Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future
Abstract
1. Introduction
2. Historical Milestones Achieved in TPGS Developments
3. TPGS Characteristics and Synthesis
3.1. Novel Properties of TPGS That Enable It to Be Used as a Promising Excipient in Nanomedicine and Targeted Drug Delivery Systems
3.2. Key Role of Vitamin E and Its Derivatives for the Protection of Body Cells
3.3. TPGS in the Role of Solubilization Promoter
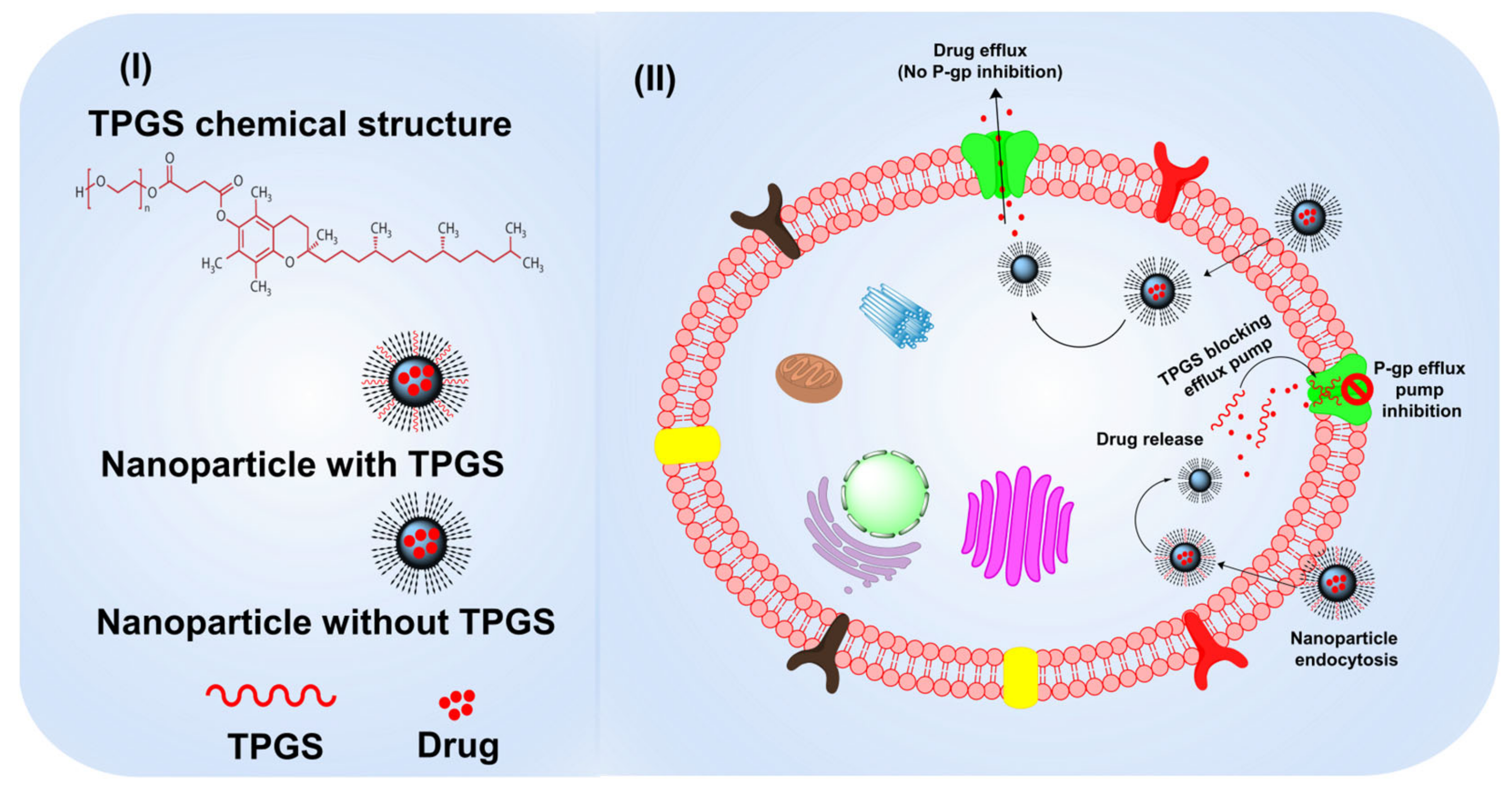
3.4. TPGS as a P-gp Modulator with Anticancer Properties
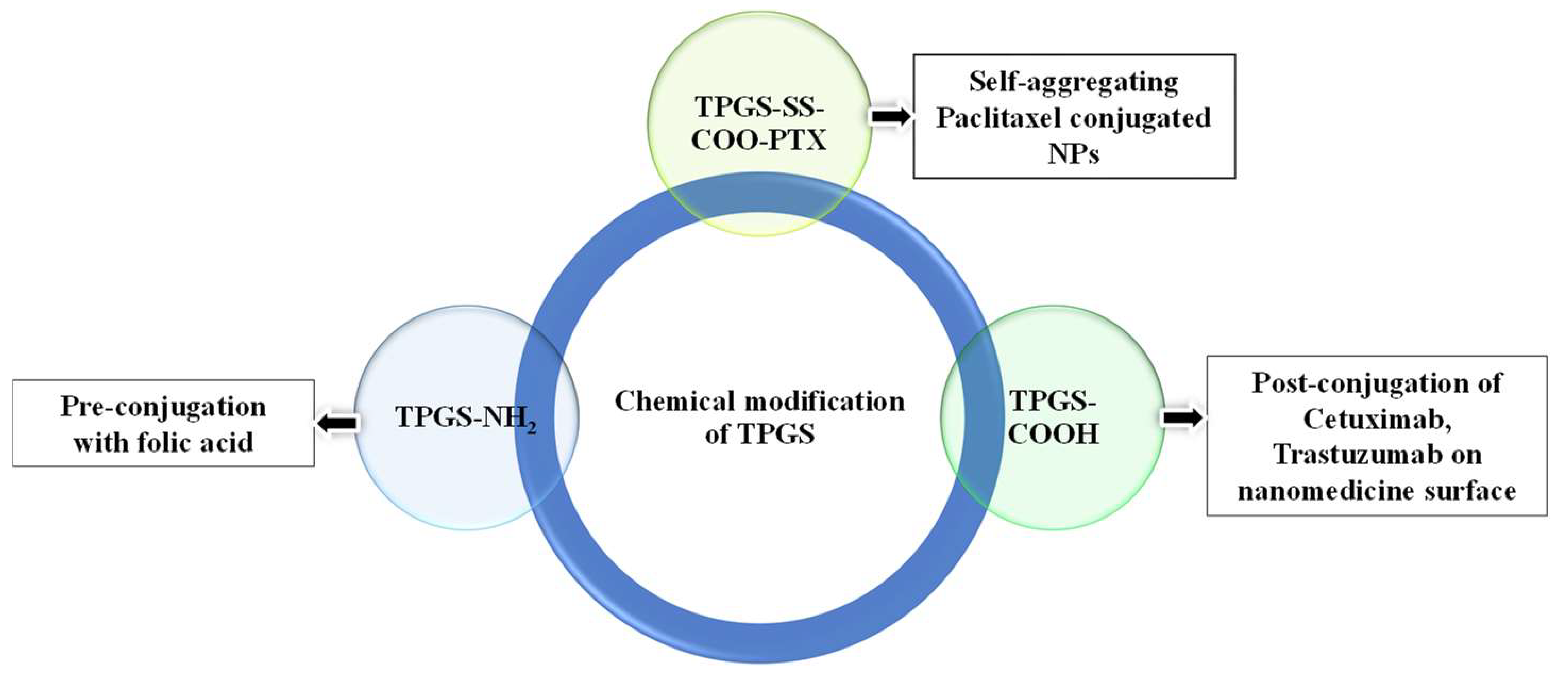
3.5. Chemical Modification of TPGS
3.6. Drug–TPGS Conjugate
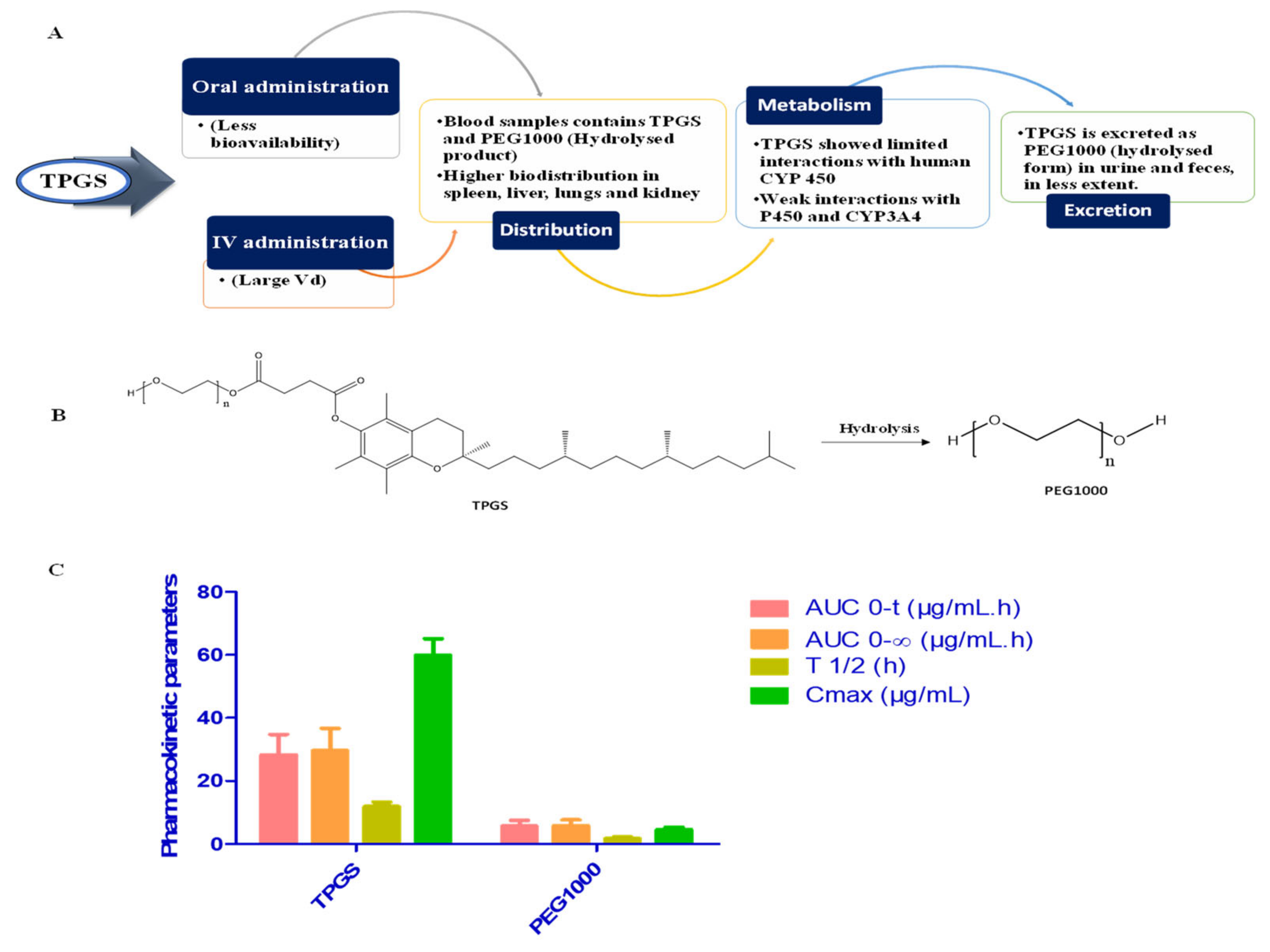
4. Pharmacokinetic and Safety Assessment of TPGS
5. Multifunctional TPGS-Copolymer-Based Nanomedicine
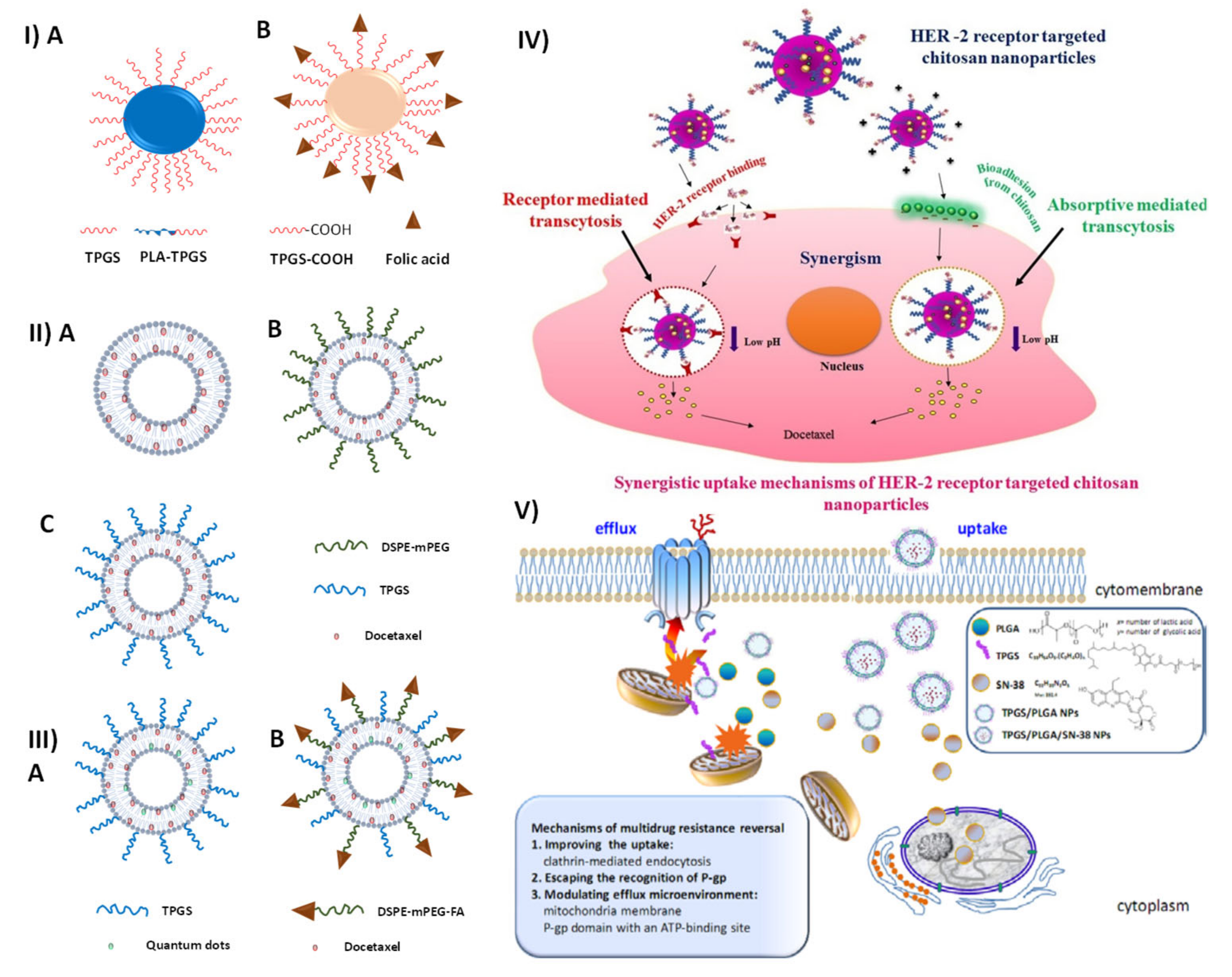
6. TPGS-Based Nanomedicine and Nanotheranostics
7. TPGS-Based Nanomaterials for Theranostics/Targeted Application
7.1. TPGS-Based Micelles for Nanotheranostics
7.2. TPGS-Based Liposomes for Nanotheranostics
7.3. TPGS-Based Polymeric Nanoparticles for Nanotheranostics
7.4. TPGS-Based Quantum Dots for Nanotheranostics
7.5. TPGS-Based Miscellaneous Agents for Nanotheranostics
8. Therapies Involving TPGS-Based Materials
8.1. Radiotherapy
8.2. Positron Emission Tomography
8.3. Photothermal Therapy
9. Randomized/Clinical Prospects of TPGS, and Its Patents
10. Regulatory Status of TPGS and Marketed Formulations
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luiz, M.T.; Di Filippo, L.D.; Alves, R.C.; Araújo, V.H.S.; Duarte, J.L.; Marchetti, J.M.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Pacl, H.T.; Tipper, J.L.; Sevalkar, R.R.; Crouse, A.; Crowder, C.; Ahmad, S.; Ahmad, A.; Holder, G.D.; Kuhlman, C.J.; Chinta, K.C.; et al. Water-soluble tocopherol derivatives inhibit SARS-CoV-2 RNA-dependent RNA polymerase. Biorxiv Prepr. Serv. Biol. 2021, 2021, 449251. [Google Scholar] [CrossRef]
- Mehata, A.K.; Viswanadh, M.K.; Solomon, V.R.; Muthu, M.S. Radionanotheranostics for breast cancer diagnosis and therapy: Recent advances and future opportunities. In Targeted Nanomedicine for Breast Cancer Therapy; Academic Press: Cambridge, MA, USA, 2022; pp. 465–508. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, S.; Feng, S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Mei, L.; Feng, S.S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef]
- Liu, Z.; Lansley, A.B.; Duong, T.N.; Smart, J.D.; Pannala, A.S. Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules 2022, 12, 1739. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kulkarni, S.A.; Raju, A.; Feng, S.S. Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 2012, 33, 3494–3501. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Kosuru, R.; Singh, S.K.; Prasad, C.B.; Narayan, G.; Muthu, M.S.; Singh, S. Resveratrol loaded PLGA: D-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016, 6, 74254–74268. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J.; Life, S. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. 2020, 24, 100503. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef]
- Pawde, D.M.; Viswanadh, M.K.; Mehata, A.K.; Sonkar, R.; Narendra; Poddar, S.; Burande, A.S.; Jha, A.; Yellappa Vajanthri, K.; Kumar Mahto, S.; et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi. Pharm. J. 2020, 28, 1616–1625. [Google Scholar] [CrossRef]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef]
- Rout, S.K.; Priya, V.; Setia, A.; Mehata, A.K.; Mohan, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Makeen, H.A.; Tambuwala, M.M.; et al. Mitochondrial targeting theranostic nanomedicine and molecular biomarkers for efficient cancer diagnosis and therapy. Biomed. Pharmacother. 2022, 153, 113451. [Google Scholar] [CrossRef]
- Mehata, A.K.; Dehari, D.; Gupta, A.; Rabin, D.C.; Miya, A. Multifunctional liquid crystal nanoparticles for cancer therapy. Curr. Nanomater. 2021, 6, 4–16. [Google Scholar] [CrossRef]
- Ventola, C.L. The nanomedicine revolution: Part 2: Current and future clinical applications. P T A Peer-Rev. J. Formul. Manag. 2012, 37, 582–591. [Google Scholar]
