A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives
Abstract
1. Introduction
2. Treatments of Dry Eye Disease
2.1. Weakness of Existing Treatments
2.2. Contact Lenses as an Alternative DED Treatment
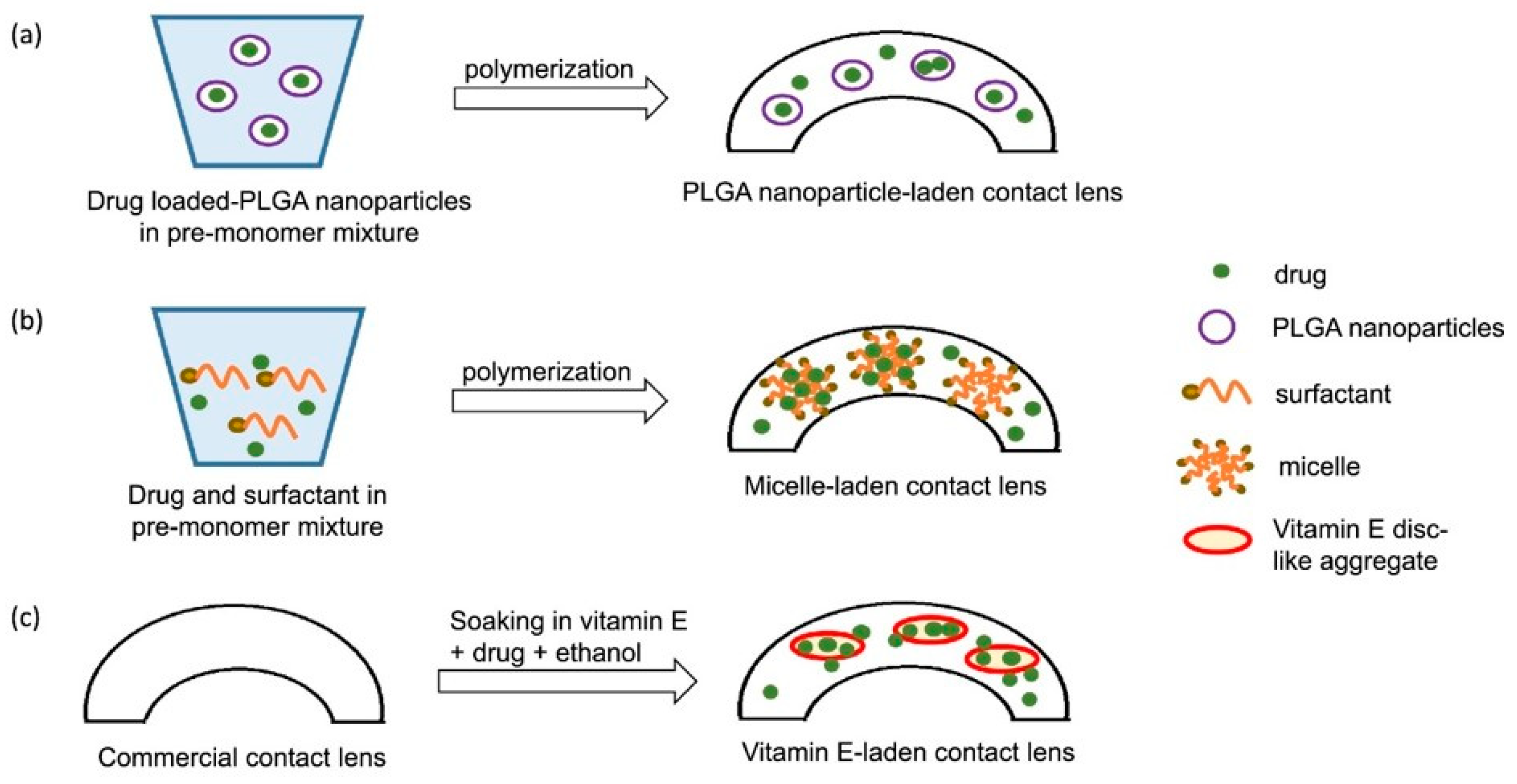
| Drug Molecules | Contact Lens Type | Drug Loading Method | Duration | References |
|---|---|---|---|---|
| Cyclosporine A | Hydrogel and silicone hydrogel | Soaking | 1 day (hydrogel) 15 days (silicone hydrogel). Pre-soaking with vit. E increases time release to 30 days | [73] |
| Hyaluronic acid | Hydrogel and silicone hydrogel | Soaking | 1 h | [74] |
| Phospholipids | Silicone hydrogel | Soaking | 10 h | [75] |
| Dexamethasone | Silicone hydrogel | Soaking | 2 weeks–3 months | [76] |
| Dexamethasone | Silicone hydrogel | Soaking | 7 days | [77,78] |
| Ap4A (Secretagoge) | Hydrogel and silicone hydrogel | Soaking | 5–6 h | [41,79] |
| Betaine (Osmoprotectant) | Silicone hydrogel | Soaking | 10 h | [41,80] |
| Polyvinilpyrrolidone | Hydrogel | Polymerization | 30 days | [81] |
| Hyaluronic acid | Hydrogel and silicone hydrogel | Polymerization | 21 days (hydrogel), 49 days (silicone hydrogel) | [82] |
| Polyvinilpyrrolidone | Hydrogel | Polymerization | 30 days | [81] |
| Diclofenac | Hydrogel | Copolymerization | 7 days | [83] |
| Dexamethasone | Hydrogel | Copolymerization | 50 h | [84] |
| Diclofenac | Hydrogel | Copolymerization | 14 days | [85] |
| Cyclosporine A | Hydrogel | Nanoparticles (Brij surfactants) | 20–30 days | [86,87,88] |
| Dexamethasone | Hydrogel | Nanoparticles/soaking | 50 h | [89] |
| Hydroxypropyl methylcellulose | Silicone hydrogel | Molecular imprinting | 60 days | [90] |
| Hyaluronic acid | Hydrogel and silicone hydrogel | Molecular imprinting | 24 h | [91] |
| Diclofenac | Hydrogel | Molecular imprinting | 6 days | [79] |
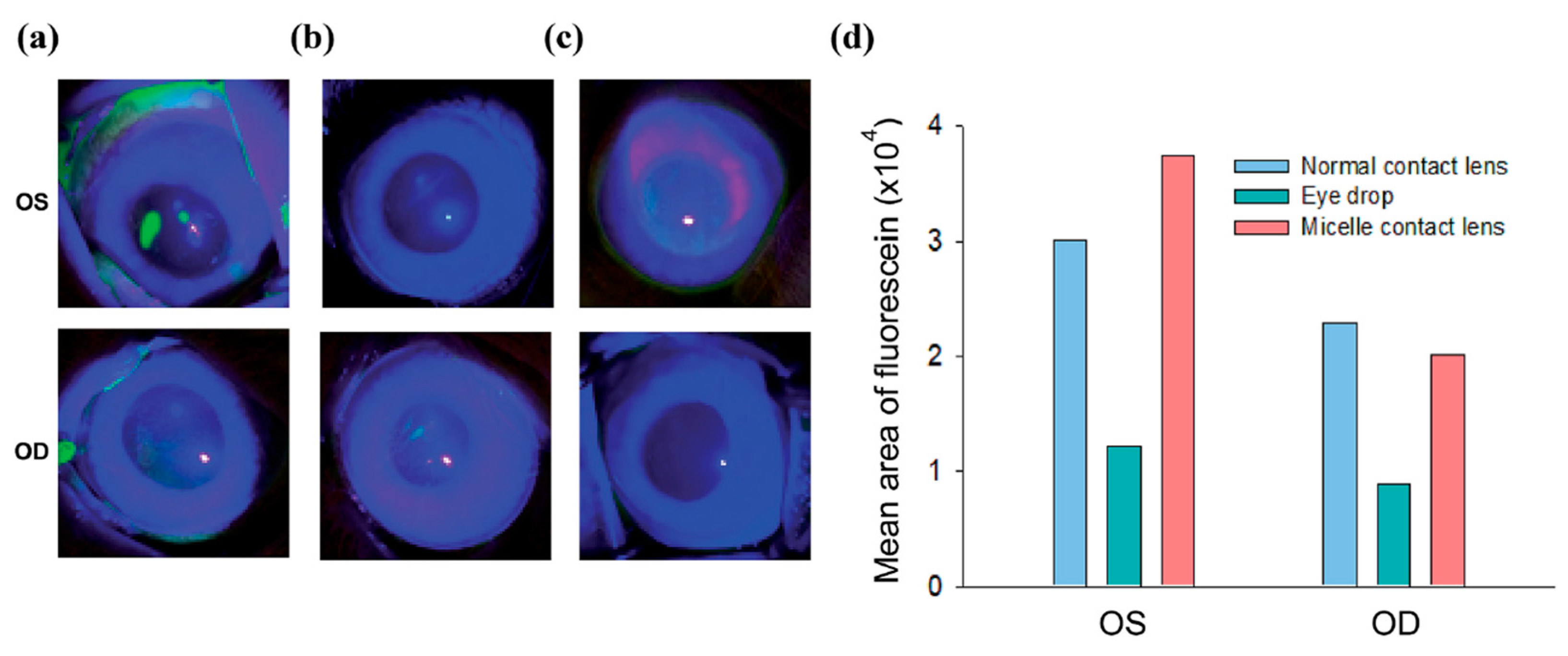
2.3. Contact Lens-Based Drug Delivery for DED
2.3.1. Advantages of CsA
2.3.2. In Vitro CsA Release from Contact Lenses
2.3.3. In Vivo Biological Activity of CsA Contact Lenses
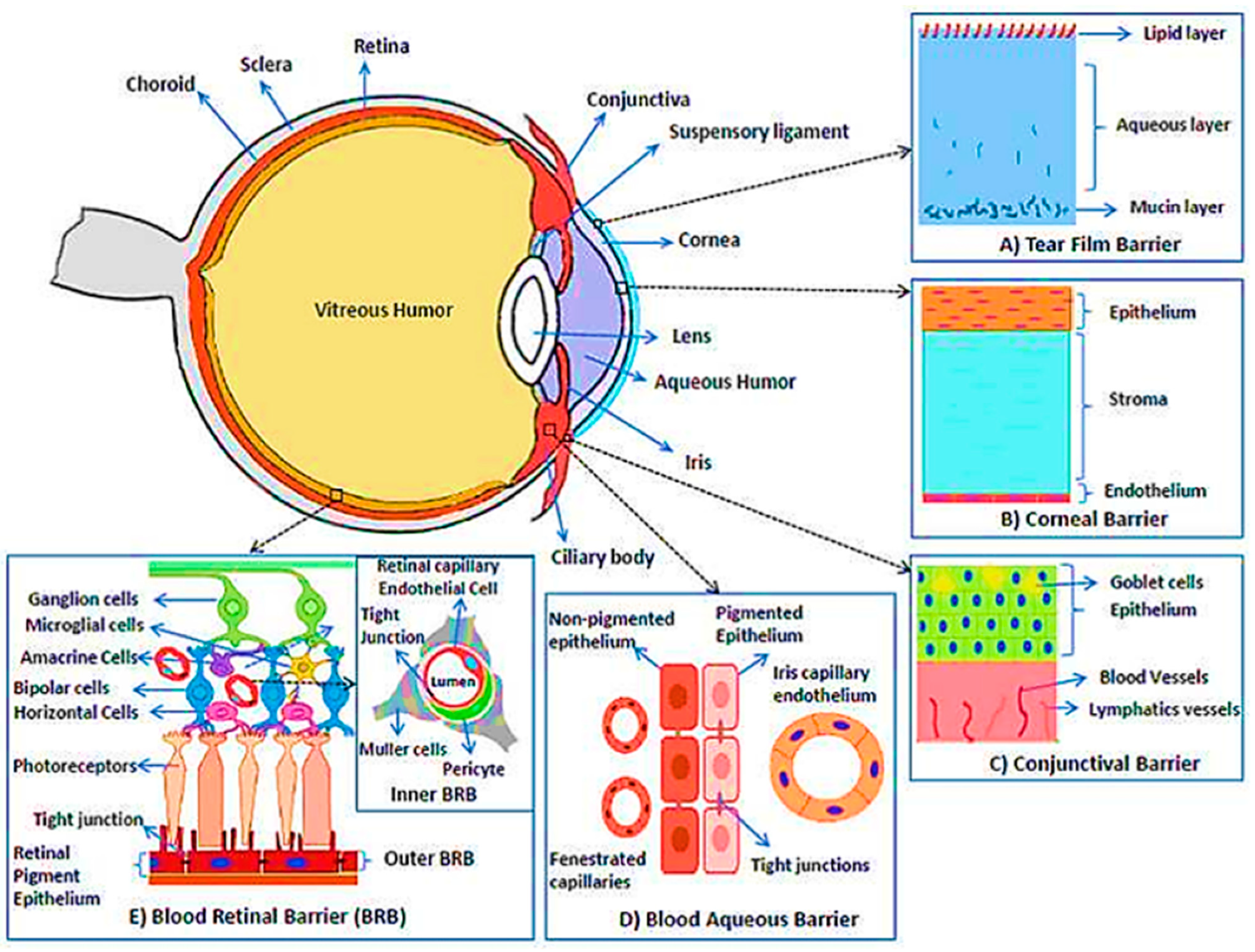
3. Challenges of Contact Lenses in Drug Delivery
4. Biosensors Integrated Contact Lens
4.1. Tear Film Biomarkers for DED
4.2. Contact Lens Sensors for Sensing of Tear Fluid Biomarkers in DED
5. The Future Perspective of Biosensor Fused Contact Lens
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phadatare, S.P.; Momin, M.; Nighojkar, P.; Askarkar, S.; Singh, K.K. A Comprehensive Review on Dry Eye Disease: Diagnosis, Medical Management, Recent Developments, and Future Challenges. Adv. Pharm. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; et al. The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar] [CrossRef]
- Marshall, L.L.; Roach, J.M. Treatment of Dry Eye Disease. Consult. Pharm. 2016, 31, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Shilpi, J.A.; Mondal, H.; Hossain, F.; Anisuzzman, M.; Hasan, M.M.; Cordell, G.A. Ethnomedicinal, Phytochemical, and Pharmacological Profile of the Genus Dalbergia L.(Fabaceae). Phytopharmacology 2013, 4, 291–346. [Google Scholar]
- Messmer, E.M. Pathophysiology, Diagnosis and Treatment of Dry Eye. Dtsch. Arzteblatt Int. 2015, 112, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Farrand, K.F.; Fridman, M.; Stillman, I.Ö.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L.; Syed, B.A. Pharmacoeconomics of Anterior Ocular Inflammatory Disease. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 537–542. [Google Scholar] [CrossRef]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jha, G. Drug Delivery through Soft Contact Lenses: An Introduction. Chron. Young Sci. 2011, 2, 3. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for Drug Delivery to the Anterior Segment of the Eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Shaikh, A.A.; Lakdawala, D.H.; Desai, A.R.; Pandya, M.M.; Singhania, S.S.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Design and Optimization of a Novel Implantation Technology in Contact Lenses for the Treatment of Dry Eye Syndrome: In Vitro and in Vivo Evaluation. Acta Biomater. 2017, 53, 211–221. [Google Scholar] [CrossRef]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained Drug Release by Contact Lenses for Glaucoma Treatment-a Review. J. Control. Release Off. J. Control. Release Soc. 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in Ocular Drug Delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma Therapy by Extended Release of Timolol from Nanoparticle Loaded Silicone-Hydrogel Contact Lenses. J. Control. Release Off. J. Control. Release Soc. 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended Drug Delivery by Contact Lenses for Glaucoma Therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jung Jung, H.; Chauhan, A. Ophthalmic Drug Delivery by Contact Lenses. Expert Rev. Ophthalmol. 2012, 7, 199–201. [Google Scholar] [CrossRef]
- Hsu, K.H.; Gause, S.; Chauhan, A. Review of Ophthalmic Drug Delivery by Contact Lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R.; et al. Smart Contact Lenses for Biosensing Applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Chen, G.Z.; Chan, I.S.; Leung, L.K.K.; Lam, D.C.C. Soft Wearable Contact Lens Sensor for Continuous Intraocular Pressure Monitoring. Med. Eng. Phys. 2014, 36, 1134–1139. [Google Scholar] [CrossRef]
