Smart Polymeric Nanoparticles in Cancer Immunotherapy
Abstract
1. Introduction
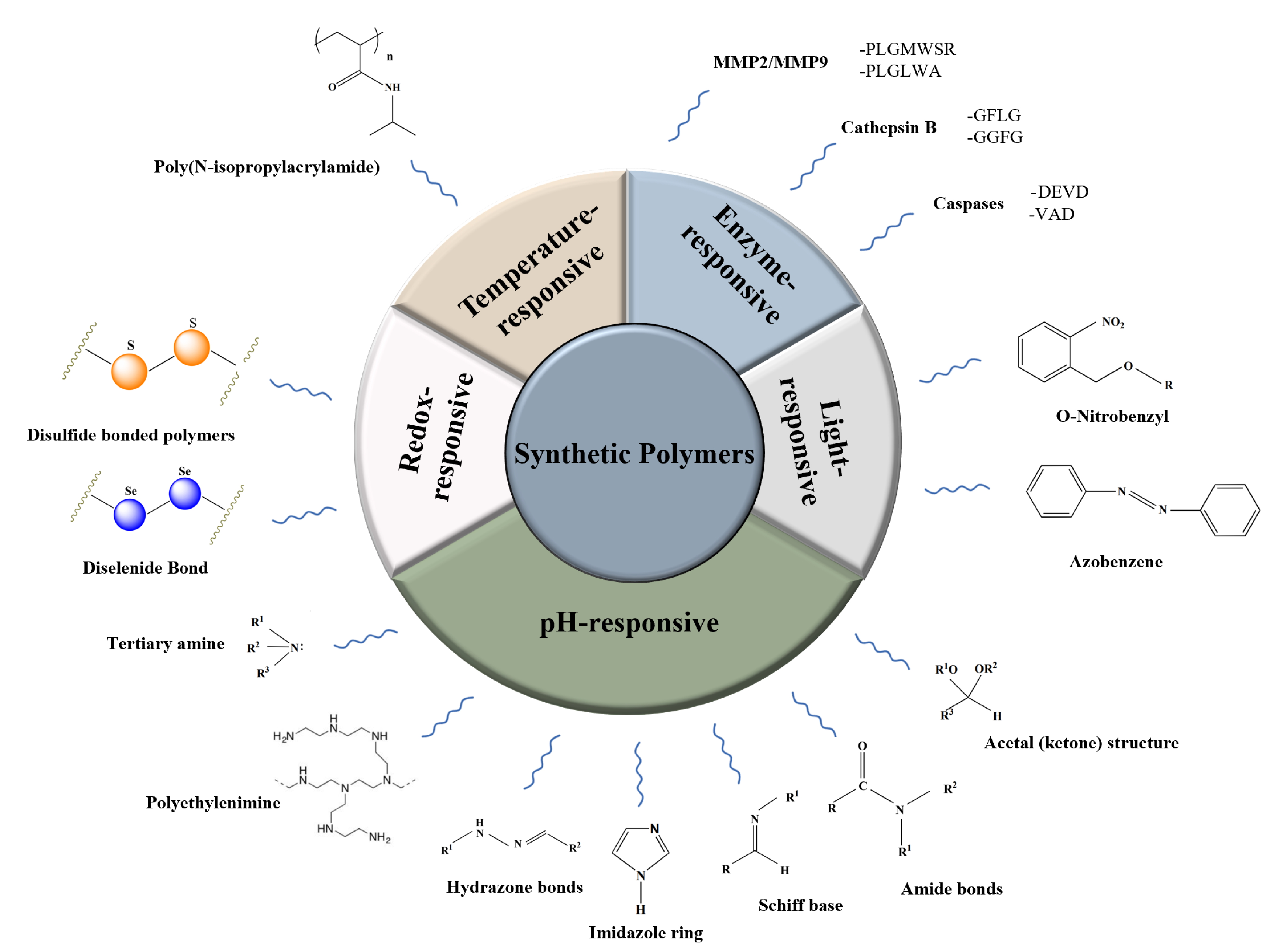
2. Synthetic Polymers
2.1. Enzyme-Responsive Polymers
2.1.1. MMP
2.1.2. Cathepsin B
2.1.3. Caspases
2.2. pH-Responsive Polymers
2.2.1. Schiff Bases
2.2.2. Polyethyleneimine
2.2.3. Imidazole Ring
2.2.4. Hydrazone Bonds
2.2.5. Tertiary Amine
2.2.6. Amide Bonds
2.2.7. Acetal (Ketone) Structure
2.3. Redox-Responsive Polymers
2.3.1. Disulfide Bonded Polymers
2.3.2. Diselenide Bond
2.4. Temperature-Responsive Polymeric Delivery Systems
2.5. Light-Responsive Polymeric Nanoparticles
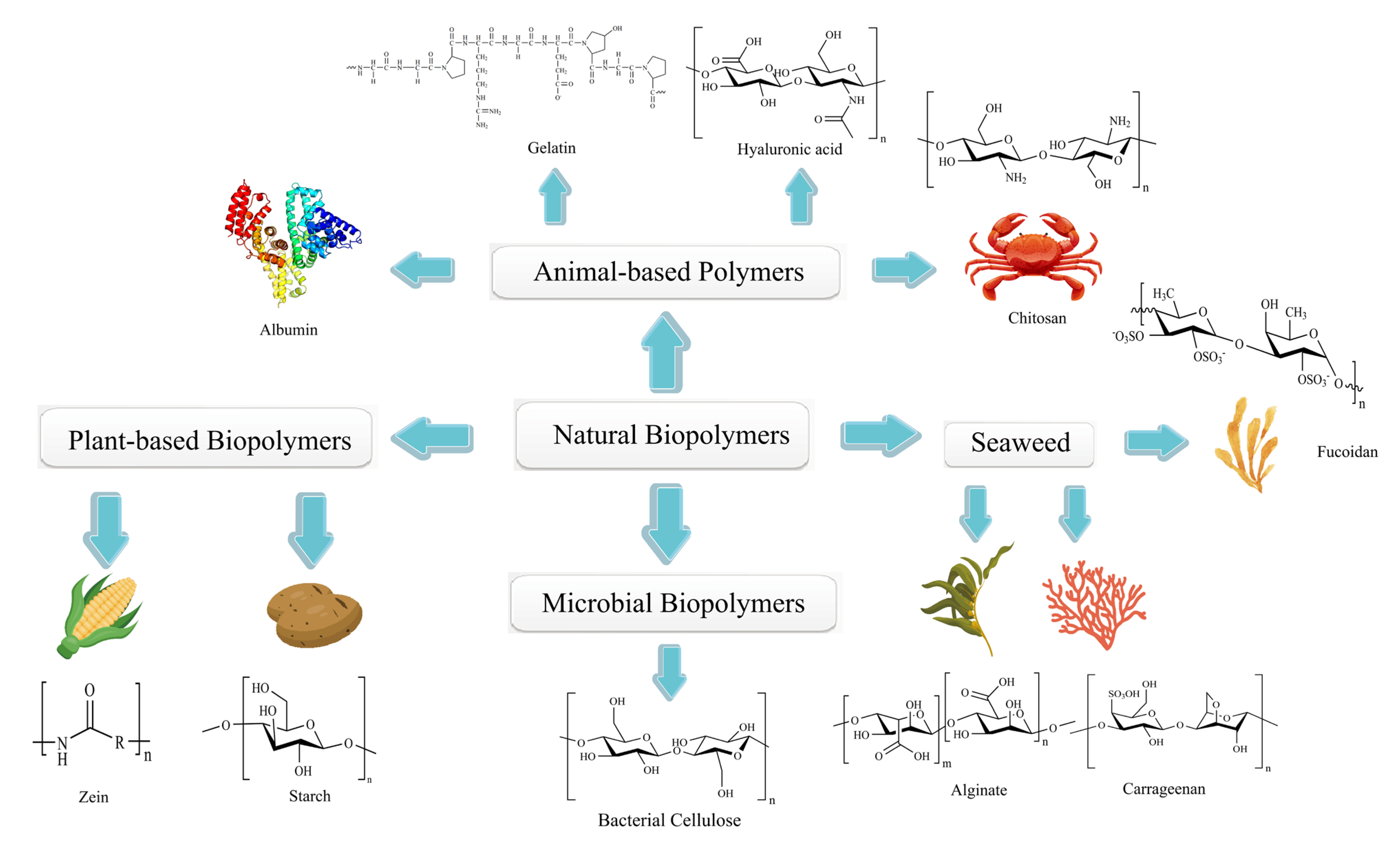
3. Natural Polymers
3.1. Animal-Based Biopolymers
3.1.1. Albumin
3.1.2. Gelatin
3.1.3. Hyaluronic Acid
3.1.4. Chitosan
3.2. Plant-Based Biopolymers
3.2.1. Starch
3.2.2. Zein
3.3. Microbial Biopolymers
3.4. Biopolymers from Seaweed
3.4.1. Fucoidan
3.4.2. Alginate
3.4.3. Carrageenan
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ·OH | Hydroxyl radicals |
| 1MT | 1-methyltryptophan |
| 1O2 | Singlet oxygen |
| αPD-L1 | Anti-PD-L1 antibody |
| ALG | Alginate |
| CAD | Cis-aconityl-doxorubicin |
| CDN | Cyclic dinucleotide |
| CGN | Carrageenan |
| CpG | Cytosine–phosphate–guanine |
| CpG8 | Cytosine–phosphate–guanine |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein-4 |
| Cyt-SeSe-Cyt | Cytosine-containing diselenides |
| DCs | Dendritic cells |
| DEVD | Asp-Glu-Val-Asp |
| DMXAA | 5,6-dimethylxantheonone-4-acetic acid |
| DOX | Doxorubicin |
| ECM | Extracellular matrix |
| EMA | European Medicines Agency |
| en-srNP | Endo-stimuli-responsive nanoparticle |
| FDA | US Food and Drug Administration |
| GFLG | Gly-Phe-Leu-Gly |
| GGFG | Gly-Gly-Phe-Gly |
| GITR | Glucocorticoid-induced TNF receptor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRGDS | Gly-Arg-Gly-Asp-Ser |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| HA | Hyaluronic acid |
| HLA-E | Human leukocyte antigen E |
| HNSCCs | Head and neck squamous cell carcinomas |
| HPMA | N-(2-hydroxypropyl) methacrylamide |
| ICD | Immunogenic cell death |
| ICG | Indocyanine green |
| IL-2 | Interleukin-2 |
| IMQ | Imiquimod |
| IMT | Imatinib |
| iNKT | Invariant natural killer T cell |
| LBL hNPs | Layer-by-layer hybrid nanoparticles |
| mAb | Monoclonal antibody |
| MAN | Mannose |
| MDSCs | Myeloid-derived suppressor cells |
| MMP | Matrix metalloproteinase |
| mPEG-b-PLA | Methoxy poly(ethylene glycol)-b-poly(L-lactide) |
| mPEG-TK-Ad | Methoxy poly(ethylene glycol)-thioxo-adamantane |
| Nano-QUT | Quercetin-encapsulated PLGA-PEG nanoparticles |
| NK | Natural killer |
| NY-ESO-1 | New York Esophageal Squamous Cell Carcinoma-1 |
| OVA | Ovalbumin |
| PAMAM-TK-Ad | Polyamido-thioxo-adamantane |
| PCL | Polycaprolactone |
| PcM | Morpholine-modified silicone phthalocyanine |
| PDA | Polydopamine |
| PD-L1 | Programmed death-ligand 1 |
| PDT | Photodynamic therapeutic |
| PEG | Polyethylene glycol |
| PEG-b-PLG | Poly(ethylene glycol)-block-poly(L-glutamic acid) |
| PEG-PCL-PLA-PCL-PEG | Polyethylene glycol -polycaprolactone -poly(propyleneglycol) -poly(caprolactone-poly(ethylene glycol) |
| PEI | Polyethyleneimine |
| PFP | Perfluoropentane |
| PIAN | Programmable immune activating nanodrug |
| PIT | Photoimmunotherapy |
| PLA | Polypropylene glycol |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLGLWA | Pro-Leu-Gly-Leu-Trp-Ala |
| PLGMWSR | Pro-Leu-Gly-Met-Trp-Ser-Arg |
| PLH | Poly-L-histidine |
| PPCD | Poly[(N-2-hydroxyethyl)-asparagine]-Pt(IV)/β-cyclodextrin |
| PpIX | Protoporphyrin IX |
| PTT | Photothermal therapy |
| PTX | Paclitaxel |
| ROS | Reactive oxygen species |
| RSQ | Resiquimod |
| STAT3 | Signal transducer activator of transcription 3 |
| ThrCer6, IMM60 | Threitolceramide-6 |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| VAD | Val-Ala-Asp |
| VP | Verteporfin |
References
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chin. Med. J. (Engl.) 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ackerknecht, E.H. The History of Cancer Therapy. Gesnerus 1980, 37, 189–197. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Helmy, K.Y.; Patel, S.A.; Nahas, G.R.; Rameshwar, P. Cancer Immunotherapy: Accomplishments to Date and Future Promise. Ther. Deliv. 2013, 4, 1307–1320. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic Targets and Biomarkers of Tumor Immunotherapy: Response Versus Non-Response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Demaria, S.; Rodriguez-Ruiz, M.E.; Zarour, H.M.; Melero, I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1845–1855. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A Novel Targeted Drug Delivery System for Cancer. Med. Oncol. (Northwood Lond. Engl.) 2022, 39, 240. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as Promising Theranostic Tools in Nanomedicine and Their Applications in Clinical Disease Diagnosis and Treatment. Nanomaterials 2021, 11, 3346. [Google Scholar] [CrossRef]
- Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.S.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. [Google Scholar] [CrossRef]
- Gupta, A.; Costa, A.P.; Xu, X.; Burgess, D.J. Continuous Processing of Paclitaxel Polymeric Micelles. Int. J. Pharm. 2021, 607, 120946. [Google Scholar] [CrossRef]