- Sharma, M.; Dube, T.; Chibh, S.; Kour, A.; Mishra, J.; Panda, J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 113–128. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Lobine, D.; Koay, A.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 649395. [Google Scholar] [CrossRef]
- Jo, S.D.; Ku, S.H.; Won, Y.Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzinska, M.; Leporatti, S.; Anissimov, Y.; Zamyatnin, A.A., Jr. Smart Nanotheranostics Responsive to Pathological Stimuli. Front. Bioeng. Biotechnol. 2020, 8, 503. [Google Scholar] [CrossRef]
- Verleger, R.; Hagenah, J.; Weiss, M.; Ewers, T.; Heberlein, I.; Pramstaller, P.P.; Siebner, H.R.; Klein, C. Responsiveness to distracting stimuli, though increased in Parkinson’s disease, is decreased in asymptomatic PINK1 and Parkin mutation carriers. Neuropsychologia 2010, 48, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Mehata, A.K.; Viswanadh, M.K. Upconversion nanotheranostics: Emerging designs for integration of diagnosis and therapy. Nanomedicine 2017, 12, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.; Yoon, J.; Chen, X. Cancer-Associated, Stimuli-Driven, Turn on Theranostics for Multimodality Imaging and Therapy. Adv. Mater. 2017, 29, 1606857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Jasim, A.M.; Jawad, M.J. Pharmaceutical Applications of Vitamin E TPGS. In Vitamin E in Health and Disease—Interactions, Diseases and Health Aspects; IntechOpen: London, UK, 2021; pp. 1–20. [Google Scholar]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Krasavage, W.J.; Terhaar, C.J. d-alpha-Tocopheryl poly(ethylene glycol) 1000 succinate. Acute toxicity, subchronic feeding, reproduction, and teratologic studies in the rat. J. Agric. Food Chem. 1977, 25, 273–278. [Google Scholar] [CrossRef]
- Sokol, R.J.; Heubi, J.E.; Butler-Simon, N.; McClung, H.J.; Lilly, J.R.; Silverman, A. Treatment of vitamin E deficiency during chronic childhood cholestasis with oral d-alpha-tocopheryl polyethylene glycol-1000 succinate. Gastroenterology 1987, 93, 975–985. [Google Scholar] [CrossRef]
- Chang, T.; Benet, L.Z.; Hebert, M.F. The effect of water-soluble vitamin E on cyclosporine pharmacokinetics in healthy volunteers. Clin. Pharmacol. Ther. 1996, 59, 297–303. [Google Scholar] [CrossRef]
- Savla, R.; Browne, J.; Plassat, V.; Wasan, K.M.; Wasan, E.K. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev. Ind. Pharm. 2017, 43, 1743–1758. [Google Scholar] [CrossRef]
- Robin, Y. Using tocophersolan for drug delivery. Pharm. Technol. 2015, 39, 48–52. [Google Scholar]
- De Melo-Diogo, D.; Pais-Silva, C.; Costa, E.C.; Louro, R.O.; Correia, I.J. D-α-tocopheryl polyethylene glycol 1000 succinate functionalized nanographene oxide for cancer therapy. Nanomedicine 2017, 12, 443–456. [Google Scholar] [CrossRef]
- Ke, W.T.; Lin, S.Y.; Ho, H.O.; Sheu, M.T. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J. Control. Release Off. J. Control. Release Soc. 2005, 102, 489–507. [Google Scholar] [CrossRef]
- Barakat, N.S. Etodolac-liquid-filled dispersion into hard gelatin capsules: An approach to improve dissolution and stability of etodolac formulation. Drug Dev. Ind. Pharm. 2006, 32, 865–876. [Google Scholar] [CrossRef]
- Yu, L.; Bridgers, A.; Polli, J.; Vickers, A.; Long, S.; Roy, A.; Winnike, R.; Coffin, M. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm. Res. 1999, 16, 1812–1817. [Google Scholar] [CrossRef]
- Sampath, M.; Pichaimani, A.; Kumpati, P.; Sengottuvelan, B. The remarkable role of emulsifier and chitosan, dextran and PEG as capping agents in the enhanced delivery of curcumin by nanoparticles in breast cancer cells. Int. J. Biol. Macromol. 2020, 162, 748–761. [Google Scholar] [CrossRef]
- Yuan, Z.; Yuan, Y.; Han, L.; Qiu, Y.; Huang, X.; Gao, F.; Fan, G.; Zhang, Y.; Tang, X.; He, X.; et al. Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/RGD conjugated TPGS mixed micelles demonstrated improved antitumor activity against drug-resistant colon cancer. Int. J. Nanomed. 2018, 13, 7533–7548. [Google Scholar] [CrossRef]
