Ex Vivo Visualization of Distribution of Intravitreal Injections in the Porcine Vitreous and Hydrogels Simulating the Vitreous
Abstract
1. Introduction
2. Materials and Methods
2.1. Artificial Vitreous Substitutes
2.2. Ex Vivo Material
2.3. Physicochemical Characterisation
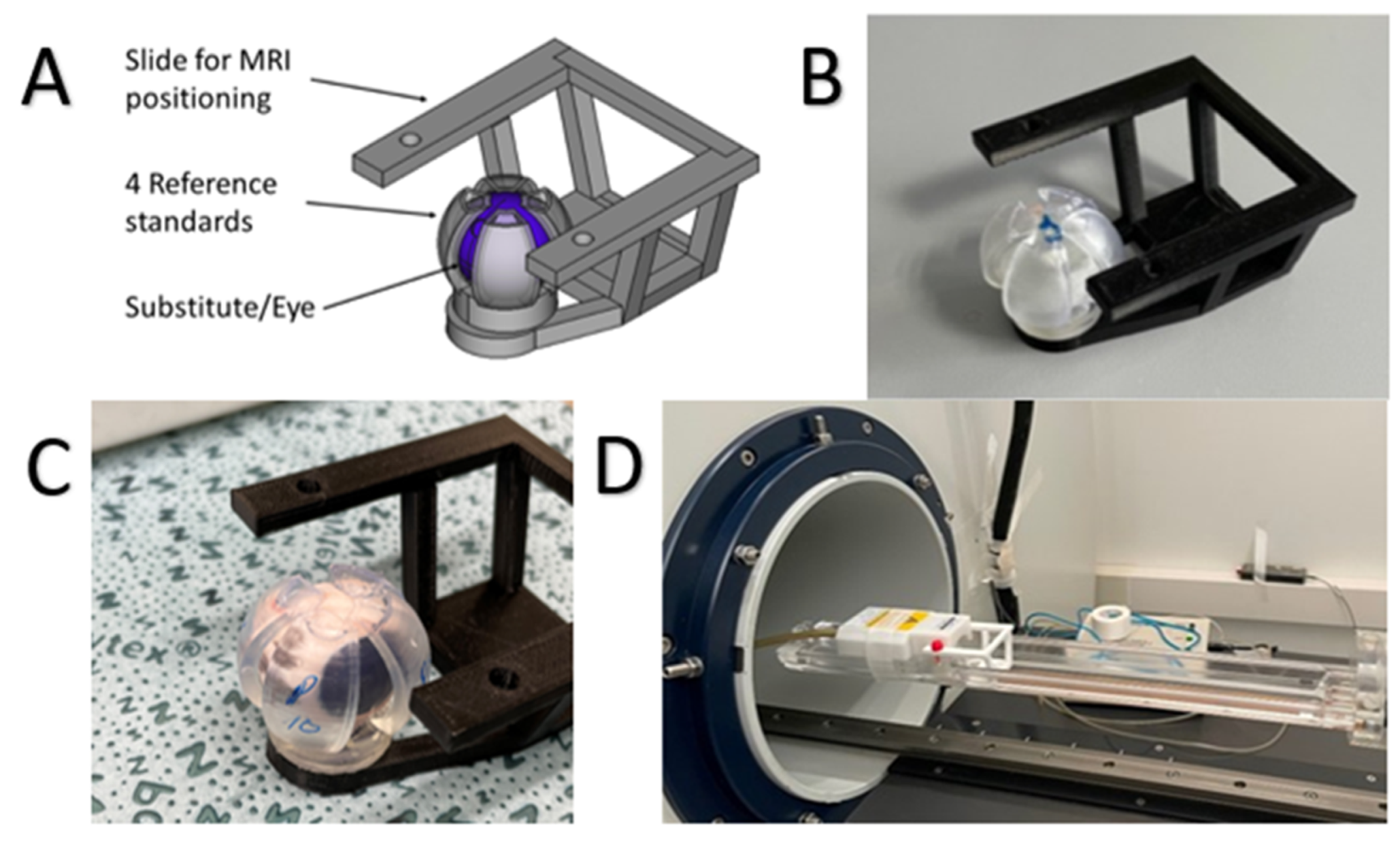
2.4. MRI Measurements
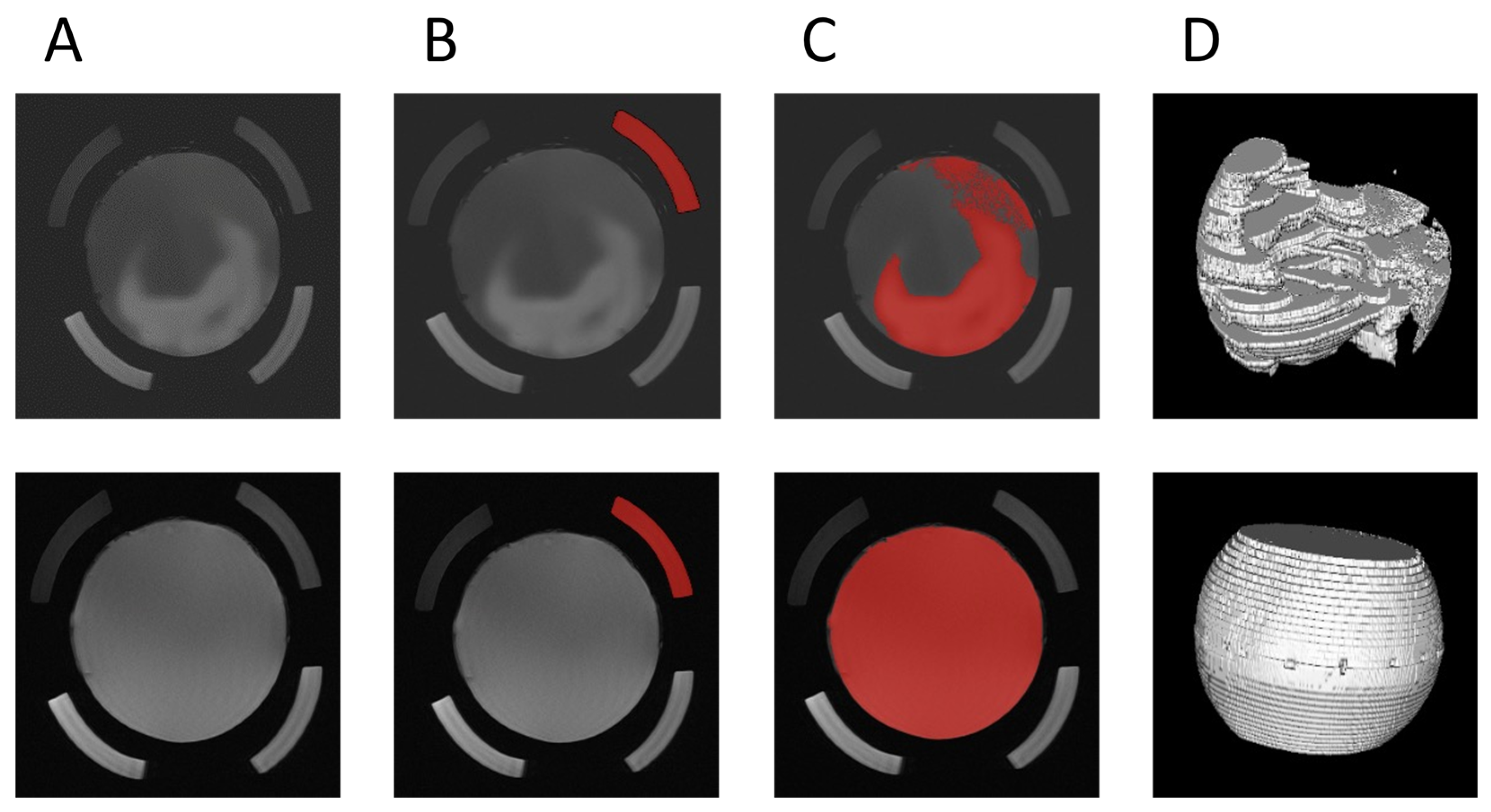
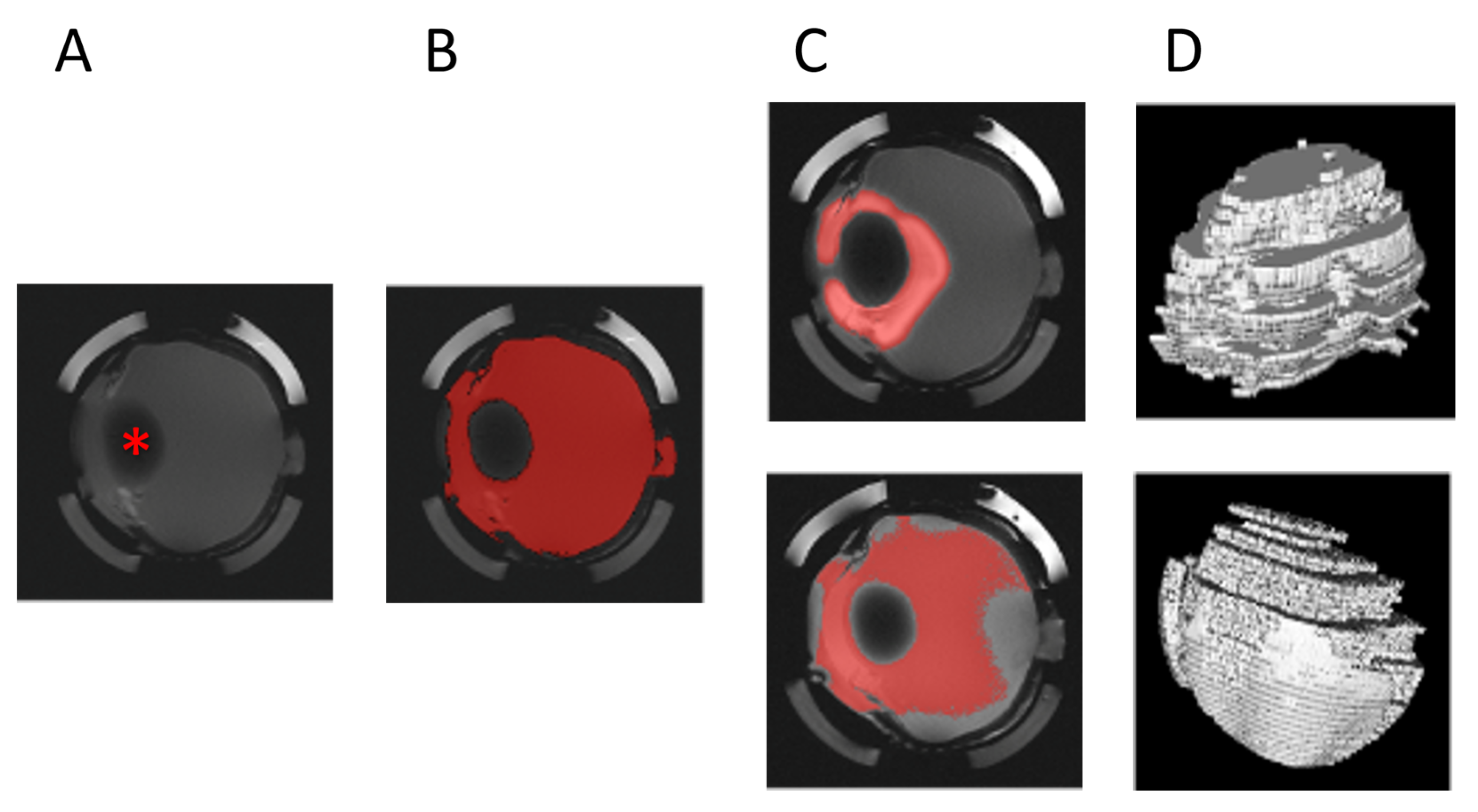
2.5. MRI Image Processing
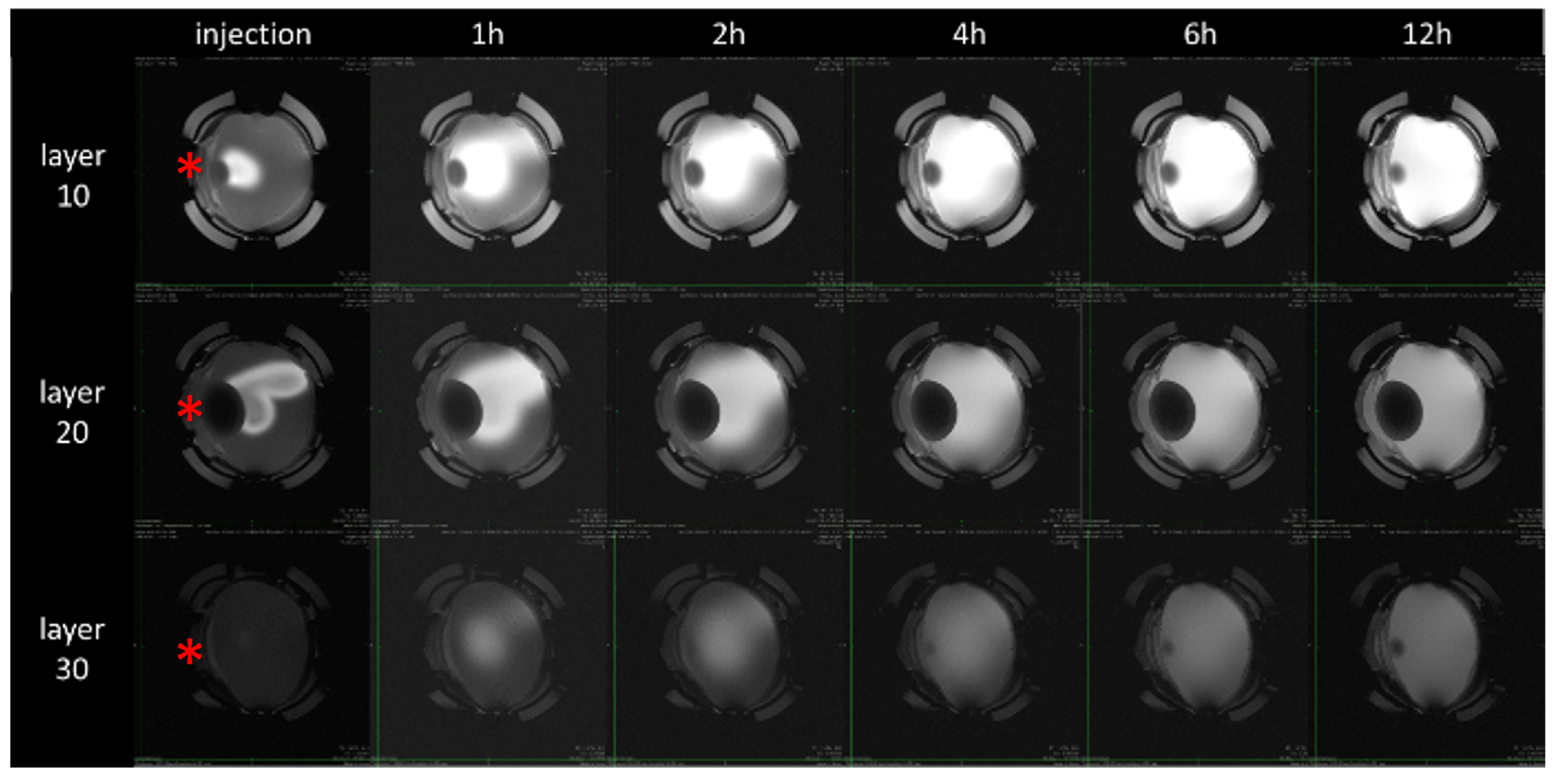
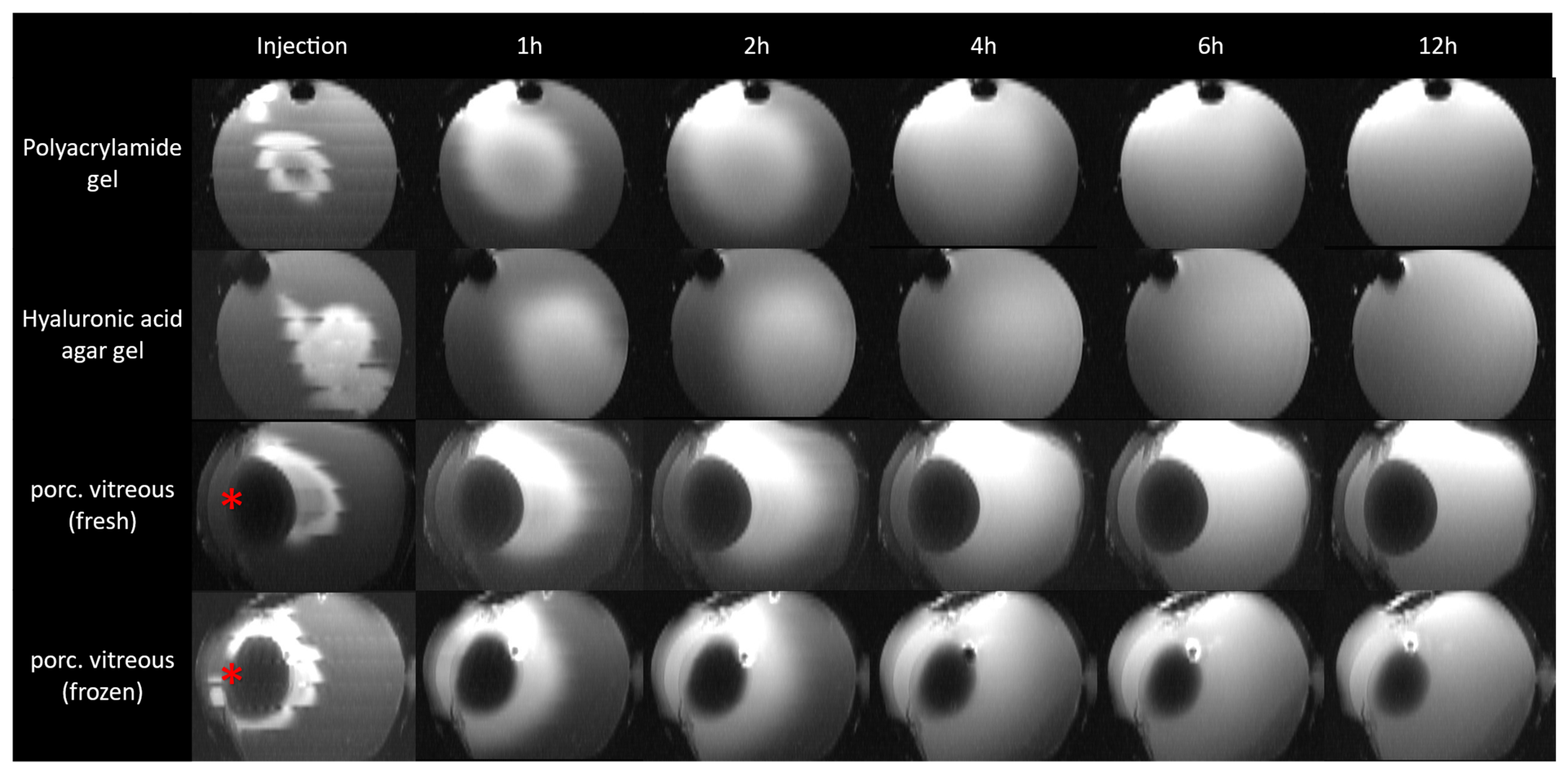
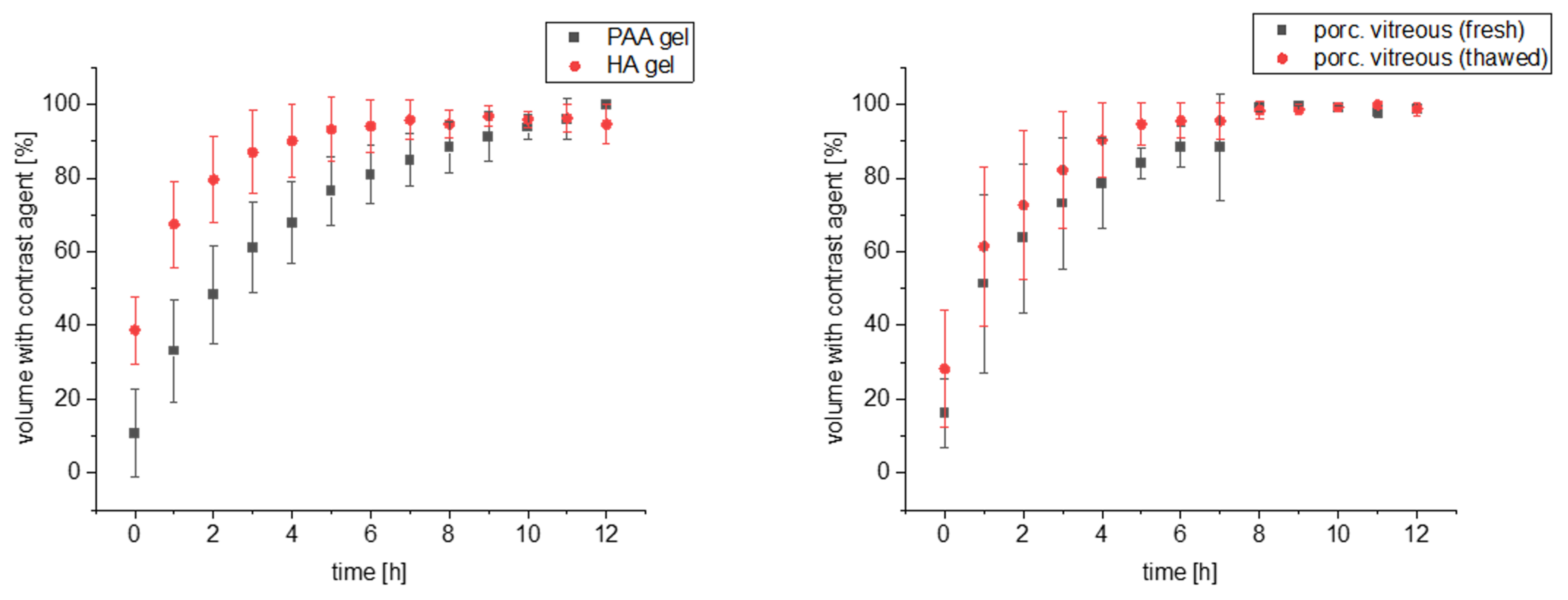
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Shikari, H.; Samant, P. Intravitreal Injections: A Review of Pharmacological Agents and Techniques. J. Clin. Ophthalmol. Res. 2016, 4, 51. [Google Scholar] [CrossRef]
- Silva, A.F.; Alves, M.A.; Oliveira, M.S.N. Rheological Behaviour of Vitreous Humour. Rheol. Acta 2017, 56, 377–386. [Google Scholar] [CrossRef]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, 148039. [Google Scholar] [CrossRef]
- Kokavec, J.; Min, S.H.; Tan, M.H.; Gilhotra, J.S.; Newland, H.S.; Durkin, S.R.; Grigg, J.; Casson, R.J. Biochemical Analysis of the Living Human Vitreous. Clin. Exp. Ophthalmol. 2016, 44, 597–609. