A Wall Fragment of Cutibacterium acnes Preserves Junctional Integrity Altered by Staphylococcus aureus in an Ex Vivo Porcine Skin Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Growth, Bacterial Suspensions, and Free Cells Supernatant (FCS) Preparation
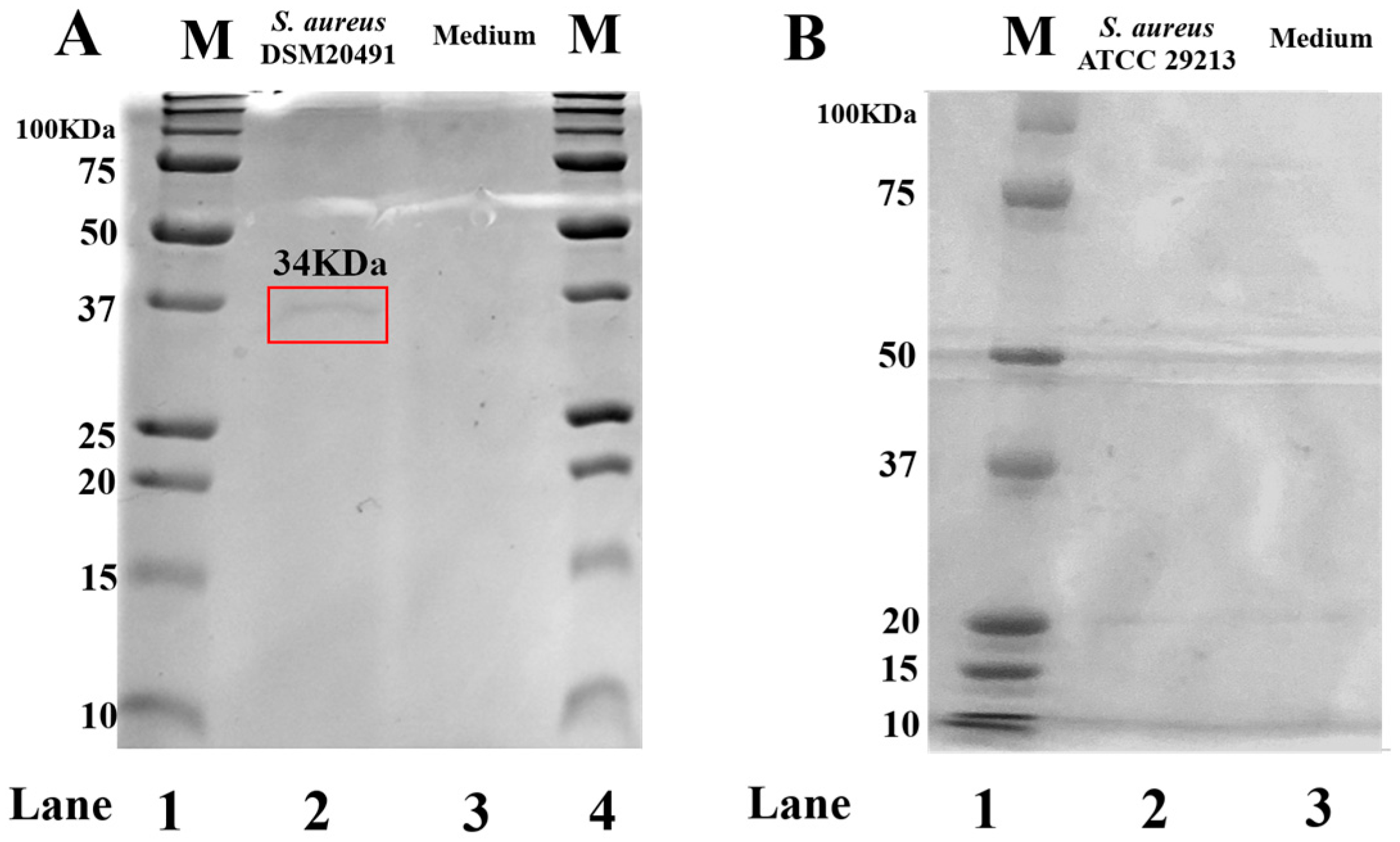
2.3. FCS Qualitative Analysis
2.4. Explant Preparation
2.5. Experimental Design
2.5.1. Preincubation
2.5.2. Co-Incubation
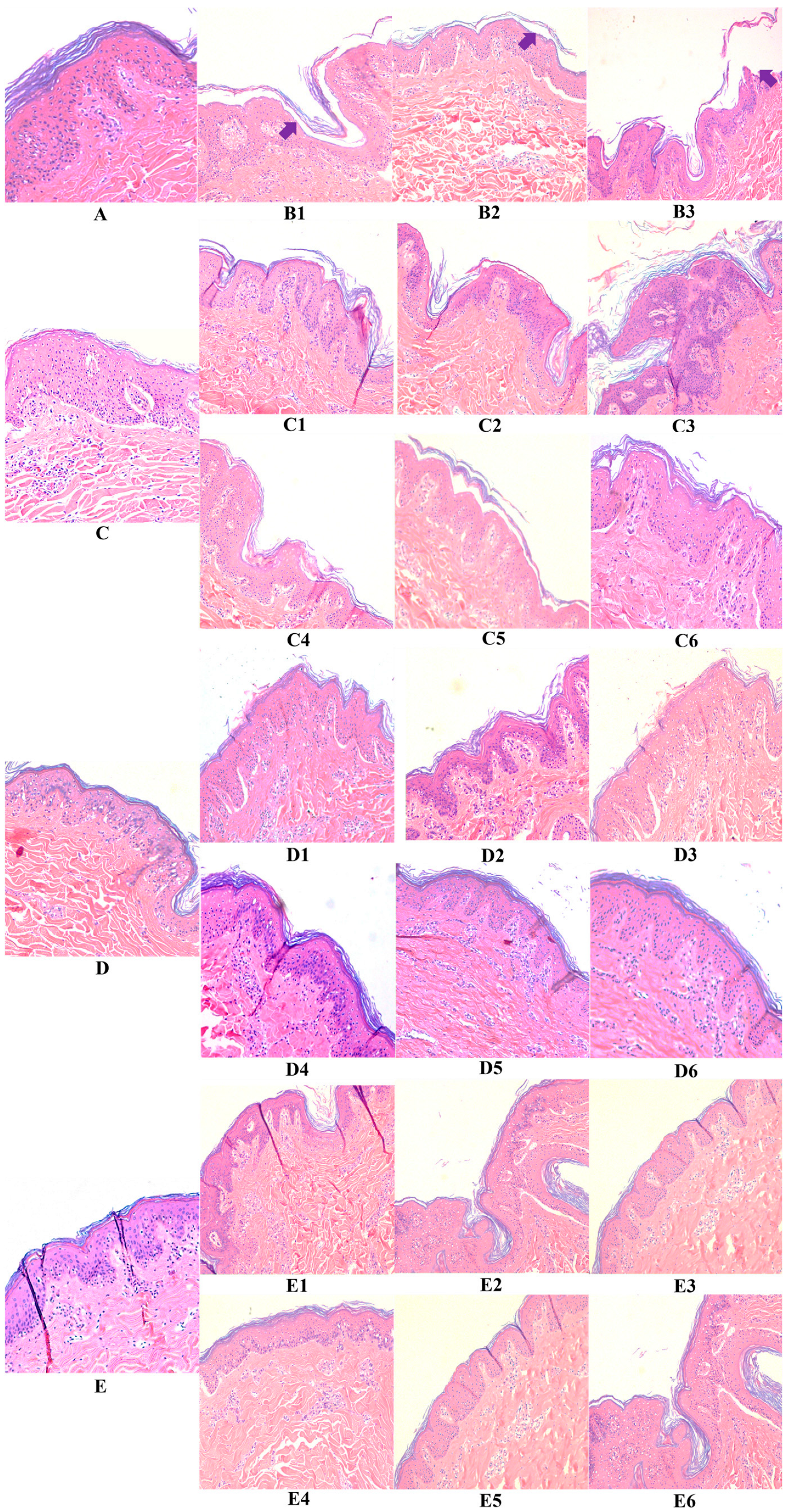
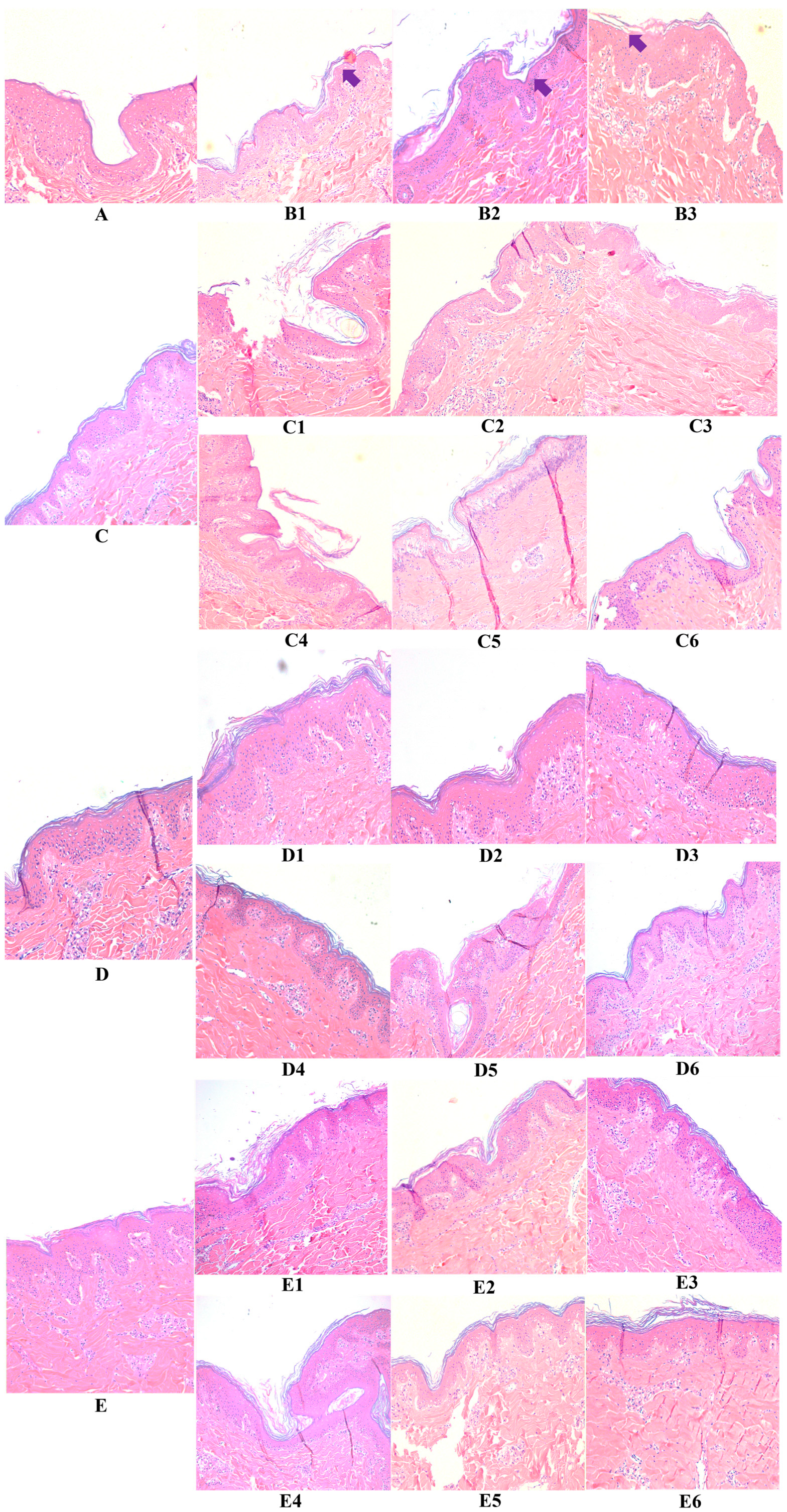
2.6. Hematoxylin and Eosin Stain
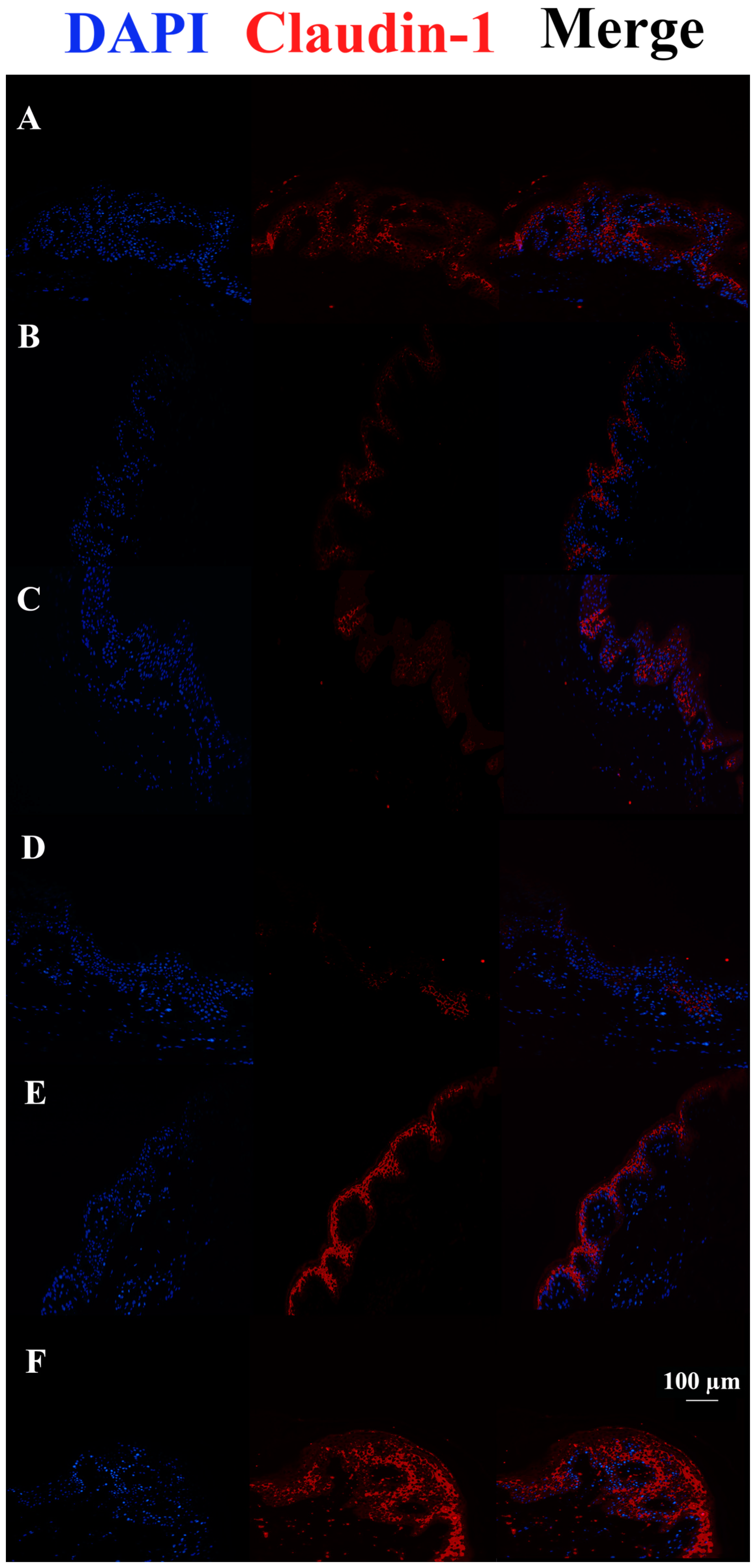
2.7. Immunofluorescence
2.8. Microscopic Observation
3. Results
3.1. FCS Qualitative Analysis
3.2. Hyaluronic Acid, c40 Fragment Alone and Conjugated with Hyaluronic Acid on Hematoxylin-Eosin-Stained Sections of Animal Explants
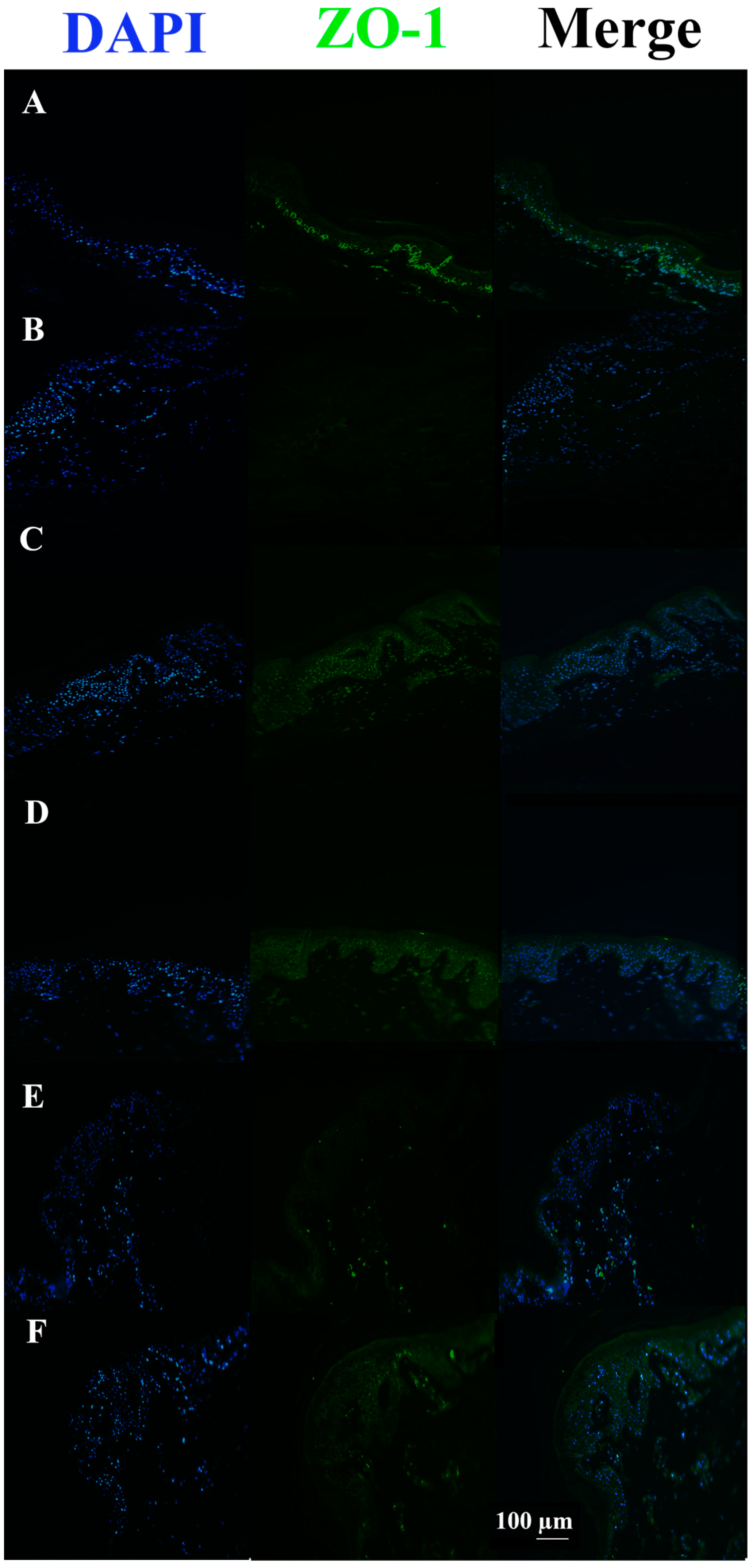
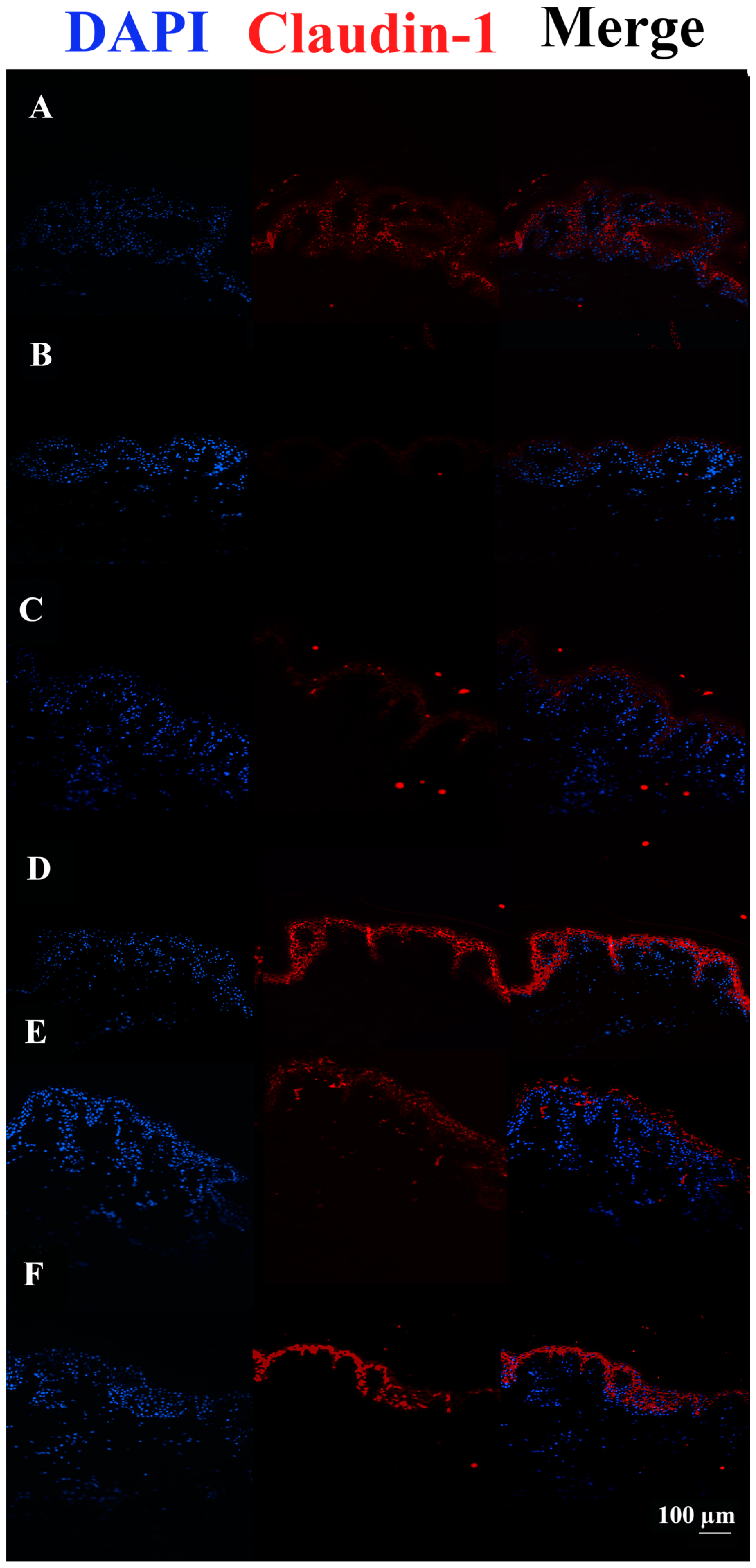
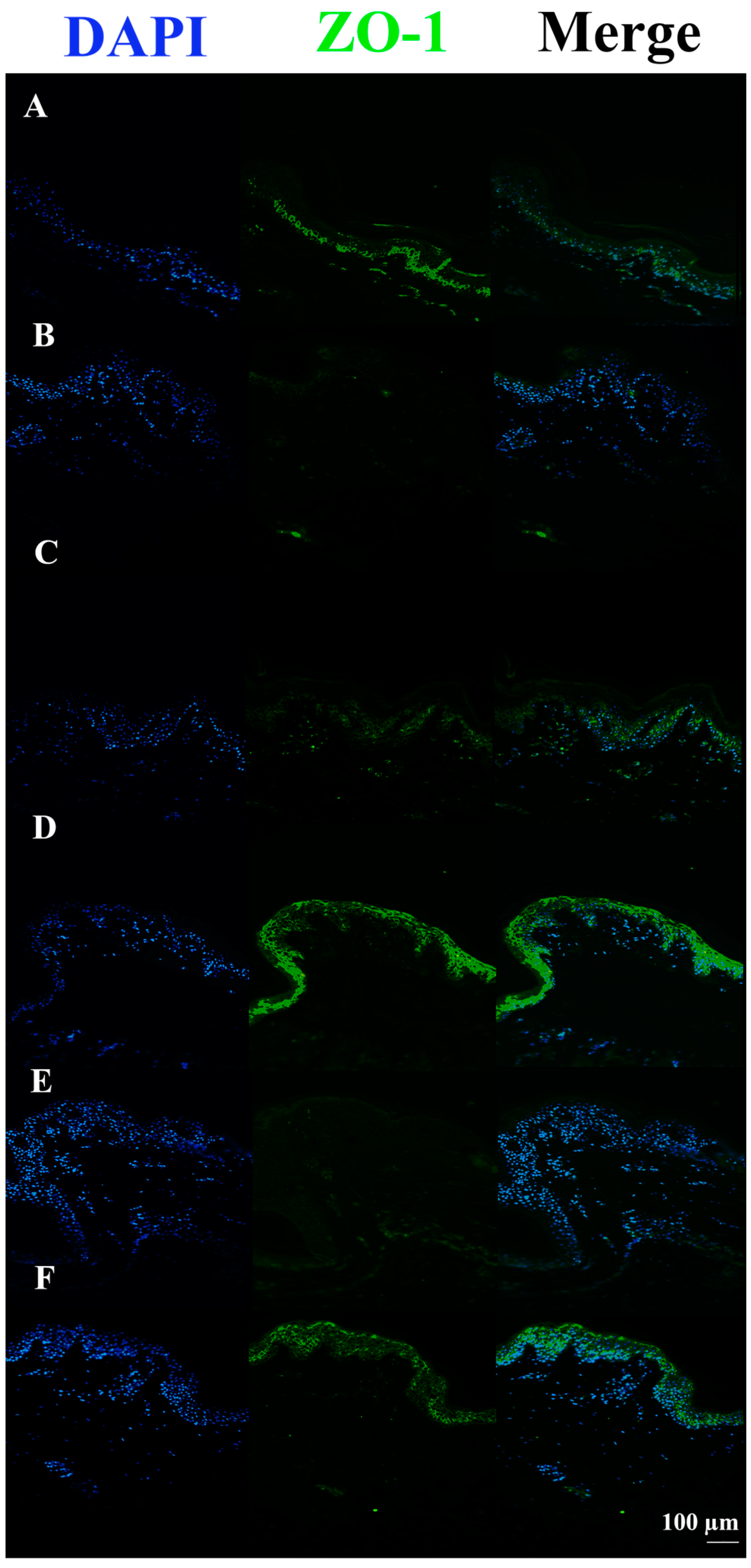
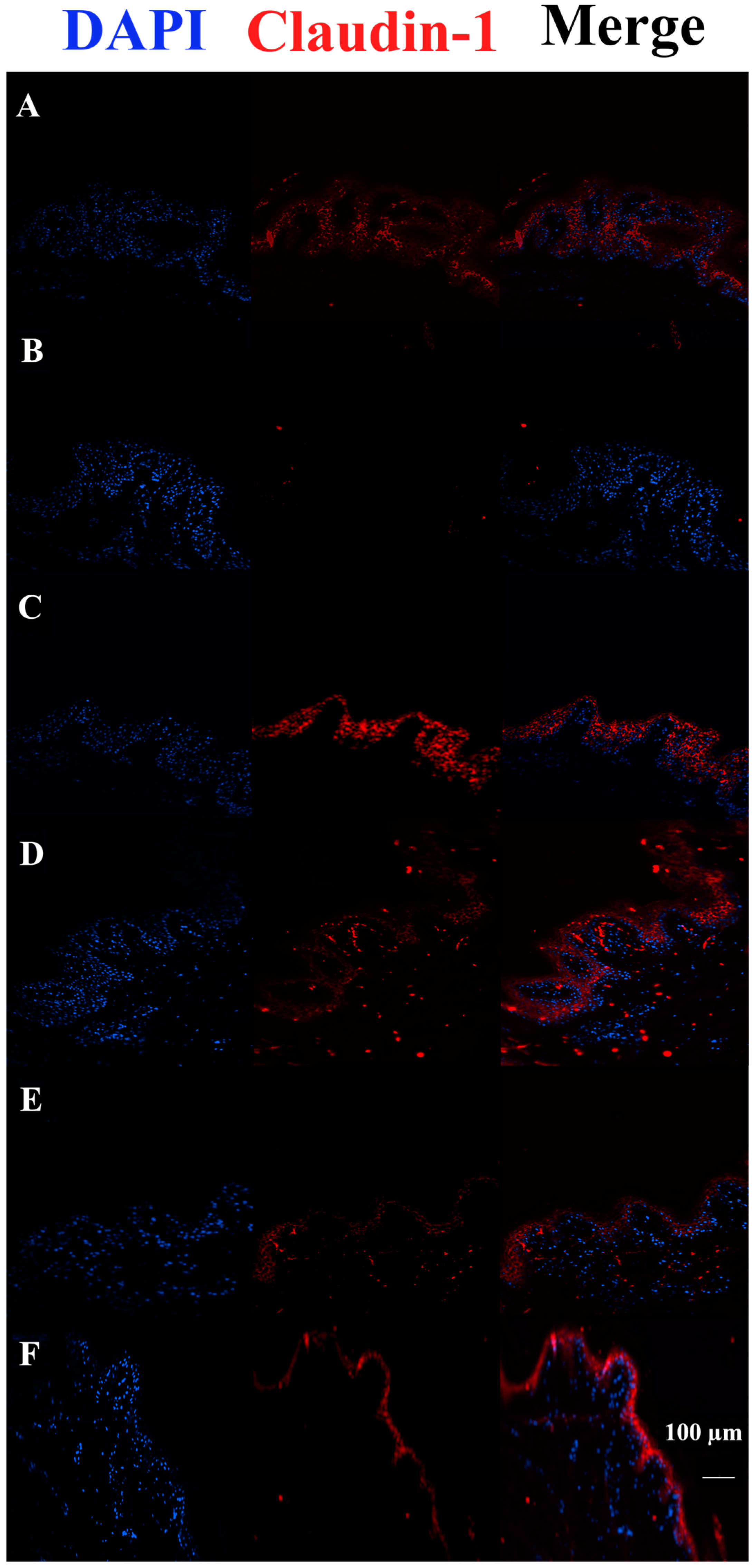
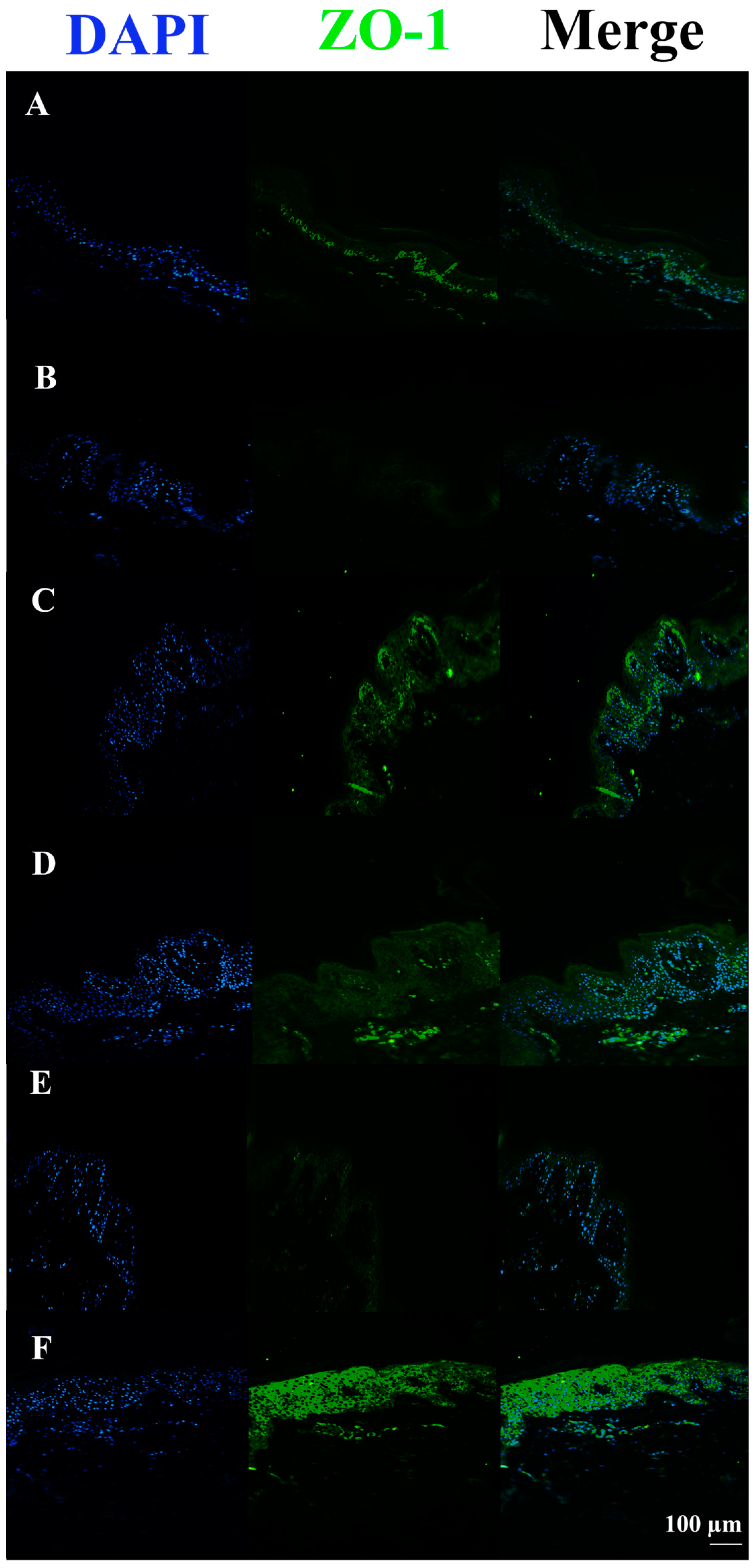
3.3. c40, and HAc40 Activity on Animal Explant Sections Subjected to Immunofluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brandner, J.M. Importance of tight junctions in relation to skin barrier function. Curr. Probl. Dermatol. 2016, 49, 27–37. [Google Scholar] [PubMed]
- Svoboda, M.; Bílková, Z.; Muthný, T. Could tight junctions regulate the barrier function of the aged skin? J. Dermatol. Sci. 2016, 81, 147–152. [Google Scholar] [CrossRef]
