Abstract
As major public health concerns associated with a rapidly growing aging population, neurodegenerative diseases (NDDs) and neurological diseases are important causes of disability and mortality. Neurological diseases affect millions of people worldwide. Recent studies have indicated that apoptosis, inflammation, and oxidative stress are the main players of NDDs and have critical roles in neurodegenerative processes. During the aforementioned inflammatory/apoptotic/oxidative stress procedures, the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway plays a crucial role. Considering the functional and structural aspects of the blood–brain barrier, drug delivery to the central nervous system is relatively challenging. Exosomes are nanoscale membrane-bound carriers that can be secreted by cells and carry several cargoes, including proteins, nucleic acids, lipids, and metabolites. Exosomes significantly take part in the intercellular communications due to their specific features including low immunogenicity, flexibility, and great tissue/cell penetration capabilities. Due to their ability to cross the blood–brain barrier, these nano-sized structures have been introduced as proper vehicles for central nervous system drug delivery by multiple studies. In the present systematic review, we highlight the potential therapeutic effects of exosomes in the context of NDDs and neurological diseases by targeting the PI3K/Akt/mTOR signaling pathway.
Keywords:
exosome; neurological disease; neurodegenerative disease; targeted delivery; PI3K; Akt; mTOR 1. Introduction
Neurodegenerative diseases (NDDs) are progressive and chronic diseases characterized by imperceptible changes in neuronal structure [1,2]. NDDs are accompanied by a decrease in the population of specific neurons leading to disability, impaired normal functioning, dementia, and reduced life expectancy of patients. NDDs and neurological diseases are classified based on various parameters such as anatomical distribution, main clinical features, and important molecular abnormalities. Multiple sclerosis (MS), Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) are common NDDs/neurological diseases that result in the progressive deterioration of neurons [3,4]. The prevalence of neurodegenerative disorders is rapidly increasing alongside the increase in the aging global population. It is estimated that the number of dementia cases will increase from 13.5 million in 2000 to 36.7 million in 2050, which is alarming [5,6].
Although the pathogenesis of NDDs has not yet been precisely appreciated, numerous studies have underscored the undeniable contributions of inflammation, oxidative stress, apoptosis, and protein aggregation in some cases. The production of free radicals during both pathological and physiological processes plays a considerable role in several signaling pathways including phagocytosis, activation of enzymes, and regulation of the cell cycle. Excessive production of reactive oxygen species (ROS) causes various noxious effects such as protein and deoxyribonucleic acid (DNA) damage and lipid peroxidation [6,7,8,9]. As another contributor to the neurological disease, neuroinflammation brings about complex alterations in the brain’s immune system associated with multiple cellular and molecular aspects [6,7]. Such events lead to the alteration of glial cells, as well as augmentation of the concentration, activity, and levels of several inflammatory mediators including cytokines (e.g., interleukin-1β (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α)), chemokines (e.g., CCL2, CCL5, CXCL1), in addition to the generation of reactive nitrogen species (RNS) and ROS [10,11]. Increased permeability/breakdown of the blood–brain barrier (BBB), infiltration of peripheral immune cells, and edema are some of the other harmful processes that come to pass during neuroinflammation. Neuroinflammation contributes significantly to the development and progression of NDDs and neurological diseases. Therefore, suppression of inflammation could result in the prevention and amelioration of neurological disorders [6,12].
The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway is one of the key signaling pathways that plays pivotal roles in several pathological/physiological processes such as cellular migration, proliferation, apoptosis, and angiogenesis. In addition, according to numerous studies dysregulation of the PI3K/Akt/mTOR signaling pathway is associated with pathological effects in several disorders such as cardiovascular diseases [13,14], Crohn’s disease [15], cancer [16], and especially NDDs [9,12,17]. Following the activation of PI3K and phosphorylation of phosphatidylinositol 4,5- bisphosphate (PIP2), Akt is recruited to the cell membrane. As another key mediator, mTOR is associated with physiological neuroregeneration [18]. Moreover, the mTOR pathway is engaged in neuronal response-related signals in the gastrointestinal tract [19]. Additionally, mTOR signaling could be involved in autophagy where damaged mitochondria observed in the oxidative stress process could suppress PI3K signaling downstream through regulatory pathways such as the phosphatase and tensin homologue (PTEN) pathways [20]. Notably, PI3K/Akt pathway can regulate a wide range of upstream molecules such as growth factor receptors, G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), extracellular signal-regulated kinase (ERK), and cytokines, which are involved in the attenuation of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) activation [9,18,21,22,23,24]. Multiple studies have pointed to the significant effects of PI3K/Akt/mTOR signaling on several interconnected molecules implicated in oxidative stress (e.g., superoxide dismutase (SOD), heme oxygenase-1 (HO-1), ROS, catalase (CAT), nuclear factor erythroid 2–related factor 2 (Nrf2)), apoptosis (e.g., Bax/Bcl-2, and caspases), and inflammation (e.g., nuclear factor kappa B (NF-κB), ILs, matrix metalloproteinases (MMPs), chemokines, cytokines, and cyclooxygenase (COX)) [8,9,25,26,27].