- Huang, J.F.; Zhong, J.; Chen, G.P.; Lin, Z.T.; Deng, Y.; Liu, Y.L.; Cao, P.Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef]
- Mohanto, N.; Khatun, A.; Begum, J.A.; Parvin, M.M.; Siddiqui, M.S.I.; Begum, S.; Parvin, R.; Islam, M.R.; Chowdhury, E.H. Trehalose Improves PPR Vaccine Virus Stability in Diluent. Bangladesh J. Vet. Med. BJVM 2019, 17, 117–123. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Gu, Z.; Xiu, G.; Xiao, X. Electrochemical Sensing in Contact Lenses. Electroanalysis 2021, 34, 227–236. [Google Scholar] [CrossRef]
- Moreddu, R.; Elsherif, M.; Adams, H.; Moschou, D.; Cordeiro, M.F.; Wolffsohn, J.S.; Vigolo, D.; Butt, H.; Cooper, J.M.; Yetisen, A.K. Integration of Paper Microfluidic Sensors into Contact Lenses for Tear Fluid Analysis. Lab. Chip 2020, 20, 3970–3979. [Google Scholar] [CrossRef]
- Moreddu, R.; Wolffsohn, J.S.; Vigolo, D.; Yetisen, A.K. Laser-Inscribed Contact Lens Sensors for the Detection of Analytes in the Tear Fluid. Sens. Actuators B Chem. 2020, 317, 128183. [Google Scholar] [CrossRef]
- Ray, T.R.; Ivanovic, M.; Curtis, P.M.; Franklin, D.; Guventurk, K.; Jeang, W.J.; Chafetz, J.; Gaertner, H.; Young, G.; Rebollo, S.; et al. Soft, Skin-Interfaced Sweat Stickers for Cystic Fibrosis Diagnosis and Management. Sci. Transl. Med. 2021, 13, eabd8109. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Geerling, G.; Kinoshita, S.; Lemp, M.A.; McCulley, J.; Nelson, D.; Novack, G.N.; Shimazaki, J.; Wilson, C. Management and Therapy of Dry Eye Disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 163–178. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Tong, L. Lipid-Containing Lubricants for Dry Eye. Optom. Vis. Sci. 2012, 89, 1654–1661. [Google Scholar] [CrossRef]
- Messmer, E.M. Konservierungsmittel in Der Ophthalmologie. Ophthalmologe 2012, 109, 1064–1070. [Google Scholar] [CrossRef]
- Raj, G.B.; Surendra, P.; Kim, D. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Kim, H.; Yoo, J.-W. Development of Clindamycin-Loaded Alginate/Pectin/Hyaluronic Acid Composite Hydrogel Film for the Treatment of MRSA-Infected Wounds. J. Pharm. Investig. 2021, 51, 597–610. [Google Scholar] [CrossRef]
- Kim, M.-H.; Nguyen, D.-T.; Kim, D.-D. Recent Studies on Modulating Hyaluronic Acid-Based Hydrogels for Controlled Drug Delivery. J. Pharm. Investig. 2022, 52, 397–413. [Google Scholar] [CrossRef]
- Marsh, P.; Pflugfelder, S.C. Topical Nonpreserved Methylprednisolone Therapy for Keratoconjunctivitis Sicca in Sjogren Syndrome. Ophthalmology 1999, 106, 811–816. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Maskin, S.L.; Anderson, B.; Chodosh, J.; Holland, E.J.; De Paiva, C.S.; Bartels, S.P.; Micuda, T.; Proskin, H.M.; Vogel, R. A Randomized, Double-Masked, Placebo-Controlled, Multicenter Comparison of Loteprednol Etabonate Ophthalmic Suspension, 0.5%, and Placebo for Treatment of Keratoconjunctivitis Sicca in Patients with Delayed Tear Clearance. Am. J. Ophthalmol. 2004, 138, 444–457. [Google Scholar] [CrossRef]
- Dursun, D.; Ertan, A.; Bilezikçi, B.; Akova, Y.A.; Pelit, A. Ocular Surface Changes in Keratoconjunctivitis Sicca with Silicone Punctum Plug Occlusion. Curr. Eye Res. 2003, 26, 263–269. [Google Scholar] [CrossRef]
- Matsuda, S.; Koyasu, S. Mechanisms of Action of Cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Moscovici, B.K.; Holzchuh, R.; Chiacchio, B.B.; Santo, R.M.; Shimazaki, J.; Hida, R.Y. Clinical Treatment of Dry Eye Using 0.03% Tacrolimus Eye Drops. Cornea 2012, 31, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Marco, E.; Udaondo, P.; García-Delpech, S.; Vazquez, A.; Diaz-Llopis, M. Treatment of Refractory Dry Eye Associated with Graft versus Host Disease with 0.03% Tacrolimus Eyedrops. J. Ocul. Pharmacol. Ther. 2013, 29, 776–783. [Google Scholar] [CrossRef]
- Auw-Hädrich, C.; Reinhard, T. Treatment of Chronic Blepharokeratoconjunctivitis with Local Calcineurin Inhibitors. Ophthalmologe 2009, 106, 635–638. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Fonseca, B.; Carracedo, G.; Martin-Gil, A.; Martinez-Aguila, A.; Pintor, J. Dry Eye Treatment Based on Contact Lens Drug Delivery: A Review. Eye Contact Lens 2016, 42, 280–288. [Google Scholar] [CrossRef]
- Yoo, S.E.; Lee, D.C.; Chang, M.H. The Effect of Low-Dose Doxycycline Therapy in Chronic Meibomian Gland Dysfunction. Korean J. Ophthalmol. KJO 2005, 19, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Rosenblatt, M.; Li, D.Q.; Liu, Z.; Monroy, D.; Ji, Z.; Lokeshwar, B.L.; Pflugfelder, S.C. Doxycycline Inhibition of Interleukin-1 in the Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Sadrai, Z.; Hajrasouliha, A.R.; Chauhan, S.; Saban, D.R.; Dastjerdi, M.H.; Dana, R. Effect of Topical Azithromycin on Corneal Innate Immune Responses. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2525–2531. [Google Scholar] [CrossRef]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kim, S.H.; McKay, J.W. Topical Azithromycin Therapy for Meibomian Gland Dysfunction: Clinical Response and Lipid Alterations. Cornea 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Haque, R.M.; Torkildsen, G.L.; Brubaker, K.; Zink, R.C.; Kowalski, R.P.; Mah, F.S.; Pflugfelder, S.C. Multicenter Open-Label Study Evaluating the Efficacy of Azithromycin Ophthalmic Solution 1% on the Signs and Symptoms of Subjects with Blepharitis. Cornea 2010, 29, 871–877. [Google Scholar] [CrossRef]