- Brandner, J.M.; Schulzke, J.D. Hereditary barrier-related diseases involving the tight junction: Lessons from skin and intestine. Cell Tissue Res. 2015, 360, 723–748. [Google Scholar] [CrossRef] [PubMed]
- Herbig, M.E.; Houdek, P.; Gorissen, S.; Zorn-Kruppa, M.; Wladykowski, E.; Volksdorf, T.; Grzybowski, S.; Kolios, G.; Willers, C.; Mallwitz, H.; et al. A custom tailored model to investigate skin penetration in porcine skin and its comparison with human skin. Eur. J. Pharm. Biopharm. 2015, 95, 99–109. [Google Scholar] [CrossRef]
- Ohnemus, U.; Kohrmeyer, K.; Houdek, P.; Rohde, H.; Wladykowski, E.; Vidal, S.; Horstkotte, M.A.; Aepfelbacher, M.; Kirschner, N.; Behne, M.J. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J. Investig. Dermatol. 2008, 128, 906–916. [Google Scholar] [CrossRef]
- Olivry, T.; Dunston, S.M. Expression patterns of superficial epidermal adhesion molecules in an experimental dog model of acute atopic dermatitis skin lesions. Vet. Dermatol. 2015, 26, 53–e18. [Google Scholar] [CrossRef]
- Roussel, A.; Knol, A.; Bourdeau, P.; Bruet, V. Optimization of an immunohistochemical method to assess distribution of tight junction proteins in canine epidermis and adnexae. J. Comp. Pathol. 2014, 150, 35–46. [Google Scholar] [CrossRef]
- Yuki, T.; Haratake, A.; Koishikawa, H.; Morita, K.; Miyachi, Y.; Inoue, S. Tight junction proteins in keratinocytes: Localisation and contribution to barrier function. Exp. Derm. 2007, 16, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Luna, P.C. Skin microbiome as years go by. Am. J. Clin. Dermatol. 