Although a definitive treatment has still not been offered for NDDs, rapid fundamental advances in several new fields of science including nanotechnology, artificial intelligence, proteomics, stem cell therapy, genomics, gene therapy, exosome, and extracellular vesicles (EVs) technology combined with multidisciplinary approaches have opened new horizons for the treatment of NDDs and neurological diseases [28,29,30,31,32,33]. EVs are classified into exosomes (30–150 nm), microvesicles (50–1000 nm), or apoptotic bodies (800–5000 nm) based on their size and origin [34,35]. Exosomes originate from multi-vesicular bodies, and the budding of the plasma membrane is the main source of microvesicles and apoptotic bodies, which contain ribosomal ribonucleic acid (RNA), histones, and DNA produced from cells that undergo programmed cell death [33,36,37,38,39]. It has been demonstrated that microvesicles and exosomes play pivotal roles in intercellular communication as they are able to mediate long-distance transmission of biological information via transferring microRNAs (miRNAs), lipids, membrane receptors, RNA, and proteins between cells [33,36,37,38,39]. The type of cell and the physiological condition affect exosome composition, which may be strongly correlated with the development and progression of pathological processes [40]. As shown by recent studies, exosomes produced from cancer tissues can lead to disease progression. In addition, exosomes have been demonstrated to exert detrimental effects on neuronal tissues in neurological diseases. However, healthy cell-derived exosomes may possess therapeutic benefits. As a result, therapeutic strategies aimed to inhibit the production, uptake, or release of disease-promoting exosomes are of great promise [33,36,37,38,39]. There is already no review on the effects of exosome-mediated PI3K/Akt/mTOR signaling pathway in NDDs. This is the first systematic review that critically highlights the modulatory effects of exosomes as effective/safe drug delivery vehicles in the context of neurological diseases through PI3K/Akt/mTOR pathway.
2. Study Design and Methods
The current systematic review was performed based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria. The keywords (“brain” OR “neuron” OR “Alzheimer’s disease” OR “dementia” OR “Parkinson’s disease” OR “multiple sclerosis” OR “spinal cord injury” OR “stroke” OR “depression” OR “aging” OR “seizure” OR “autism” OR “Amyotrophic lateral sclerosis” OR “ALS” OR “Huntington’s disease” OR “epilepsy”) AND (“PI3K” OR “phosphatidylinositol-3-kinase” OR “PKB” OR “Akt” OR “protein kinase B” OR “mTOR” OR “m-TOR” OR “mammalian target of rapamycin”) AND (“exosome”) were searched in [title/abstract/keywords] of the electronic databases, including Scopus, PubMed, and Web of Science. All pathways/factors related to PI3K/Akt/mTOR and exosomes were considered in the whole text. Data were collected without time limitation until September 2022. Only English language studies were included. Two independent researchers (S.Z.M. and A.I.) performed the search screening. Of the 786 articles collected via systematic search in aforementioned electronic databases, 276 and 222 articles were excluded due to being reviews and duplication, respectively. Moreover, 171 articles were excluded according to their title/abstract, 63 articles were excluded according to their full-text information, and 8 articles were excluded because they were not in English. Ultimately, 46 papers were included in this systematic review (Figure 1). In completing the search strategy, manual search of citations and reference lists falling within the authors’ expertise were also employed in the PI3K/Akt/mTOR signaling pathway as a pivotal therapeutic target in neurological diseases.
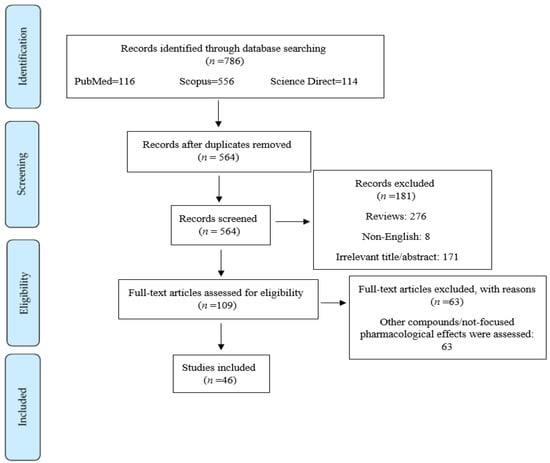
Figure 1.
Flowchart of the process of literature search and selection of relevant reports.
3. Neurodegenerative Diseases, Neurological Disorders, and Exosomes: Focusing on Pivotal Functions of PI3k/Akt/mTOR Signaling Pathway
Over the last few years, several studies demonstrated a promising future for EVs, especially exosomes for targeting neurological diseases. Concentrating on the pivotal PI3K/Akt/mTOR signaling pathway and associated factors, exosomes could combat AD, stroke, SCI, traumatic brain injury (TBI), ALS, optic nerve crush (ONC) injury, and other central nervous systems (CNS) injuries.