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Current and Future Ophthalmic Drug Delivery Systems. A Shift to the Posterior Segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Luaces-Rodríguez, A.; González-Barcia, M.; Blanco-Teijeiro, M.J.; Gil-Martínez, M.; Gonzalez, F.; Gómez-Ulla, F.; Lamas, M.J.; Otero-Espinar, F.J.; Fernández-Ferreiro, A. Review of Intraocular Pharmacokinetics of Anti-Infectives Commonly Used in the Treatment of Infectious Endophthalmitis. Pharmaceutics 2018, 10, 66. [Google Scholar] [CrossRef]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef]
- Le Goff, M.M.; Bishop, P.N. Adult Vitreous Structure and Postnatal Changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef]
- Bishop, P.N. Structural Macromolecules and Supramolecular Organisation of the Vitreous Gel. Prog. Retin. Eye Res. 2000, 19, 323–344. [Google Scholar] [CrossRef]
- Sebag, J. Ageing of the Vitreous. Eye 1987, 1, 254–262. [Google Scholar] [CrossRef]
- Kane, F.E.; Green, K.E. Ocular Pharmacokinetics of Fluocinolone Acetonide Following Iluvien Implantation in the Vitreous Humor of Rabbits. J. Ocul. Pharmacol. Ther. 2015, 31, 11–16. [Google Scholar] [CrossRef]
- Urtti, A.; Pipkin, J.D.; Rork, G.; Sendo, T.; Finne, U.; Repta, A.J. Controlled Drug Delivery Devices for Experimental Ocular Studies with Timolol 2. Ocular and Systemic Absorption in Rabbits. Int. J. Pharm. 1990, 61, 241–249. [Google Scholar] [CrossRef]
