New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect
Abstract
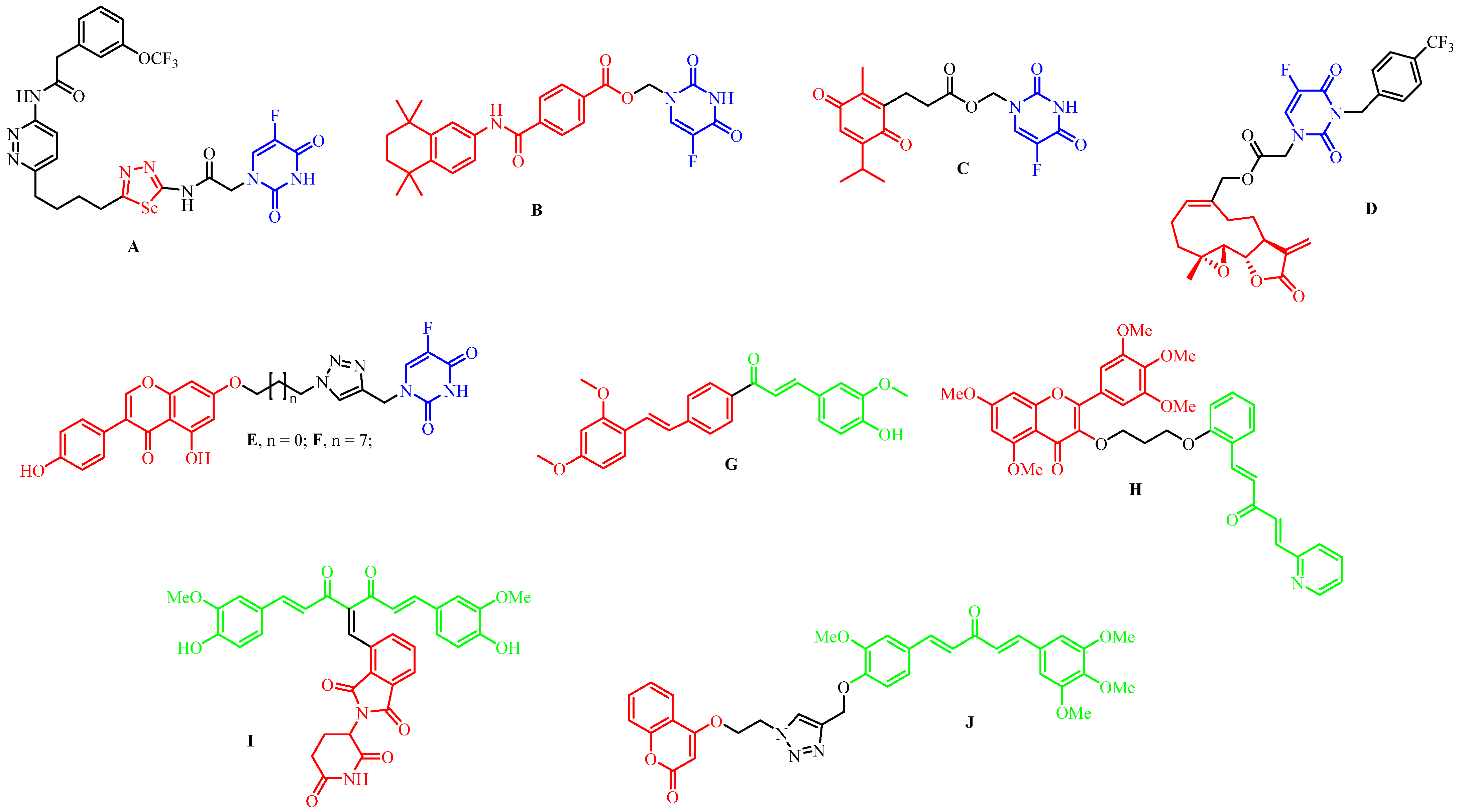
1. Introduction
2. Materials and Methods
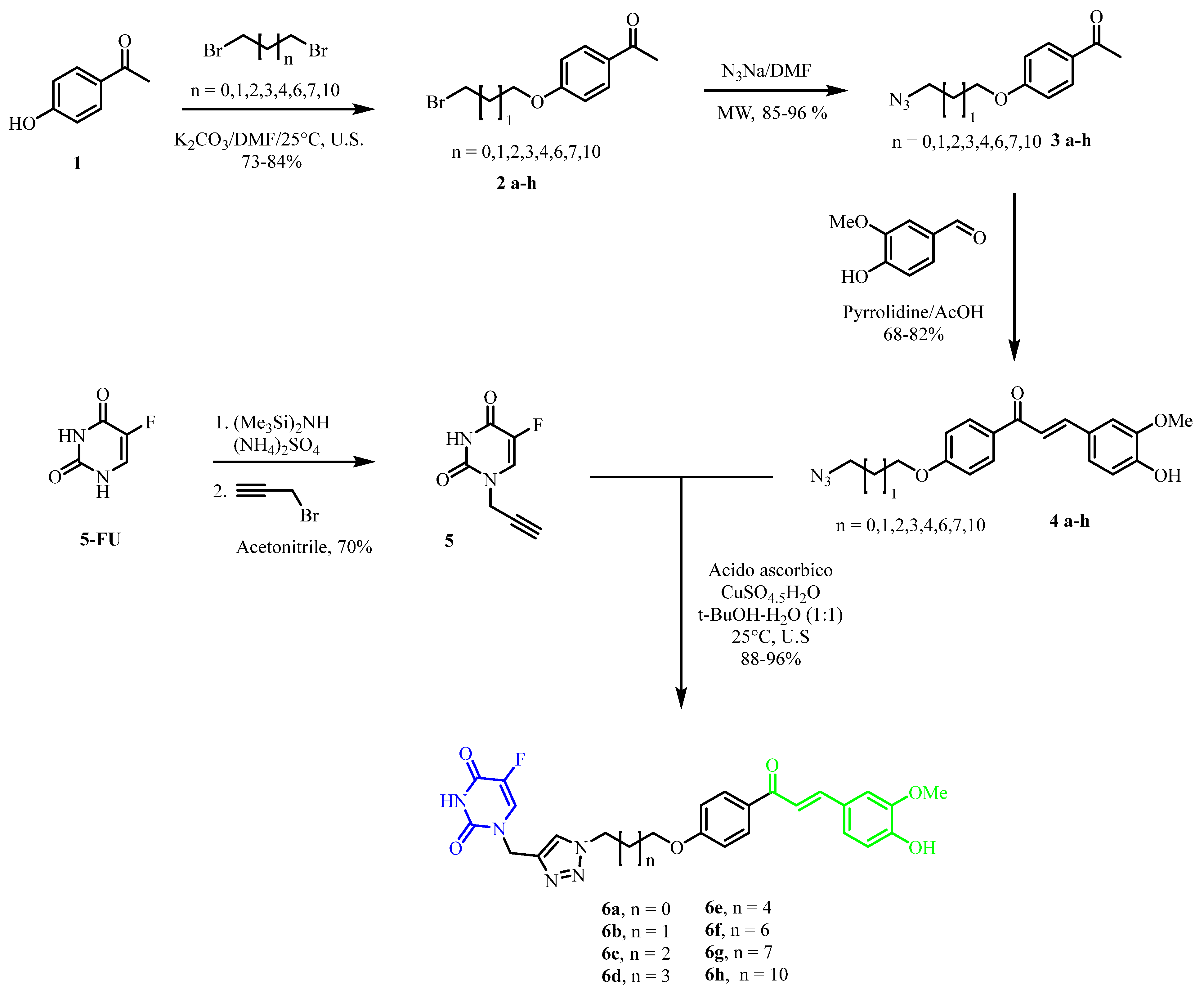
2.1. Chemical Synthesis
2.1.1. General Remarks
2.1.2. General Procedure for the Synthesis of Bromoalkyl Derivatives of 4-Hydroxyacetophenone (2a–h)
2.1.3. General Procedure for the Synthesis of Alkylazides Derivatives of the 4-Hydroxyacetophenone (3a–h)
2.1.4. General Procedure for the Synthesis of Alkylazide Derivatives of the (E)-3-(4-Hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one (4a–h)
2.1.5. Synthesis of Propargyl-5-FU (5)
2.1.6. General Procedure for the Synthesis of 5-FU-Curcumin Hybrids (6a–h)
2.2. Biological Activity Assays
2.2.1. Cell Lines and Culture Medium
2.2.2. Cell Viability
2.2.3. Antiproliferative Activity
2.2.4. Cell Cycle Analysis
2.2.5. Measurement of Mitochondrial Membrane Potential (∆Ψm)
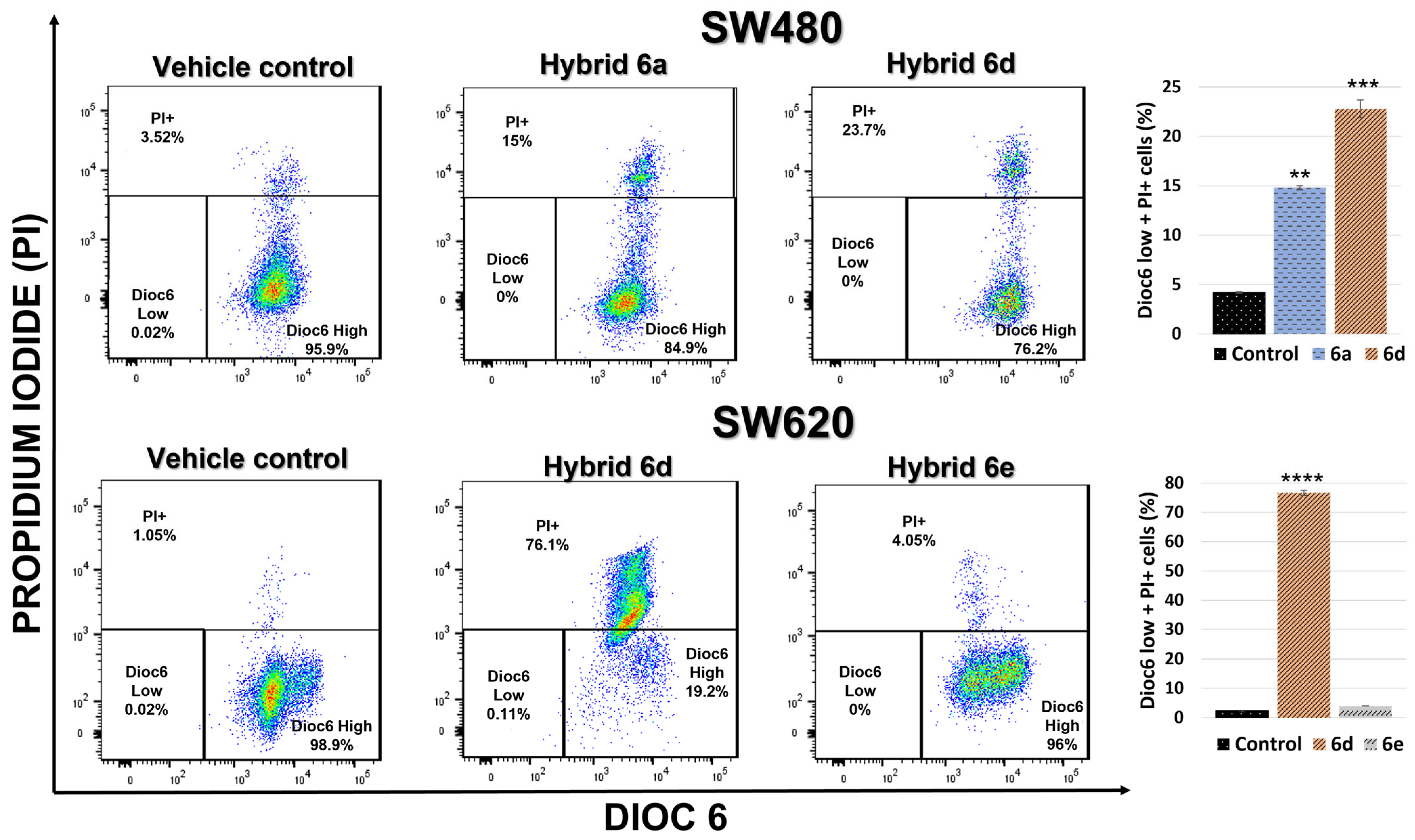
2.2.6. Cell Death Induction by 5-FU/Curcumin Hybrids
2.2.7. Determination of Apoptotic Biomarkers
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Biological Activity
3.2.1. Effect of 5-FU/Curcumin Hybrids on SW480, SW620, HaCaT, and CHO-K1 Cell Viability
3.2.2. Antiproliferative Effect of 5-FU/Curcumin Hybrids on SW480 and SW620 Cells
3.2.3. 5-FU/Curcumin Hybrids Induce Cell Cycle Arrest on SW480 and SW620 Cells
3.2.4. Changes in Mitochondrial Membrane Potential (ΔΨm) Induced by 5-FU/Curcumin Hybrids
3.2.5. Cell Death Induction by 5-FU/Curcumin Hybrids
3.2.6. Determination of Apoptotic Biomarkers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Campos, J.; Domínguez, J.F.; Gallo, M.A.; Espinosa, A. From a classic approach in cancer chemotherapy towards differentiation therapy: Acyclic and cyclic seven-membered 5-fluorouracil O, N-acetals. Curr. Pharm. Des. 2000, 6, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Cardona-G, W.; Herrera-R, A.; Castrillón-L, W.; Ramírez-Malule, H. Chemistry and Anticancer Activity of Hybrid Molecules and Derivatives Based on 5-Fluorouracil. Curr. Med. Chem. 2021, 51, 5551–5601. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Mirzaei, H.; Masoudifar, A.; Sahebkar, A.; Zare, N.; Sadri Nahand, J.; Rashidi, B.; Mehrabian, E.; Mohammadi, M.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: A novel target of curcumin in cancer therapy. J. Cell. Physiol. 2018, 233, 3004–3015. [Google Scholar] [CrossRef]
- Martinez-Cifuentes, M.; Weiss-Lopez, B.; Santos, L.S.; Araya-Maturana, R. Heterocyclic curcumin derivatives of pharmacological interest: Recent progress. Curr. Top. Med. Chem. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Zhou, G.Z.; Li, A.F.; Sun, Y.H.; Sun, G.C. A novel synthetic curcumin derivative MHMM-41 induces ROS-mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed. Pharmacother. 2018, 103, 391–398. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- de Oliveira Pedrosa, M.; Duarte da Cruz, R.M.; de Oliveira Viana, J.; de Moura, R.O.; Ishiki, H.M.; Barbosa Filho, J.M.; Diniz, M.F.; Scotti, M.T.; Scotti, L.; Bezerra Mendonca, F.J. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef]
- Keith, C.T.; Borisy, A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Xu, N.; Fang, J.; Yu, Y.; Hou, W.; Ruan, H.; Zhu, P.; Ma, R.; Lu, S.; et al. Novel 1,3,4-Selenadiazole-Containing KidneyType Glutaminase Inhibitors Showed Improved Cellular Uptake and Antitumor Activity. J. Med. Chem. 2019, 62, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, R.; Yang, Y.; Cheng, Y.; Ge, S.; Xu, G. Tamibarotene inhibit the accumulation of fibrocyte and alleviate renal fibrosis by IL-17A. Ren. Fail. 2020, 42, 1173–1183. [Google Scholar] [CrossRef]
