Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Agents
2.2. Preparation of MET Base
2.3. Preparation of Multicomponent Salts
2.4. Powder X-ray Diffraction (PXRD)
2.5. Thermal Analysis
2.6. Single-Crystal X-ray Diffraction (SXRD)
2.7. Theoretical Computation
2.8. Crystal Form Transformation Analysis
2.9. Stability Study
2.10. Hygroscopicity Study
2.11. Powder Dissolution and Solubility In Vitro
2.12. In Vivo Hypoglycemic Activity Evaluation
3. Results and Discussion
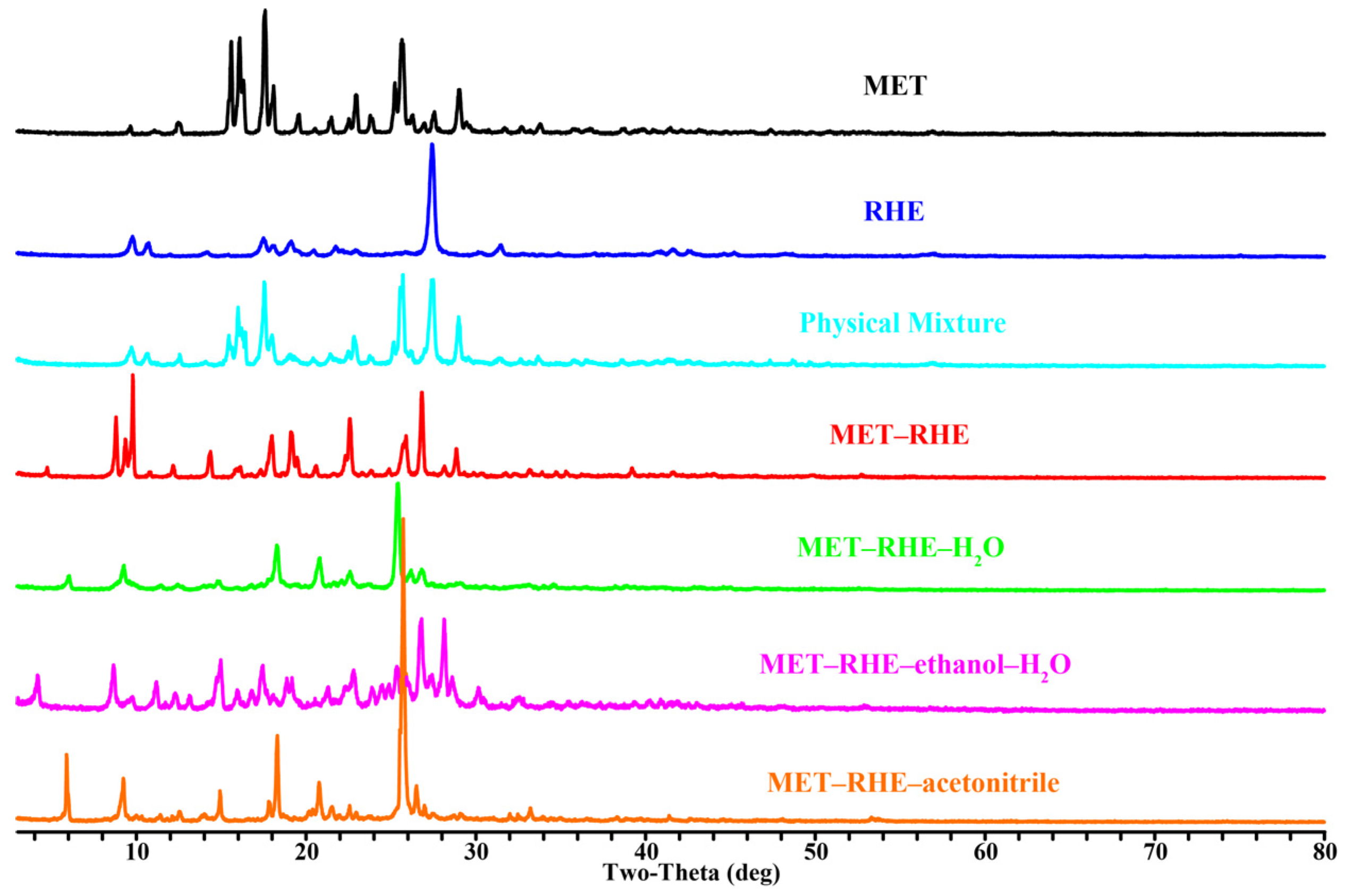
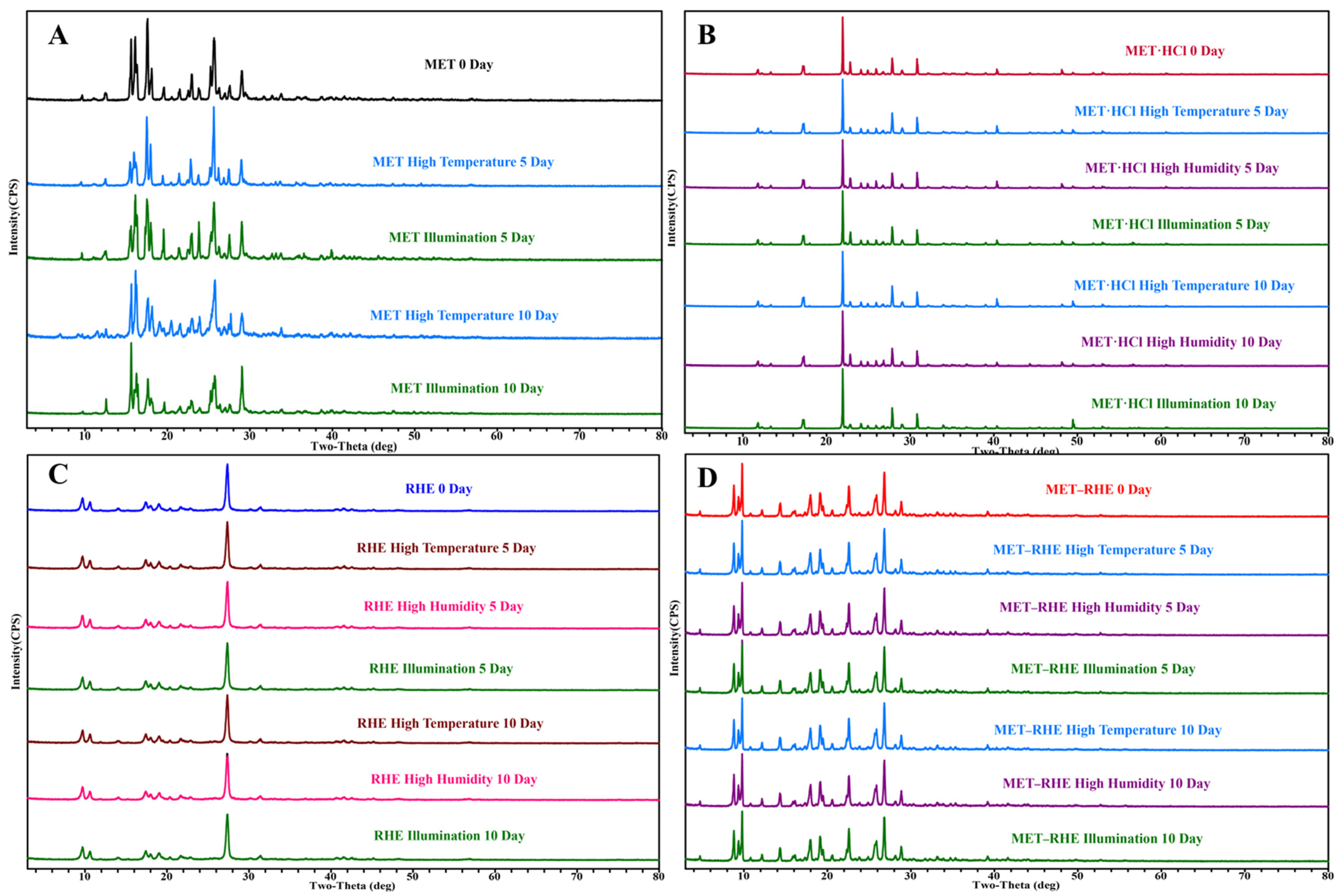
3.1. PXRD
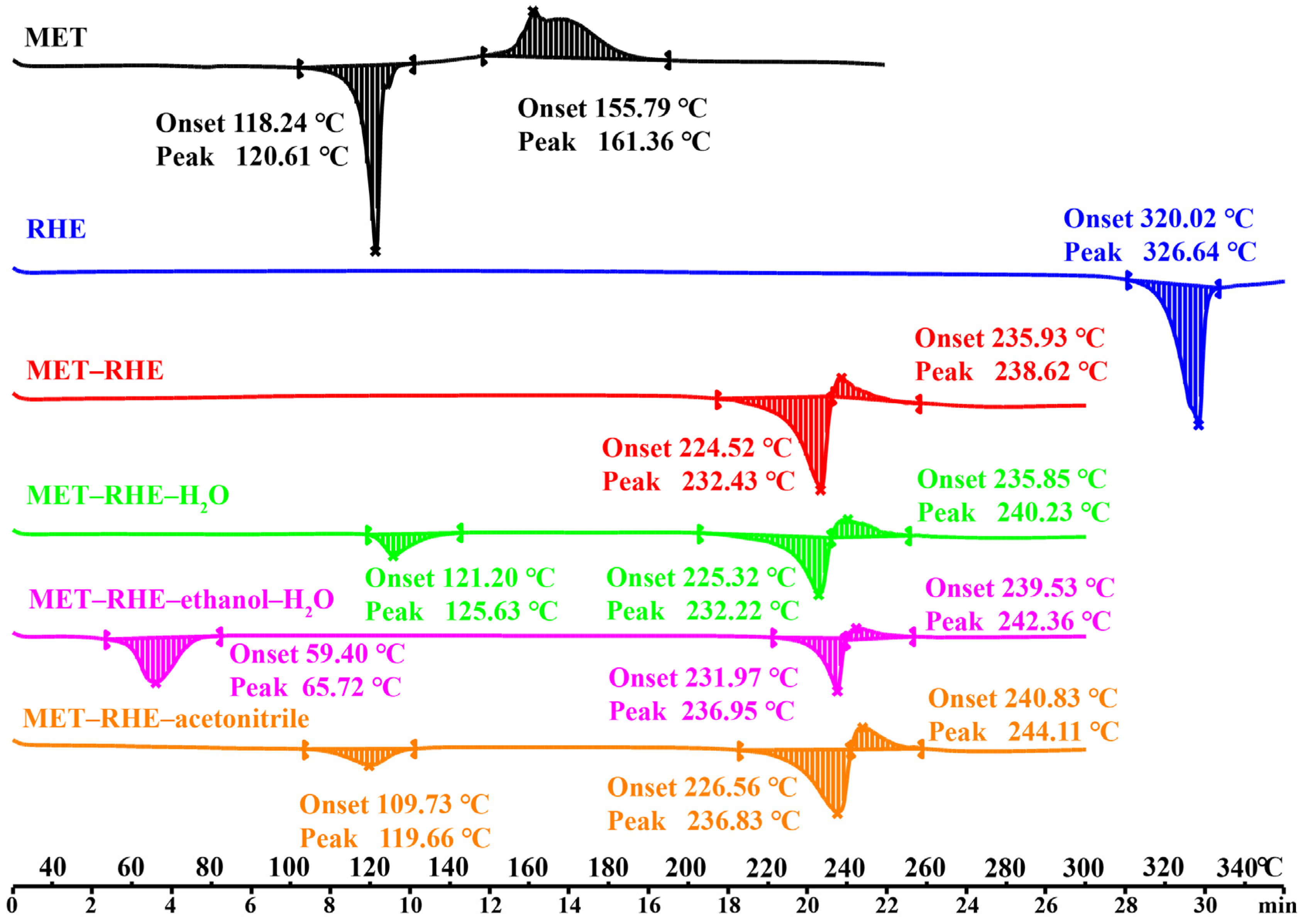
3.2. Thermal Analysis
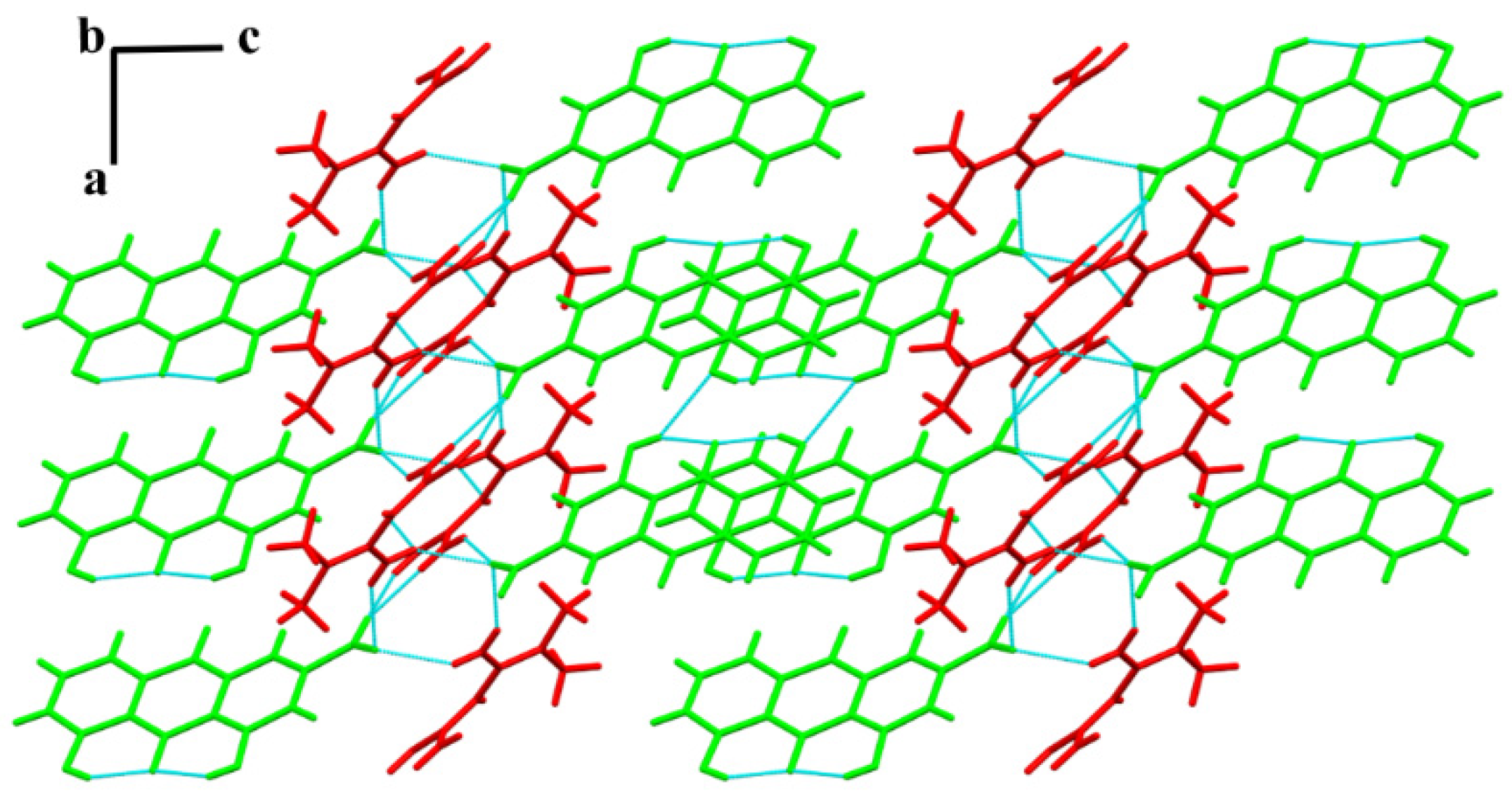
3.3. SXRD
3.4. Theoretical Computation
3.4.1. The Interaction Energies and Lattice Energy Analysis
3.4.2. The Molecular Planarity Analysis
3.5. Crystal Form Transformation Analysis
3.6. Stability Study
3.7. Hygroscopicity Study
3.8. Powder Dissolution and Solubility In Vitro
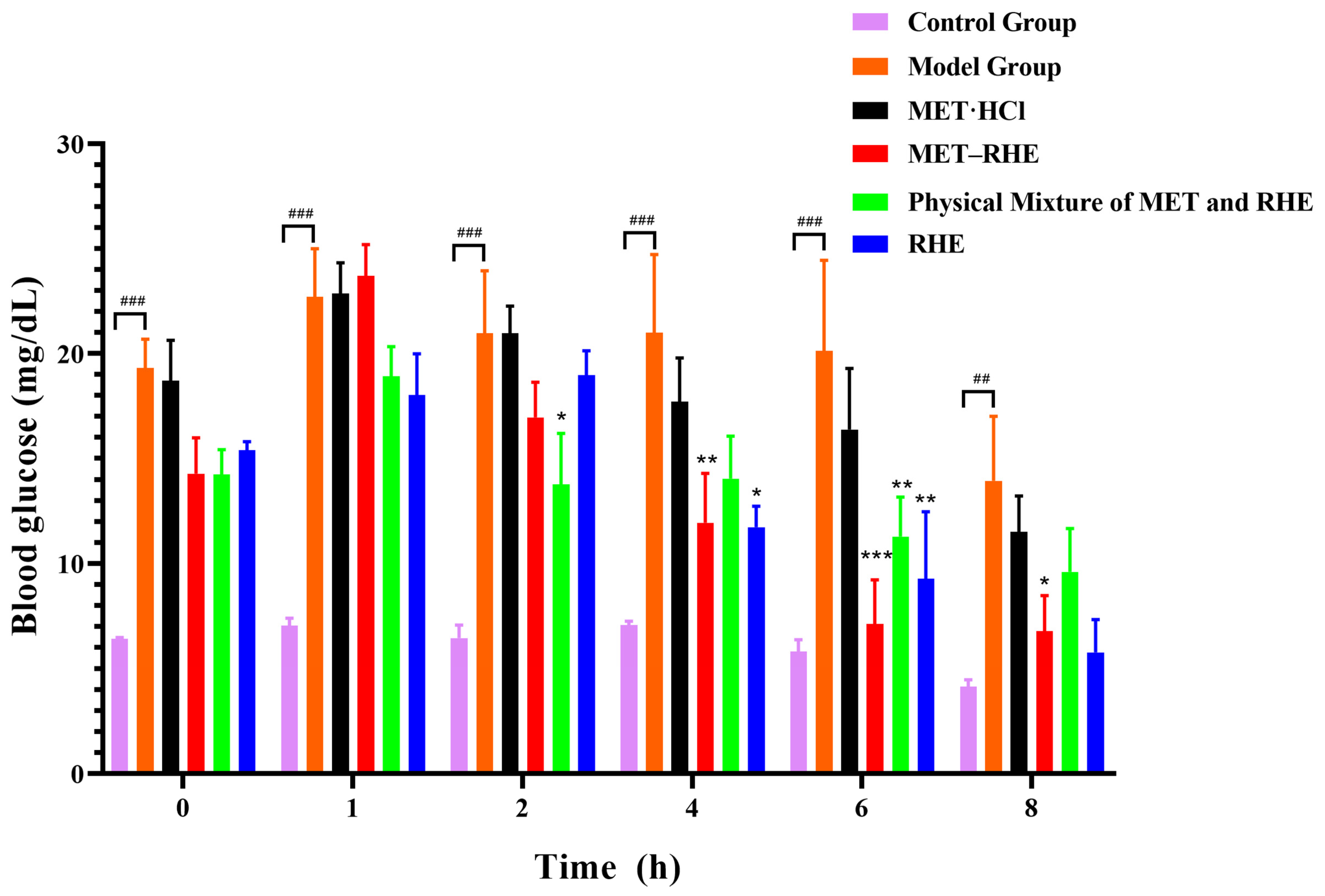
3.9. In Vivo Hypoglycemic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carracher, A.M.; Marathe, P.H.; Close, K.L. International Diabetes Federation 2017. J. Diabetes 2018, 10, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.H. Combine and Conquer: Advantages and Disadvantages of Fixed-Dose Combination. Diabetes Obes. Metab. 2013, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Webster, R. Fixed-Dose Combination Medications for Non-Communicable Diseases. Heart 2019, 105, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Simon, F. The Trouble with Making Combination Drugs. Nat. Rev. Drug Discov. 2006, 5, 881–882. [Google Scholar] [CrossRef]
- Wang, J.R.; Yu, Q.; Dai, W.; Mei, X. Drug-Drug Co-Crystallization Presents a New Opportunity for the Development of Stable Vitamins. Chem. Commun. 2016, 52, 3572–3575. [Google Scholar] [CrossRef]
- Putra, O.D.; Furuishi, T.; Yonemochi, E.; Terada, K.; Uekusa, H. Drug–Drug Multicomponent Crystals as an Effective Technique to Overcome Weaknesses in Parent Drugs. Cryst. Growth Des. 2016, 16, 3577–3581. [Google Scholar] [CrossRef]
- Putra, O.D.; Yoshida, T.; Umeda, D.; Higashi, K.; Uekusa, H.; Yonemochi, E. Crystal Structure Determination of Dimenhydrinate After More Than 60 Years: Solving Salt–Cocrystal Ambiguity Via Solid-State Characterizations and Solubility Study. Cryst. Growth Des. 2016, 16, 5223–5229. [Google Scholar] [CrossRef]