2020, 21, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R. Potential role of the skin microbiota in Inflammatory skin diseases. J. Cosmet. Dermatol. 2021, 20, 400–409. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Flohr, C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 209–213. [Google Scholar] [CrossRef]
- Meisel, J.S.; Hannigan, G.D.; Tyldsley, A.S.; SanMiguel, A.J.; Hodkinson, B.P.; Zheng, Q.; Grice, E.A. Skin microbiome surveys are strongly influenced by experimental design. J. Investig. Dermatol. 2016, 136, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.E.; Bhatia, N.; Friedman, A.; Prunty, T.; Martin, R.; Seite, S. The role of cutaneous microbiota harmony in maintaining a functional skin barrier. SKIN J. Cutan. Med. 2017, 1, s139. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Yamasaki, O.; Morizane, S.; Oono, T. Staphylococcal cutaneous infections: Invasion, evasion and aggression. J. Dermatol. Sci. 2006, 42, 203–214. [Google Scholar] [CrossRef]
- Alam, M.A. Antibacterial pyrazoles: Tackling resistant bacteria. Future Med. Chem. 2022, 14, 343–362. [Google Scholar] [CrossRef]
- Bukowski, M.; Wladyka, B.; Dubin, G. Exfoliative toxins of Staphylococcus aureus. Toxins 2010, 2, 1148–1165. [Google Scholar] [CrossRef]
- Magnifico, I.; Petronio Petronio, G.; Venditti, N.; Cutuli, M.A.; Pietrangelo, L.; Vergalito, F.; Mangano, K.; Zella, D.; Di Marco, R. Atopic dermatitis as a multifactorial skin disorder. Can the analysis of pathophysiological targets represent the winning therapeutic strategy? Pharmaceuticals 2020, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Amagai, M. Controlling skin microbiome as a new bacteriotherapy for inflammatory skin diseases. Inflamm. Regen. 