- Ndreshkjana, B.; Çapci, A.; Klein, V.; Chanvorachote, P.; Muenzner, J.K.; Huebner, K.; Steinmann, S.; Erlenbach-Wuensch, K.; Geppert, C.I.; Agaimy, A.; et al. Combination of 5-fluorouracil and thymoquinone targets stem cell gene signature in colorectal cancer cells. Cell Death Dis. 2019, 10, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A.; et al. Parthenolide Covalently Targets and Inhibits Focal Adhesion Kinase in Breast Cancer Cells. Cell Chem. Biol. 2019, 26, 1027–1035. [Google Scholar] [CrossRef]
- Moreno-Quintero, G.; Castrillón-Lopez, W.; Herrera-Ramirez, A.; Yepes-Pérez, A.F.; Quintero-Saumeth, J.; Cardona-Galeano, W. Synthesis and Chemopreventive Potential of 5-FU/Genistein Hybrids on Colorectal Cancer Cells. Pharmaceuticals 2022, 15, 1299. [Google Scholar] [CrossRef]
- Hernández, C.; Moreno, G.; Herrera-R, A.; Cardona-G, W. New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells. Molecules 2021, 26, 2661. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Wang, Y.; Tang, W.; Shi, J.B.; Liu, X.H. Novel curcumin analogue hybrids: Synthesis and anticancer activity. Eur. J. Med. Chem. 2018, 156, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, D.; Chojnacki, J.; Du, Y.; Fu, H.; Grant, S.; Zhang, S. Design and biological characterization of hybrid compounds of curcumin and thalidomide for multiple myeloma. Org. Biomol. Chem. 2013, 11, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, M.; Nepali, K.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Bedi, P.M.S. Triazole tethered C5-curcuminoid-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. Eur. J. Med. Chem. 2016, 116, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Yepes, A.F.; Arias, J.D.; Cardona-G, W.; Herrera-R, A.; Moreno, G. New class of hybrids based on chalcone and melatonin: A promising therapeutic option for the treatment of colorectal cancer. Med. Chem. Res. 2021, 30, 2240–2255. [Google Scholar] [CrossRef]