- Peng, B.; Wang, J.R.; Mei, X. Triamterene-Furosemide Salt: Structural Aspects and Physicochemical Evaluation. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 738–741. [Google Scholar] [CrossRef]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer Selection in Pharmaceutical Cocrystal Development: A Case Study of a Meloxicam Aspirin Cocrystal That Exhibits Enhanced Solubility and Pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Trimorphs of a Pharmaceutical Cocrystal Involving Two Active Pharmaceutical Ingredients: Potential Relevance to Combination Drugs. CrystEngComm 2009, 11, 1823–1827. [Google Scholar] [CrossRef]
- Wang, C.; Hu, S.; Sun, C.C. Expedited Development of a High Dose Orally Disintegrating Metformin Tablet Enabled by Sweet Salt Formation with Acesulfame. Int. J. Pharm. 2017, 532, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cornell, S. Comparison of the Diabetes Guidelines from the ADA/EASD and the AACE/ACE. J. Am. Pharm. Assoc. 2017, 57, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Rimondi, E.; Melloni, E.; Gilli, P.; Bertolasi, V.; Casciano, F.; Rigolin, G.M.; Zauli, G.; Secchiero, P. Metformin Combined with Sodium Dichloroacetate Promotes B Leukemic Cell Death by Suppressing Anti-Apoptotic Protein Mcl-1. Oncotarget 2016, 7, 18965–18977. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Morris, A.D. Metformin in Cancer Treatment and Prevention. Annu. Rev. Med. 2015, 66, 17–29. [Google Scholar] [CrossRef]
- He, H.; Ke, R.; Lin, H.; Ying, Y.; Liu, D.; Luo, Z. Metformin, an Old Drug, Brings a New Era to Cancer Therapy. Cancer J. 2015, 21, 70–74. [Google Scholar] [CrossRef]
- Bulterijs, S. Metformin as a Geroprotector. Rejuvenation Res. 2011, 14, 469–482. [Google Scholar] [CrossRef]
- Hofman, A.; Ott, A.; Breteler, M.M.B.; Bots, M.L.; Slooter, A.J.C.; van Harskamp, F.; van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, Apolipoprotein E, and Prevalence of Dementia and Alzheimer’s Disease in the Rotterdam Study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Wang, J.; Gallagher, D.; DeVito, L.M.; Cancino, G.I.; Tsui, D.; He, L.; Keller, G.M.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem Cell 2012, 11, 23–35. [Google Scholar] [CrossRef]
- Calvert, J.W.; Gundewar, S.; Jha, S.; Greer, J.J.M.; Bestermann, W.H.; Tian, R.; Lefer, D.J. Acute Metformin Therapy Confers Cardioprotection Against Myocardial Infarction Via AMPK-Enos-Mediated Signaling. Diabetes 2008, 57, 696–705. [Google Scholar] [CrossRef]