2022, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Hata, T.R.; Tong, Y.; Cheng, J.Y.; Shafiq, F.; Butcher, A.M.; Salem, S.S.; Brinton, S.L.; Rudman Spergel, A.K.; Johnson, K. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomised clinical trial. Nat. Med. 2021, 27, 700–709. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, L.; Ren, Y.; Tan, X.; Liu, W.; Liu, Z. Applications of Human Skin Microbiota in the Cutaneous Disorders for Ecology-Based Therapy. Front. Cell. Infect. Microbiol. 2020, 10, 570261. [Google Scholar] [CrossRef]
- Junca, H.; Pieper, D.H.; Medina, E. The emerging potential of microbiome transplantation on human health interventions. Comput. Struct. Biotechnol. J. 2022, 20, 615–627. [Google Scholar] [CrossRef]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From dysbiosis to healthy skin: Major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Kane, T.L.; Carothers, K.E.; Lee, S.W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug Targets 2018, 19, 111–127. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Mansour, S.C.; Liu, L.T.; Pletzer, D.; Draeger, A.; Babiychuk, E.B.; Hancock, R.E.W. Liposomal therapy attenuates dermonecrosis induced by community-associated methicillin-resistant Staphylococcus aureus by targeting α-type phenol-soluble modulins and α-hemolysin. EBioMedicine 2018, 33, 211–217. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-spectrum neutralisation of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv. Healthc. Mater. 2018, 7, 1701366. [Google Scholar] [CrossRef]