3.1. Exosomes and Alzheimer’s Disease, Cognition, Learning and Memory
AD is characterized as a common neurodegenerative disorder with a close relationship with dementia development. Reportedly, the prevalence of AD is conspicuous in the aged population and affects quality of life and social activities. In addition, AD pathology is associated with amyloid beta (Aβ) accumulation, tau protein hyperphosphorylation, neuroinflammation, and oxidative stress [41,42,43]. Based on the previous evidence, PI3K/Akt/mTOR axis is considered to be one of the most significant signaling pathways in the pathogenesis of NDDs. Indeed, this regulatory pathway displays critical functions in biological processes such as metabolism, cell proliferation, apoptosis, and angiogenesis [23]. Several lines of evidence have proposed that there is a correlation between exosome therapy and PI3K/Akt/mTOR signaling with neuronal damage. Reportedly, adipose mesenchymal stem cell-derived exosomes (ADSC-Exo) were shown to be effective in improving PC12 cell migration/proliferation and could repress apoptosis through boosting PI3K/Akt signaling pathway. In this line, ADSC-Exo treatment led to the overexpression of CD29, CD44, CD73, and CD105 as mesenchymal stem cell surface markers while reducing the expression of CD45 and HLA-DR [44]. In another study, bone marrow mesenchymal stromal cells (BMSCs)-Exo containing growth differentiation factor-15 (GDF-15) could exert a protective effect on Aβ42-induced SH-SY5Y cell injury through amelioration of the Akt/glycogen synthase kinase-3 beta (GSK-3β)/β-catenin signaling pathway. Of note, though this method of therapy promoted cell viability, it attenuated TNF-α, IL-6, IL-1β, IL-8 (as inflammatory cytokines), and apoptosis in Aβ42-induced SH-SY5Y cell damage [45]. In addition, a recent study illustrated that exosomes carrying curcumin (Exo-cur) significantly improved BBB crossing and ameliorated learning and memory deficits in okadaic acid (OA)-induced AD under both in vitro and in vivo models. Exo-cur also attenuated neural death and OA-induced tau hyperphosphorylation by stimulating the Akt/GSK-3β signaling pathway. Thus, Exo-cur represents a promising treatment through deactivation of microglia and mitigation of the OA-induced apoptosis of neuron cells. In addition, it led to improved neuronal function and alleviated AD symptoms [46]. According to an in vitro study, MSC-derived exosomal miR-223 (when applying 2 μg exosome-based on exosomal protein content per 1 × 105 recipient cells) could target PTEN and stimulated PI3K/Akt signaling pathway in an in vitro model of AD. Thus MSC-derived exosomes can increase cell migration and decrease neuronal apoptosis and inflammatory mediators including IL-6, IL-1β, and TNF-α [47]. Other studies suggested that neural stem cell (NSC)-derived exosomes (NSC-Exo) prevented high-fat diet (HFD)-dependent memory deficits in male C57BL/6 mice by restoring the cAMP response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) signaling and the expression of synaptic plasticity-associated genes. Taken together, treatment with NSC-Exo (1.5 μg per nostril, three times per week) could upregulate CREB/BDNF/TrkB signaling in the hippocampus of HFD mice, underscoring the treatment as a potential therapy for metabolic disease-related cognitive impairment [48]. Furthermore, it was shown that MSCs-miR-132-3p-Exo improved cognitive decline and synaptic dysfunction as well as promoted dendritic spine density and neuron numbers by activating the Ras/Akt/GSK-3β pathway in vascular dementia under in vitro and in vivo models [49].
Overall, exosomes play critical roles in circumventing cognitive/memory dysfunction through affecting the PI3K/Akt/mTOR pathway and its associated markers (Table 1). In summary, although more in vitro and in vivo studies are necessary to accurately prove the role of exosomes in AD, according to the mentioned reports, exosomes could be regarded to be a promising candidate for the prevention or treatment of AD, cognition, learning, and memory deficit. Additionally, exosomes could be used in combination with other drugs, which requires comprehensive pre-clinical and clinical studies. Future clinical applications should also focus on the usefulness of exosomes in predicting the emerging symptoms of AD. Additionally, application of the microfluidic technique will show the road to diagnosis of the primary symptoms of AD before the late stages of the disease. High biocompatibility, high BBB penetration, long blood circulation, prevention of degradation, and tissue targeting are additional advantages of exosomes to be used in AD [50]. Due to the capacity of exosomes in RNA transport, stability, and their BBB-crossing capability, exosomes are appropriate carriers in combating AD [51]. Additionally, neuron-derived exosomes could make Aβ conformational modifications to non-toxic fibrils and cause increased microglia uptake [52]. Altogether, exosomes are promising drug/enzyme/miRNA delivery vehicles, and also play a critical role in scavenging waste neurotoxic agents. The neuroprotective potential of exosomes is also closely linked with their ability to block NF-κB.

Table 1.
Exosomes circumvent AD and stroke via PI3K/Akt/mTOR and associated pathways.