- Johnson, N. Metformin Is a Reasonable First-Line Treatment Option for Non-Obese Women with Infertility Related to Anovulatory Polycystic Ovary Syndrome—A Meta-Analysis of Randomised Trials. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 125–129. [Google Scholar] [CrossRef]
- Smieszek, A.; Basinska, K.; Chrzastek, K.; Marycz, K. In Vitro and In Vivo Effects of Metformin on Osteopontin Expression in Mice Adipose-Derived Multipotent Stromal Cells and Adipose Tissue. J. Diabetes Res. 2015, 2015, 814896. [Google Scholar] [CrossRef] [PubMed]
- Anedda, A.; Rial, E.; Gonzalez-Barroso, M.M. Metformin Induces Oxidative Stress in White Adipocytes and Raises Uncoupling Protein 2 Levels. J. Endocrinol. 2008, 199, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Harumi, T.; Watanabe, J.; Fujita, Y.; Honjo, J.; Shimizu, N.; Makino, Y.; Haneda, M. Tubular Injury in a Rat Model of Type 2 Diabetes is Prevented by Metformin: A Possible Role Of HIF-1alpha Expression and Oxygen Metabolism. Diabetes 2011, 60, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin Inhibits Advanced Glycation End Products (Ages)-Induced Renal Tubular Cell Injury by Suppressing Reactive Oxygen Species Generation via Reducing Receptor for Ages (RAGE) Expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef]
- Zhai, L.; Gu, J.; Yang, D.; Hu, W.; Wang, W.; Ye, S. Metformin Ameliorates Podocyte Damage by Restoring Renal Tissue Nephrin Expression in Type 2 Diabetic Rats. J. Diabetes 2017, 9, 510–517. [Google Scholar] [CrossRef]
- Maniar, K.; Moideen, A.; Mittal, A.; Patil, A.; Chakrabarti, A.; Banerjee, D. A Story of Metformin-Butyrate Synergism to Control Various Pathological Conditions as a Consequence of gut Microbiome Modification: Genesis of a Wonder Drug? Pharmacol. Res. 2017, 117, 103–128. [Google Scholar] [CrossRef]
- Jakobsen, D.F.; Frokjaer, S.; Larsen, C.; Niemann, H.; Buur, A. Application of Isothermal Microcalorimetry in Preformulation. I. Hygroscopicity of Drug Substances. Int. J. Pharm. 1997, 156, 67–77. [Google Scholar] [CrossRef]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Coates, D.A.; Stahly, B.C.; Stahly, G.P. A Metastable Polymorph of Metformin Hydrochloride: Isolation and Characterization Using Capillary Crystallization and Thermal Microscopy Techniques. Cryst. Growth Des. 2004, 4, 441–449. [Google Scholar] [CrossRef]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija-Asimi, Z.; Pearson, E.R.; Semiz, S. Organic Cation Transporter 1 Variants and Gastrointestinal Side Effects of Metformin in Patients with Type 2 Diabetes. Diabet. Med. 2016, 33, 511–514. [Google Scholar] [CrossRef]