- Preciado, P.; Moreno, G.; Cardona, W.; Yepes, A.F. Discovery of novel trihybrids based on salicylic acid/isoleucine/N-acylhydrazone: A promising therapeutic opportunity in colorectal cancer. J. Appl. Pharm. Sci. 2022, 12, 10–20. [Google Scholar] [CrossRef]
- Herrera-Ramirez, A.; Moreno, G.; Araque, P.; Vásquez, I.; Naranjo, E.; Alzate, F.; Cardona-G, W. In-vitro chemopreventive potential of a chromone from bomarea setacea (Alstroemeriaceae) against colorectal cancer. Iran. J. Pharm. Res. 2021, 20, 254–267. [Google Scholar]
- Moreno-Q, G.; Herrera-R, A.; Yepes, A.F.; Naranjo, T.W.; Cardona-G, W. Proapoptotic Effect and Molecular Docking Analysis of Curcumin–Resveratrol Hybrids in Colorectal Cancer Chemoprevention. Molecules 2022, 27, 3486. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ramirez, A.; Yepes-Pérez, A.F.; Quintero-Saumeth, J.; Moreno-Quintero, G.; Naranjo, T.W.; Cardona-Galeano, W. Colorectal Cancer Chemoprevention by S-Allyl Cysteine–Caffeic Acid Hybrids: In vitro Biological Activity and In Silico Studies. Sci. Pharm. 2022, 90, 40. [Google Scholar] [CrossRef]
- Otero, E.; García, E.; Palacios, G.; Yepes, L.M.; Carda, M.; Agut, R.; Vélez, I.D.; Cardona, W.I.; Robledo, S.M. Triclosan-caffeic acid hybrids: Synthesis, leishmanicidal, trypanocidal and cytotoxic activities. Eur. J. Med. Chem. 2017, 141, 73–83. [Google Scholar] [CrossRef]
- García, E.; Coa, J.C.; Otero, E.; Carda, M.; Vélez, I.D.; Robledo, S.M.; Cardona, W.I. Synthesis and antiprotozoal activity of furanchalcone–quinoline, furanchalcone–chromone and furanchalcone–imidazole hybrids. Med. Chem. Res. 2018, 27, 497–511. [Google Scholar] [CrossRef]
- Ding, Y.; Li, S.; Ge, W.; Liu, Z.; Zhang, X.; Wang, M.; Chen, T.; Chen, Y.; Zhang, Q. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 183, 111706. [Google Scholar] [CrossRef] [PubMed]