- Iandiev, I.; Francke, M.; Makarov, F.; Hollborn, M.; Uhlmann, S.; Wurm, A.; Savvinov, A.; Kohen, L.; Reichenbach, A.; Wiedemann, P.; et al. Effects of Intravitreal Bevacizumab (Avastin) on the Porcine Retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1821–1829. [Google Scholar] [CrossRef]
- Schraermeyer, U.; Julien, S. Effects of Bevacizumab in Retina and Choroid after Intravitreal Injection into Monkey Eyes. Expert Opin. Biol. Ther. 2013, 13, 157–167. [Google Scholar] [CrossRef]
- Brodie, F.L.; Ruggiero, J.; Ghodasra, D.H.; Eftekhari, K.; Hui, J.Z.; Brucker, A.J.; Vanderbeek, B.L. A Novel Method for the Measurement of Reflux from Intravitreal Injections: Data from 20 Porcine Eyes. Curr. Eye Res. 2014, 39, 752–757. [Google Scholar] [CrossRef]
- Laude, A.; Tan, L.E.; Wilson, C.G.; Lascaratos, G.; Elashry, M.; Aslam, T.; Patton, N.; Dhillon, B. Intravitreal Therapy for Neovascular Age-Related Macular Degeneration and Inter-Individual Variations in Vitreous Pharmacokinetics. Prog. Retin. Eye Res. 2010, 29, 466–475. [Google Scholar] [CrossRef]
- Awwad, S.; Henein, C.; Ibeanu, N.; Khaw, P.T.; Brocchini, S. Preclinical Challenges for Developing Long Acting Intravitreal Medicines. Eur. J. Pharm. Biopharm. 2020, 153, 130–149. [Google Scholar] [CrossRef]
- Adrianto, M.F.; Annuryanti, F.; Wilson, C.G.; Sheshala, R.; Thakur, R.R.S. In Vitro Dissolution Testing Models of Ocular Implants for Posterior Segment Drug Delivery. Drug Deliv. Transl. Res. 2021, 12, 1355–1375. [Google Scholar] [CrossRef]