- Bhatt, J.A.; Bahl, D.; Morris, K.; Stevens, L.L.; Haware, R.V. Structure-Mechanics and Improved Tableting Performance of the Drug-Drug Cocrystal Metformin: Salicylic Acid. Eur. J. Pharm. Biopharm. 2020, 153, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Jiang, L.; Gan, Z.; Guan, X.; Zhang, L.; Cai, L.; Hu, X. A Glimepiride-Metformin Multidrug Crystal: Synthesis, Crystal Structure Analysis, and Physicochemical Properties. Molecules 2019, 24, 3786. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Jiang, L.; Zhou, J.; Guan, X.; Wang, J.; Xiang, P.; Pan, J.; Hu, X. Improving Dissolution and Cytotoxicity by Forming Multidrug Crystals. Molecules 2020, 25, 1343. [Google Scholar] [CrossRef] [PubMed]
- Chong-Canto, S.; Garcia-Baez, E.V.; Martinez-Martinez, F.J.; Ramos-Organillo, A.A.; Padilla-Martinez, I.I. Mechanochemical Synthesis and Structure of the Tetrahydrate and Mesoporous Anhydrous Metforminium(2+)-N,N′-1,4-Phenylenedioxalamic Acid (1:2) Salt: The Role of Hydrogen Bonding and n-->pi * Charge Assisted Interactions. Pharmaceutics 2020, 12, 998. [Google Scholar] [CrossRef]
- Diniz, L.F.; Carvalho, P.S.; Gonçalves, J.E.; Diniz, R.; Fernandes, C. Solid-State Landscape and Biopharmaceutical Implications of Novel Metformin-Based Salts. New J. Chem. 2022, 46, 13725–13737. [Google Scholar] [CrossRef]
- Feng, W.Q.; Wang, L.Y.; Gao, J.; Zhao, M.Y.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. Solid State and Solubility Study of a Potential Anticancer Drug-Drug Molecular Salt of Diclofenac and Metformin. J. Mol. Struct. 2021, 1234, 130166. [Google Scholar] [CrossRef]
- Ghasemi, F.; Ghasemi, K.; Rezvani, A.R.; Shokrollahi, A.; Refahi, M.; García-Granda, S.; Mendoza-Meroño, R. A Novel Salt of Antidiabetic Drug Metformin Resulting from a Proton Transfer Reaction: Synthesis, Characterization, Crystal Structure and Solution Studies. J. Mol. Struct. 2017, 1131, 30–35. [Google Scholar] [CrossRef]
- Jia, L.; Wu, S.; Gong, J. A Tolbutamide-Metformin Salt Based on Antidiabetic Drug Combinations: Synthesis, Crystal Structure Analysis and Pharmaceutical Properties. Acta Crystallogr. C. Struct. Chem. 2019, 75, 1250–1258. [Google Scholar] [CrossRef]
- Manjunatha, N.K.; Mahesha; Gayathri, B.H.; Lokanath, N.K.; Swamy, M.T.; Chandra Nayaka, S.; Siddaraju, B.P.; Ragini, N.; Al-Ghorbani, M.; Kannika, B.R.; et al. A Solid State Analysis of Metformin Picrate Salt. Chem. Data Collect. 2020, 30, 100577. [Google Scholar] [CrossRef]