- Barabino, S.; Rolando, M.; Camicione, P.; Ravera, G.; Zanardi, S.; Giuffrida, S.; Calabria, G. Systemic Linoleic and γ-Linolenic Acid Therapy in Dry Eye Syndrome with an Inflammatory Component. Cornea 2003, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Guillon, M.; Maissa, C.; Wong, S. Eyelid Margin Modification Associated with Eyelid Hygiene in Anterior Blepharitis and Meibomian Gland Dysfunction. Eye Contact Lens 2012, 38, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Dogru, M.; Goto, E.; Ishida, R.; Kojima, T.; Onguchi, T.; Yagi, Y.; Shimazaki, J.; Tsubota, K. Efficacy of a New Warm Moist Air Device on Tear Functions of Patients with Simple Meibomian Gland Dysfunction. Cornea 2006, 25, 644–650. [Google Scholar] [CrossRef]
- Olson, M.C.; Korb, D.R.; Greiner, J.V. Increase in Tear Film Lipid Layer Thickness Following Treatment with Warm Compresses in Patients with Meibomian Gland Dysfunction. Eye Contact Lens 2003, 29, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Purslow, C. Evaluation of the Ocular Tolerance of a Novel Eyelid-Warming Device Used for Meibomian Gland Dysfunction. Contact Lens Anterior Eye 2013, 36, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.J. Punctal Occlusion. Arch. Ophthalmol. 1999, 117, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.C.; Cosar, C.B.; Cohen, E.J.; Rapuano, C.J.; Laibson, P.R. The Clinical Efficacy of Silicone Punctal Plug Therapy. Cornea 2002, 21, 135–139. [Google Scholar] [CrossRef]
- Commissioner, O. of the FDA Approves New Medication for Dry Eye Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-dry-eye-disease (accessed on 13 December 2022).
- Donnenfeld, E.D.; Perry, H.D.; Nattis, A.S.; Rosenberg, E.D. Lifitegrast for the Treatment of Dry Eye Disease in Adults. Expert Opin. Pharmacother. 2017, 18, 1517–1524. [Google Scholar] [CrossRef]
- Bron, A.J.; Mengher, L.S. The Ocular Surface in Keratoconjunctivitis Sicca. Eye Basingstoke 1989, 3, 428–437. [Google Scholar] [CrossRef]
- McCusker, M.M.; Durrani, K.; Payette, M.J.; Suchecki, J. An Eye on Nutrition: The Role of Vitamins, Essential Fatty Acids, and Antioxidants in Age-Related Macular Degeneration, Dry Eye Syndrome, and Cataract. Clin. Dermatol. 2016, 34, 276–285. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Z.; Li, X.; Jia, H.; Sun, L.; Tian, C.; Jia, L.; Liu, J. α-Tocopherol Is an Effective Phase II Enzyme Inducer: Protective Effects on Acrolein-Induced Oxidative Stress and Mitochondrial Dysfunction in Human Retinal Pigment Epithelial Cells. J. Nutr. Biochem. 2010, 21, 1222–1231. [Google Scholar] [CrossRef]
- Nagai, N.; Otake, H. Novel Drug Delivery Systems for the Management of Dry Eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising Therapeutic Drug Delivery Systems for Glaucoma: A Comprehensive Review. Ther. Adv. Ophthalmol. 2020, 12, 251584142090574. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A.; Eladawy, S.A.; El-Enin, A.S.M.A.; Hussein, Z.M. Development and Investigation of Timolol Maleate Niosomal Formulations for the Treatment of Glaucoma. J. Pharm. Investig. 2020, 50, 59–70. [Google Scholar] [CrossRef]
- Davis, S.A.; Sleath, B.; Carpenter, D.M.; Blalock, S.J.; Muir, K.W.; Budenz, D.L. Drop Instillation and Glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Patil, B.; Shah, B.M.; Bali, S.J.; Mishra, S.K.; Dada, T. Evaluating Eye Drop Instillation Technique in Glaucoma Patients. J. Glaucoma 2012, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Fonn, D.; Bruce, A.S. A Review of the Holden-Mertz Criteria for Critical Oxygen Transmission. Eye Contact Lens 2005, 31, 247–251. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.T.; Zadnik, K. Twenty-Five Years of Contact Lenses: The Impact on the Cornea and Ophthalmic Practice. Cornea 2000, 19, 730–740. [Google Scholar] [CrossRef]
- Brennan, N.A.; Coles, M.L.C.; Comstock, T.L.; Levy, B. A 1-Year Prospective Clinical Trial of Balafilcon A (PureVision) Silicone-Hydrogel Contact Lenses Used on a 30-Day Continuous Wear Schedule. Ophthalmology 2002, 109, 1172–1177. [Google Scholar] [CrossRef]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone Hydrogel Contact Lenses and the Ocular Surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef]
- Dixon, P.; Ghosh, T.; Mondal, K.; Konar, A.; Chauhan, A.; Hazra, S. Controlled Delivery of Pirfenidone through Vitamin E-Loaded Contact Lens Ameliorates Corneal Inflammation. Drug Deliv. Transl. Res. 2018, 8, 1114–1126. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Bengani, L.; Chauhan, A. Are Contact Lenses the Solution for Effective Ophthalmic Drug Delivery? Future Med. Chem. 2012, 4, 2141–2143. [Google Scholar] [CrossRef]