- Borges, G.S.M.; Lima, F.A.; Carneiro, G.; Goulart, G.A.C.; Ferreira, L.A.M. All-Trans Retinoic Acid in Anticancer Therapy: How Nanotechnology Can Enhance Its Efficacy and Resolve Its Drawbacks. Expert Opin. Drug Deliv. 2021, 18, 1335–1354. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of Fda-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Sartor, O. Eligard: Leuprolide Acetate in a Novel Sustained-Release Delivery System. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Dinndorf, P.A.; Gootenberg, J.; Cohen, M.H.; Keegan, P.; Pazdur, R. Fda Drug Approval Summary: Pegaspargase (Oncaspar) for the First-Line Treatment of Children with Acute Lymphoblastic Leukemia (All). Oncologist 2007, 12, 991–998. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, X.; Zeng, L.; Liu, J.; Zhang, Z. A Randomized Multicenter Phase II Clinical Trial of Mitoxantrone-Loaded Nanoparticles in the Treatment of 108 Patients with Unresected Hepatocellular Carcinoma. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 419–423. [Google Scholar] [CrossRef]
- Barraud, L.; Merle, P.; Soma, E.; Lefrançois, L.; Guerret, S.; Chevallier, M.; Dubernet, C.; Couvreur, P.; Trépo, C.; Vitvitski, L. Increase of Doxorubicin Sensitivity by Doxorubicin-Loading into Nanoparticles for Hepatocellular Carcinoma Cells In Vitro and In Vivo. J. Hepatol. 2005, 42, 736–743. [Google Scholar] [CrossRef]
- Nowotnik, D.P.; Cvitkovic, E. Prolindac (Ap5346): A Review of the Development of an HPMA DACH Platinum Polymer Therapeutic. Adv. Drug Deliv. Rev. 2009, 61, 1214–1219. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Q.; Duan, T.; Hu, R.; Tang, J. The Fate of Nanoparticles in Vivo and the Strategy of Designing Stealth Nanoparticle for Drug Delivery. Curr. Drug Targets 2021, 22, 922–946. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Olatunji, O.; Bhusan Das, D.; Vladisavljević, G. Pharmaceutical Applications of Natural Polymers. In Natural Polymers: Industry Techniques and Applications; Olatunji, O., Ed.; Springer International Publishing: Cham, The Netherlands, 2016; pp. 263–313. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Kanaani, L.; Ebrahimi Far, M.; Kazemi, S.M.; Choupani, E.; Mazloumi Tabrizi, M.; Ebrahimi Shahmabadi, H.; Akbarzadeh Khiyavi, A. General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Vincent, M.P.; Navidzadeh, J.O.; Bobbala, S.; Scott, E.A. Leveraging Self-Assembled Nanobiomaterials for Improved Cancer Immunotherapy. Cancer Cell 2022, 40, 255–276. [Google Scholar] [CrossRef]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric Nanoparticles for Drug Delivery. Methods Mol. Biol. (Clifton N.J.) 2010, 624, 163–175. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in Cancer Therapeutics: Bioconjugated Nanoparticles for Drug Delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; Darweesh, R.S.; Al-Shriem, L.A.; Al-Qawasmi, F.S.; Emran, S.O.; Khafajah, A.S.; Abu-Dalo, M.A. Polymeric Nanoparticles for Inhaled Vaccines. Polymers 2022, 14, 4450. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B.Y.S. Nanotechnology Platforms for Cancer Immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1590. [Google Scholar] [CrossRef]
- Jia, Y.; Omri, A.; Krishnan, L.; McCluskie, M.J. Potential Applications of Nanoparticles in Cancer Immunotherapy. Hum. Vaccines Immunother. 2017, 13, 63–74. [Google Scholar] [CrossRef]
- Craparo, E.F.; Bondì, M.L. Application of Polymeric Nanoparticles in Immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 658–664. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv. Sci. 2022, 9, e2103444. [Google Scholar] [CrossRef]
- Tong, R.; Langer, R. Nanomedicines Targeting the Tumor Microenvironment. Cancer J. (Sudbury Mass.) 2015, 21, 314–321. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Y.; Chen, D.; Qiu, F.; Ma, C.; Jin, X.; Zhu, X.; Zhou, G.; Zhang, Z. pH-Responsive Aerobic Nanoparticles for Effective Photodynamic Therapy. Theranostics 2017, 7, 4537–4550. [Google Scholar] [CrossRef]
- Koide, H. Design of Synthetic Polymer Nanoparticles That Capture and Neutralize Target Molecules. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2021, 141, 1079–1086. [Google Scholar] [CrossRef]
- Chuanjun Liu, C.Z.; Zhang, X. Application and Progress of Functional Peptides in Tumor Therapy. Univ. Chem. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Berthier, C.; Marti, H.P. Metzincins, Including Matrix Metalloproteinases and Meprin, in Kidney Transplantation. Swiss Med. Wkly. 2006, 136, 789–794. [Google Scholar] [CrossRef]
- Madsen, M.A.; Deryugina, E.I.; Niessen, S.; Cravatt, B.F.; Quigley, J.P. Activity-Based Protein Profiling Implicates Urokinase Activation as a Key Step in Human Fibrosarcoma Intravasation. J. Biol. Chem. 2006, 281, 15997–16005. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Matrix Metalloproteinases (MMPs) in Health and Disease: An Overview. Front. Biosci. A J. Virtual Libr. 2006, 11, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix Metalloproteinases in Tumor Invasion and Metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Patterns of MMP-2 and MMP-9 Expression in Human Cancer Cell Lines. Oncol. Rep. 2009, 21, 1323–1333. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Zhou, Y.; Lin, J.; Gao, W.; Yang, T.; Jin, J.; Zhang, L.; Nagle, D.G.; Zhang, W.; et al. Polymer Chimera of Stapled Oncolytic Peptide Coupled with Anti-PD-L1 Peptide Boosts Immunotherapy of Colorectal Cancer. Theranostics 2022, 12, 3456–3473. [Google Scholar] [CrossRef]