- Awwad, S.; Lockwood, A.; Brocchini, S.; Khaw, P.T. The PK-Eye: A Novel in Vitro Ocular Flow Model for Use in Preclinical Drug Development. J. Pharm. Sci. 2015, 104, 3330–3342. [Google Scholar] [CrossRef]
- Awwad, S.; Day, R.M.; Khaw, P.T.; Brocchini, S.; Fadda, H.M. Sustained Release Ophthalmic Dexamethasone: In Vitro in Vivo Correlations Derived from the PK-Eye. Int. J. Pharm. 2017, 522, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Nagel, S.; Guthoff, R.; Seidlitz, A.; Weitschies, W. The Vitreous Model—A New in Vitro Test Method Simulating the Vitreous Body. Biomed. Tech. 2012, 57, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Bogdahn, M.; Stein, S.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Simulation of Drug Distribution in the Vitreous Body after Local Drug Application into Intact Vitreous Body and in Progress of Posterior Vitreous Detachment. J. Pharm. Sci. 2014, 103, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Bogdahn, M.; Rosenbaum, C.; Weitschies, W.; Seidlitz, A. Distribution of Fluorescein Sodium and Triamcinolone Acetonide in the Simulated Liquefied and Vitrectomized Vitreous Model with Simulated Eye Movements. Eur. J. Pharm. Sci. 2017, 109, 233–243. [Google Scholar] [CrossRef]
- Ruiz-Ederra, J.; García, M.; Hernández, M.; Urcola, H.; Hernández-Barbáchano, E.; Araiz, J.; Vecino, E. The Pig Eye as a Novel Model of Glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef]