- Nanubolu, J.B.; Sridhar, B.; Ravikumar, K.; Sawant, K.D.; Naik, T.A.; Patkar, L.N.; Cherukuvada, S.; Sreedhar, B. Polymorphism in Metformin Embonate Salt-Recurrence of Dimeric and Tetrameric Guanidinium–Carboxylate Synthons. CrystEngComm 2013, 15, 4448–4464. [Google Scholar] [CrossRef]
- Pérez-Fernández, R.; Fresno, N.; Goya, P.; Elguero, J.; Menéndez-Taboada, L.; García-Granda, S.; Marco, C. Structure and Thermodynamical Properties of Metformin Salicylate. Cryst. Growth Des. 2013, 13, 1780–1785. [Google Scholar] [CrossRef]
- Sun, J.; Jia, L.; Wang, M.; Liu, Y.; Li, M.; Han, D.; Gong, J. Novel Drug–Drug Multicomponent Crystals of Epalrestat–Metformin: Improved Solubility and Photostability of Epalrestat and Reduced Hygroscopicity of Metformin. Cryst. Growth Des. 2022, 22, 1005–1016. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Maršík, P.; Münzbergová, Z. Active Constituents in Rheum Acuminatum and Rheum Australe (Polygonaceae) Roots: A Variation between Cultivated and Naturally Growing Plants. Biochem. Syst. Ecol. 2012, 41, 83–90. [Google Scholar] [CrossRef]
- Jang, S.J.; Kuk, Y.I. Effects of Different Fractions of Rheum Palmatum Root Extract and Anthraquinone Compounds on Fungicidal, Insecticidal, and Herbicidal Activities. J. Plant Dis. Prot. 2018, 125, 451–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Hu, N.; Gu, M.; Chu, C.; Li, Y.; Lu, X.; Huang, C. Rhein Reduces Fat Weight in Db/Db Mouse and Prevents Diet-Induced Obesity in C57Bl/6 Mouse Through the Inhibition of Pparγ Signaling. PPAR Res. 2012, 2012, 374936. [Google Scholar]
- Lin, Y.J.; Zhen, Y.Z.; Wei, J.B.; Wei, J.; Dai, J.; Gao, J.L.; Li, K.J.; Hu, G. Rhein Lysinate Protects Renal Function in Diabetic Nephropathy of KK/Hlj Mice. Exp. Ther. Med. 2017, 14, 5801–5808. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.X.; Wang, Z.F.; Qin, Q.P.; Zou, B.Q.; Liang, H. Complexes of Oxoplatin with Rhein and Ferulic Acid Ligands as Platinum(Iv) Prodrugs with High Anti-Tumor Activity. Dalton Trans. 2020, 49, 1613–1619. [Google Scholar]
- Wang, H.; Yang, D.; Li, L.; Yang, S.; Du, G.; Lu, Y. Anti-Inflammatory Effects and Mechanisms of Rhein, an Anthraquinone Compound, and Its Applications in Treating Arthritis: A Review. Nat. Prod. Bioprospect. 2020, 10, 445–452. [Google Scholar] [CrossRef]
- Cong, X.D.; Ding, M.J.; Dai, D.Z.; Wu, Y.; Zhang, Y.; Dai, Y. ER stress, p66shc, and p-Akt/Akt mediate Adjuvant-Induced Inflammation, which Is Blunted by Argirein, a Supermolecule and Rhein in Rats. Inflammation 2012, 35, 1031–1040. [Google Scholar] [CrossRef]