- Mort, J.S.; Buttle, D.J. Cathepsin, B. J. Biochem. Cell Biol. 1997, 29, 715–720. [Google Scholar] [CrossRef]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple Roles in Cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-Responsive Peptide Dendrimer-Gemcitabine Conjugate as a Controlled-Release Drug Delivery Vehicle with Enhanced Antitumor Efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, D.; Luo, K.; Li, L.; Gu, Z. Biodegradable and Amphiphilic Block Copolymer-Doxorubicin Conjugate as Polymeric Nanoscale Drug Delivery Vehicle for Breast Cancer Therapy. Biomaterials 2013, 34, 8430–8443. [Google Scholar] [CrossRef]
- Chen, X.; Lee, D.; Yu, S.; Kim, G.; Lee, S.; Cho, Y.; Jeong, H.; Nam, K.T.; Yoon, J. In vivo near-Infrared Imaging and Phototherapy of Tumors Using a Cathepsin B-Activated Fluorescent Probe. Biomaterials 2017, 122, 130–140. [Google Scholar] [CrossRef]
- Du, H.; Zhao, S.; Wang, Y.; Wang, Z.; Chen, B.; Yan, Y.; Yin, Q.; Liu, D.; Wan, F.; Zhang, Q.; et al. pH/Cathepsin B Hierarchical-Responsive Nanoconjugates for Enhanced Tumor Penetration and Chemo-Immunotherapy. Adv. Funct. Mater. 2020, 30, 2003757. [Google Scholar] [CrossRef]
- Hug, H.; Los, M.; Hirt, W.; Debatin, K.M. Rhodamine 110-Linked Amino Acids and Peptides as Substrates to Measure Caspase Activity Upon Apoptosis Induction in Intact Cells. Biochemistry 1999, 38, 13906–13911. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and Their Substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Barnett, E.M.; Zhang, X.; Maxwell, D.; Chang, Q.; Piwnica-Worms, D. Single-Cell Imaging of Retinal Ganglion Cell Apoptosis with a Cell-Penetrating, Activatable Peptide Probe in an in Vivo Glaucoma Model. Proc. Natl. Acad. Sci. USA 2009, 106, 9391–9396. [Google Scholar] [CrossRef]
- Song, W.; Kuang, J.; Li, C.X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.Z. Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Chen, W.; Meng, F.; Li, F.; Ji, S.J.; Zhong, Z. pH-Responsive Biodegradable Micelles Based on Acid-Labile Polycarbonate Hydrophobe: Synthesis and Triggered Drug Release. Biomacromolecules 2009, 10, 1727–1735. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Zhao, T.; Li, Y.; Su, L.C.; Wang, Z.; Huang, G.; Sumer, B.D.; Gao, J. Ultra-pH-Sensitive Nanoprobe Library with Broad pH Tunability and Fluorescence Emissions. J. Am. Chem. Soc. 2014, 136, 11085–11092. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and Its Measurement. J. Nucl. Med. Off. Public Soc. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef]
- Kellum, J.A. Determinants of Blood pH in Health and Disease. Crit. Care 2000, 4, 6–14. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef]
- Pang, X.; Jiang, Y.; Xiao, Q.; Leung, A.W.; Hua, H.; Xu, C. pH-Responsive Polymer-Drug Conjugates: Design and Progress. J. Control. Release Off. J. Control. Release Soc. 2016, 222, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhao, R.; Wang, B.; Sun, X.; Guo, X.; Tan, S.; Liu, W. Advanced Nano-Carriers for Anti-Tumor Drug Loading. Front. Oncol. 2021, 11, 758143. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal Escape Pathways for Delivery of Biologicals. J. Control. Release Off. J. Control. Release Soc. 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive Polymers: Synthesis, Properties and Applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, X.; Feng, T.; Lin, L.; Guo, Z.; Xia, J.; Tian, H.; Chen, X. Charge-Conversional Zwitterionic Copolymer as pH-Sensitive Shielding System for Effective Tumor Treatment. Acta Biomater. 2015, 26, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Uthaman, S.; John, J.V.; Lee, H.R.; Lee, S.J.; Park, H.; Park, I.K.; Suh, H.; Kim, I. Poly(Pega)-B-Poly(L-Lysine)-B-Poly(L-Histidine) Hybrid Vesicles for Tumoral pH-Triggered Intracellular Delivery of Doxorubicin Hydrochloride. ACS Appl. Mater. Interfaces 2015, 7, 21770–21779. [Google Scholar] [CrossRef]
- Liao, J.; Peng, H.; Liu, C.; Li, D.; Yin, Y.; Lu, B.; Zheng, H.; Wang, Q. Dual pH-Responsive-Charge-Reversal Micelle Platform for Enhanced Anticancer Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111527. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Wu, X.; Yu, G.; Luo, C.; Maeda, A.; Zhang, N.; Sun, D.; Zhou, Z.; Puntel, A.; Palczewski, K.; Lu, Z.R. Synthesis and Evaluation of a Nanoglobular Dendrimer 5-Aminosalicylic Acid Conjugate with a Hydrolyzable Schiff Base Spacer for Treating Retinal Degeneration. ACS Nano 2014, 8, 153–161. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Xiang, Z.; Zhang, H.; Wang, Y.; Zhang, X. A pH-Responsive Polymer Linked with Immunomodulatory Drugs: Synthesis, Characteristics and in Vitro Biocompatibility. J. Appl. Toxicol. JAT 2021, 41, 724–735. [Google Scholar] [CrossRef]