- Baino, F. Towards an Ideal Biomaterial for Vitreous Replacement: Historical Overview and Future Trends. Acta Biomater. 2011, 7, 921–935. [Google Scholar] [CrossRef]
- Yadav, I.; Purohit, S.D.; Singh, H.; Bhushan, S.; Yadav, M.K.; Velpandian, T.; Chawla, R.; Hazra, S.; Mishra, N.C. Vitreous Substitutes: An Overview of the Properties, Importance, and Development. J. Biomed. Mater. Res. 2021, 109, 1156–1176. [Google Scholar] [CrossRef]
- Mondelo-García, C.; Bandín-Vilar, E.; García-Quintanilla, L.; Castro-Balado, A.; del Amo, E.M.; Gil-Martínez, M.; Blanco-Teijeiro, M.J.; González-Barcia, M.; Zarra-Ferro, I.; Fernández-Ferreiro, A.; et al. Current Situation and Challenges in Vitreous Substitutes. Macromol. Biosci. 2021, 21, 2100066. [Google Scholar] [CrossRef]
- Kleinberg, T.T.; Tzekov, R.T.; Stein, L.; Ravi, N.; Kaushal, S. Vitreous Substitutes: A Comprehensive Review. Surv. Ophthalmol. 2011, 56, 300–323. [Google Scholar] [CrossRef]
- Donati, S.; Caprani, S.M.; Airaghi, G.; Vinciguerra, R.; Bartalena, L.; Testa, F.; Mariotti, C.; Porta, G.; Simonelli, F.; Azzolini, C. Vitreous Substitutes: The Present and the Future. BioMed Res. Int. 2014, 2014, 351804. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Dinser, S.; Nelson, B.J. Artificial Vitreous Humor for In Vitro Experiments. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Lyon, France, 22–26 August 2007. [Google Scholar]