- Li, C.C.; Chauhan, A. Modeling Ophthalmic Drug Delivery by Soaked Contact Lenses. Ind. Eng. Chem. Res. 2006, 45, 3718–3734. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-Based Ocular Drug Delivery Systems for Hydrophobic Drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chauhan, A. Extended Cyclosporine Delivery by Silicone-Hydrogel Contact Lenses. J. Control. Release 2011, 154, 267–274. [Google Scholar] [CrossRef]
- Scheuer, C.A.; Fridman, K.M.; Barniak, V.L.; Burke, S.E.; Venkatesh, S. Retention of Conditioning Agent Hyaluronan on Hydrogel Contact Lenses. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2010, 33 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Pitt, W.G.; Jack, D.R.; Zhao, Y.; Nelson, J.L.; Pruitt, J.D. Loading and Release of a Phospholipid from Contact Lenses. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2011, 88, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Conway, A.; Chauhan, A. Extended Delivery of Ophthalmic Drugs by Silicone Hydrogel Contact Lenses. Biomaterials 2008, 29, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Kim, J.; Chauhan, A. Extended Delivery of Hydrophilic Drugs from Silicone-Hydrogel Contact Lenses Containing Vitamin E Diffusion Barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Peng, C.C.; Chauhan, A. Extended Release of Dexamethasone from Silicone-Hydrogel Contact Lenses Containing Vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef]
- Dominguez-Godinez, C.O.; Martin-Gil, A.; Carracedo, G.; Guzman-Aranguez, A.; González-Méijome, J.M.; Pintor, J. In Vitro and in Vivo Delivery of the Secretagogue Diadenosine Tetraphosphate from Conventional and Silicone Hydrogel Soft Contact Lenses. J. Optom. 2013, 6, 205–211. [Google Scholar] [CrossRef]
- Hsu, K.-H.; de la Jara, P.L.; Ariyavidana, A.; Watling, J.; Holden, B.; Garrett, Q.; Chauhan, A. Release of Betaine and Dexpanthenol from Vitamin E Modified Silicone-Hydrogel Contact Lenses. Curr. Eye Res. 2015, 40, 267–273. [Google Scholar] [CrossRef]
- Yañez, F.; Concheiro, A.; Alvarez-Lorenzo, C. Macromolecule Release and Smoothness of Semi-Interpenetrating PVP-PHEMA Networks for Comfortable Soft Contact Lenses. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2008, 69, 1094–1103. [Google Scholar] [CrossRef]
- Weeks, A.; Subbaraman, L.N.; Jones, L.; Sheardown, H. Physical Entrapment of Hyaluronic Acid during Synthesis Results in Extended Release from Model Hydrogel and Silicone Hydrogel Contact Lens Materials. Eye Contact Lens 2013, 39, 179–185. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.-F.R.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.-J.; Concheiro, A. Soft Contact Lenses Functionalized with Pendant Cyclodextrins for Controlled Drug Delivery. Biomaterials 2009, 30, 1348–1355. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended Delivery of an Anionic Drug by Contact Lens Loaded with a Cationic Surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Tieppo, A.; Pate, K.M.; Byrne, M.E. In Vitro Controlled Release of an Anti-Inflammatory from Daily Disposable Therapeutic Contact Lenses under Physiological Ocular Tear Flow. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2012, 81, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-Laden Soft Contact Lenses for Extended Delivery of Ophthalmic Drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Chauhan, A. Drug and Surfactant Transport in Cyclosporine A and Brij 98 Laden P-HEMA Hydrogels. J. Colloid Interface Sci. 2008, 322, 624–633. [Google Scholar] [CrossRef]
- Kapoor, Y.; Chauhan, A. Ophthalmic Delivery of Cyclosporine A from Brij-97 Microemulsion and Surfactant-Laden p-HEMA Hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the Loading and Release of NSAIDs from PHEMA Hydrogels by Copolymerization with Functionalized Monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar] [CrossRef]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended Release of High Molecular Weight Hydroxypropyl Methylcellulose from Molecularly Imprinted, Extended Wear Silicone Hydrogel Contact Lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Byrne, M.E. Controlled Release of High Molecular Weight Hyaluronic Acid from Molecularly Imprinted Hydrogel Contact Lenses. Pharm. Res. 2009, 26, 714–726. [Google Scholar] [CrossRef]
- Kymionis, G. Treatment of Chronic Dry Eye: Focus on Cyclosporine. Clin. Ophthalmol. 2008, 2, 829. [Google Scholar] [CrossRef] [PubMed]
- Bushley, K.E.; Raja, R.; Jaiswal, P.; Cumbie, J.S.; Nonogaki, M.; Boyd, A.E.; Owensby, C.A.; Knaus, B.J.; Elser, J.; Miller, D.; et al. The Genome of Tolypocladium Inflatum: Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster. PLoS Genet. 2013, 9, e1003496. [Google Scholar] [CrossRef]
- Borel, J.F.; Feurer, C.; Magnée, C.; Stähelin, H. Effects of the New Anti-Lymphocytic Peptide Cyclosporin A in Animals. Immunology 1977, 32, 1017–1025. [Google Scholar] [PubMed]
- Mondal, H.; Saha, S.; Awang, K.; Hossain, H.; Ablat, A.; Islam, K.; Jahan, I.A.; Sadhu, S.; Hossain, G.; Shilpi, J.; et al. Central-Stimulating and Analgesic Activity of the Ethanolic Extract of Alternanthera Sessilis in Mice. BMC Complement. Altern. Med. 2014, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A Eyedrops with Self-Nanoemulsifying Drug Delivery Systems Have Improved Physicochemical Properties and Efficacy against Dry Eye Disease in a Murine Dry Eye Model. PLoS ONE 2019, 14, e0224805. [Google Scholar] [CrossRef]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A Review of the Mechanism of Action of Cyclosporine a: The Role of Cyclosporine a in Dry Eye Disease and Recent Formulation Developments. Clin. Ophthalmol. 2020, 14, 4187–4200. [Google Scholar] [CrossRef]