- Kauffman, A.C.; Piotrowski-Daspit, A.S.; Nakazawa, K.H.; Jiang, Y.; Datye, A.; Saltzman, W.M. Tunability of Biodegradable Poly(Amine- Co-Ester) Polymers for Customized Nucleic Acid Delivery and Other Biomedical Applications. Biomacromolecules 2018, 19, 3861–3873. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Statz, A.R.; Rho, J.; Park, T.G.; Messersmith, P.B. Substrate-Independent Layer-by-Layer Assembly by Using Mussel-Adhesive-Inspired Polymers. Adv. Mater. 2008, 20, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Fahira, A.I.; Amalia, R.; Barliana, M.I.; Gatera, V.A.; Abdulah, R. Polyethyleneimine (PEI) as a Polymer-Based Co-Delivery System for Breast Cancer Therapy. Breast Cancer (Dove Med. Press) 2022, 14, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Jiang, X.; Yang, T.; Xu, H.; Xie, Q.; Hu, M.; Yang, C.; Kong, L.; Zhang, Z. Enhancing Cancer Chemo-Immunotherapy by Biomimetic Nanogel with Tumor Targeting Capacity and Rapid Drug-Releasing in Tumor Microenvironment. Acta Pharm. Sinica. B 2022, 12, 2550–2567. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, J.; He, Y.; Cresswell, G.M.; Lanman, N.A.; Lyle, L.T.; Ratliff, T.L.; Yeo, Y. A Single Local Delivery of Paclitaxel and Nucleic Acids Via an Immunoactive Polymer Eliminates Tumors and Induces Antitumor Immunity. Proc. Natl. Acad. Sci. USA 2022, 119, e2122595119. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, W.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. Sequentially pH-Responsive Drug-Delivery Nanosystem for Tumor Immunogenic Cell Death and Cooperating with Immune Checkpoint Blockade for Efficient Cancer Chemoimmunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 43963–43974. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Synthesis and Therapeutic Potential of Imidazole Containing Compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Chen, J.X.; Wang, M.; Tian, H.H.; Chen, J.H. Hyaluronic Acid and Polyethylenimine Self-Assembled Polyion Complexes as pH-Sensitive Drug Carrier for Cancer Therapy. Colloids Surf. B Biointerfaces 2015, 134, 81–87. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as Potential Anticancer Agents. MedChemComm 2017, 8, 1742–1773. [Google Scholar] [CrossRef]
- Maravajjala, K.S.; Swetha, K.L.; Roy, A. pH-Responsive Nanoparticles for Multidimensional Combined Chemo-Immunotherapy of Cancer. J. Pharm. Sci. 2022, 111, 2353–2368. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone Linkages in pH Responsive Drug Delivery Systems. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Howard, M.D.; Ponta, A.; Eckman, A.; Jay, M.; Bae, Y. Polymer Micelles with Hydrazone-Ester Dual Linkers for Tunable Release of Dexamethasone. Pharm. Res. 2011, 28, 2435–2446. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lee, T.Y.; Fu, P.C.; Lo, C.L.; Chiang, Y.T. Multifunctional Polymer Nanoparticles for Dual Drug Release and Cancer Cell Targeting. Polymers 2017, 9, 213. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Qiao, Z.-Y.; Wang, H. Polymers with Tertiary Amine Groups for Drug Delivery and Bioimaging. Sci. China Chem. 2016, 59, 991–1002. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Wang, X.; Lin, Z.; Yan, M.; Wang, W.; Dong, A.; Zhang, J.; Huang, P.; Wang, C. pH-Sensitive Polycations for Sirna Delivery: Effect of Asymmetric Structures of Tertiary Amine Groups. Macromol. Biosci. 2021, 21, e2100025. [Google Scholar] [CrossRef]
- Ueda, H.; Wakabayashi, S.; Kikuchi, J.; Ida, Y.; Kadota, K.; Tozuka, Y. Anomalous Role Change of Tertiary Amino and Ester Groups as Hydrogen Acceptors in Eudragit E Based Solid Dispersion Depending on the Concentration of Naproxen. Mol. Pharm. 2015, 12, 1050–1061. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, B.; Wang, D.; Sun, F.; Song, R.; Shao, Q.; Wang, H.; Yu, H.; Li, Y. Engineering Polymeric Prodrug Nanoplatform for Vaccination Immunotherapy of Cancer. Nano Lett. 2020, 20, 4393–4402. [Google Scholar] [CrossRef]
- Banks, S.R.; Enck, K.; Wright, M.; Opara, E.C.; Welker, M.E. Chemical Modification of Alginate for Controlled Oral Drug Delivery. J. Agric. Food Chem. 2019, 67, 10481–10488. [Google Scholar] [CrossRef]
- Liu, B.; Thayumanavan, S. Substituent Effects on the pH Sensitivity of Acetals and Ketals and Their Correlation with Encapsulation Stability in Polymeric Nanogels. J. Am. Chem. Soc. 2017, 139, 2306–2317. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, X.; Jia, L.; Ma, C.; Song, R.; Deng, Y.; Hu, X.; Sun, W. Acetal-Linked Paclitaxel Polymeric Prodrug Based on Functionalized mPEG-PCL Diblock Polymer for pH-Triggered Drug Delivery. Polymers 2017, 9, 698. [Google Scholar] [CrossRef]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.; Lambrecht, B.N.; et al. pH-Degradable Imidazoquinoline-Ligated Nanogels for Lymph Node-Focused Immune Activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiang, R.; Wen, Y.; Xu, G.; Wang, C.; Luo, S.; Yin, T.; Wei, X.; Shao, B.; Liu, N.; et al. A Whole-Cell Tumor Vaccine Modified to Express Fibroblast Activation Protein Induces Antitumor Immunity against Both Tumor Cells and Cancer-Associated Fibroblasts. Sci. Rep. 2015, 5, 14421. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent Advances in the Redox-Responsive Drug Delivery Nanoplatforms: A Chemical Structure and Physical Property Perspective. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111536. [Google Scholar] [CrossRef] [PubMed]