- Stein, S.; Hadlich, S.; Langner, S.; Biesenack, A.; Zehm, N.; Kruschke, S.; Oelze, M.; Grimm, M.; Mahnhardt, S.; Weitschies, W.; et al. 7.1 T MRI and T2 Mapping of the Human and Porcine Vitreous Body Post Mortem. Eur. J. Pharm. Biopharm. 2018, 131, 82–91. [Google Scholar] [CrossRef]
- Noulas, A.V.; Theocharis, A.D.; Feretis, E.; Papageorgakopoulou, N.; Karamanos, N.K.; Theocharis, D.A. Pig Vitreous Gel: Macromolecular Composition with Particular Reference to Hyaluronan-Binding Proteoglycans. Biochimie 2002, 84, 295–302. [Google Scholar] [CrossRef]
- Lee, B.; Litt, M.; Buchsbaum, A.C. Rheology of the vitreous body: Part 2. Viscoelasticity of bovine and porcine vitreous. Biorheology 1994, 31, 327–338. [Google Scholar] [CrossRef]
- Allmendinger, A.; Butt, Y.L.; Mueller, C. Intraocular Pressure and Injection Forces during Intravitreal Injection into Enucleated Porcine Eyes. Eur. J. Pharm. Biopharm. 2021, 166, 87–93. [Google Scholar] [CrossRef]
- Friedrich, S.; Cheng, Y.L.; Saville, B. Drug Distribution in the Vitreous Humor of the Human Eye: The Effects of Intravitreal Injection Position and Volume. Curr. Eye Res. 1997, 16, 663–669. [Google Scholar] [CrossRef]