- Gao, J.; Sana, R.; Calder, V.; Calonge, M.; Lee, W.; Wheeler, L.A.; Stern, M.E. Mitochondrial Permeability Transition Pore in Inflammatory Apoptosis of Human Conjunctival Epithelial Cells and T Cells: Effect of Cyclosporin A. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4717–4733. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; De Paiva, C.S.; Moore, Q.L.; Volpe, E.A.; Li, D.-Q.; Gumus, K.; Zaheer, M.L.; Corrales, R.M. Aqueous Tear Deficiency Increases Conjunctival Interferon-γ (IFN-γ) Expression and Goblet Cell Loss. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Djalilian, A. Topical Calcineurin Inhibitors: Expanding Indications for Corneal and Ocular Surface Inflammation. J. Ophthalmic Vis. Res. 2019, 14, 398. [Google Scholar] [CrossRef]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Mun, J.; Mok, J.W.; Jeong, S.; Cho, S.; Joo, C.K.; Hahn, S.K. Drug-Eluting Contact Lens Containing Cyclosporine-Loaded Cholesterol-Hyaluronate Micelles for Dry Eye Syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef]
- Desai, D.T.; Maulvi, F.A.; Desai, A.R.; Shukla, M.R.; Desai, B.V.; Khadela, A.D.; Shetty, K.H.; Shah, D.O.; Willcox, M.D.P. In Vitro and in Vivo Evaluation of Cyclosporine-Graphene Oxide Laden Hydrogel Contact Lenses. Int. J. Pharm. 2022, 613, 121414. [Google Scholar] [CrossRef]
- Bowman, F.W. The Sterility Testing of Pharmaceuticals. J. Pharm. Sci. 1969, 58, 1301–1308. [Google Scholar] [CrossRef]
- Yu, F.; Liu, X.; Zhong, Y.; Guo, X.; Li, M.; Mao, Z.; Xiao, H.; Yang, S. Sodium Hyaluronate Decreases Ocular Surface Toxicity Induced by Benzalkonium Chloride-Preserved Latanoprost: An in Vivo Study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3385–3393. [Google Scholar] [CrossRef]
- Elshaer, A.; Ghatora, B.; Mustafa, S.; Alany, R.G. Contact Lenses as Drug Reservoirs & Delivery Systems: The Successes & Challenges. Ther. Deliv. 2014, 5, 1085–1100. [Google Scholar] [CrossRef]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple Drug Delivery from the Drug-Implants-Laden Silicone Contact Lens: Addressing the Issue of Burst Drug Release. Mater. Sci. Eng. C 2020, 112, 110885. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wu, K.; Li, C.; Wang, H.; Sun, Z.; Xi, D.; Zhang, S.; Ding, W.; Zaghloul, M.E.; Wang, C.; et al. Integrated Contact Lens Sensor System Based on Multifunctional Ultrathin MoS2 Transistors. Matter 2021, 4, 969–985. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Khalilian, A.; Khan, M.R.R.; Kang, S.W. Highly Sensitive and Wide-Dynamic-Range Side-Polished Fiber-Optic Taste Sensor. Sens. Actuators B Chem. 2017, 249, 700–707. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications, 1st ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1987; ISBN 0198547242. [Google Scholar]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear Fluid Biomarkers in Ocular and Systemic Disease: Potential Use for Predictive, Preventive and Personalised Medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chen, C.-C.; Hsu, S.-M.; Chuang, H.-S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef]
- Aluru, S.V.; Agarwal, S.; Srinivasan, B.; Iyer, G.K.; Rajappa, S.M.; Tatu, U.; Padmanabhan, P.; Subramanian, N.; Narayanasamy, A. Lacrimal Proline Rich 4 (LPRR4) Protein in the Tear Fluid Is a Potential Biomarker of Dry Eye Syndrome. PLoS ONE 2012, 7, e51979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W.; Choi, M.C.; Shao, Z.Z.; Xiao, R.L.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of Tear Fluid Biomarkers in Dry Eye Syndrome Using ITRAQ Quantitative Proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Micera, A.; Sacchetti, M.; Cortes, M.; Mantelli, F.; Bonini, S. Alterations of Tear Neuromediators in Dry Eye Disease. Arch. Ophthalmol. Chic. Ill 1960 2011, 129, 981–986. [Google Scholar] [CrossRef]
- Chhadva, P.; Lee, T.; Sarantopoulos, C.D.; Hackam, A.S.; McClellan, A.L.; Felix, E.R.; Levitt, R.C.; Galor, A. Human Tear Serotonin Levels Correlate with Symptoms and Signs of Dry Eye. Ophthalmology 2015, 122, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Boehm, N.; Funke, S.; Wiegand, M.; Wehrwein, N.; Pfeiffer, N.; Grus, F.H. Alterations in the Tear Proteome of Dry Eye Patients--a Matter of the Clinical Phenotype. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2385–2392. [Google Scholar] [CrossRef]
- Argüeso, P.; Balaram, M.; Spurr-Michaud, S.; Keutmann, H.T.; Dana, M.R.; Gipson, I.K. Decreased Levels of the Goblet Cell Mucin MUC5AC in Tears of Patients with Sjögren Syndrome. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1004–1011. [Google Scholar]