- Doskey, C.M.; Buranasudja, V.; Wagner, B.A.; Wilkes, J.G.; Du, J.; Cullen, J.J.; Buettner, G.R. Tumor Cells Have Decreased Ability to Metabolize H2O2: Implications for Pharmacological Ascorbate in Cancer Therapy. Redox Biol. 2016, 10, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tao, R.; Ding, D.; Kong, D.; Fan, A.; Wang, Z.; Zhao, Y. Ratiometric Co-Delivery of Multiple Chemodrugs in a Single Nanocarrier. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 107, 16–23. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Chitrapriya, N.; Poudal, B.K.; Choi, J.Y.; Kim, S.T.; Youn, Y.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; et al. Engineering of Cell Microenvironment-Responsive Polypeptide Nanovehicle Co-Encapsulating a Synergistic Combination of Small Molecules for Effective Chemotherapy in Solid Tumors. Acta Biomater. 2017, 48, 131–143. [Google Scholar] [CrossRef]
- Fu, S.; Rempson, C.M.; Puche, V.; Zhao, B.; Zhang, F. Construction of Disulfide Containing Redox-Responsive Polymeric Nanomedicine. Methods (San Diego Calif.) 2022, 199, 67–79. [Google Scholar] [CrossRef]
- Xiao, C.; Ding, J.; Ma, L.; Yang, C.; Zhuang, X.; Chen, X. Synthesis of Thermal and Oxidation Dual Responsive Polymer for Reactive Oxygen Species (ROS)-Triggered Drug Release. Polym. Chem. 2014, 6, 738–747. [Google Scholar] [CrossRef]
- Mirhadi, E.; Mashreghi, M.; Faal Maleki, M.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-Sensitive Nanoscale Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of Gellan-Cholesterol Nanohydrogels Embedding Baicalin and Evaluation of Their Wound Healing Activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef]
- Gong, C.; Shan, M.; Li, B.; Wu, G. A pH and Redox Dual Stimuli-Responsive Poly(Amino Acid) Derivative for Controlled Drug Release. Colloids Surf. B Biointerfaces 2016, 146, 396–405. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, R.F. Enhancing Cancer Immunotherapy by Intracellular Delivery of Cell-Penetrating Peptides and Stimulation of Pattern-Recognition Receptor Signaling. Adv. Immunol. 2012, 114, 151–176. [Google Scholar] [CrossRef]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; et al. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, R.; Huang, Y.; Zhao, W.; Li, J.; Zhang, X.; Wang, P.; Venkataramanan, R.; Fan, J.; Xie, W.; et al. An Immunostimulatory Dual-Functional Nanocarrier That Improves Cancer Immunochemotherapy. Nat. Commun. 2016, 7, 13443. [Google Scholar] [CrossRef]
- Sun, J.J.; Chen, Y.C.; Huang, Y.X.; Zhao, W.C.; Liu, Y.H.; Venkataramanan, R.; Lu, B.F.; Li, S. Programmable Co-Delivery of the Immune Checkpoint Inhibitor NLG919 and Chemotherapeutic Doxorubicin Via a Redox-Responsive Immunostimulatory Polymeric Prodrug Carrier. Acta Pharmacol. Sin. 2017, 38, 823–834. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; et al. Disulfide Bond-Driven Oxidation- and Reduction-Responsive Prodrug Nanoassemblies for Cancer Therapy. Nano Lett. 2018, 18, 3643–3650. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the Impact of Sulfur/Selenium/Carbon Linkages on Prodrug Nanoassemblies for Cancer Therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef]
- Li, T.; Pan, S.; Gao, S.; Xiang, W.; Sun, C.; Cao, W.; Xu, H. Diselenide-Pemetrexed Assemblies for Combined Cancer Immuno-, Radio-, and Chemotherapies. Angew. Chem. 2020, 59, 2700–2704. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the Nk Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef]
- Bikram, M.; West, J.L. Thermo-Responsive Systems for Controlled Drug Delivery. Expert Opin. Drug Deliv. 2008, 5, 1077–1091. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Liu, P. Possibility of Active Targeting to Tumor by Local Hyperthermia with Temperature-Sensitive Nanoparticles. Med. Hypotheses 2008, 71, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Bobbala, S.; Tamboli, V.; McDowell, A.; Mitra, A.K.; Hook, S. Novel Injectable Pentablock Copolymer Based Thermoresponsive Hydrogels for Sustained Release Vaccines. AAPS J. 2016, 18, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide Loaded Thermo-Responsive Hydrogel System Synergize with a Hydrogel Cancer Vaccine to Amplify Cancer Immunotherapy in a Prime-Boost Manner. Bioact. Mater. 2021, 6, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Chou, H.Y.; Chuang, S.H.; Lai, J.Y.; Chen, Y.S.; Wen, Y.H.; Yu, L.Y.; Lo, C.L. Preparation of Immunotherapy Liposomal-Loaded Thermal-Responsive Hydrogel Carrier in the Local Treatment of Breast Cancer. Polymers 2019, 11, 1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Duan, H.; Xu, W.; Sheng, G.; Sun, Z.; Chu, H. Light-Activated Nanomaterials for Tumor Immunotherapy. Front. Chem. 2022, 10, 1031811. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Baghaee-Ravari, S.; Ghazadeh, M.; Mirshekari, H.; Hamblin, M.R. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. J. Am. Chem. Soc. 2017, 139, 4584–4610. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Combined CD44- and CD25-Targeted near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. Res. 2020, 8, 345–355. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Jiang, G.; Liang, M.; Chen, C.; Yang, Z.; Zou, L. Progress in Advanced Nanotherapeutics for Enhanced Photodynamic Immunotherapy of Tumor. Theranostics 2022, 12, 5272–5298. [Google Scholar] [CrossRef]
- Nakajima, K.; Miyazaki, F.; Terada, K.; Takakura, H.; Suzuki, M.; Ogawa, M. Comparison of Low-Molecular-Weight Ligand and Whole Antibody in Prostate-Specific Membrane Antigen Targeted near-Infrared Photoimmunotherapy. Int. J. Pharm. 2021, 609, 121135. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Ou, W.; Jiang, L.; Thapa, R.K.; Soe, Z.C.; Poudel, K.; Chang, J.H.; Ku, S.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Combination of NIR Therapy and Regulatory T Cell Modulation Using Layer-by-Layer Hybrid Nanoparticles for Effective Cancer Photoimmunotherapy. Theranostics 2018, 8, 4574–4590. [Google Scholar] [CrossRef]