- Karginov, V.A.; Nestorovich, E.M.; Schmidtmann, F.; Robinson, T.M.; Yohannes, A.; Fahmi, N.E.; Bezrukov, S.M.; Hecht, S.M. Inhibition of S. aureus α-hemolysin and B. anthracis lethal toxin by β-cyclodextrin derivatives. Bioorg. Med. Chem. 2007, 15, 5424–5431. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, J.; Wang, J.; Li, H.; Dai, X.; Zhang, Y.; Wang, X.; Tan, W.; Niu, X.; Deng, X. Apigenin alleviates the symptoms of Staphylococcus aureus pneumonia by inhibiting the production of alpha-hemolysin. FEMS Microbiol. Lett. 2013, 338, 124–131. [Google Scholar] [CrossRef]
- Jiang, L.; Yi, T.; Shen, Z.; Teng, Z.; Wang, J. Aloe-emodin Attenuates Staphylococcus aureus Pathogenicity by Interfering with the Oligomerization of α-Toxin. Front. Cell. Infect. Microbiol. 2019, 9, 157. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Liu, S.; Li, G.; Shi, L.; Dong, J.; Li, W.; Deng, X.; Niu, X. Morin hydrate attenuates Staphylococcus aureus virulence by inhibiting the self-assembly of α-hemolysin. J. Appl. Microbiol. 2015, 118, 753–763. [Google Scholar] [CrossRef]
- Qiu, J.; Niu, X.; Dong, J.; Wang, D.; Wang, J.; Li, H.; Luo, M.; Li, S.; Feng, H.; Deng, X. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of α-hemolysin. J. Infect. Dis. 2012, 206, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin Attenuates Staphylococcus aureus-Induced Lung Cell Injury by Inhibiting Alpha-Hemolysin Expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. PTR 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, L.; Magnifico, I.; Guerrera, A.; Cutuli, M.A.; Petronio, G.P.; Venditti, N.; Covelli, M.; Buccieri, N.; Garofalo, S.; Di Marco, R.; et al. LimpiAD foam and the potential control of the pressure ulcers onset. Biomed. Pharmacother. 2021, 144, 112327. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, L.; Dattola, A.; Magnifico, I.; Petronio Petronio, G.; Cutuli, M.A.; Venditti, N.; Guarnieri, A.; Wollenberg, A.; Pellacani, G.; Di Marco, R. Efficacy and Microbiota Modulation Induced by LimpiAL 2.5%, a New Medical Device for the Inverse Psoriasis Treatment. Int. J. Mol. Sci. 2023, 24, 6339. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.M. Ex Vivo Live Full-Thickness Porcine Skin Model as a Versatile In Vitro Testing Method for Skin Barrier Research. Int. J. Mol. Sci. 2021, 22, 657. [Google Scholar] [CrossRef] [PubMed]
- Lind, I.; Ahnert-Hilger, G.; Fuchs, G.; Gratzl, M. Purification of alpha-toxin from Staphylococcus aureus and application to cell permeabilisation. Anal. Biochem. 1987, 164, 84–89. [Google Scholar] [CrossRef]
- Lazar, I., Jr.; Lazar, I., Sr. GelAnalyzer 19.1. 2023. Available online: www.gelanalyzer.com (accessed on 1 February 2023).