- Boehm, N.; Riechardt, A.I.; Wiegand, M.; Pfeiffer, N.; Grus, F.H. Proinflammatory Cytokine Profiling of Tears from Dry Eye Patients by Means of Antibody Microarrays. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- López-Miguel, A.; Tesón, M.; Martín-Montañez, V.; Enríquez-De-Salamanca, A.; Stern, M.E.; González-García, M.J.; Calonge, M. Clinical and Molecular Inflammatory Response in Sjögren Syndrome-Associated Dry Eye Patients Under Desiccating Stress. Am. J. Ophthalmol. 2016, 161, 133–141.e2. [Google Scholar] [CrossRef]
- Lam, S.M.; Tong, L.; Reux, B.; Duan, X.; Petznick, A.; Yong, S.S.; Khee, C.B.S.; Lear, M.J.; Wenk, M.R.; Shui, G. Lipidomic Analysis of Human Tear Fluid Reveals Structure-Specific Lipid Alterations in Dry Eye Syndrome. J. Lipid Res. 2014, 55, 299–306. [Google Scholar] [CrossRef]
- Galbis-Estrada, C.; Martinez-Castillo, S.; Morales, J.M.; Vivar-Llopis, B.; Monleón, D.; Díaz-Llopis, M.; Pinazo-Durán, M.D. Differential Effects of Dry Eye Disorders on Metabolomic Profile by 1H Nuclear Magnetic Resonance Spectroscopy. BioMed Res. Int. 2014, 2014, 542549. [Google Scholar] [CrossRef]
- Willshire, C.; Buckley, R.J.; Bron, A.J. Estimating Basal Tear Osmolarity in Normal and Dry Eye Subjects. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2018, 41, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered Cytokine Balance in the Tear Fluid and Conjunctiva of Patients with Sjögren’s Syndrome Keratoconjunctivitis Sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ishida, R.; Kojima, T.; Goto, E.; Matsumoto, Y.; Watanabe, K.; Ishida, N.; Nakata, K.; Takeuchi, T.; Tsubota, K. Abnormal Protein Profiles in Tears with Dry Eye Syndrome. Am. J. Ophthalmol. 2003, 136, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef]
- Chotikavanich, S.; de Paiva, C.S.; Li, D.Q.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and Activity of Matrix Metalloproteinase-9 on the Ocular Surface Increase in Dysfunctional Tear Syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef]
- Aragona, P.; Aguennouz, M.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; De Pasquale, M.G.; Pisani, A.; Puzzolo, D. Matrix Metalloproteinase 9 and Transglutaminase 2 Expression at the Ocular Surface in Patients with Different Forms of Dry Eye Disease. Ophthalmology 2015, 122, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tesón, M.; González-García, M.J.; López-Miguel, A.; Enríquez-de-Salamanca, A.; Martín-Montañez, V.; Benito, M.J.; Mateo, M.E.; Stern, M.E.; Calonge, M. Influence of a Controlled Environment Simulating an In-Flight Airplane Cabin on Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- López-Miguel, A.; Tesón, M.; Martín-Montañez, V.; Enríquez-de-Salamanca, A.; Stern, M.E.; Calonge, M.; González-García, M.J. Dry Eye Exacerbation in Patients Exposed to Desiccating Stress under Controlled Environmental Conditions. Am. J. Ophthalmol. 2014, 157, 788–798.e2. [Google Scholar] [CrossRef]
- Mondal, H.; Hossain, H.; Awang, K.; Saha, S.; Mamun-Ur-Rashid, S.; Islam, K.; Rahman, M.S.; Jahan, I.A.; Rahman, M.M.; Shilpi, J.A. Anthelmintic Activity of Ellagic Acid, a Major Constituent of Alternanthera Sessilis against Haemonchus Contortus. Pak. Vet. J. 2015, 35, 58–62. [Google Scholar]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Santiago, J.L.; Fernandez-Vigo, J.I.; Espino-Paisán, L.; Sarriá, B.; García-Feijoo, J.; Martinez-de-la-Casa, J.M. Hypercytokinemia in COVID-19: Tear Cytokine Profile in Hospitalized COVID-19 Patients. Exp. Eye Res. 2020, 200, 108253. [Google Scholar] [CrossRef]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart PH-Responsive Nanomedicines for Disease Therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kyun Kim, H.; et al. Wearable Smart Sensor Systems Integrated on Soft Contact Lenses for Wireless Ocular Diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef]
- Yin, R.; Xu, Z.; Mei, M.; Chen, Z.; Wang, K.; Liu, Y.; Tang, T.; Priydarshi, M.K.; Meng, X.; Zhao, S.; et al. Soft Transparent Graphene Contact Lens Electrodes for Conformal Full-Cornea Recording of Electroretinogram. Nat. Commun. 2018, 9, 2334. [Google Scholar] [CrossRef]
- Park, S.; Heo, S.W.; Lee, W.; Inoue, D.; Jiang, Z.; Yu, K.; Jinno, H.; Hashizume, D.; Sekino, M.; Yokota, T.; et al. Self-Powered Ultra-Flexible Electronics via Nano-Grating-Patterned Organic Photovoltaics. Nature 2018, 561, 516–521. [Google Scholar] [CrossRef]
- Hong, G.; Lieber, C.M. Author Correction: Novel Electrode Technologies for Neural Recordings. Nat. Rev. Neurosci. 2019, 20, 376. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.; Jones, L.W. The Use of Contact Lenses as Biosensors. Optom. Vis. Sci. 2016, 93, 419–425. [Google Scholar] [CrossRef]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless Smart Contact Lens for Diabetic Diagnosis and Therapy. Sci. Adv. 2020, 6, 1–13. [Google Scholar] [CrossRef]






| Medication | Description | Mechanism of Action | References |
|---|---|---|---|
| Artificial tears | Polyvinyl alcohol, povidone, hydroxypropyl guar, cellulose derivatives, and hyaluronic acid | Increase tear film stability. Reduce ocular surface stress. Improve contrast sensitivity and the optical quality of the surface. | [28,29,30,31,32,33] |
| Topical corticosteroids (loteprednol 0.5%) | Unpreserved corticosteroid eyedrops, instilled over a period of 2 to 4 weeks, improve the symptoms and clinical signs of moderate to severe dry eye disease. | Corticosteroids act by the induction of phospholipase A2 inhibitory proteins and inhibiting the release of arachidonic acid. | [34,35,36] |