- Zhang, N.; Song, J.; Liu, Y.; Liu, M.; Zhang, L.; Sheng, D.; Deng, L.; Yi, H.; Wu, M.; Zheng, Y.; et al. Photothermal Therapy Mediated by Phase-Transformation Nanoparticles Facilitates Delivery of Anti-PD1 Antibody and Synergizes with Antitumor Immunotherapy for Melanoma. J. Control. Release 2019, 306, 15–28. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-Derived Biopolymers: Potential Platforms for Enzyme Immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Caillol, S. Special Issue “Natural Polymers and Biopolymers II”. Molecules 2020, 26, 112. [Google Scholar] [CrossRef]
- Castro, J.G.; Chin-Beckford, N. Crofelemer for the Symptomatic Relief of Non-Infectious Diarrhea in Adult Patients with HIV/AIDS on Anti-Retroviral Therapy. Expert Rev. Clin. Pharmacol. 2015, 8, 683–690. [Google Scholar] [CrossRef]
- Garcia-Martinez, R.; Caraceni, P.; Bernardi, M.; Gines, P.; Arroyo, V.; Jalan, R. Albumin: Pathophysiologic Basis of Its Role in the Treatment of Cirrhosis and Its Complications. Hepatology 2013, 58, 1836–1846. [Google Scholar] [CrossRef]
- Rozga, J.; Piątek, T.; Małkowski, P. Human Albumin: Old, New, and Emerging Applications. Ann. Transplant. 2013, 18, 205–217. [Google Scholar] [CrossRef]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A., Jr. Albumin Nanovectors in Cancer Therapy and Imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef]
- Tiwari, R.; Sethiya, N.K.; Gulbake, A.S.; Mehra, N.K.; Murty, U.S.N.; Gulbake, A. A Review on Albumin as a Biomaterial for Ocular Drug Delivery. Int. J. Biol. Macromol. 2021, 191, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kim, K.H.; Yoo, J.; Li, X.; Kwon, N.; Jeon, Y.H.; Shin, S.K.; Han, S.S.; Lee, D.S.; Yoon, J. A Nanostructured Phthalocyanine/Albumin Supramolecular Assembly for Fluorescence Turn-on Imaging and Photodynamic Immunotherapy. ACS Nano 2022, 16, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.M.; Poudel, K.; Ou, W.; Phung, C.D.; Nguyen, H.T.; Nguyen, B.L.; Karmacharya, P.; Pandit, M.; Chang, J.H.; Jeong, J.H.; et al. Combination Chemotherapeutic and Immune-Therapeutic Anticancer Approach Via Anti-PD-L1 Antibody Conjugated Albumin Nanoparticles. Int. J. Pharm. 2021, 605, 120816. [Google Scholar] [CrossRef]
- Boran, G.; Regenstein, J.M. Fish Gelatin. Adv. Food Nutr. Res. 2010, 60, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Estevez, J.; Yang, J.D.; Leong, J.; Nguyen, P.; Giama, N.H.; Zhang, N.; Ali, H.A.; Lee, M.H.; Cheung, R.; Roberts, L.; et al. Clinical Features Associated with Survival Outcome in African-American Patients with Hepatocellular Carcinoma. Am. J. Gastroenterol. 2019, 114, 80–88. [Google Scholar] [CrossRef]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive Hydrogel Releasing Nitric Oxide Donor and Anti-CTLA-4 Micelles for Anti-Tumor Immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.L.; Wang, H.Q.; Pan, Y.; Chen, L.; Wu, H.; Wu, X.; Zhao, C.; Rao, L.; Liu, B.; Sun, Z.J. Gelatinase-Sensitive Nanoparticles Loaded with Photosensitizer and Stat3 Inhibitor for Cancer Photothermal Therapy and Immunotherapy. J. Nanobiotechnology 2021, 19, 379. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Duan, S.; Davies, N.M.; Forrest, M.L. CD44-Tropic Polymeric Nanocarrier for Breast Cancer Targeted Rapamycin Chemotherapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Chen, T.; Huang, Y.; Xiao, Z.; Jin, Y. Chemo-Photothermal Immunotherapy for Eradication of Orthotopic Tumors and Inhibition of Metastasis by Intratumoral Injection of Polydopamine Versatile Hydrogels. Acta Pharm. Sinica B 2022, 12, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, J.; Mao, Y.; Zhao, Q.; Chen, C.; Han, J.; Han, M.; Yuan, H.; Wang, S. A Mutually Beneficial Macrophages-Mediated Delivery System Realizing Photo/Immune Therapy. J. Control. Release 2022, 347, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosio, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-Mediated Stimulation of Macrophage Function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Alizadeh, L.; Zarebkohan, A.; Salehi, R.; Ajjoolabady, A.; Rahmati-Yamchi, M. Chitosan-Based Nanotherapeutics for Ovarian Cancer Treatment. J. Drug Target. 2019, 27, 839–852. [Google Scholar] [CrossRef]
- Mushtaq, A.; Li, L.; Anitha, A.; Grøndahl, L. Chitosan Nanomedicine in Cancer Therapy: Targeted Delivery and Cellular Uptake. Macromol. Biosci. 2021, 21, e2100005. [Google Scholar] [CrossRef]
- Yang, X.; Yu, T.; Zeng, Y.; Lian, K.; Zhou, X.; Ke, J.; Li, Y.; Yuan, H.; Hu, F. pH-Responsive Biomimetic Polymeric Micelles as Lymph Node-Targeting Vaccines for Enhanced Antitumor Immune Responses. Biomacromolecules 2020, 21, 2818–2828. [Google Scholar] [CrossRef]
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure-Immunomodulatory Activity Relationships of Dietary Polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341. [Google Scholar] [CrossRef]
- Geshi, N.; Petersen, B.L.; Scheller, H.V. Toward Tailored Synthesis of Functional Polysaccharides in Plants. Ann. N. Y. Acad. Sci. 2010, 1190, 50–57. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Varghese, S.; Joseph, M.M.; Aravind, S.R.; Unnikrishnan, B.S.; Pillai, K.R.; Sreelekha, T.T. Immunostimulatory Plant Polysaccharides Impede Cancer Progression and Metastasis by Avoiding Off-Target Effects. Int. Immunopharmacol. 2019, 73, 280–292. [Google Scholar] [CrossRef]