- Gómez-R, L.; Moreno-Q, G.; Herrera-R, A.; Castrillón-L, W.; Yepes, A.F.; Cardona-G, W. New Hybrid Scaffolds Based on ASA/Genistein: Synthesis, Cytotoxic Effect, Molecular Docking, Drug-likeness and in silico ADME/tox Modeling. J. Appl. Pharm. Sci. 2022, 12, 15–30. [Google Scholar]
- Pessel, F.; Billault, I.; Scherrmann, M.C. Total synthesis of triazole-linked C-glycosyl flavonoids in alternative solvents and environmental assessment in terms of reaction, workup and purification. Green Chem. 2016, 18, 5558–5568. [Google Scholar] [CrossRef]
- Belkharchach, S.; Ighachane, H.; Lachgar, A.; Ait Ali, M.; Lazrek, H.B. Efficient and selective catalytic N-Alkylation of pyrimidine by ammonium Sulfate@Hydro-thermal carbone under eco-friendly conditions. J. Chem. Sci. 2020, 132, 78. [Google Scholar] [CrossRef]
- Shinde, V.; Mhaske, P.C.; Singh, A.; Sarkar, D.; Mahulikar, P. Synthesis and biological evaluation of new 4-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl)-2-phenyl thiazole derivatives. J. Heterocycl. Chem. 2019, 56, 3093–3101. [Google Scholar] [CrossRef]
- Zhang, R.; Song, X.Q.; Liu, R.P.; Ma, Z.Y.; Xu, J.Y. Fuplatin: An Efficient and Low-Toxic Dual-Prodrug. J. Med. Chem. 2019, 62, 4543–4554. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, M.K.; Saxena, A.K.; Bedi, P.M. Triazole linked mono carbonyl curcumin-isatin bifunctional hybrids as novel anti tubulin agents: Design, synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 7165–7180. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G. Medicinal Chemistry: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Goldhahn, K.; Hintersteininger, M.; Steiner, G.; Erker, T.; Kloesch, B. Enhanced Antiproliferative and Pro-apoptotic Activities of a Novel Curcumin-related Compound in Jurkat Leukemia T-Cells. Anticancer Res. 2015, 35, 2675–2680. [Google Scholar]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M.; et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef]