- Leonard, K.C.; Worden, N.; Boettcher, M.L.; Dickinson, E.; Hartstone-Rose, A. Effects of Freezing and Short-Term Fixation on Muscle Mass, Volume, and Density. Anat. Rec. 2022, 305, 199–208. [Google Scholar] [CrossRef]
- Petrovic, L.; Grujic, R.; Petrovic, M. Definition of the Optimal Freezing Ratc 2. Investigation of the Physico-Chemical Properties of Beef M. Iongissimus Dorsi Frozen at Different Freezing Rates. Meat Sci. 1993, 33, 319–331. [Google Scholar] [CrossRef]
- Landers, M.B., 3rd; Watson, J.S.; Ulrich, J.N.; Quiroz-Mercado, H. Determination of retinal and vitreous temperature in vitrectomy. Retina 2012, 32, 172–176. [Google Scholar] [CrossRef]
| Polyacrylamide Gel | Hyaluronic Acid Agar Gel | ||
|---|---|---|---|
| Rotiphorese (37.5:1) | 6.69 g | Hyaluronic acid | 0.15 g |
| APS-Solution (10%) | 1 g | Agar | 0.1875 g |
| TEMED | 0.1 g | PBS pH 7.4 | Ad 100.0 g |
| PBS pH 7.4 | Ad 100.0 g | ||
| Parameter | Value |
|---|---|
| Field of view | 40 mm |
| Slice thickness | 0.5 mm |
| Interslice gap | 0 mm |
| Slices | 40 |
| Voxel size | 0.089 × 0.089 × 0.5 mm3 |
| Repetition time (TR) | 845 ms |
| Echo time (TE) | 14 ms |
| Flip angle | 180° |
| Aquisition time | 13:35 min |
| VS | pH Value | Density (g/cm3) | Viscosity (mPa·s) |
|---|---|---|---|
| Human vitreous [26,27] | 7.4–7.52 | 1.0053–1.0089 | 300–2000 |
| Porcine vitreous body | 7.42 ± 0.02 | 1.0041 ± 0.0027 | 43.1 ± 5.2 |
| Polyacrylamide gel | 7.32 ± 0.01 | 1.0110 ± 0.0009 | 660 ± 18.1 |