- Azelmat, J.; Larente, J.F.; Grenier, D. The Anthraquinone Rhein Exhibits Synergistic Antibacterial Activity in Association with Metronidazole or Natural Compounds and Attenuates Virulence Gene Expression in Porphyromonas Gingivalis. Arch. Oral Biol. 2015, 60, 342–346. [Google Scholar] [CrossRef]
- Lea-Banks, H.; Wu, S.K.; Lee, H.; Hynynen, K. Ultrasound-Triggered Oxygen-Loaded Nanodroplets Enhance and Monitor Cerebral Damage from Sonodynamic Therapy. Nanotheranostics 2022, 6, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xu, Z.; Wang, J.; Zheng, S.; Cai, Y. C-Peptide and Islet Transplantation Improve Glomerular Filtration Barrier in Diabetic Nephropathy Rats. Transpl. Immunol. 2020, 62, 101322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Zhang, Y.; Zhang, M.; Zhu, X.; Fan, Y.; Shi, S.; Zen, K.; Liu, Z. Rhein Protects Pancreatic Beta-Cells from Dynamin-Related Protein-1-Mediated Mitochondrial Fission and Cell Apoptosis Under Hyperglycemia. Diabetes 2013, 62, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; Garcia-Perez, J. Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Rao, L.V.; Tan, S.H.; Candasamy, M.; Bhattamisra, S.K. Diabetic Nephropathy: An Update on Pathogenesis and Drug Development. Diabetes Metab. Syndr. 2019, 13, 754–762. [Google Scholar]
- Chen, Y.; Mu, L.; Xing, L.; Li, S.; Fu, S. Rhein Alleviates Renal Interstitial Fibrosis by Inhibiting Tubular Cell Apoptosis in Rats. Biol. Res. 2019, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, Z.; Sun, J.; Luo, J.; Wei, Y.; Zou, J. Deoxycholic Acid-Functionalised Nanoparticles for Oral Delivery of Rhein. Eur. J. Pharm. Sci. 2021, 159, 105713. [Google Scholar] [CrossRef]
- Luo, J.; Sun, J.; Luo, X.; Wei, Y.; Zheng, H.; Mu, C.; Yao, W. Low Molecular Weight Chitosan-Based Conjugates for Efficient Rhein Oral Delivery: Synthesis, Characterization, and Pharmacokinetics. Drug Dev. Ind. Pharm. 2019, 45, 96–104. [Google Scholar] [CrossRef]
- Shan, N.; Zaworotko, M.J. The Role of Cocrystals in Pharmaceutical Science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharmaceutics 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Ravindran, S.; Kuruvilla, V.; Wilbur, K.; Munusamy, S. Nephroprotective Effects of Metformin in Diabetic Nephropathy. J. Cell. Physiol. 2017, 232, 731–742. [Google Scholar] [PubMed]