| Cyclosporin A (CsA) | Topical application of CsA leads to increased production of tear fluid, possibly via local release of parasympathetic neuro transmitters. CsA eyedrops 0.05% (Restasis) were approved for the topical treatment of dry eye by the FDA in 2002. | CsA is an immunosuppressant that inhibits the calcineurin–phosphatase pathway by complex formation with cyclophilin, and thus reduces the transcription of T-cell-activating cytokines such as interleukin-2 (IL-2). | [37,38,39,40,41] |
| Tacrolimus/pimecrolimus | Appear to be as effective as CsA and are used in patients who cannot tolerate CsA | Inhibition of interleukin-2 gene transcription, nitric oxide synthase activation, cell degranulation, and apoptosis. | [5] |
| Tetracyclines | Bacteriostatic antibiotics with anti-inflammatory effect. | They reduce the synthesis and activity of matrix metalloproteinases, the production of interleukin-1 (IL-1) and tumor necrosis factor, collagenase activity, and B-cell activation. | [5,42,43] |
| Macrolides | Azithromycin 1% has been successfully used to improve meibomian gland function and symptoms, a reduction in bacterial colonization of the eyelid margins, and normalization of the meibomian gland secretion lipid profile. | Inhibition of bacterial protein biosynthesis by preventing peptidyltransferase from adding the growing peptide attached to tRNA to the next amino acid and also inhibiting bacterial ribosomal translation. | [44,45,46] |
| Omega fatty acids | Omega-3 and omega-6 are essential fatty acids for ocular surface homeostasis. | Omega-3 fatty acids work by blocking pro-inflammatory eicosanoids and reducing cytokines through anti-inflammatory activity. | [47] |
| Eyelid hygiene | Hot compresses, eyelid warming masks or goggles, infrared heaters, and eyelid massage improve eyelid margin morphology with a reduction in blocked meibomian gland excretory ducts, and an increase in tear film stability and lipid layer thickness of the tear film. | [48,49,50,51] | |
| Punctal plugs | Temporary occlusion of the tear ducts by small collagen or silicone plugs (punctal plugs) is effective in patients with severe aqueous-deficient dry eye disease. | [36,52,53] | |
| Lifitegrast (Xidra) | The U.S. Food and Drug Administration approved Xiidra (lifitegrast ophthalmic solution) for the treatment of signs and symptoms of dry eye disease, on Monday, 11 July 2016. Xiidra is the first medication in a new class of drugs, called lymphocyte function-associated antigen 1 (LFA-1) antagonist, approved by the FDA for dry eye disease. Xiidra is manufactured by Shire US Inc., of Lexington, Massachusetts. | Lifitegrast blocks the interaction of cell surface proteins LFA-1 and intercellular adhesion molecule-1 (ICAM-1), and is believed to inhibit T-cell-mediated inflammation in DED. | [54,55] |
| Vitamin A | Vitamin A is an essential nutrient present naturally in tear film of healthy eyes. Vitamin A plays an important role in production of the mucin layer, the most innermost lubricating layer of tear film that is crucial for a healthy tear film. Vitamin A deficiency leads to loss of mucin layer and goblet cell atrophy. | Vitamin A drops protect the eyes from free radicals, toxins, allergens, and inflammation. | [1,28,56] |
| Vitamin E | Vitamin E is a fat-soluble antioxidant that prevents the oxidation of fatty acids by reactive oxygen species. The retina is a lipid-rich environment and is bombarded by ultraviolet radiation. In cell culture, vitamin E has been found to enhance the antioxidant ability of lutein to protect retinal pigment epithelial cells from acrolein-induced oxidation. | [57,58] | |
| Functions | Drug | Stage |
|---|---|---|
| A mucin-like glycoprotein | Lacritin | Phase II |
| Lubricin | Phase II | |
| Anti-inflammatory and/or immunosuppressive | Loteprednol etabonate 0.25% suspension | FDA-approved |
| OCS-O2 | Phase II | |
| A higher concentration of Cyclosporine | Phase III | |
| Tacrolimus (0.03%) eye drops | Phase IV | |
| Rapamycin (sirolimus) | Phase I | |
| EBI-005 | Phase III | |
| Resolvin E1 analogues | Phase II | |
| Biological components | Albumin 5% | Phase II |
| Estradiol | Phase II | |
| N-acetylcysteine | Phase II | |
| Thymosin b4 | Phase II | |
| Amniotic membrane extract eye drops | Phase I/II | |
| Mesenchymal stem cells | Phase I/II | |
| Mucin secretagogues | Tavilermide (MIM-D3, 1% or 5%) | Phase II |
| Ecabet sodium | Phase III | |
| Mycophenolate mofetil | Phase II | |
| 15(s)-HETE or Icomucret | Phase III/II | |
| Other’s products | Visomitin (SkQ1) | Phase II/III |
| Tivanisiran (SYL1001) | Phase III |
| Dosage Form | Contact Lens Material | Loading Method | Drug Release Duration | References |
|---|---|---|---|---|
| Contact lens | hydroxyethyl methacrylate (HEMA), cholesterol-hyaluronate (C-HA) micelle | mixing | 12 days | [103] |
| Contact lens | poly-hydroxyethyl methacrylate (p-HEMA), Brij 97, Brij 78 and Brij 700 | mixing | Brij 97—20 days, Brij 78—50 days, Brij 700—20 days | [86] |