3.2. Exosomes and Stroke
Stroke is considered the second leading cause of mortality and the third leading cause of disability worldwide with an increasing growth globally [68,69]. As a socioeconomic problem, stroke has a high morbidity and mortality rate [70]. Thus, it could decrease the life quality of patients and impose high economic costs on patients and healthcare systems [69]. Its occurrence is predicted to reach 23 million people in the world by 2030 [71]. There are three different types of stroke. Ischemic stroke and hemorrhagic stroke are the major types of strokes [69,70]. A transient ischemic attack (TIA) or mini-stroke is another type of stroke in which symptoms last less than 24 h and can be a warning sign for future strokes [68]. Ischemic stroke or brain ischemia is the most common type of stroke and occurs due to a blockage of blood flow to the brain. Obstruction is usually caused by blood clots and results in hypoxia, nutrient deprivation, and the induction of inflammation and oxidative stress [69,70,72]. Hemorrhagic stroke is caused by a blood vessel rupture, leading to intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH) [73].
The persistent bleeding from a hemorrhagic stroke causes oxidative stress, neuroinflammation, apoptosis, and BBB disruption [56,70]. In addition, the PI3K/Akt/mTOR pathway can modulate multiple cellular and molecular events including oxidative stress factors, inflammatory responses, programmed cell death, and cell survival, and it protects neurons and the brain from ischemic damages [9,74,75]. Hence, this pathway can be considered as one a significant signaling pathway and therapeutic target for neuroprotection against stroke.
Xin et al. illustrated that MSCs-miR-17-92+-Exo enhanced PI3K/Akt/mTOR activation by reducing PTEN expression, which modulated the stroke injury induced by middle cerebral artery occlusion (MCAO) in rats. In addition, they demonstrated that Exo-miR-17-92+ significantly elevated corticospinal tract (CST) axonal remodeling, myelination, neurological recovery, and reversed MCAO-induced behavioral dysfunctionality [53]. In another similar model, Exo-miR-17-92+ represented protective effects on brain injury and increased functional recovery, axonal density, neurogenesis, oligodendrogenesis, and spine and dendritic plasticity through inhibition of PTEN activity and increases in p-Akt, p-mTOR, and p-GSK-3β activity [54]. Healthy rat serum-derived exosomes have also shown neuroprotective effects in combating stroke injury in in vitro (50 μg/mL) and in vivo (800 μg/kg, i.v.) models through elevation of the p-Akt/Akt ratio, claudin-5, zonula occludens (ZO)-1, the Bcl-2/Bax ratio, and sequestosome 1 (SQSTM1)/p62 expression while reducing BBB leakage, cell apoptosis, MMP-9, cleaved caspase-3, and LC3B-II/LC3B-I ratio. Healthy rat serum-derived exosomes also improved the results of behavioral tests [55]. MSCs-miR-132-3p-Exo showed a notable amelioration of infarct volume, BBB dysfunction, neurological deficit scores (NDS), brain edema, and injury in focal ischemic stroke induced by transient MCAO in C57BL/6 mice through suppressing the expression of RASA1, reducing apoptosis and the ROS levels, and upregulating Ras, ZO-1, claudin-5, and the Ras/PI3K/Akt/endothelial nitric oxide synthesis (eNOS) pathway [56].
In a rat model of ICH, Duan and colleagues showed that BMSC-miR-146a-5p-Exos ameliorated neurological function through decreasing oxidative stress and inflammatory mediators interconnected to the PI3K/Akt/mTOR pathway including COX-2, malondialdehyde (MDA), inducible nitric oxide synthase (iNOS), TNF-α, myeloperoxidase (MPO)-positive cells, monocyte chemoattractant protein-1 (MCP-1), IL-1β, and IL-6 and suppression of microglial M1 polarization. These effects were associated with the downregulation of nuclear factor of activated T cells 5 (NFAT5) and IL-1 receptor-associated kinase 1 (IRAK1) expression [57]. In another rat model of ICH, MSC exosome-transferred miR-133b (100 μg via the tail vein, i.v., 72 h after ICH) reduced neuronal apoptosis and neurodegeneration by RhoA downregulation and ERK1/2/CREB pathway activation [58]. A recent report by Wang and colleagues showed that ADSC-Exos could decline neurological deficits and promote cell viability through suppression of insulin-like growth factor-binding protein 5 (IGFBP5) and increase in the expression of PI3K/Akt signaling pathway components in in vitro and in vivo models of SAH [59]. Recent studies have shown that BDNF could confer protection against apoptosis and neuronal injury by activating the ERK and/or PI3K/Akt pathway to stimulate the phosphorylation of CREB [76,77]. In another study, miR-206-knockdown exosomes from human umbilical cord-derived mesenchymal stem cells (hucMSCs) demonstrated significant in vivo therapeutic effects in SAH-induced early brain injury (EBI) through the BDNF/TrkB/CREB signaling pathway. BDNF/TrkB/CREB signaling pathway activation inhibited neuronal death and minimized brain edema and neurological dysfunction. In addition, hucMSCs-derived miR-206-knockdown exosomes ameliorated Bcl-2/Bax ratio and reduced cleaved caspase-8 induced by brain injury [60].