- Ali, S.; Fonseca, V. Overview of Metformin: Special Focus on Metformin Extended Release. Expert Opin. Pharmacother. 2012, 13, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Liu, Q.; Yang, D.; Chen, T.; Zhang, B.; Xing, C.; Zhang, L.; Lu, Y.; Du, G. Insights into the Solubility and Structural Features of Four Praziquantel Cocrystals. Cryst. Growth Des. 2021, 21, 6321–6331. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of Equilibrium Solubility Measurement by Saturation Shake-Flask Method Using Hydrochlorothiazide as Model Compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Avdeef, A.; Fuguet, E.; Llinàs, A.; Ràfols, C.; Bosch, E.; Völgyi, G.; Verbic, T.; Boldyreva, E.; Takács-Novák, K. Equilibrium Solubility Measurement of Ionizable Drugs-Consensus Recommendations for Improving Data Quality. ADMET DMPK 2016, 4, 117–178. [Google Scholar] [CrossRef]
- Tiwari, M.; Chawla, G.; Bansal, A.K. Quantification of Olanzapine Polymorphs Using Powder X-Ray Diffraction Technique. J. Pharm. Biomed. Anal. 2007, 43, 865–872. [Google Scholar] [PubMed]
- Thorat, S.H.; Sahu, S.K.; Patwadkar, M.V.; Badiger, M.V.; Gonnade, R.G. Drug-Drug Molecular Salt Hydrate of an Anticancer Drug Gefitinib and a Loop Diuretic Drug Furosemide: An Alternative for Multidrug Treatment. J. Pharm. Sci. 2015, 104, 4207–4216. [Google Scholar] [CrossRef]
- Deng, J.H.; Lu, T.B.; Sun, C.C.; Chen, J.M. Dapagliflozin-Citric Acid Cocrystal Showing Better Solid State Properties than Dapagliflozin. Eur. J. Pharm. Sci. 2017, 104, 255–261. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Yan, Y.; Lu, T. Improving the Solubility of 6-Mercaptopurine Via Cocrystals and Salts. Cryst. Growth Des. 2012, 12, 6004–6011. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; Diego, H.L. Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Aakeroy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Dose it Really Matter? Mol. Pharm. 2006, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, D.; Zhang, W.; Song, J.; Gong, N.; Yu, M.; Yang, S.; Zhang, B.; Liu, Q.; Du, G.; et al. An Innovative Rhein-Matrine Cocrystal: Synthesis, Characterization, Formation Mechanism and Pharmacokinetic Study. Chinese Chin. Chem. Lett. 2023, 34, 107258. [Google Scholar] [CrossRef]
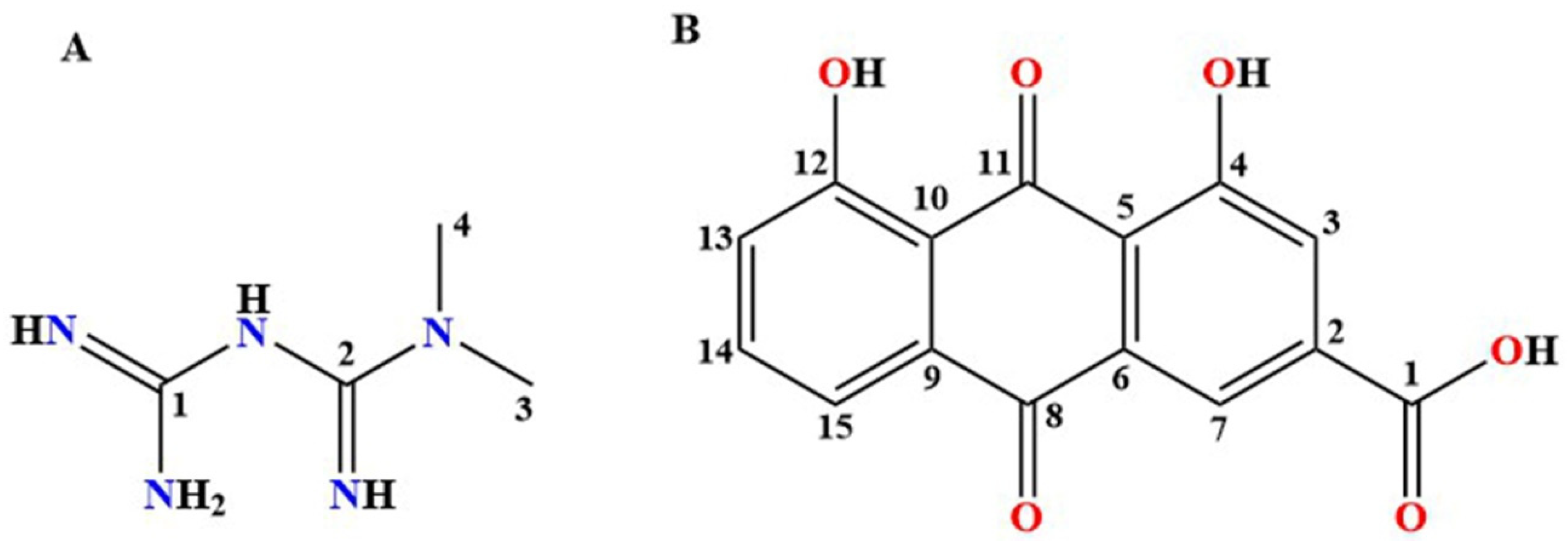
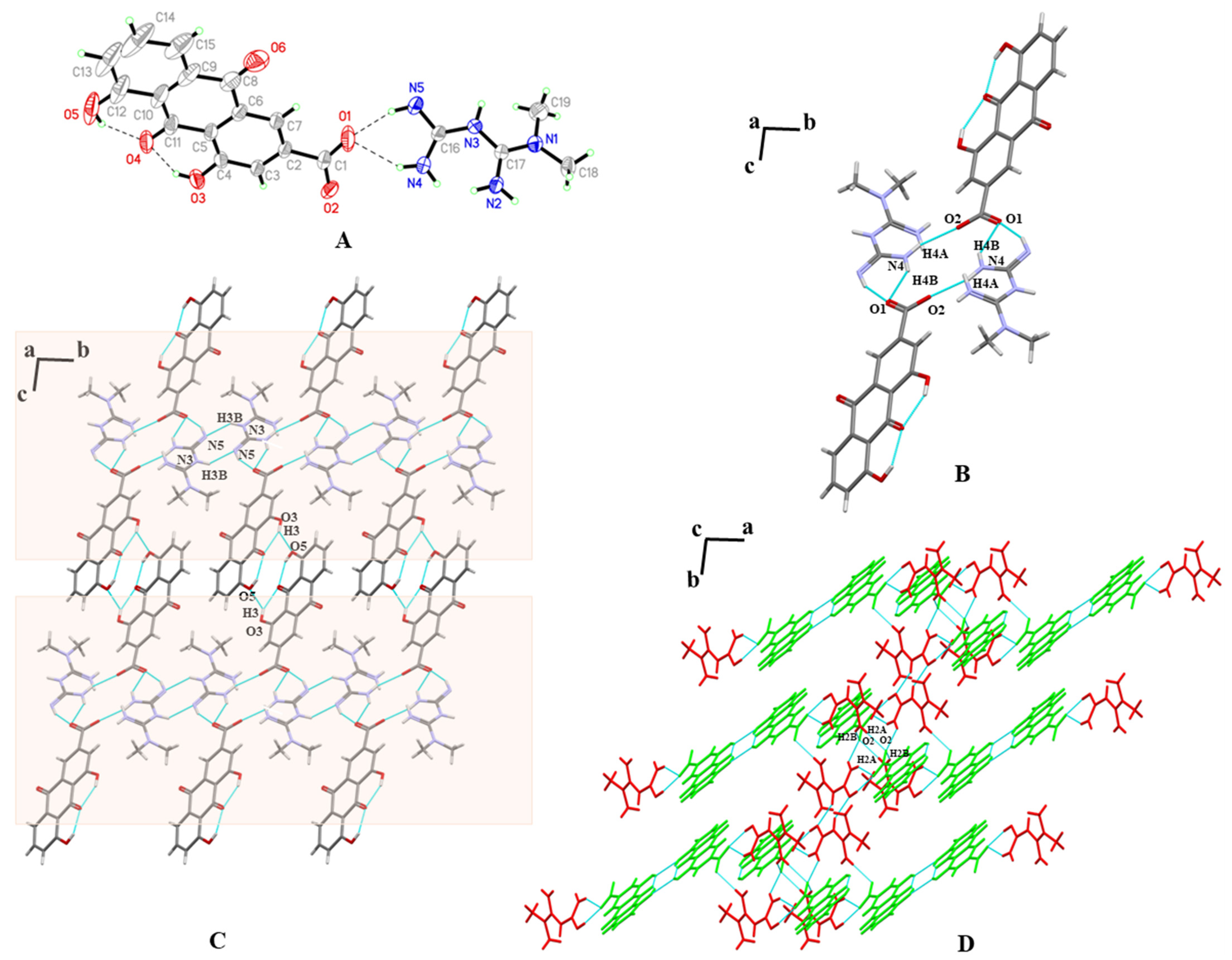
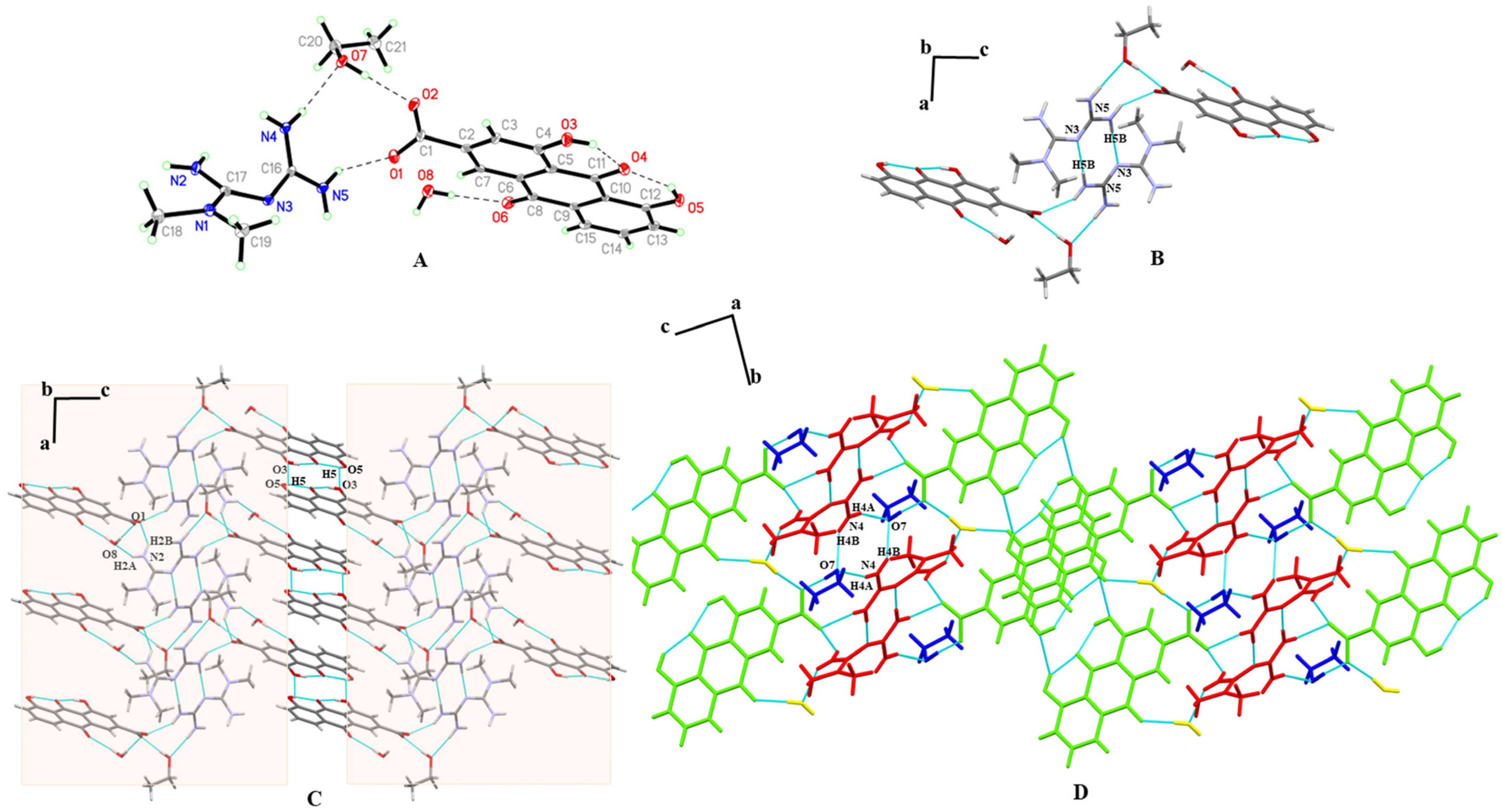
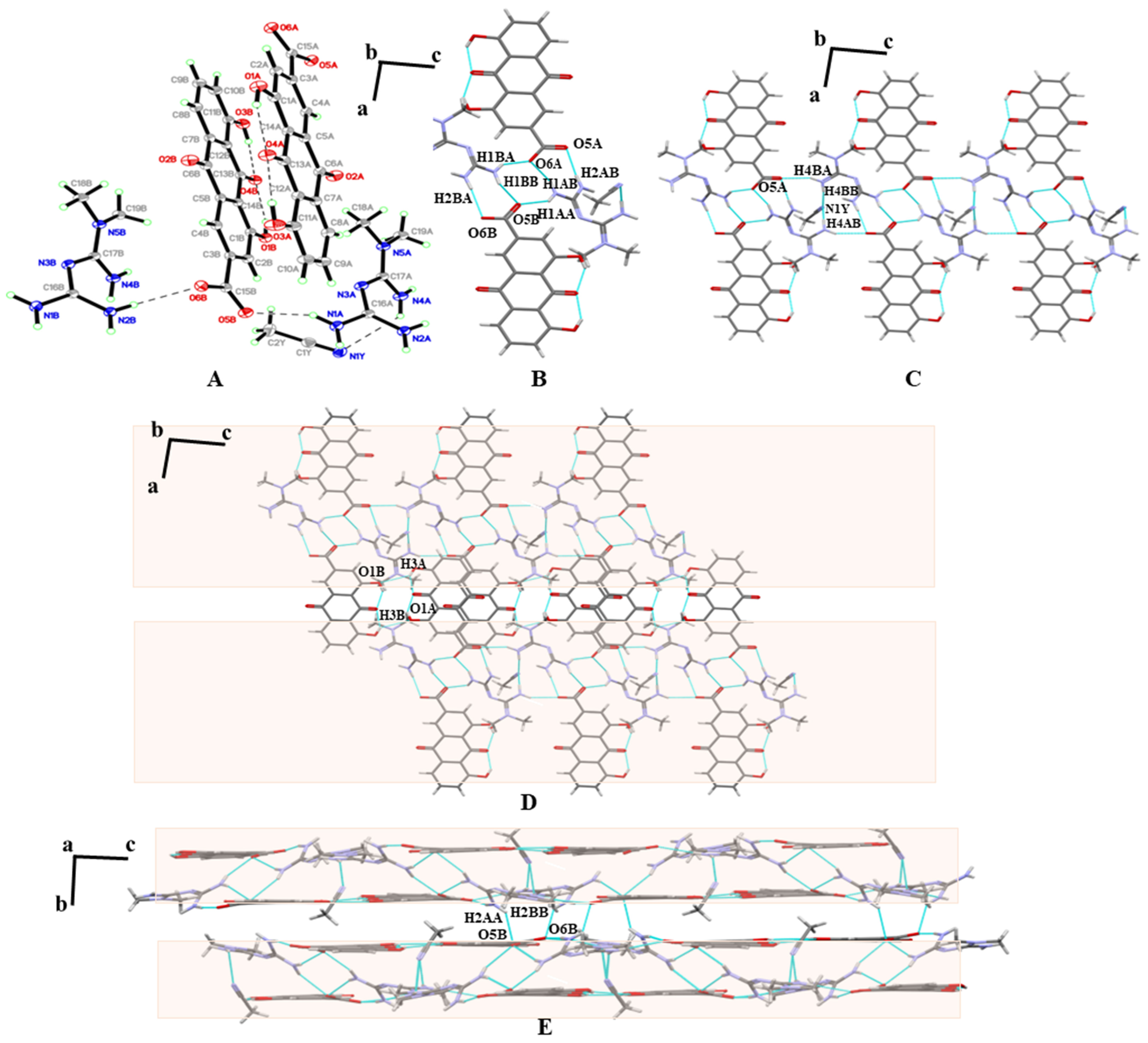
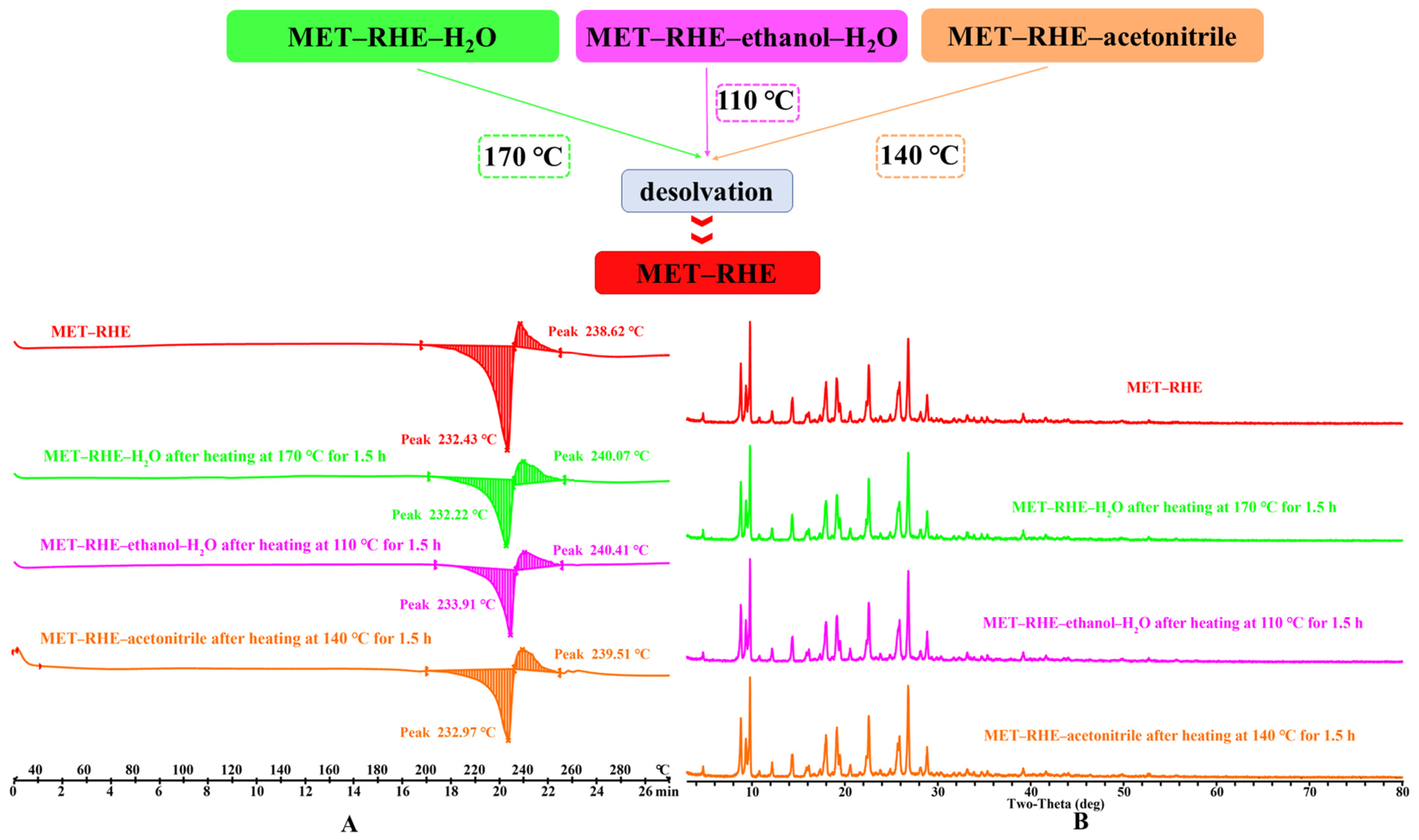


| Parameters | MET–RHE | MET–RHE–Ethanol–H2O | MET–RHE–Acetonitrile |
|---|---|---|---|
| Empirical formula | C19H19N5O6 | C21H27N5O8 | C40H41N11O12 |
| Formula weight | 413.39 | 477.47 | 867.84 |