- Jain, A.K.; Sahu, H.; Mishra, K.; Thareja, S. Mannose Conjugated Starch Nanoparticles for Preferential Targeting of Liver Cancer. Curr. Drug Deliv. 2021, 18, 369–380. [Google Scholar] [CrossRef]
- Yassaroh, Y.; Woortman, A.J.J.; Loos, K. Physicochemical Properties of Heat-Moisture Treated, Stearic Acid Complexed Starch: The Effect of Complexation Time and Temperature. Int. J. Biol. Macromol. 2021, 175, 98–107. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Parrot-Lopez, H.; Perret, F.; Bertino-Ghera, B. Amphiphilic Cyclodextrins and Their Applications. Preparation of Nanoparticles Based on Amphiphilic Cyclodextrins for Biomedical Applications. Ann. Pharm. Fr. 2010, 68, 12–26. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-Based Delivery Systems for Chemotherapeutic Anticancer Drugs: A Review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.; Liu, X.; Xu, Y.; Zhao, J.; Si, X.; Li, H.; Huang, Z.; Wang, Z.; Tang, Z.; et al. Supramolecular Assembled Programmable Nanomedicine as in Situ Cancer Vaccine for Cancer Immunotherapy. Adv. Mater. 2021, 33, e2007293. [Google Scholar] [CrossRef] [PubMed]
- Paraman, I.; Lamsal, B.P. Recovery and Characterization of A-Zein from Corn Fermentation Coproducts. J. Agric. Food Chem. 2011, 59, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.; Freag, M.; Mamdouh, H.; Elkhodairy, K. Zein-Based Nanocarriers as Potential Natural Alternatives for Drug and Gene Delivery: Focus on Cancer Therapy. Curr. Pharm. Des. 2017, 23, 5261–5271. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Duan, W.; Lee, B.J.; Tran, T.T.D. The Use of Zein in the Controlled Release of Poorly Water-Soluble Drugs. Int. J. Pharm. 2019, 566, 557–564. [Google Scholar] [CrossRef]
- Lee, S.; Alwahab, N.S.; Moazzam, Z.M. Zein-Based Oral Drug Delivery System Targeting Activated Macrophages. Int. J. Pharm. 2013, 454, 388–393. [Google Scholar] [CrossRef]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.Y.; Wong, M.S.; Wang, Y. Doxorubicin-Loaded Biodegradable Self-Assembly Zein Nanoparticle and Its Anti-Cancer Effect: Preparation, in Vitro Evaluation, and Cellular Uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef]
- Soe, Z.C.; Ou, W.; Gautam, M.; Poudel, K.; Kim, B.K.; Pham, L.M.; Phung, C.D.; Jeong, J.H.; Jin, S.G.; Choi, H.G.; et al. Development of Folate-Functionalized Pegylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel. Pharmaceutics 2019, 11, 562. [Google Scholar] [CrossRef]
- Qi, Z.; Pei, P.; Zhang, Y.; Chen, H.; Yang, S.; Liu, T.; Zhang, Y.; Yang, K. 131I-αDP-L1 Immobilized by Bacterial Cellulose for Enhanced Radio-Immunotherapy of Cancer. J. Control. Release 2022, 346, 240–249. [Google Scholar] [CrossRef]
- Takahashi, R.Y.U.; Castilho, N.A.S.; Silva, M.; Miotto, M.C.; Lima, A.O.S. Prospecting for Marine Bacteria for Polyhydroxyalkanoate Production on Low-Cost Substrates. Bioengineering 2017, 4, 60. [Google Scholar] [CrossRef]
- Iliou, K.; Kikionis, S.; Ioannou, E.; Roussis, V. Marine Biopolymers as Bioactive Functional Ingredients of Electrospun Nanofibrous Scaffolds for Biomedical Applications. Mar. Drugs 2022, 20, 314. [Google Scholar] [CrossRef]
- Rahman, M.A. Marine Skeletal Biopolymers and Proteins and Their Biomedical Application. Mar. Drugs 2021, 19, 389. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Nishiya, N.; Oku, Y.; Ishikawa, C.; Fukuda, T.; Dan, S.; Mashima, T.; Ushijima, M.; Furukawa, Y.; Sasaki, Y.; Otsu, K.; et al. Lamellarin 14, a Derivative of Marine Alkaloids, Inhibits the T790m/C797s Mutant Epidermal Growth Factor Receptor. Cancer Sci. 2021, 112, 1963–1974. [Google Scholar] [CrossRef]
- Park, H.B.; Hwang, J.; Lim, S.M.; Zhang, W.; Jin, J.O. Dendritic Cell-Mediated Cancer Immunotherapy with Ecklonia cava Fucoidan. Int. J. Biol. Macromol. 2020, 159, 941–947. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Yadav, D.; An, E.K.; Kwak, M.; Lee, P.C.; Jin, J.O. Enhancement of Immune Checkpoint Inhibitor-Mediated Anti-Cancer Immunity by Intranasal Treatment of Ecklonia cava Fucoidan against Metastatic Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9125. [Google Scholar] [CrossRef]
- Chung, C.H.; Lu, K.Y.; Lee, W.C.; Hsu, W.J.; Lee, W.F.; Dai, J.Z.; Shueng, P.W.; Lin, C.W.; Mi, F.L. Fucoidan-Based, Tumor-Activated Nanoplatform for Overcoming Hypoxia and Enhancing Photodynamic Therapy and Antitumor Immunity. Biomaterials 2020, 257, 120227. [Google Scholar] [CrossRef]
- Su, X.; Cao, Y.; Liu, Y.; Ouyang, B.; Ning, B.; Wang, Y.; Guo, H.; Pang, Z.; Shen, S. Localized Disruption of Redox Homeostasis Boosting Ferroptosis of Tumor by Hydrogel Delivery System. Mater. Today. Bio. 2021, 12, 100154. [Google Scholar] [CrossRef]
- Smith, A.M.; Senior, J.J. Alginate Hydrogels with Tuneable Properties. Adv. Biochem. Eng. Biotechnol. 2021, 178, 37–61. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted Antigen Delivery to Dendritic Cell Via Functionalized Alginate Nanoparticles for Cancer Immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef]