Stem cell-derived exosomes (SC-Exos) attenuated neuronal apoptosis, augmented interferon-gamma (IFN-γ) and Bcl-2, and decreased IL-1α, IL-2, TNF-α, Bax, cytochrome C (CytC), and cleaved caspase-3 and caspase-9 production in rats with cerebral ischemia/reperfusion (I/R) injury. These mechanisms could be linked to PI3K/Akt pathway-mediated mitochondrial apoptosis [61]. Wu et al. elucidated the in vitro and in vivo neuroprotective effects of astrocyte-derived exosome (ATC-Exos)-contained miR-34c in terms of the protection of Neuro 2A (N2a) mouse neuroblastoma cells by increasing toll-like receptor 7 (TLR7) expression and downregulating the NF-κB/mitogen-activated protein kinase (MAPK) pathways. It has been shown that treatment with ATC-Exos significantly reduces infarction volume; brain edema; inflammatory mediators including IL-6, IL-8 and TNF-α; apoptotic factors such as Bax, cleaved caspase-3, and cleaved PARP; and ameliorates neurological deficits caused by cerebral I/R injury [62]. Furthermore, 10 μg/mL BMSC-Exos conferred protective effects on oxygen–glucose deprivation/reperfusion (OGD/R)-induced injury in PC12 cells and promoted cell viability by targeting the AMP-activated protein kinase (AMPK)/mTOR pathway [63]. In another study, Bu and colleagues showed the neuroprotective advantages of ATC-Exos contained miR-361 under in vitro (30 μg/mL) and 2 mL exosomes (30 μg/mL) via the caudal vein, twice a week for 2 weeks) models of cerebral I/R injury through attenuation of the AMPK/mTOR signaling pathway via targeting cathepsin B (CTSB). In addition, infarct volume, cerebral edema, cleaved poly adenosine diphosphate-ribose polymerase (PARP), cleaved caspase-3, and Bax exhibited a significant decrease in the ATC-Exo-treated groups, while neuronal viability increased [64].
In addition, BMSC-derived exosomal miR-29b-3p [65], exosomes from different H9 human embryonic stem cell (hES)-derived cells [66], and miR-126 enriched endothelial progenitor cells (EPCs)-released exosomes [67] are among other exosomes with promising protective activities in combating brain injury through modulation of the PI3K/Akt/mTOR signaling pathway and the related mediators.
Altogether, the above-mentioned studies highlight the promising protective effects of exosomes against different types of stroke by employing different mechanisms such as the modulation of inflammatory cytokines, autophagic molecules, and oxidative and apoptotic factors. These functions of exosomes typically pass through the PI3K/Akt/mTOR signaling pathway (Table 1). In addition, these nano-sized structures display different advantages such as low immunogenicity and high BBB penetration capacity over other therapeutic modalities. However, there exist several challenges against exploiting EVs for therapeutic applications including drug interactions with EV components, a lack of controlled drug release mechanisms, and a lack of specific biomarkers. Exosome therapy still has various limitations and more studies are needed to find ways to elevate their circulation half-life, increase the quantity of bioactive molecules loaded in exosomes, enhance their stay at the disease site, and use them for targeted delivery to highlight their eligibility in clinical trials to combat stroke. Thus, further studies including extensive in vitro and in vivo experimentations as well as comprehensive pre-clinical and clinical trials on exosomes are necessary to introduce exosomes as potential agents for modulating stroke. Multiple in vitro and in vivo reports have proven that exosomes could increase functional recovery, angiogenesis, neurovascular remodeling, and synaptic plasticity and could be neurorestorative after stroke through transfer of different types of cargoes such as miRNAs, proteins, lipids, and phytochemicals [78,79].
3.3. Exosomes and Spinal Cord Injury
SCI is a critical insult to the spinal cord that causes temporary or permanent motor and sensory impairment and disability [80,81]. SCI affects most of the body’s functions and can diminish patients’ quality of life [80]. SCI is classified as non-traumatic or traumatic SCI, according to its etiology [82]. Recent studies have shown that the PI3K/Akt/mTOR pathway plays critical roles in the recovery of the spinal cord after injury via regulation of the release of proinflammatory cytokines, oxidative stress, cell death, neuron growth, differentiation, and formation of glial scar [75,83]. Thus, it is critical to consider the roles of exosomes in the context of SCI via modulation of the PI3K/Akt/mTOR signaling pathway.