- Tang, J.; Fu, Q.; Wang, Y.; Racette, K.; Wang, D.; Liu, F. Vitamin E reverses multidrug resistance in vitro and in vivo. Cancer Lett. 2013, 336, 149–157. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, C.; Sun, W.; Cao, X.; Choi, G.; Choy, J.H.; Shi, X.; Guo, R. Doxorubicin Encapsulated in TPGS-Modified 2D-Nanodisks Overcomes Multidrug Resistance. Chemistry 2020, 26, 2470–2477. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.; Liu, W.; Gao, F.; Chen, Y. Targeting integrin-rich tumors with temoporfin-loaded vitamin-E-succinate-grafted chitosan oligosaccharide/d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles to enhance photodynamic therapy efficiency. Int. J. Pharm. 2017, 528, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, M.; Pei, Y.; Huang, Y.; Chen, Y.; Ma, F.; Lu, H.; Chen, J. Core-matched nanoassemblies for targeted co-delivery of chemotherapy and photosensitizer to treat drug-resistant cancer. Acta Biomater. 2019, 88, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Jia, H.R.; Duan, Q.Y.; Wu, F.G. Nanomedicines for combating multidrug resistance of cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1715. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P.; Han, J.; Davis, S.S. Advances in the use of tocols as drug delivery vehicles. Pharm. Res. 2006, 23, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Duhem, N.; Danhier, F.; Préat, V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 182, 33–44. [Google Scholar] [CrossRef]
- Chen, Y.; Yue, Q.; De, G.; Wang, J.; Li, Z.; Xiao, S.; Yu, H.; Ma, H.; Sui, F.; Zhao, Q. Inhibition of breast cancer metastasis by paclitaxel-loaded pH responsive poly(β-amino ester) copolymer micelles. Nanomedicine 2017, 12, 147–164. [Google Scholar] [CrossRef]
- Yan, H.; Du, X.; Wang, R.; Zhai, G. Progress in the study of D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) reversing multidrug resistance. Colloids Surf. B Biointerfaces 2021, 205, 111914. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current trends in the use of vitamin E-based micellar nanocarriers for anticancer drug delivery. Expert Opin. Drug Deliv. 2017, 14, 715–726. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Constantinou, C.; Papageorgis, P.; Constantinou, A.I. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem. Pharmacol. 2014, 89, 31–42. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Kappl, R.; Hüttermann, J.; Hyatt, J.A.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.M. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: Influence on ATPase activity and membrane fluidity. Mol. Pharm. 2007, 4, 465–474. [Google Scholar] [CrossRef]
- Gao, J.; Feng, S.S.; Guo, Y. Nanomedicine against multidrug resistance in cancer treatment. Nanomedicine 2012, 7, 465–468. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Chen, Q.; Hao, T.; Qiao, M.; Zhao, H.; Zhang, J.; Hu, H.; Zhao, X.; Chen, D.; et al. pH-sensitive nanoparticles of poly(L-histidine)-poly(lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef]
- Rege, B.D.; Kao, J.P.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Schaefer, U.F.; Edgar, K.J.; Wempe, M.F.; Lehr, C.M. Vitamin E TPGS P-glycoprotein inhibition mechanism: Influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010, 7, 642–651. [Google Scholar] [CrossRef]
- Hao, T.; Chen, D.; Liu, K.; Qi, Y.; Tian, Y.; Sun, P.; Liu, Y.; Li, Z. Micelles of d-α-Tocopheryl Polyethylene Glycol 2000 Succinate (TPGS 2K) for Doxorubicin Delivery with Reversal of Multidrug Resistance. ACS Appl. Mater. Interfaces 2015, 7, 18064–18075. [Google Scholar] [CrossRef]
- Wang, A.T.; Liang, D.S.; Liu, Y.J.; Qi, X.R. Roles of ligand and TPGS of micelles in regulating internalization, penetration and accumulation against sensitive or resistant tumor and therapy for multidrug resistant tumors. Biomaterials 2015, 53, 160–172. [Google Scholar] [CrossRef]
- Zhu, X.; Tsend-Ayush, A.; Yuan, Z.; Wen, J.; Cai, J.; Luo, S.; Yao, J.; Bian, J.; Yin, L.; Zhou, J.; et al. Glycyrrhetinic acid-modified TPGS polymeric micelles for hepatocellular carcinoma-targeted therapy. Int. J. Pharm. 2017, 529, 451–464. [Google Scholar] [CrossRef]
- Vikas; Viswanadh, M.K.; Mehata, A.K.; Sharma, V.; Priya, V.; Varshney, N.; Mahto, S.K.; Muthu, M.S. Bioadhesive chitosan nanoparticles: Dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr. Polym. 2021, 274, 118617. [Google Scholar] [CrossRef]
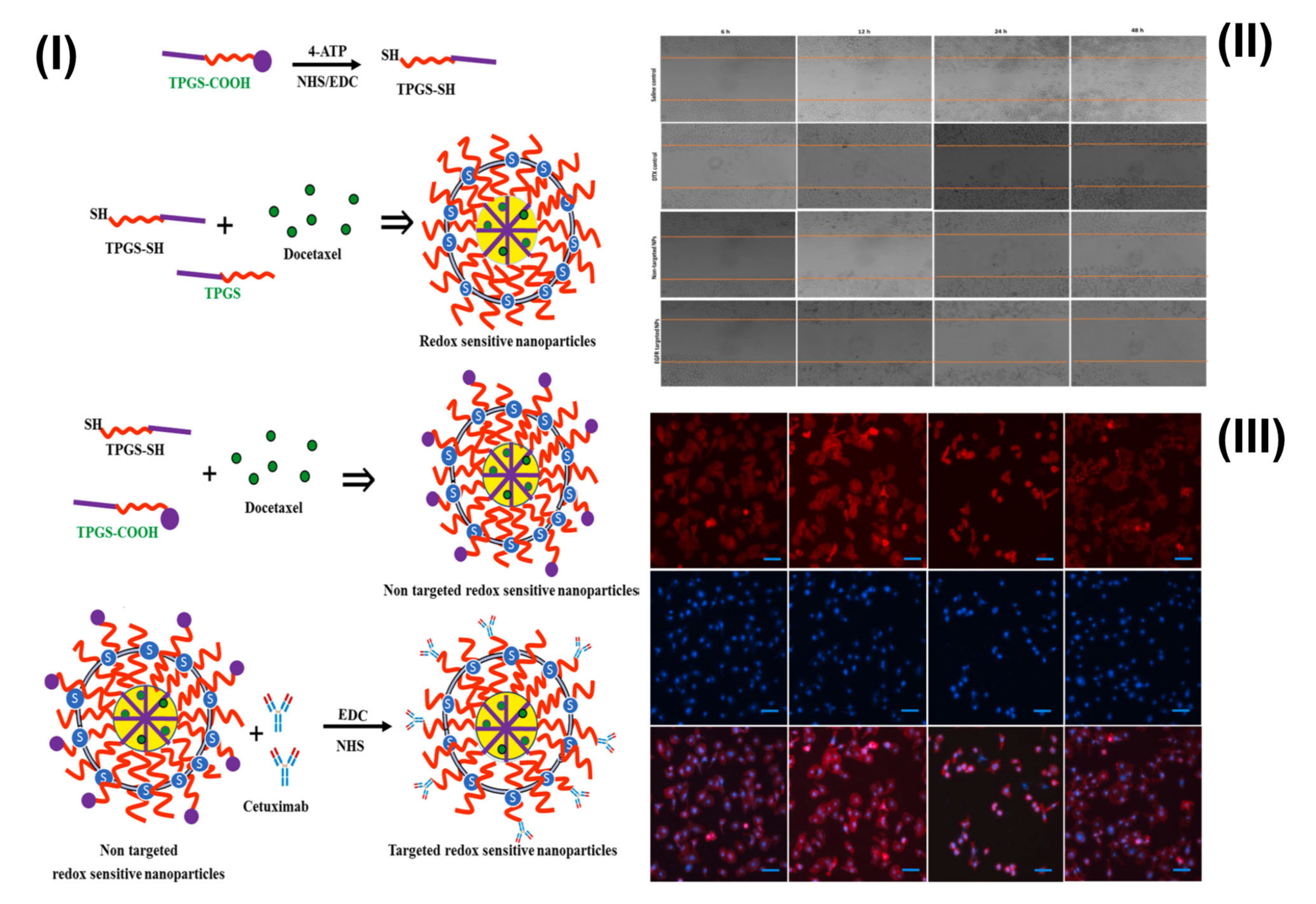