- Rahim, N.F.C.; Hussin, Y.; Aziz, M.N.M.; Mohamad, N.E.; Yeap, S.K.; Masarudin, M.J.; Abdullah, R.; Akhtar, M.N.; Alitheen, N.B. Cytotoxicity and Apoptosis Effects of Curcumin Analogue (2E,6E)-2,6-Bis(2,3-Dimethoxybenzylidine) Cyclohexanone (DMCH) on Human Colon Cancer Cells HT29 and SW620 In Vitro. Molecules 2021, 26, 1261. [Google Scholar] [CrossRef]
- Herrera-R, A.; Naranjo, T.W.; Maldonado, M.E.; Moreno-Q, G.; Yepes, A.; Cardona, G.W. Styrylcoumarin 7-SC2 induces apoptosis in SW480 human colon adenocarcinoma cells and inhibits azoxymethane-induced aberrant crypt foci formation in BALB/c mice. Med. Chem. Res. 2020, 29, 377–395. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Weng, Q.; Fu, L.; Chen, G.; Hui, J.; Song, J.; Feng, J.; Shi, D.; Cai, Y.; Ji, J.; Liang, G. Design, synthesis, and anticancer evaluation of long-chain alkoxylated mono-carbonyl analogues of curcumin. Eur. J. Med. Chem. 2015, 103, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Hill, R.; Yaffe, P.B.; Greenshields, A.; Walsh, M.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]











| Compound | IC50 (µM) CHO-K1 | IC50 (µM) HaCaT | IC50 (µM) SW480 | SI CHO-K1/SW480 | SI HaCaT/SW480 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 6a | 82.84 ± 6.43 | 73.70 ± 1.07 | 71.77 ± 4.22 | 27.90 ± 2.53 | 75.30 ± 4.65 | 17.37 ± 1.16 | 1.10 | 4.24 | 0.95 | 1.61 |
| 6b | 51.34 ± 2.86 | 46.50 ± 1.34 | 58.07 ± 3.30 | 18.45 ± 1.10 | 48.36 ± 2.82 | 17.14 ± 0.80 | 1.06 | 2.71 | 1.20 | 1.07 |
| 6c | 26.30 ± 2.99 | 30.54 ± 2.18 | 45.44 ± 4.18 | 19.10 ± 1.68 | 30.42 ± 2.69 | 16.39 ± 0.97 | 0.86 | 1.86 | 1.49 | 1.17 |
| 6d | 12.43 ± 1.34 | 28.10 ± 1.95 | 42.64 ± 6.93 | 20.32 ± 2.66 | 15.73 ± 1.91 | 2.43 ± 0.33 | 0.79 | 11.56 | 2.71 | 8.36 |