In 2021, Chen et al. investigated the advantages of BMSC-miR-26a-Exos in SCI in in vitro and in vivo models. The results demonstrated that interference of the PTEN/Akt/mTOR pathway is the major neuroprotective mechanism governing BMSC-miR-26a-Exo-mediated functional recovery, neurogenesis, axonal regeneration, and attenuation of astrocyte inflammation, autophagy, and glial scarring [84]. In another report, miR-338-5p overexpressing BMSC-derived exosomes showed neuroprotective activities by elevating neuronal survival, modulating oxidative stress factors, and suppressing SCI-induced cell death in both in vitro and in vivo experiments. These effects were attributed to the PI3K/Akt pathway via downregulation of cannabinoid receptor 1 (Cnr1) and cAMP-mediated Rap1 activation [85]. Furthermore, exosome-shuttled miR-216a-5p from hypoxic BMSCs (200 μg/mL) was shown to deviate microglia/macrophage polarization from M1 pro-inflammatory phenotype to M2 anti-inflammatory phenotype, increase IL-4, and IL-10, and decrease iNOS, TNF-α, IL-1β, and IL-6 through activation of the PI3K/Akt pathway and TLR4/NF-κB pathway suppression. These pathophysiological signaling pathways led to improved functional, gait, and motor recovery in a C57BL/6 mice model of SCI [86]. Luo and colleagues showed that exosomes from G protein-coupled receptor kinase 2 interacting protein 1 (GIT1)-overexpressing BMSCs promoted neural regeneration, functional behavioral recovery, and antiapoptotic factors (e.g., Bcl-2) while reducing glial scar formation, inflammatory mediators (e.g., IL-1β, IL-6, and TNF-α), and proapoptotic factors (e.g., Bax and cleaved caspase-3 and -9) which led to the alleviation of apoptosis and neuroinflammation as evaluated by in vitro and in vivo experiments. Upregulation of the PI3K/Akt signaling pathway could be presumed to be one of the major protective mechanisms adopted by these exosomes in combating traumatic SCI [87].
Wang et al. showed that MSCs-Exo conferred neuroprotection by anti-inflammatory activities through downregulation of the nuclear translocation of NF-κB p65, TNF-α, IL-1α, IL-1β, and p-IKBα as demonstrated by in vitro and in vivo experiments [88]. In another study, MSCs-miR-126-Exo enhanced neurogenesis, angiogenesis, functional recovery, connectivity value, and blood vessel numbers and diminished apoptosis and lesion volume after SCI. Such effects were mediated through inhibition of sprouty-related EVH1 domain-containing protein 1 (SPRED1) and PI3K regulatory subunit 2 (PIK3R2) [89]. Of other reports on the exosome-mediated regulation of PI3K/Akt/mTOR, neuron-derived exosomes transmitting miR-124-3p could remarkably attenuate axonal damage, lesion volume, M1 microglia and A1 astrocytes activation. In addition, it minimized pro-inflammatory cytokines (TNF-α, IL-1α, IL-6, and IL-1β), iNOS, and improved functional/gait recovery through the regulation of PI3K/Akt/NF-κB signaling pathways as assessed by in vitro and in vivo experiments [90]. Chen et al. investigated the therapeutic effect of FTY720-loaded exosomes derived from nerve stem cells (NSCs) (FTY720-NSCs-Exos) in SCI using in vitro and in vivo models. In this line, FTY720-NSC-Exos increased p-Akt, Bcl-2, claudin-5, ZO-1, and locomotor function while reducing PTEN, SCI lesion, edema formation, inflammatory cell infiltration, and apoptosis of neuronal cells via regulation of the PTEN/Akt pathway which led to neuroprotective effects [91]. Pan and colleagues demonstrated that primary Schwann cell-derived exosomes (SCDEs) could promote recovery after SCI through modulation of NF-κB/PI3K [92] and epidermal growth factor receptor (EGFR)/Akt/mTOR signaling pathways as elucidated by in vitro and in vivo assays [93]. Human urine stem cell (HUSC)-ANGPTL3-Exo exhibited a neuroprotective activity against SCI and increased angiogenesis, spinal cord regeneration, sensory improvement, vessel volume fraction, and recovery of neurological functional by interference with the PI3K/Akt signaling pathway as confirmed by in vitro and in vivo experiments [94]. According to in vitro and in vivo evidence, hucMSCs-miR-199a-3p/145-5p-Exos diminished neurological symptoms via decreasing the inflammation and apoptotic cells and increasing TrkA, p-Akt, and p-Erk, and interconnected to nerve growth factor (NGF)/TrkA pathway [95]. Additional studies discovered that peripheral macrophage (PM)-Exos [96], resveratrol-primed exosomes secreted by primary microglia [97], and pericyte exosomes [98] possessed neuroprotective properties in combating SCI through PI3K/Akt/mTOR signaling pathway and related factors.
Therefore, exosomes could be contemplated as possible therapeutic tools for SCI by modulating neuroapoptosis, neuronal oxidative stress, and neuroinflammation through the PI3K/Akt/mTOR signaling pathway. Considering the challenges mentioned for exosomes, it seems that more in vivo studies are needed to conduct clinical trials to determine the role of exosomes in neurological diseases such as SCI. Taken together, these reports suggest exosomes as new tools for modulating SCI. As natural carriers of biologically active cargoes, exosomes could not only remarkably ameliorate functional recovery, neural regeneration, and angiogenesis of animals with SCI, but also notably elevate the expression of antioxidant factors, anti-apoptotic protein Bcl-2, and anti-inflammatory mediators including IL-4, IL-10, and IL-13. Exosomes markedly reduced pro-inflammatory factors such as IL-1β, IL-6, and TNF-α and the expression of the apoptotic protein Bax. These effects are in a near linkage with their ability to regulate the PI3K/Akt/mTOR signaling pathway.