- Viswanadh, M.K.; Agrawal, N.; Azad, S.; Jha, A.; Poddar, S.; Mahto, S.K.; Muthu, M.S. Novel redox-sensitive thiolated TPGS based nanoparticles for EGFR targeted lung cancer therapy. Int. J. Pharm. 2021, 602, 120652. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Zhu, J.; Shen, L.; Zhang, Q.Y.; Zhu, H. Folic acid modified TPGS as a novel nano-micelle for delivery of nitidine chloride to improve apoptosis induction in Huh7 human hepatocellular carcinoma. BMC Pharmacol. Toxicol. 2021, 22, 1. [Google Scholar] [CrossRef]
- Cao, N.; Feng, S.S. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS): Conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials 2008, 29, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Guo, Y.; Zhuang, X.; Li, D.; Cheng, B.; Tan, S.; Zhang, Z. D-α-tocopherol polyethylene glycol succinate-based redox-sensitive paclitaxel prodrug for overcoming multidrug resistance in cancer cells. Mol. Pharm. 2014, 11, 3196–3209. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhao, J.; Feng, S.S. Vitamin E TPGS prodrug micelles for hydrophilic drug delivery with neuroprotective effects. Int. J. Pharm. 2012, 438, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mehata, A.; Bharti, S.; Singh, P.; Viswanadh, M.K.; Kumari, L.; Agrawal, P.; Singh, S.; Koch, B.; Muthu, M.S. Trastuzumab decorated TPGS-g-chitosan nanoparticles for targeted breast cancer therapy. Colloids Surf. B Biointerfaces 2019, 173, 366–377. [Google Scholar] [CrossRef] [PubMed]
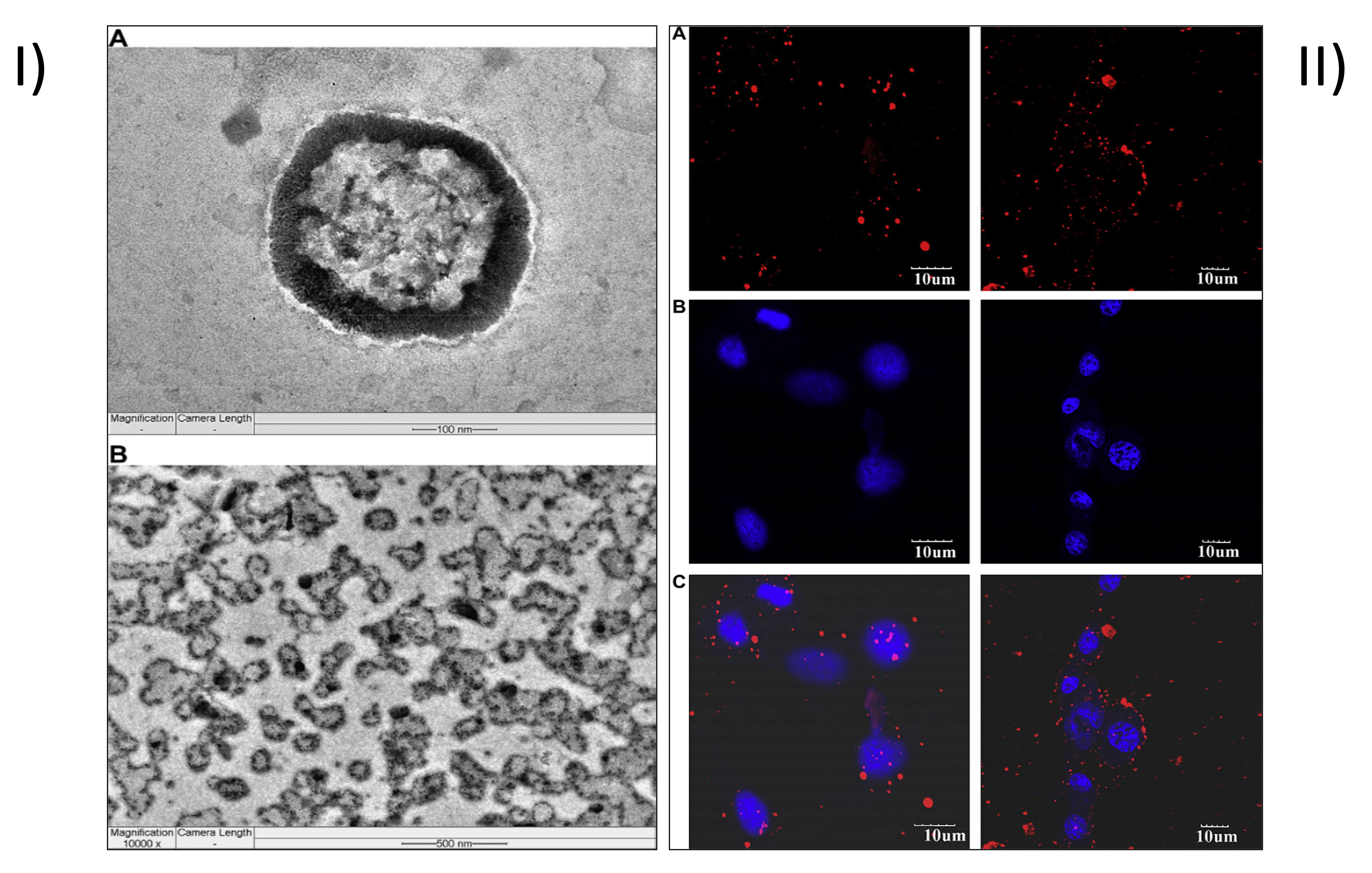
- Agrawal, P.; Sonali; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef]
- Agrawal, P.; Singh, R.P.; Sonali; Kumari, L.; Sharma, G.; Koch, B.; Rajesh, C.V.; Mehata, A.K.; Singh, S.; Pandey, B.L.; et al. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Engineering. C Mater. Biol. Appl. 2017, 74, 167–176. [Google Scholar] [CrossRef]
- Sonali; Agrawal, P.; Singh, R.P.; Rajesh, C.V.; Singh, S.; Vijayakumar, M.R.; Pandey, B.L.; Muthu, M.S. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: Preparation, characterization and brain distribution in rats. Drug Deliv. 2016, 23, 1788–1798. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, S.S. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: Synthesis, formulation, characterization and in vitro drug release. Biomaterials 2006, 27, 262–270. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V. Advances and applications of block-copolymer-based nanoformulations. Drug Discov. Today 2018, 23, 1139–1151. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, B.; Feng, S.S. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials 2010, 31, 9145–9155. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.; Guo, Y.; Feng, S.S. Nanomedicine strategies for sustained, controlled and targeted treatment of cancer stem cells. Nanomedicine 2016, 11, 3261–3282. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, S.H.; Gan, C.W.; Feng, S.S. In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm. Res. 2008, 25, 1925–1935. [Google Scholar] [CrossRef]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release Off. J. Control. Release Soc. 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 269–286. [Google Scholar] [CrossRef]
- Pan, J.; Feng, S.S. Targeted delivery of paclitaxel using folate-decorated poly(lactide)-vitamin E TPGS nanoparticles. Biomaterials 2008, 29, 2663–2672. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kulkarni, S.A.; Xiong, J.; Feng, S.S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int. J. Pharm. 2011, 421, 332–340. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Lu, Y.; Ding, L.Y.; Ron, W.T.; Liu, Y.Q.; Song, F.F.; Yu, S.Q. Alpha-tocopheryl polyethylene glycol succinate-emulsified poly(lactic-co-glycolic acid) nanoparticles for reversal of multidrug resistance in vitro. Nanotechnology 2012, 23, 495103. [Google Scholar] [CrossRef]
- Yong, H.; Bi, F.; Liu, J.; Qin, Y.; Bai, R.; Liu, J. Preparation and characterization of antioxidant packaging by chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and baicalein. Int. J. Biol. Macromol. 2020, 153, 836–845. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Zhu, Y.; Sun, C.; Adu-Frimpong, M.; Deng, W.; Yu, J.; Xu, X. Enhanced oral bioavailability of self-assembling curcumin-vitamin E prodrug-nanoparticles by co-nanoprecipitation with vitamin E TPGS. Drug Dev. Ind. Pharm. 2020, 46, 1800–1808. [Google Scholar] [CrossRef]