| 6e | 33.80 ± 2.20 | 34.51 ± 1.37 | 76.86 ± 5.87 | 19.65 ± 1.99 | 38.79 ± 5.46 | 18.18 ± 0.88 | 0.87 | 1.90 | 1.98 | 1.08 |
| 6f | >100 | >100 | >100 | 35.10 ± 4.74 | >100 | 29.71 ± 2.43 | NA | >3 | NA | 1.18 |
| 6g | >100 | >100 | >100 | >100 | >100 | >100 | NA | NA | NA | NA |
| 6h | >100 | >100 | >100 | >100 | >100 | >100 | NA | NA | NA | NA |
| Mixture 5FU + Curcumin * | 87.64 ± 12.55 | 17.66 ± 2.28 | 88.63 ± 6.37 | 7.83 ± 1.30 | 95.92 ± 3.37 | 27.23 ± 1.21 | 0.91 | 0.65 | 0.92 | 0.44 |
| Curcumin | 20.18 ± 2.41 | 36.29 ± 4.17 | >100 | 28.78 ± 2.33 | 84.64 ± 6.60 | 90.44 ± 1.00 | 0.24 | 0.40 | >1 | 0.32 |
| 5-FU | 543.50 ± 52.94 | 173.20 ± 14.61 | >1000 | 118.67 ± 2.84 | 1544.01 ± 127.90 | 174.30 ± 19.10 | 0.35 | 0.99 | NA | 0.68 |
| Compound | IC50 (µM) CHO-K1 | IC50 (µM) HaCaT | IC50 (µM) SW620 | SI CHO-K1/SW620 | SI HaCaT/SW620 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 6a | 82.84 ± 6.43 | 73.70 ± 1.07 | 71.77 ± 4.22 | 27.90 ± 2.53 | >100 | 45.10 ± 0.98 | <1 | 1.63 | <1 | 0.62 |
| 6b | 51.34 ± 2.86 | 46.50 ± 1.34 | 58.07 ± 3.30 | 18.45 ± 1.10 | 90.21 ± 9.33 | 33.73 ± 1.03 | 0.57 | 1.38 | 0.64 | 0.55 |
| 6c | 26.30 ± 2.99 | 30.54 ± 2.18 | 45.44 ± 4.18 | 19.10 ± 1.68 | 83.90 ± 8.08 | 28.07 ± 1.85 | 0.31 | 1.09 | 0.54 | 0.68 |
| 6d | 12.43 ± 1.34 | 28.10 ± 1.95 | 42.64 ± 6.93 | 20.32 ± 2.66 | 37.08 ± 3.05 | 7.51 ± 1.47 | 0.34 | 3.74 | 1.15 | 2.71 |
| 6e | 33.80 ± 2.20 | 34.51 ± 1.37 | 76.86 ± 5.87 | 19.65 ± 1.99 | >100 | 14.52 ± 1.31 | <1 | 1.77 | <1 | 1.36 |
| 6f | >100 | >100 | >100 | 35.10 ± 4.74 | NI | >100 | NA | NA | NA | <1 |
| 6g | >100 | >100 | >100 | >100 | NI | >100 | NA | NA | NA | NA |
| 6h | >100 | >100 | >100 | >100 | NI | >100 | NA | NA | NA | NA |
| Mixture 5FU + Curcumin * | 87.64 ± 12.55 | 17.66 ± 2.28 | 88.63 ± 6.37 | 7.83 ± 1.30 | >100 | 63.92 ± 2.05 | <1 | 0.28 | <1 | 0.12 |
| Curcumin | 20.18 ± 2.41 | 36.29 ± 4.17 | >100 | 28.78 ± 2.33 | 163.60 ± 5.88 | 22.21 ± 2.37 | 0.12 | 1.63 | NA | 1.30 |