| Hyaluronic acid-agar gel | 7.31 ± 0.01 | 1.0077 ± 0.0000 | 62.3 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auel, T.; Scherke, L.P.; Hadlich, S.; Mouchantat, S.; Grimm, M.; Weitschies, W.; Seidlitz, A. Ex Vivo Visualization of Distribution of Intravitreal Injections in the Porcine Vitreous and Hydrogels Simulating the Vitreous. Pharmaceutics 2023, 15, 786. https://doi.org/10.3390/pharmaceutics15030786
Auel T, Scherke LP, Hadlich S, Mouchantat S, Grimm M, Weitschies W, Seidlitz A. Ex Vivo Visualization of Distribution of Intravitreal Injections in the Porcine Vitreous and Hydrogels Simulating the Vitreous. Pharmaceutics. 2023; 15(3):786. https://doi.org/10.3390/pharmaceutics15030786
Chicago/Turabian StyleAuel, Tobias, Lara Paula Scherke, Stefan Hadlich, Susan Mouchantat, Michael Grimm, Werner Weitschies, and Anne Seidlitz. 2023. "Ex Vivo Visualization of Distribution of Intravitreal Injections in the Porcine Vitreous and Hydrogels Simulating the Vitreous" Pharmaceutics 15, no. 3: 786. https://doi.org/10.3390/pharmaceutics15030786
APA StyleAuel, T., Scherke, L. P., Hadlich, S., Mouchantat, S., Grimm, M., Weitschies, W., & Seidlitz, A. (2023). Ex Vivo Visualization of Distribution of Intravitreal Injections in the Porcine Vitreous and Hydrogels Simulating the Vitreous. Pharmaceutics, 15(3), 786. https://doi.org/10.3390/pharmaceutics15030786