Overall, because exosomes can effectively cross the BBB, they could be used for the treatment and diagnosis of several neurological disorders, such as SCI. In addition, more studies are required to clarify the specific role of exosomes in SCI and bring hope for clinical treatment of SCI.
3.4. Exosomes and Traumatic Brain Injury
TBI is defined as a mechanical injury to the parenchymal tissues and meninges of the brain associated with inflammatory and oxidative responses [99,100]. Based on in vitro and in vivo studies, endothelial colony-forming cells (ECFCs)-derived exosomes rescued the expression of tight junction (TJ) proteins by targeting the PTEN/Akt pathway. Indeed, theses exosomes decreased PTEN expression and activated Akt phosphorylation. On the other hand, pretreatment with exosomes showed beneficial effects in declining MMP-9 expression, Evans blue dye extravasation, and TJ protein degradation in mice with TBI [101]. To suppress TBI-related neuroinflammation, microglial exosomes-contained miR-124-3p could be considered as a potential therapeutic molecule in preventing neuronal inflammation following TBI through targeting phosphodiesterase 4B (PDE4B) and ultimately repressing the mTOR signaling pathway. Furthermore, elevated miR-124-3p in microglial exosome-mediated inhibition of neuronal inflammation was associated with improved anti-inflammatory M2 polarization of microglial cells [102]. Growing evidence has indicated that hADSC-Exo shows neuroprotective effects against in vivo TBI models via suppression of the classical NF-κB and MAPK signaling pathways to restrain microglia/macrophage activation. Overall, hADSC-Exo administration caused sensorimotor functional recovery in TBI rats, improved hippocampal neurogenesis, suppressed neuroinflammation, and mitigated neuronal apoptosis. Notably, in vitro application of hADSC-Exo markedly inhibited M1 microglial polarization and increased M2 microglial polarization. Hence, intracerebroventricular hADSC-Exo administration could serve as a precious therapeutic modality against CNS diseases [103]. Further in-depth studies are needed to confirm the effectiveness of exosomes in TBI. Collectively, exosomes have exhibited a promising future in combating TBI through modulating the PI3K/Akt/mTOR pathway (Table 2).

Table 2.
Exosomes circumvent SCI and TBI via PI3K/Akt/mTOR and associated pathways.
Exosomes could be considered powerful biomarkers for the diagnosis of TBI. Exosome therapies are effective approaches for improving neurological and functional recovery via increasing neurite growth and neurogenesis by delivery of gene or pharmacological agents after TBI [104,105]. Moreover, there are still some challenges with the design, separation, and purification procedure of exosomes [106]. Therefore, future research should explore a cheap, quick, and simple standardized method for the generation of exosomes and establish the best strategies for exosome modulation of TBI toward promoting the translation of preclinical reports outcomes to clinical studies. In conclusion, the evidence shows that exosomes might have great potential in neurorestorative therapy for TBI.
3.5. Exosomes and Other Neurological Disorders
It is worth mentioning that various types of exosomes could ameliorate several other neurological diseases via the PI3K/Akt/mTOR pathway and other interconnected signaling pathways [107]. Emerging reports suggest that the PI3K/Akt signaling pathway and the associated proteins can be targeted by ADSC-Exo administration in ALS. Notably, it has been reported that treatment with adipose stem cell (ACS) exosomes reduces pro-apoptotic proteins such as cleaved caspase-3 and Bax while increasing the anti-apoptotic protein Bcl-2 in an in vitro model of ALS. In addition, Western blot analysis revealed increased p-Akt and SOD1 expression in ASC exosome-treated cells [108]. Moreover, another study provided evidence on the effects of fibroblast-derived exosomes (FD-Exo) (50 ng/mL) on the promotion of axonal regeneration in the injured CNS by recruiting Wnt10b toward lipid rafts and subsequently activating mTOR signaling through GSK-3β and tuberous sclerosis complex 2 (TSC2) [109]. In an experimental study, researchers found that FD-miR-673-5p-Exo is associated with peripheral neuron myelination of Schwann cells via stimulation of the TSC2/mTOR complex 1 (mTORC1)/sterol-regulatory element binding protein 2 (SREBP2) axis. Indeed, FD-miR-673-5p-Exo can enhance peripheral neuron myelination in newborn rats and myelin gene expression in Schwann cells [110]. In this context, MSC-exosomes caused improved axonal growth of cortical recipient neurons and increased the length of distal axons through augmenting p-mTOR and p-GSK-3β and reducing PTEN; it was also found that elevation of miR-17-92 cluster in the exosomes (miR-17-92 exosomes) further promoted axonal growth [111]. According to the reported studies, exosomes derived from ADSCs (50 ng/mL) show inhibitory effects on lipopolysaccharide (LPS)-induced injury in SH-SY5Y and BV-2 cells by reducing TNF-α, IL-1β, IL-6, COX-2, iNOS, p-P38, p-P65, p-ERK, and p-JNK. In addition, ADSC-exosomes mitigated LPS cytototoxic effects and suppressed neuroinflammation by repressing the NF-κB and MAPK signaling pathways. In conclusion, ADSC-exosomes reveal therapeutic effects on neural injury induced by microglia activation [112]. Evidence indicates that exosomes released by human ADSC (hAMSCs) seem to be a promising therapeutic approach against neural injury induced by glutamate in PC12 cells by upregulating PI3K/Akt signaling pathway [113]. Based on the research, MSC-Exo in a rat model of ONC injury decreased the expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, TNF-α, and MCP-1, whereas it increased the anti-inflammatory mediator IL-10. In addition, MSC-Exo decreased the ONC-induced apoptosis of the retinal ganglion cells (RGCs) via promotion of the Bcl-2/Bax ratio and reduction in caspase-3 activity, and Akt phosphorylation/activation was observed following intravitreal MSC-Exo administration [114]. Altogether, the authors demonstrated that exosomes could serve as potential therapies in the management/treatment of other neurological diseases via regulating PI3K/Akt/mTOR and associated signaling pathways (Table 3).