- Mohyeldin, S.M.; Samy, W.M.; Ragab, D.; Abdelmonsif, D.A.; Aly, R.G.; Elgindy, N.A. Hybrid lipid core chitosan-TPGS shell nanocomposites as a promising integrated nanoplatform for enhanced oral delivery of sulpiride in depressive disorder therapy. Int. J. Biol. Macromol. 2021, 188, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Anbharasi, V.; Cao, N.; Feng, S.S. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol succinate and folic acid as a prodrug for targeted chemotherapy. J. Biomed. Mater. Research. Part A 2010, 94, 730–743. [Google Scholar] [CrossRef]
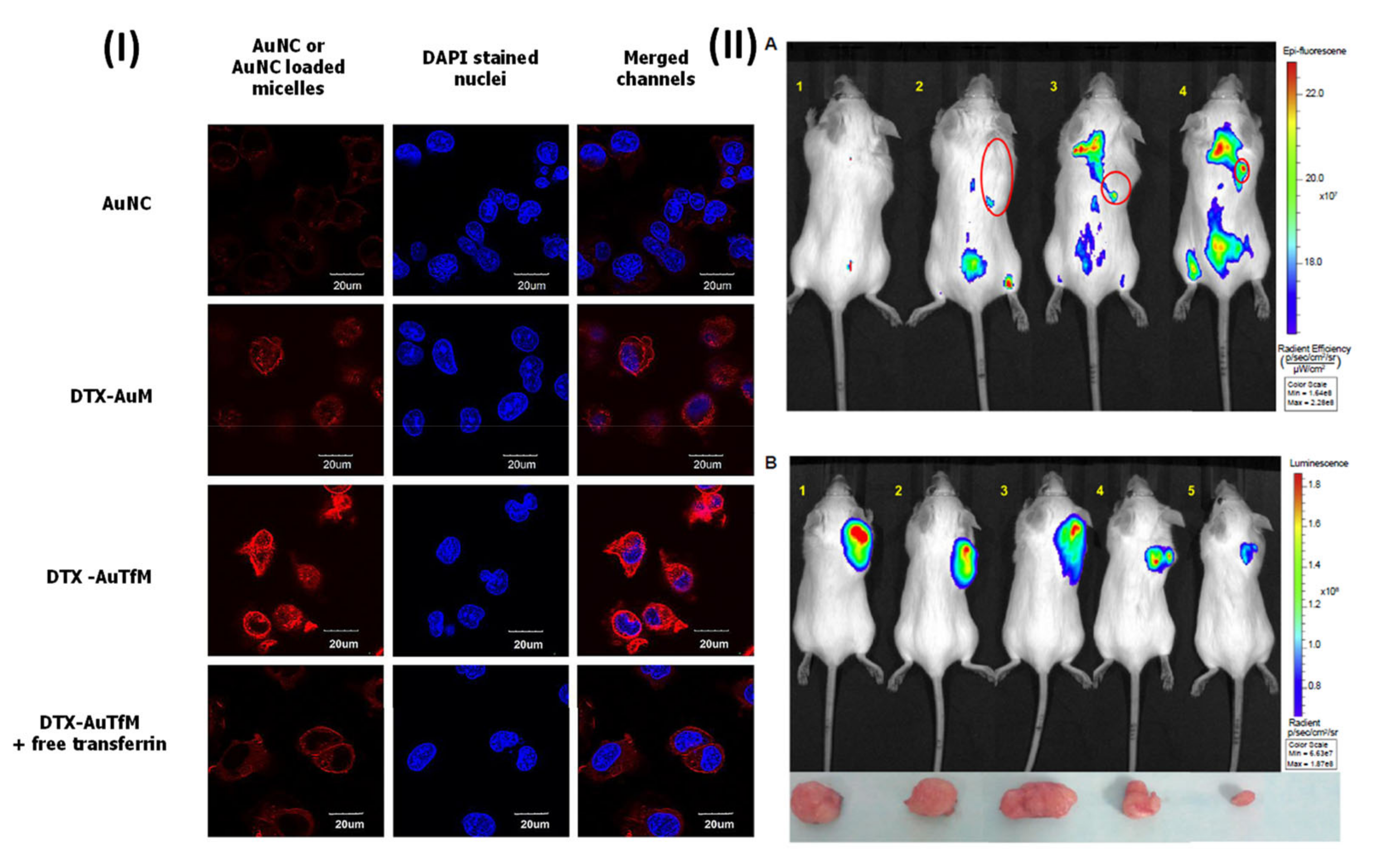
- Muthu, M.S.; Kutty, R.V.; Luo, Z.; Xie, J.; Feng, S.S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Kulkarni, S.A.; Liu, Y.; Feng, S.S. Development of docetaxel-loaded vitamin E TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nanomedicine 2012, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.V.; Chia, S.L.; Setyawati, M.I.; Muthu, M.S.; Feng, S.S.; Leong, D.T. In vivo and ex vivo proofs of concept that cetuximab conjugated vitamin E TPGS micelles increases efficacy of delivered docetaxel against triple negative breast cancer. Biomaterials 2015, 63, 58–69. [Google Scholar] [CrossRef]
- Raju, A.; Muthu, M.S.; Feng, S.S. Trastuzumab-conjugated vitamin E TPGS liposomes for sustained and targeted delivery of docetaxel. Expert Opin. Drug Deliv. 2013, 10, 747–760. [Google Scholar] [CrossRef]
- Muthu, M.S.; Ashish, K.S.; Sonali; Allabakshi, A.; Dhansukh, K.; Chellappa, V.R.; Sanjay, S.; Bajarangprasad, L.P. Solubilized delivery of paliperidone palmitate by D-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv. 2016, 23, 230–237. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, biodistribution, in vitro cytotoxicity and biocompatibility of Vitamin E TPGS coated trans resveratrol liposomes. Colloids Surf. B Biointerfaces 2016, 145, 479–491. [Google Scholar] [CrossRef]
- Vikas; Mehata, A.K.; Suseela, M.N.L.; Behera, C.; Kumari, P.; Mahto, S.K.; Muthu, M.S. Chitosan-alginate nanoparticles of cabazitaxel: Design, dual-receptor targeting and efficacy in lung cancer model. Int. J. Biol. Macromol. 2022, 221, 874–890. [Google Scholar] [CrossRef]
- Sonali; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Mehata, A.K.; Muthu, M. Development of Supramolecules in the Field of Nanomedicines. In Pharmaceutical Applications of Supramolecules; Springer: Berlin/Heidelberg, Germany, 2023; pp. 211–239. [Google Scholar]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Moretton, M.A.; Chiappetta, D.A. Deoxycholate-TPGS mixed nanomicelles for encapsulation of methotrexate with enhanced in vitro cytotoxicity on breast cancer cell lines. J. Drug Deliv. Sci. Technol. 2019, 50, 293–304. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Pinto-Alphandary, H.; Andremont, A.; Couvreur, P. Targeted delivery of antibiotics using liposomes and nanoparticles: Research and applications. Int. J. Antimicrob. Agents 2000, 13, 155–168. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T A Peer-Rev. J. Formul. Manag. 2017, 42, 742–755. [Google Scholar]
- Burande, A.S.; Viswanadh, M.K.; Jha, A.; Mehata, A.K.; Shaik, A.; Agrawal, N.; Poddar, S.; Mahto, S.K.; Muthu, M.S. EGFR Targeted Paclitaxel and Piperine Co-loaded Liposomes for the Treatment of Triple Negative Breast Cancer. AAPS PharmSciTech 2020, 21, 151. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-S.; Zeng, W.; Lim, Y.T.; Zhao, L.; Win, K.Y.; Oakley, R.; Teoh, S.H.; Lee, R.C.H.; Pan, S. Vitamin E TPGS-emulsified poly(lactic-co-glycolic acid) nanoparticles for cardiovascular restenosis treatment. Nanomedicine 2007, 2, 333–344. [Google Scholar] [CrossRef]
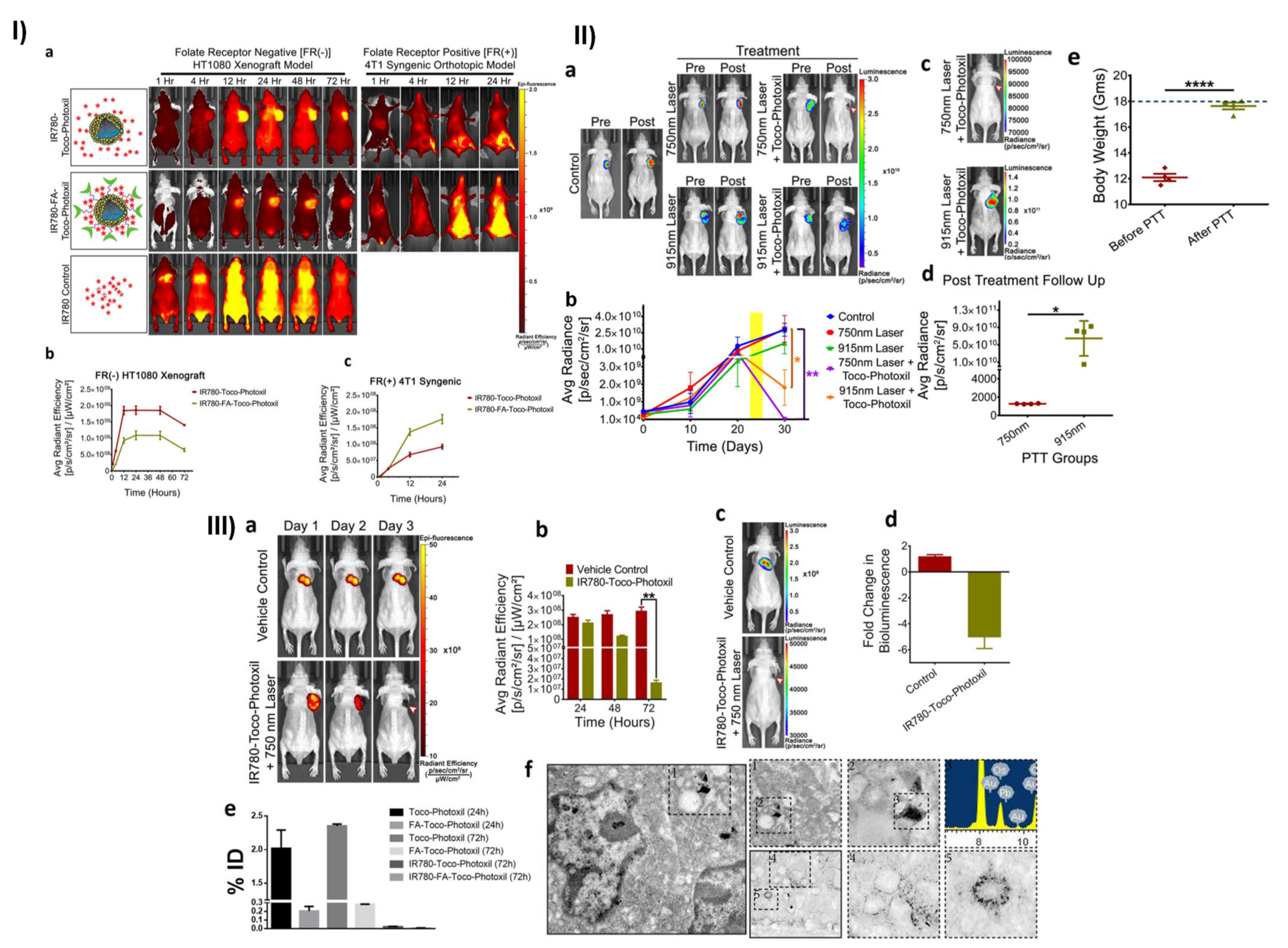
- Chauhan, D.S.; Bukhari, A.B.; Ravichandran, G.; Gupta, R.; George, L.; Poojari, R.; Ingle, A.; Rengan, A.K.; Shanavas, A.; Srivastava, R.; et al. Enhanced EPR directed and Imaging guided Photothermal Therapy using Vitamin E Modified Toco-Photoxil. Sci. Rep. 2018, 8, 16673. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C.Y. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Mansur, H.S. Quantum dots and nanocomposites. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 113–129. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef]