| 5-FU | 543.5 ± 52.94 | 173.20 ± 14.61 | >1000 | 118.67 ± 2.84 | 898.80 ± 60.74 | 180.90 ± 18.80 | 0.60 | 0.96 | <1 | 0.66 |
| Cell Line | Hybrids | Concentration (µM) | Time after Plating (Days) | |||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||
| Cell Viability (%) ± SE | ||||||
| SW480 | 6a | 2.5 | 97.91 ± 1.55 | 81.21 ± 2.80 | 59.53 ± 3.45 | 42.83 ± 1.30 |
| 40 | 16.24 ± 2.51 | 8.00 ± 1.81 | 2.04 ± 0.35 | 0.00 | ||
| 6d | 0.625 | 90.11 ± 1.45 | 55.47 ± 2.21 | 46.36 ± 0.18 | 32.34 ± 0.77 | |
| 10 | 7.35 ± 1.45 | 0.75 ± 0.20 | 0.68 ± 0.14 | 0.00 | ||
| SW620 | 6d | 1.25 | 90.95 ± 2.85 | 85.19 ± 3.23 | 67.34 ± 2.32 | 54.41 ± 2.76 |
| 20 | 42.47 ± 4.57 | 7.10 ± 0.95 | 0.00 | 0.00 | ||
| 6e | 2.5 | 95.57 ± 4.08 | 60.65 ± 0.46 | 56.53 ± 2.09 | 48.93 ± 3.46 | |
| 40 | 8.77 ± 1.85 | 0.00 | 0.00 | 0.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Quintero, G.; Betancur-Zapata, E.; Herrera-Ramírez, A.; Cardona-Galeano, W. New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics 2023, 15, 1221. https://doi.org/10.3390/pharmaceutics15041221
Moreno-Quintero G, Betancur-Zapata E, Herrera-Ramírez A, Cardona-Galeano W. New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics. 2023; 15(4):1221. https://doi.org/10.3390/pharmaceutics15041221
Chicago/Turabian StyleMoreno-Quintero, Gustavo, Emmanuel Betancur-Zapata, Angie Herrera-Ramírez, and Wilson Cardona-Galeano. 2023. "New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect" Pharmaceutics 15, no. 4: 1221. https://doi.org/10.3390/pharmaceutics15041221
APA StyleMoreno-Quintero, G., Betancur-Zapata, E., Herrera-Ramírez, A., & Cardona-Galeano, W. (2023). New Hybrid Scaffolds Based on 5-FU/Curcumin: Synthesis, Cytotoxic, Antiproliferative and Pro-Apoptotic Effect. Pharmaceutics, 15(4), 1221. https://doi.org/10.3390/pharmaceutics15041221