| Silicone contact lenses | ethylene glycol dimethacrylate (EGDMA) | soaking | 2 weeks, with vitamin E—1 month | [73] |
| Contact lens | poly-hydroxy ethyl methacrylate (p-HEMA), Brij 98 | mixing | 25 days | [87] |
| Contact lens | poly (2-hydroxyethyl methacrylate) (p-HEMA), Brij 97 | mixing | 20 days | [73] |
| Contact lens | graphene oxide | soaking | - | [104] |
| Analyte | Tear Fluid Concentration [mM] | Blood Concentration [mM] | Diagnostic Application |
|---|---|---|---|
| Glucose | 0.013–0.051 | 3.3–6.5 | Diabetes management |
| Lactate | 2.0–5.0 | 0.36–0.75 | Ischemia, sepsis, liver disease, and cancer |
| Na+ | 120–165 | 130–145 | Hyper/hyponatremia |
| K+ | 20–40 | 3.5–5.0 | Hyper/hypokalemia and an indicator of ocular disease |
| Ca2+ | 0.4–1.1 | 2.0–2.6 | Hyper/hypocalcemia |
| Mg2+ | 0.5–0.9 | 0.7–1.1 | Hyper/hypomagnesemia |
| Cl− | 118–135 | 95–125 | Hyper/hypochloremia |
| HCO3− | 20–26 | 24–30 | Respiratory quotient indicator |
| Urea | 3.0–6.0 | 3.3–6.5 | Renal function |
| Pyruvate | 0.05–0.35 | 0.1–0.2 | Genetic disorders of mitochondrial energy metabolism |
| Ascorbate | 0.22–1.31 | 0.04–0.06 | Diabetes |
| Total Protein | ≈7 g/L | ≈70 g/L | Dry eye conditions, ocular insult, and inflammation |
| Dopamine | 0.37 | 475 × 10−9 | Glaucoma |
| Types of Biomarker Molecule | Biomarkers | References |
|---|---|---|
| Proteins | Lysozyme, lactoferrin, lysozyme proline-rich protein 4 (LPRR4), calgranulin A/S100 A8, lysozyme proline-rich protein 3 (LPRR3), nasopharyngeal carcinoma-associated PRP 4, α-1 antitrypsin α-enolase, α-1 acid glycoprotein 1, S100 A4, S100 A11 (calgizzarin), S100 A9/calgranulin B, lipocalin-1 (LCN-1), mammaglobin B, lipophilin A, beta-2 microglobulin (B2M), S100A6, annexin A1 annexin A11, cystatin S (CST4), phospholipase A2-activating protein (PLAA), transferrin, defensin-1, clusterin, lactotransferrin, cathepsin S, anti-SS-A, anti-SS-B, anti-α-fodrin antibodies, malate dehydrogenase (MDH) 2, palate lung nasal clone-PLUNC | [115,121] |
| Mucins | (MUC)5AC | [122] |
| Neuromediators | Nerve growth factor (NGF), calcitonin gene related peptide (CGRP), neuropeptide Y (NPY), vasointestinal peptide (VIP), serotonin, substance P | [119] |
| Cytokines/chemokines | Interleukin-1(IL-1), interleukin-2 (IL-2), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin 8 (IL-8) or chemokine (C-X-C motif) ligand 8 (CXCL8), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-16 (IL-16), interleukin-33 (IL-33), GCSF, monocyte chemoattractant protein 1 (MCP1)/chemokine (C-C motif) ligand 2 (CCL2), MIP5/chemokine (C-C motif) ligand 15 (CCL15), C-X-C motif chemokine 5 (CXCL5 or ENA78), soluble interleukin-1 receptor Type I (sIL-1RI), soluble interleukin-6 receptor (sIL-6R), soluble gp130 (sgp130), soluble vascular endothelial growth factor receptor 1 (sVEGFR1), soluble epidermal growth factor receptor (sEGFR), soluble tumor necrosis factor receptor I (sTNFR I), interleukin-17A (IL-17A), interleukin-21 (IL-21), interleukin-22 (IL-22), interleukin-1 receptor antagonist (IL-1RA), chemokine (C-X-C motif) ligand 9 (CXCL9)/monokine induced by gamma interferon (MIG), interferon-inducible T-cell alpha chemoattractant (I-TAC)/C-X-C motif chemokine 11 (CXCL11), C–X–C motif chemokine 10 (CXCL10)/interferon γ-induced protein 10 kDa (IP-10), ligand 4 (CCL4)/macrophage inflammatory protein-1β (MIP-1β), chemokine (C-C motif) ligand 5 (also CCL5)/regulated on activation, normal T cell expressed and secreted (RANTES), epidermal growth factor (EGF), tumor necrosis factor alpha (TNF-α), interferon gamma (IFNγ), matrix metallopeptidase 9 (MMP-9), macrophage inflammatory protein-1 alpha (MIP-1α/CCL3), vascular endothelial growth factor (VEGF), fractalkine | [115,123,124] |
| Lipids | (O-acyl) ω-hydroxy fatty acids (OAHFAs), lysophospholipids, PUFA-containing diacylglyceride species, hexanoyl-lysine (HEL), 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA) | [125] |
| Metabolites | Cholesterol, N-acetylglucosamine, glutamate, creatine, amino-n-butyrate, choline, acetylcholine, arginine, phosphoethanolamine, glucose, phenylalanine | [126] |
| Tear solutes | Osmolarity | [127] |
| Biomarkers | Sensor Type |
|---|---|
| Glucose | Enzymatic biosensor; amperometric |
| Osmolarity | Impedimetric |
| MMP-9 | Electrochemical immunosensors |
| Urea | Voltammetric |
| Serum | Electrochemical immunosensors |
| TNF-α | Electrochemical aptamer sensor |
| Mucins | Electrochemical immunosensors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, H.; Kim, H.-J.; Mohanto, N.; Jee, J.-P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. https://doi.org/10.3390/pharmaceutics15030990
Mondal H, Kim H-J, Mohanto N, Jee J-P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics. 2023; 15(3):990. https://doi.org/10.3390/pharmaceutics15030990
Chicago/Turabian StyleMondal, Himangsu, Ho-Joong Kim, Nijaya Mohanto, and Jun-Pil Jee. 2023. "A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives" Pharmaceutics 15, no. 3: 990. https://doi.org/10.3390/pharmaceutics15030990
APA StyleMondal, H., Kim, H.-J., Mohanto, N., & Jee, J.-P. (2023). A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics, 15(3), 990. https://doi.org/10.3390/pharmaceutics15030990