- Su, Y.L.; Yu, T.W.; Chiang, W.H.; Chiu, H.C.; Chang, C.H.; Chiang, C.S.; Hu, S.H. Hierarchically targeted and penetrated delivery of drugs to tumors by size-changeable graphene quantum dot nanoaircrafts for photolytic therapy. Adv. Funct. Mater. 2017, 27, 1700056. [Google Scholar] [CrossRef]
- Svenson, S. Theranostics: Are we there yet? Mol. Pharm. 2013, 10, 848–856. [Google Scholar] [CrossRef]
- Zhu, D.; Tao, W.; Zhang, H.; Liu, G.; Wang, T.; Zhang, L.; Zeng, X.; Mei, L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016, 30, 144–154. [Google Scholar] [CrossRef]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv. Mater. 2019, 31, e1802244. [Google Scholar] [CrossRef]
- Zhao, J.; Mi, Y.; Feng, S.S. siRNA-based nanomedicine. Nanomedicine 2013, 8, 859–862. [Google Scholar] [CrossRef]
- Guo, T.; Lu, J.; Fan, Y.; Zhang, Y.; Yin, S.; Sha, X.; Feng, N. TPGS assists the percutaneous administration of curcumin and glycyrrhetinic acid coloaded functionalized ethosomes for the synergistic treatment of psoriasis. Int. J. Pharm. 2021, 604, 120762. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J. Neuro-Oncol. 2015, 124, 13–22. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 279–285. [Google Scholar] [CrossRef]
- Du, J.; Zheng, X.; Yong, Y.; Yu, J.; Dong, X.; Zhang, C.; Zhou, R.; Li, B.; Yan, L.; Chen, C.; et al. Design of TPGS-functionalized Cu(3)BiS(3) nanocrystals with strong absorption in the second near-infrared window for radiation therapy enhancement. Nanoscale 2017, 9, 8229–8239. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Niu, G.; Chen, X. PET imaging of inflammation biomarkers. Theranostics 2013, 3, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Papathanassiou, D.; Bruna-Muraille, C.; Liehn, J.C.; Nguyen, T.D.; Curé, H. Positron Emission Tomography in oncology: Present and future of PET and PET/CT. Crit. Rev. Oncol. Hematol. 2009, 72, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Judenhofer, M.S.; Pfannenberg, C. Multimodal imaging approaches: PET/CT and PET/MRI. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 109–132. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Zhang, S.; Chen, N.; Sun, Y.; Ma, K.; Hong, D.; Li, L.; Du, Y.; Lu, X.; et al. TPGS-based and S-thanatin functionalized nanorods for overcoming drug resistance in Klebsiella pneumonia. Nat. Commun. 2022, 13, 3731. [Google Scholar] [CrossRef]
- Akimov, A.B.; Seregin, V.E.; Rusanov, K.V.; Tyurina, E.G.; Glushko, T.A.; Nevzorov, V.P.; Nevzorova, O.F.; Akimova, E.V. Nd: YAG interstitial laser thermotherapy in the treatment of breast cancer. Lasers Surg. Med. 1998, 22, 257–267. [Google Scholar] [CrossRef]
- Chen, F.; Cai, W. Nanomedicine for targeted photothermal cancer therapy: Where are we now? Nanomedicine 2015, 10, 1–22. [Google Scholar] [CrossRef]
- Omidi, Y.; Barar, J. Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines. BioImpacts BI 2014, 4, 55–67. [Google Scholar] [CrossRef]
- Carroll, L.; Humphreys, T.R. LASER-tissue interactions. Clin. Dermatol. 2006, 24, 2–7. [Google Scholar] [CrossRef]
- Tunc, B.; Gulsoy, M. Tm:fiber laser ablation with real-time temperature monitoring for minimizing collateral thermal damage: Ex vivo dosimetry for ovine brain. Lasers Surg. Med. 2013, 45, 48–56. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Reis, C.A.; Moreira, A.F.; Ferreira, P.; Correia, I. Optimization of gold core-mesoporous silica shell functionalization with TPGS and PEI for cancer therapy. Microporous Mesoporous Mater. 2019, 285, 1–12. [Google Scholar] [CrossRef]
- Caruso, C.; Epstein, R.L.; Troiano, P.; Napolitano, F.; Scarinci, F.; Costagliola, C. Topo-Pachimetric Accelerated Epi-on Cross-Linking Compared to the Dresden Protocol Using Riboflavin with Vitamin E TPGS: Results of a 2-Year Randomized Study. J. Clin. Med. 2021, 10, 3799. [Google Scholar] [CrossRef]
- Ozates, S.; Elgin, K.U.; Yilmaz, N.S.; Demirel, O.O.; Sen, E.; Yilmazbas, P. Evaluation of oxidative stress in pseudo-exfoliative glaucoma patients treated with and without topical coenzyme Q10 and vitamin E. Eur. J. Ophthalmol. 2019, 29, 196–201. [Google Scholar] [CrossRef]