| Crystal size/mm | 0.15 × 0.22 × 0.27 | 0.35 × 0.30 × 0.13 | 0.25 × 0.23 × 0.21 |
| Description | block | block | block |
| Crystal system | triclinic | triclinic | monoclinic |
| Space group | P-1 | P-1 | P21/c |
| a (Å) | 5.492 (1) | 7.295 (1) | 14.151 (1) |
| b (Å) | 9.856 (1) | 7.619 (1) | 13.658 (1) |
| c (Å) | 17.707 (1) | 19.721 (3) | 21.051 (2) |
| α (°) | 98.989 (3) | 86.327 (8) | 90 |
| β (°) | 90.299 (3) | 87.895 (7) | 98.372 (1) |
| γ (°) | 96.771 (4) | 87.742 (6) | 90 |
| Volume (Å3) | 939.9 (1) | 1092.4 (2) | 4025.6 (2) |
| Z | 2 | 2 | 4 |
| F(000) | 432 | 504 | 1816 |
| Density (g·cm−3) | 1.461 | 1.452 | 1.432 |
| Reflections with I > 2σ (I) | 2759 | 3648 | 5645 |
| Rindexs (I > 2σI) | R1 = 0.0926 wR2 = 0.2759 | R1 = 0.0468 wR2 = 0.1091 | R1 = 0.0592 wR2 = 0.1673 |
| S | 1.045 | 0.985 | 1.037 |
| Interaction Style | Interaction Energy (kcal/mol) |
|---|---|
| MET–RHE 1 | −76.54 |
| MET–RHE 2 | −83.27 |
| MET–RHE 3 | −90.19 |
| MET–RHE–ethanol–H2O 1 | −79.34 |
| MET–RHE–ethanol–H2O 2 | −79.68 |
| MET–RHE–acetonitrile 1 | −96.79 |
| MET–RHE–acetonitrile 2 | −77.85 |
| MET–RHE–acetonitrile 3 | −73.52 |
| Salt | MPP | SDP | Interaction Energy (kcal/mol) | H-Bond Distance (Å) |
|---|---|---|---|---|
| MET–RHE | 0.1642 | 1.1686 | −4.68 | 2.96 |
| MET–RHE–ethanol–H2O | 0.1938 | 1.1437 | −4.43 | 2.88 |
| MET–RHE–acetonitrile | 0.1250 | 0.6812 | −5.23 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Liang, M.; An, Q.; Wang, W.; Zhang, B.; Yang, S.; Zhou, J.; Yang, X.; Yang, D.; Zhang, L.; et al. Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design. Pharmaceutics 2023, 15, 1196. https://doi.org/10.3390/pharmaceutics15041196
Yu M, Liang M, An Q, Wang W, Zhang B, Yang S, Zhou J, Yang X, Yang D, Zhang L, et al. Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design. Pharmaceutics. 2023; 15(4):1196. https://doi.org/10.3390/pharmaceutics15041196
Chicago/Turabian StyleYu, Mingchao, Meidai Liang, Qi An, Wenwen Wang, Baoxi Zhang, Shiying Yang, Jian Zhou, Xiuying Yang, Dezhi Yang, Li Zhang, and et al. 2023. "Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design" Pharmaceutics 15, no. 4: 1196. https://doi.org/10.3390/pharmaceutics15041196
APA StyleYu, M., Liang, M., An, Q., Wang, W., Zhang, B., Yang, S., Zhou, J., Yang, X., Yang, D., Zhang, L., Du, G., & Lu, Y. (2023). Versatile Solid Modifications of Multicomponent Pharmaceutical Salts: Novel Metformin–Rhein Salts Based on Advantage Complementary Strategy Design. Pharmaceutics, 15(4), 1196. https://doi.org/10.3390/pharmaceutics15041196