- Selam, B.; Kayisli, U.A.; Mulayim, N.; Arici, A. Regulation of Fas ligand expression by estradiol and progesterone in human endometrium. Biol. Reprod. 2001, 65, 979–985. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis-back to the future? Gut. Pathog. 2011, 3, 1–11. [Google Scholar] [CrossRef]
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Factories 2022, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.S.; Alqam, M.L.; Jones, B.C.; Phadungpojna, S.; Day, D.; Hitchcock, T.M. Characterization of a live Cutibacterium acnes subspecies defendens strain XYCM42 and clinical assessment as a topical regimen for general skin health and cosmesis. J. Cosmet. Dermatol. 2022, 22, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.P.M.; Lood, R. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Tsilochristou, O.; du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Radulovic, S.; Basting, M.; Plaut, M.; et al. Association of Staphylococcus aureus colonisation with food allergy occurs independently of eczema severity. Clin. Immunol. 2019, 144, 494–503. [Google Scholar]
- Blicharz, L.; Rudnicka, L.; Samochocki, Z.; Alergologii, A. Staphylococcus aureus: An underestimated factor in the pathogenesis of atopic dermatitis? Postep. Derm. Alergol. 2019, 36, 11. [Google Scholar] [CrossRef]
- Seite, S.; Bieber, T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2015, 8, 479. [Google Scholar] [CrossRef]
- Richert, S.; Schrader, A.; Schrader, K. Transdermal delivery of two antioxidants from different cosmetic formulations. Int. J. Cosmet. Sci. 2003, 25, 