- Jandzinski, R.; Papas, A.; Papas, K.; Neophytou, C. Vitamin E TPGS Concentrated Fluid with a Low Water Content. U.S. Patent US20060O88558A1, 26 October 2005. [Google Scholar]
- Hyatt, J.A.; Chester Zima, G.; Edgar, K.J.; Navarro, L.T.; Lehr, C.-M.; Collnot, E.-M.; Singleton, A.H.; Baldes, C.; Schafer, U.F.; Wempe, M.F. Pharmaceutical Formulations Containing Vitamin E Tpgs Molecules That Solubilize Lipophilic Drugs without Significant Efflux Inhibition, and Use of Such Formulations. WO Patent WO2006039268A3, 29 September 2005. [Google Scholar]
- Xia, E.; Green, G. New Viscoelastic Composition Comprising Alginate and Vitamin E Tpgs or Tpgsa. European Patent EP-1796628-B1, 23 July 2008. [Google Scholar]
- Volker, B. Compositions Comprising TPGS-750-M. U.S. Patent US20160340332A1, 27 May 2016. [Google Scholar]
- Faith, D.; Peter, H.; Thomas, K.; Drazen, K.; Bernd, L.; Anna, M.; Mirko, P.; Karin, R. Consistently Effective Pharmaceutical Formulations. U.S. Patent US 2015O141351A1, 18 September 2013. [Google Scholar]
- Lambert, K.; Constantinides, P.; Quay, S. Poorly Soluble Medications Are Transported in Emulsion Carriers. U.S. Patent US 2003OO27858A1, 5 January 1998. [Google Scholar]
- Amselem, S. Effective Bioavailability of Lipophilic Substances Using Solid Coprecipitates. U.S. Patent USOO5891469A, 2 April 1997. [Google Scholar]
- Berl, V. Assemblies and Synthesis of Surfactants. U.S. Patent US 2011 O184194A1, 17 January 2011. [Google Scholar]
- Feng, S.-S. Nanoparticle Coating for Drug Delivery. U.S. Patent US 2006O1885.43A1, 31 January 2006. [Google Scholar]
- Sadler, B.M.; Stein, D.S. Clinical pharmacology and pharmacokinetics of amprenavir. Ann. Pharmacother. 2002, 36, 102–118. [Google Scholar] [CrossRef]
- Varma, M.V.; Panchagnula, R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: Effect on solubility and permeability in vitro, in situ and in vivo. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2005, 25, 445–453. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Chavan, R.B.; Kumar, D.; Modugula, S.; Shastri, N.R. Micellar carriers for the delivery of multiple therapeutic agents. Colloids Surf. B Biointerfaces 2015, 135, 291–308. [Google Scholar] [CrossRef]
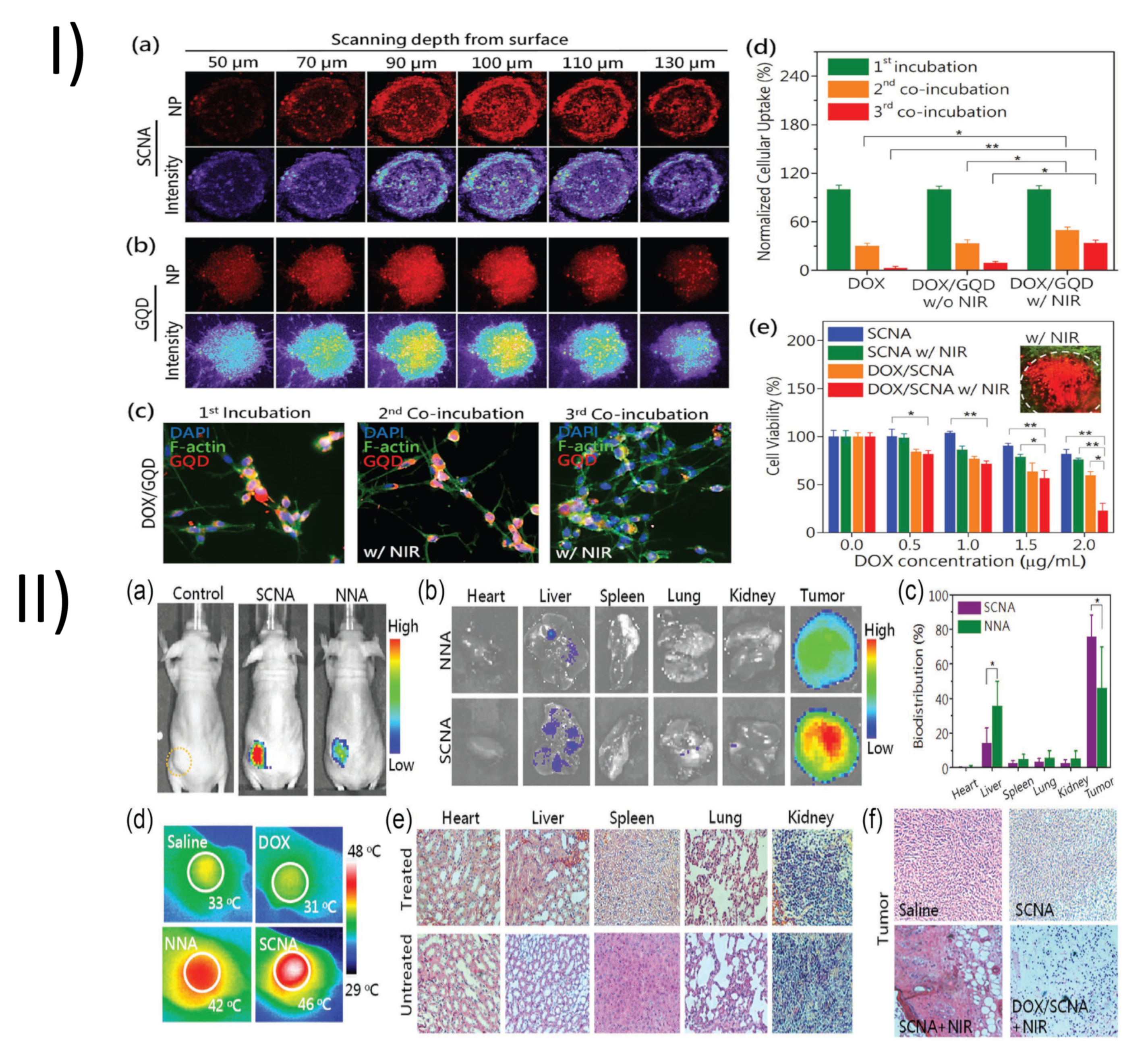
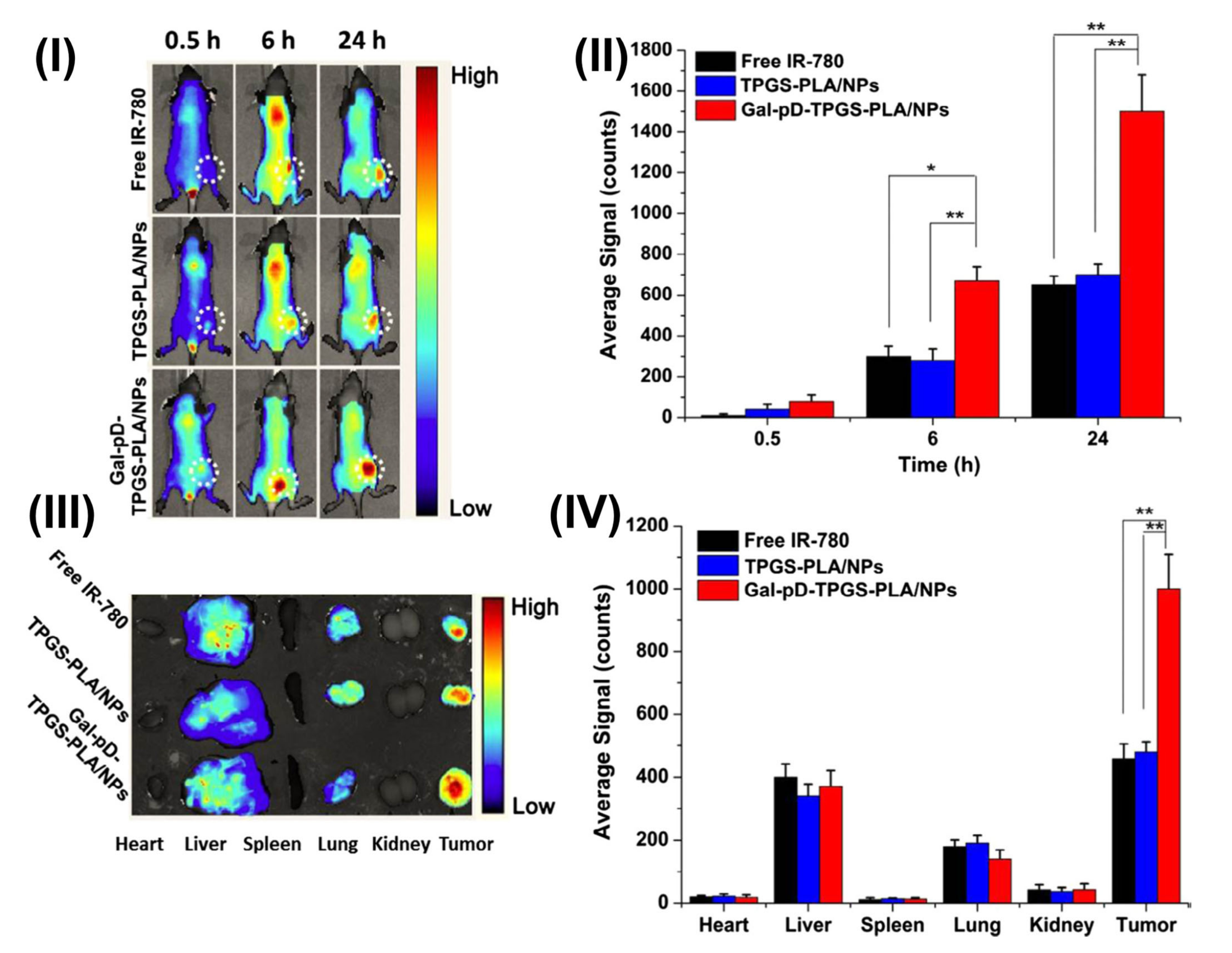
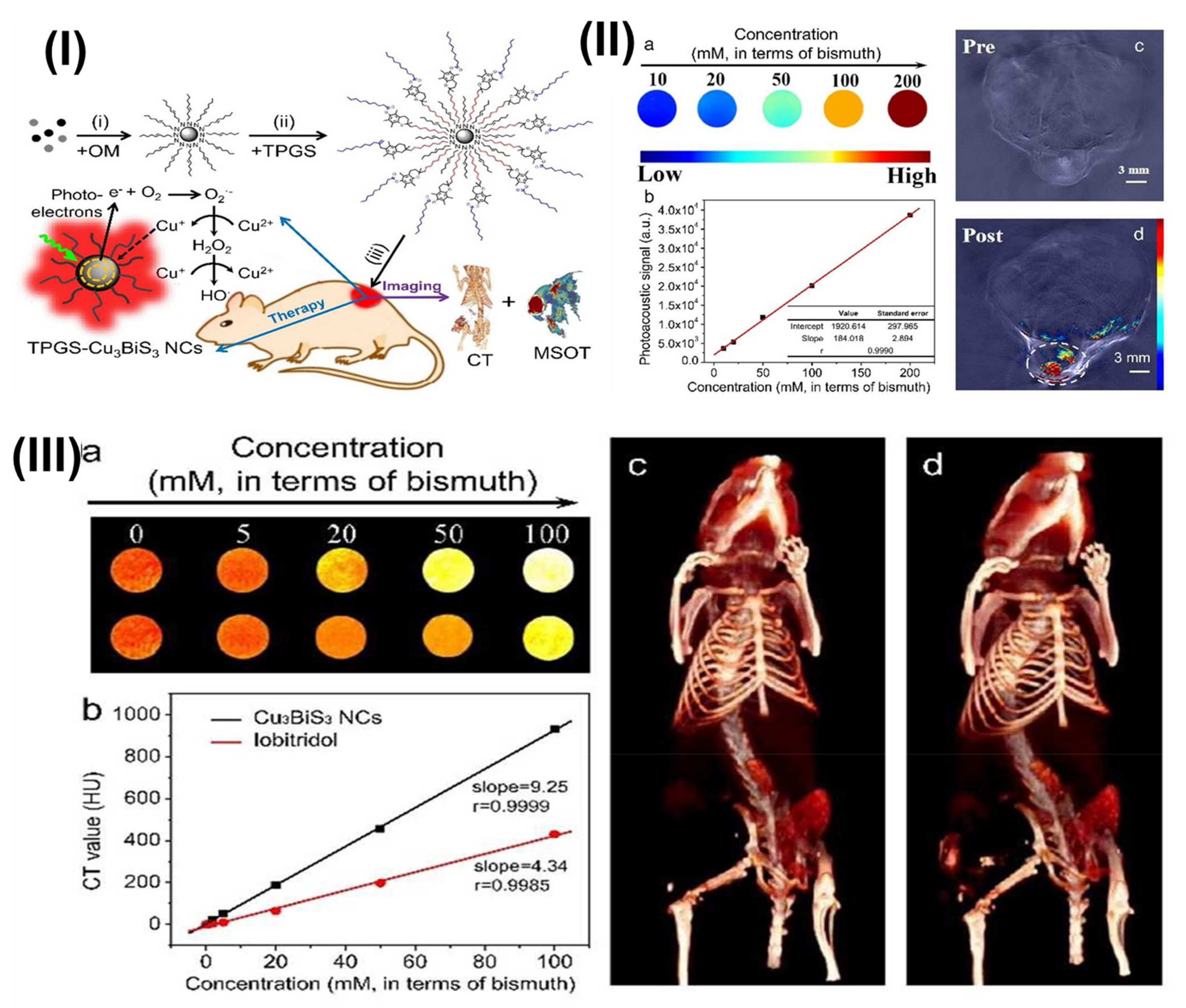
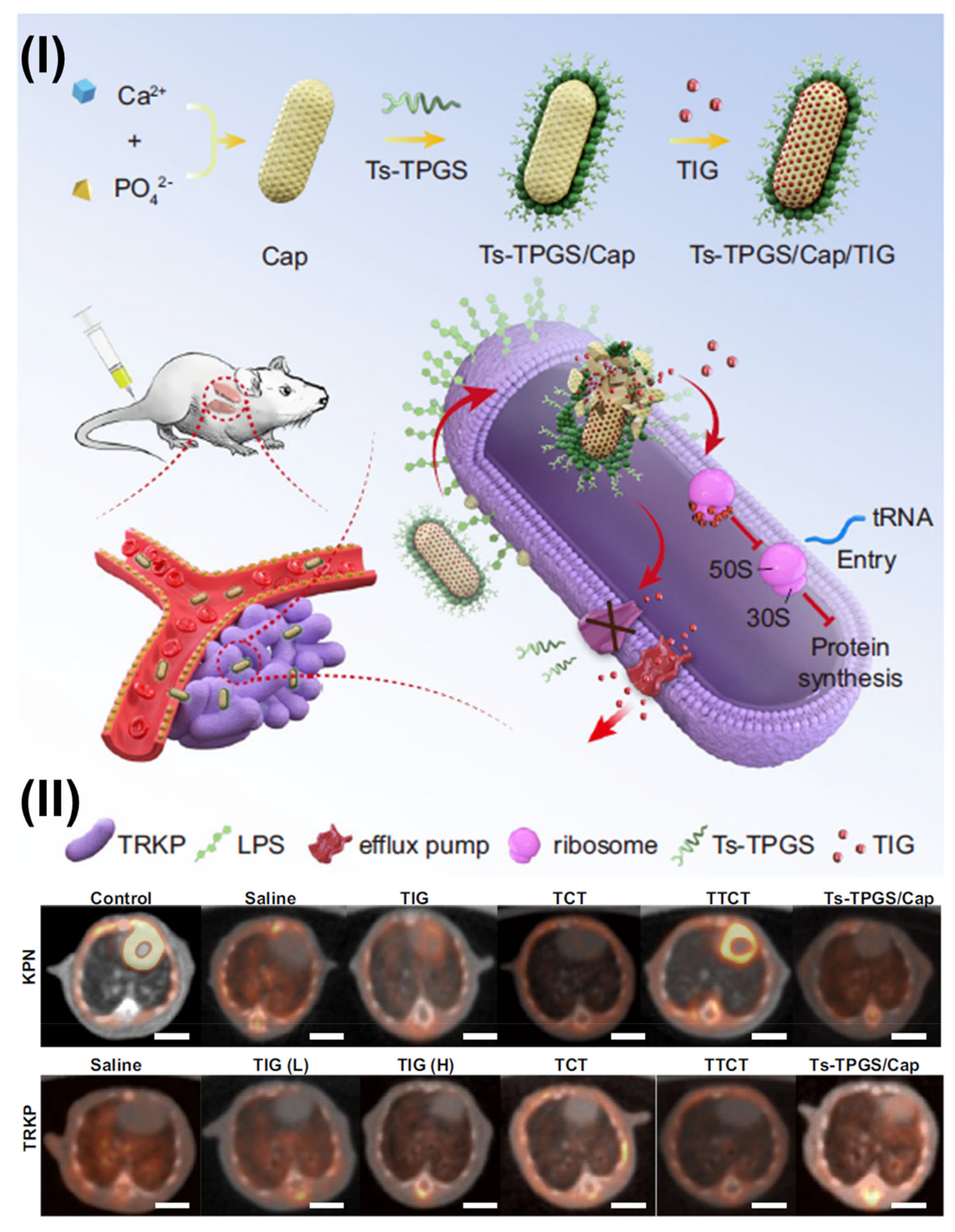

| TPGS-Modified Nanomedicines | In-Vivo Pharmacokinetic Studies | Outcomes |
|---|---|---|
| Trastuzumab targeted TPGS-Chitosan nanoparticles [66] | Animal model: Charles Foster (CF) rats | AUC and MRT of targeted formulation were improved, 2.82-times enhancement in relative and achieved 5.94 folds longer half-life in comparison to Docel™ |
| Transferrin receptor targeted TPGS bioadhesive micelles [67] | Animal model: Charles Foster (CF) rats | AUC and MRT of targeted formulation were improved by 4.08 and 14 times, respectively. |
| Transferrin receptor targeted TPGS-chitosan cross-linked Chitosan nanoparticles [68] | Animal model: Charles Foster (CF) rats | AUC and MRT of targeted formulation were improved by 4.10 and 10 times, respectively. |
| Transferrin conjugated TPGS micelles for brain cancer therapy [69] | Animal model: Male Sprague–Dawley rats | After 0.5 h of treatment, it is 18.68 times more efficient than the docetaxel control, and after 2 h of treatment, it is 23.09 times more effective. |
| Authors | Nanotherapeutics | Synthesis Method | Drug | Potential Outcomes | Ref. |
|---|---|---|---|---|---|
| Muthu et al., 2015 | Micelles | Solvent casting method | Docetaxel |
| [86] |
| Sonali et al., 2015 | Micelles | Solvent casting method | Docetaxel |
| [69] |
| Muthu et al., 2012 | Micelles | Solvent exchange method | Docetaxel |
| [87] |
| Kutty et al., 2015 | Micelles | Solvent casting method | Docetaxel |
| [88] |
| Raju et al., 2013 | Liposomes | Solvent injection method | Docetaxel |
| [89] |
| Muthu et al., 2012 | Liposomes | Solvent injection method | Docetaxel |
| [9] |
| Muthu et al., 2011 | Liposomes | Solvent injection method | Docetaxel |
| [78] |