- Pei, M.; Li, H.; Zhu, Y.; Lu, J.; Zhang, C. In Vitro Evidence of Oncofetal Antigen and TLR-9 Agonist Co-Delivery by Alginate Nanovaccines for Liver Cancer Immunotherapy. Biomater. Sci. 2022, 10, 2865–2876. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An Overview on Red Algae Bioactive Compounds and Their Pharmaceutical Applications. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Hung, C.F.; Wu, T.C. Carrageenan as an Adjuvant to Enhance Peptide-Based Vaccine Potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Aipire, A.; Li, J.; Zhu, H.; Wang, Y.; Guo, W.; Li, X.; Yang, J.; Liu, C. Λ-Carrageenan Improves the Antitumor Effect of Dendritic Cellbased Vaccine. Oncotarget 2017, 8, 29996–30007. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Ibrahim, O.M.; Mohamed, M.A.; Farag, M.M.S.; Farrag, A.A.; El-Aassar, M.R. Alginate/κ-Carrageenan Oral Microcapsules Loaded with Agaricus bisporus Polysaccharides Mh751906 for Natural Killer Cells Mediated Colon Cancer Immunotherapy. Int. J. Biol. Macromol. 2022, 205, 385–395. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic Polymersomes Increase the Activity of Cyclic Dinucleotide STING Agonists to Enhance Cancer Immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zhou, B.; Zhou, F.; Murray, C.; Towner, R.A.; Smith, N.; Saunders, D.; Xie, G.; Chen, W.R. Pegylated Reduced-Graphene Oxide Hybridized with Fe3O4 Nanoparticles for Cancer Photothermal-Immunotherapy. J. Mater. Chem. B 2019, 7, 7406–7414. [Google Scholar] [CrossRef]
- Urbina, F.; Morales-Pison, S.; Maldonado, E. Enzymatic Protein Biopolymers as a Tool to Synthetize Eukaryotic Messenger Ribonucleic Acid (mRNA) with Uses in Vaccination, Immunotherapy and Nanotechnology. Polymers 2020, 12, 1633. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applicaions. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In Vitro Biosafety of Pro-Ecological Chitosan-Based Hydrogels Modified with Natural Substances. J. Biomed. Mater. Res. Part A 2019, 107, 2501–2511. [Google Scholar] [CrossRef]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-Specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, J.; Sun, H.; Li, J.; Wang, X. Polymer-Based Hydrogels with Local Drug Release for Cancer Immunotherapy. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 137, 111333. [Google Scholar] [CrossRef]


| Polymeric Platform | Active Component | Drug Product | Indication | Status | Reference or Identifier |
|---|---|---|---|---|---|
| Micelles | Paclitaxel | Genexol-PM | Breast cancer and small cell lung cancer | Marketed in Europe, Republic of Korea | [12] |
| Paclitaxel | Apealea | First relapse of platinum-sensitive epithelial ovarian cancer, primary peritoneal cancer, and fallopian tube cancer | Approved by EMA 1 | [13] | |
| Paclitaxel | Abraxane | Pancreatic cancer, NSCL and breast cancer | Approved by US FDA | [14] | |
| Docetaxel | - | Advanced solid tumors | Phase 2 (Not yet recruiting) | NCT05254665 | |
| Polymeric Nanoparticles | Leuprolide acetate | Eligard | Prostate cancer | Approved by US FDA 2 | [15] |
| iNKT 3 activator (ThrCer6, IMM60) 4; NY-ESO-1 5 cancer-testis antigen peptides | PRECIOUS-01 * | Advanced solid tumor | Phase 1 (Recruiting) | NCT04751786 | |
| Tumor lysate 6; GM-CSF 7; CpG 8 | WDVAX * | Melanoma | Phase 1(Active, not recruiting) | NCT01753089 | |
| Polymer–protein conjugate | Asparaginase | Pegaspargase (Oncaspar) | Acute lymphoblastic leukemia | Approved by US FDA | [16] |
| IL-15 receptor agonist | NKTR-255 * | Non-Hodgkin lymphoma; relapsed/refractory diffuse large B-cell lymphoma | Phase 2/3 (Recruiting) | NCT05664217 | |
| Polymer–drug conjugate | Paclitaxel | FID-007 | Advanced malignant solid neoplasm; refractory malignant solid neoplasm | Phase 1 (Recruiting) | NCT03537690 |
| Lidocaine | ST-01 | Postoperative pain; postsurgical pain | Phase 2 (Recruiting) | NCT05193227 | |
| Rotigotine | SER-214 | Parkinson’s disease | Phase 1 (Active, not recruiting) | NCT02579473 | |
| Ammonium molybdate | BP-C2 | Prostate cancer | Phase 1 (Not yet recruiting) | NCT04186585 | |
| Quercetin | Nano-QUT 9 | Oral cancer | Phase 2 (Not yet recruiting) | NCT05456022 | |
| Triptorelin pamoate | Decapeptyl | Prostate cancer | Phase 3 (Recruiting) | NCT05458856 | |
| Mitoxantrone | DHAD-PBCA- NPs | Hepatocellular carcinoma | Phase 2 (Recruiting) | [17] | |
| Doxorubicin | BA-003 | Hepatocellular carcinoma | Phase 3 (Recruiting) | [18] | |
| Docetaxel | ABI-008 | Prostate cancer | Phase 1/2 (Recruiting) | NCT00477529 | |
| DACHPt | ProLindac | Advanced ovarian cancer | Phase 2/3 (Recruiting) | [19] | |
| Rapamycin | ABI-009 | Solid tumors | Phase 1/2 (Completed) | NCT02494570 | |
| Paclitaxel | Nanotax | Peritoneal and neoplasms | Phase 1 (Completed) | NCT00666991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. https://doi.org/10.3390/pharmaceutics15030775
Yu Z, Shen X, Yu H, Tu H, Chittasupho C, Zhao Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics. 2023; 15(3):775. https://doi.org/10.3390/pharmaceutics15030775
Chicago/Turabian StyleYu, Zhecheng, Xingyue Shen, Han Yu, Haohong Tu, Chuda Chittasupho, and Yunqi Zhao. 2023. "Smart Polymeric Nanoparticles in Cancer Immunotherapy" Pharmaceutics 15, no. 3: 775. https://doi.org/10.3390/pharmaceutics15030775
APA StyleYu, Z., Shen, X., Yu, H., Tu, H., Chittasupho, C., & Zhao, Y. (2023). Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics, 15(3), 775. https://doi.org/10.3390/pharmaceutics15030775