5–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnifico, I.; Perna, A.; Cutuli, M.A.; Medoro, A.; Pietrangelo, L.; Guarnieri, A.; Foderà, E.; Passarella, D.; Venditti, N.; Vergalito, F.; et al. A Wall Fragment of Cutibacterium acnes Preserves Junctional Integrity Altered by Staphylococcus aureus in an Ex Vivo Porcine Skin Model. Pharmaceutics 2023, 15, 1224. https://doi.org/10.3390/pharmaceutics15041224
Magnifico I, Perna A, Cutuli MA, Medoro A, Pietrangelo L, Guarnieri A, Foderà E, Passarella D, Venditti N, Vergalito F, et al. A Wall Fragment of Cutibacterium acnes Preserves Junctional Integrity Altered by Staphylococcus aureus in an Ex Vivo Porcine Skin Model. Pharmaceutics. 2023; 15(4):1224. https://doi.org/10.3390/pharmaceutics15041224
Chicago/Turabian StyleMagnifico, Irene, Angelica Perna, Marco Alfio Cutuli, Alessandro Medoro, Laura Pietrangelo, Antonio Guarnieri, Emanuele Foderà, Daniela Passarella, Noemi Venditti, Franca Vergalito, and et al. 2023. "A Wall Fragment of Cutibacterium acnes Preserves Junctional Integrity Altered by Staphylococcus aureus in an Ex Vivo Porcine Skin Model" Pharmaceutics 15, no. 4: 1224. https://doi.org/10.3390/pharmaceutics15041224
APA StyleMagnifico, I., Perna, A., Cutuli, M. A., Medoro, A., Pietrangelo, L., Guarnieri, A., Foderà, E., Passarella, D., Venditti, N., Vergalito, F., Petronio Petronio, G., & Di Marco, R. (2023). A Wall Fragment of Cutibacterium acnes Preserves Junctional Integrity Altered by Staphylococcus aureus in an Ex Vivo Porcine Skin Model. Pharmaceutics, 15(4), 1224. https://doi.org/10.3390/pharmaceutics15041224






_Di_Marco.png)