| Sonali et al., 2016 | Liposomes | Solvent injection method | Docetaxel |
| [90] |
| Agarwal et al., 2017 | Polymeric nanoparticles | Solvent evaporation method | Docetaxel |
| [68] |
| Mehata et al., 2019 | Chitosan-based nanoparticles | Emulsification, solvent evaporation, and ionic gelation method | Docetaxel |
| [66] |
| Vijayakumar et al., 2016 | Polymeric nanoparticles | Film hydration method | Resveratrol |
| [91] |
| Vikas et al., 2021 | Chitosan nanoparticles | Emulsification, solvent evaporation, and ionic gelation method | Docetaxel |
| [60] |
| Vikas et al., 2022 | Chitosan-algiante nanoparticles | Ionic gelation technique | Cabazitaxel |
| [92] |
| Vijayakumar et al., 2016 | Polymeric nanoparticles | Single-emulsion solvent-evaporation technique | Resveratrol |
| [10] |
| Singh et al., 2016 | Carbon nanotubes | Nano-extraction method | Docetaxel |
| [93] |
| Viswanadh et al., 2021 | Polymeric nanoparticles | Dialysis method | Docetaxel |
| [61] |
| Clinical Trials of TPGS | ||
|---|---|---|
| Title | Phase, Status | NCT Number |
| Riboflavin and vitamin E expedite topo-pachimetric Epi-on cross-linking significantly more than the Dresden protocol | Not Applicable, Completed | NCT05019768 |
| Evaluation of the OZ439 + TPGS Formulation of Oral Piperaquine in the Fasted State in Healthy Volunteers | Phase 1, Completed | NCT01853475 |
| Piperaquine and OZ439: An Open-Labeled Pharmacokinetic Study of OZ439+TPGS Granules for Oral Suspension Given Alone or in Combination with Piperaquine Phosphate Tablets or Granules for Oral Solution in Healthy Volunteers | Phase 1, Completed | NCT01958619 |
| Berberine Absorption in Humans, a Bioactive Compound | Not Applicable, Completed | NCT03438292 |
| DSM265’s Effect on Malaria and Food Bioavailability | Phase 1, Completed | NCT03637517 |
| Summary of TPGS-based materials | ||
| Title | Inventor Name | Patent No |
| Vitamin E TPGS concentrated fluid with a low water content | Jandzinski et al. [137] | US20060O88558A1 |
| Vitamin E TPGS molecules, which are present in pharmaceutical formulations, have been shown to facilitate the metabolism of lipophilic drugs without causing a noticeable decrease in the drug’s efficacy. | Hyatt et al. [138] | WO2006039268A3 |
| A new class of surfactant-like materials comprising vitamin E TPGS and water-soluble polymer | Li et al. [135] | EP 1 799 194 B1 |
| Innovative viscoelastic material based on alginate and TPGS/TPGSA | Xia et al. [139] | EP 1 796 628 B1 |
| TPGS-750-M-Containing Compositions | Volker Berl [140] | US20160340332A1 |
| Consistently effective pharmaceutical formulations | Durak et al. [141] | US 2015O141351A1 |
| Poorly soluble medications are transported in emulsion carriers | Lambert et al. [142] | US 2003OO27858A1 |
| Effective bioavailability of lipophilic substances using solid coprecipitates | Amselem et al. [143] | USOO5891469A |
| Assemblies and synthesis of surfactants | Volker Berl [144] | US 2011 O184194A1 |
| Efficient Drug Delivery Through Nanoparticle Coating | Si-Shen Feng [145] | US 2006O1885.43A1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehata, A.K.; Setia, A.; Vikas; Malik, A.K.; Hassani, R.; Dailah, H.G.; Alhazmi, H.A.; Albarraq, A.A.; Mohan, S.; Muthu, M.S. Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future. Pharmaceutics 2023, 15, 722. https://doi.org/10.3390/pharmaceutics15030722
Mehata AK, Setia A, Vikas, Malik AK, Hassani R, Dailah HG, Alhazmi HA, Albarraq AA, Mohan S, Muthu MS. Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future. Pharmaceutics. 2023; 15(3):722. https://doi.org/10.3390/pharmaceutics15030722
Chicago/Turabian StyleMehata, Abhishesh Kumar, Aseem Setia, Vikas, Ankit Kumar Malik, Rym Hassani, Hamad Ghaleb Dailah, Hassan A. Alhazmi, Ahmed A. Albarraq, Syam Mohan, and Madaswamy S. Muthu. 2023. "Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future" Pharmaceutics 15, no. 3: 722. https://doi.org/10.3390/pharmaceutics15030722
APA StyleMehata, A. K., Setia, A., Vikas, Malik, A. K., Hassani, R., Dailah, H. G., Alhazmi, H. A., Albarraq, A. A., Mohan, S., & Muthu, M. S. (2023). Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future. Pharmaceutics, 15(3), 722. https://doi.org/10.3390/pharmaceutics15030722











