesicles from antibiotic-resistant Staphylococcus aureus transfer antibiotic-resistance to antibiotic-susceptible Escherichia coli. J. Appl. Microbiol. 2022, 132, 2746–2759. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. Outer membrane vesicles from β-lactam-resistant Escherichia coli enable the survival of β-lactam-susceptible E. coli in the presence of β-lactam antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [PubMed]
- Cloet, T.; Momenbeitollahi, N.; Li, H. Recent advances on protein-based quantification of extracellular vesicles. Anal. Biochem. 2021, 622, 114168. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Godeux, A.-S.; Svedholm, E.; Barreto, S.; Potron, A.; Venner, S.; Charpentier, X.; Laaberki, M.-H. Interbacterial Transfer of Carbapenem Resistance and Large Antibiotic Resistance Islands by Natural Transformation in Pathogenic Acinetobacter. mBio 2022, 13, e0263121. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Jang, K.M.; Lee, J.H.; Kang, L.W.; Lee, S.H. Transmission of antibiotic resistance genes through mobile genetic elements in Acinetobacter baumannii and gene-transfer prevention. Sci. Total Environ. 2023, 857, 159497. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Oliver, A.; Weigel, L.M.; Rasheed, J.K.; McGowan, J.E., Jr.; Raney, P.; Tenover, F.C. Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob. Agents Chemother. 2002, 46, 3829–3836. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Sondak, T.; Kim, K.-s. Characterization of Increased Extracellular Vesicle-Mediated Tigecycline Resistance in Acinetobacter baumannii. Pharmaceutics 2023, 15, 1251. https://doi.org/10.3390/pharmaceutics15041251
Cho H, Sondak T, Kim K-s. Characterization of Increased Extracellular Vesicle-Mediated Tigecycline Resistance in Acinetobacter baumannii. Pharmaceutics. 2023; 15(4):1251. https://doi.org/10.3390/pharmaceutics15041251
Chicago/Turabian StyleCho, Hyejin, Tesalonika Sondak, and Kwang-sun Kim. 2023. "Characterization of Increased Extracellular Vesicle-Mediated Tigecycline Resistance in Acinetobacter baumannii" Pharmaceutics 15, no. 4: 1251. https://doi.org/10.3390/pharmaceutics15041251
APA StyleCho, H., Sondak, T., & Kim, K.-s. (2023). Characterization of Increased Extracellular Vesicle-Mediated Tigecycline Resistance in Acinetobacter baumannii. Pharmaceutics, 15(4), 1251. https://doi.org/10.3390/pharmaceutics15041251