Table 3.
Exosomes circumvent other neurological diseases via PI3K/Akt/mTOR and associated pathways.
4. Conclusions and Future Perspective
A growing number of studies have highlighted the pivotal role of the PI3K/Akt/mTOR signaling pathway in the CNS and associated dysregulations. In addition, recent reports are demonstrating the modulatory roles of PI3K/Akt/mTOR pathway and the related inflammatory mediators (e.g., NF-κB, TNF-α, ILs, CRP, COX-2, and MMP-9); oxidative/antioxidative factors (e.g., GSH, SOD, CAT, Nrf2/HO-1, and iNOS); apoptotic factors (e.g., Bax, Bcl-2, caspase-3, and caspase-9); and the interlinked pathways (e.g., MAPK, CREB/BDNF, GSK-3β, ERK1/2, and JAK/STAT) in the progression/treatment of NDDs and other neurological diseases (Figure 2). Considering the side effects and resistance mechanisms to conventional neuroprotective drugs, it is of great importance to provide alternative therapies in combating neurological diseases. Exosomes can be considered to be promising therapies owing to their specific properties including low immunogenicity, flexibility, high BBB penetration capacity, and the capability of being used as drug delivery vehicles that could provide a long-lasting concentration of medication in the CNS while having few side effects and especially regulating the aforementioned inflammatory, oxidative, and apoptotic pathways/factors. To combat such pathophysiological mechanisms, it is necessary to consider novel approaches to drug delivery into the CNS using natural entities with fewer side effects. Exosomes strategically carry drugs and possess suitable stability and half-life. Understanding the roles that exosomes play in communication between various cell types at diverse sites provides a critical step forward in revealing the details of cell communication [115]. However, exosome-based therapeutic strategies and research progress in the field of exosomes face some challenges including difficulties in characterization, lack of controlled drug release mechanisms, inefficient isolation methods, drug interaction with exosome/EV components, and lack of specific biomarkers. Exosomes are suitable carriers for various cargoes including proteins, drugs, and natural products. There are now limitations in the manipulation of exosomes to be used in diseases. Separation, identification, and diagnosis of exosomes are critical steps requiring future research [50]. The optimization of operational procedures is necessary, and the characterization of exosome cargoes’ mediating therapeutic effects is warranted. New low-cost techniques to obtain a large amount of high-purity exosomes need to be provided. Furthermore, increasing the half-life of exosomes and targeting the ability of exosomes could provide clinical-grade exosomes as promising therapeutic approaches for future studies [116].
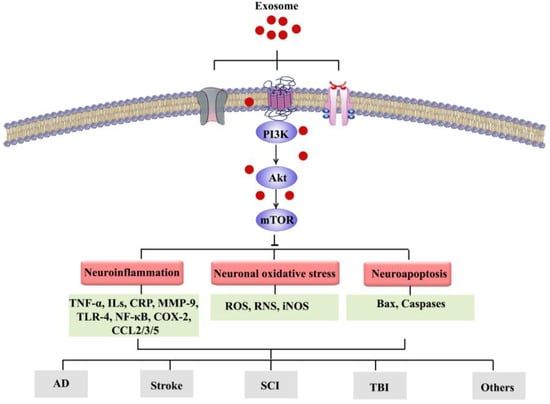
Figure 2.
Exosomes can target the PI3K/Akt/mTOR signaling pathway, thereby modulating various interconnected events involved in NDDs towards therapeutic application.
Accordingly, considering the critical role of PI3K/Akt/mTOR in NDDs and the multiple advantages of EVs in the context of effective and safe drug delivery, exosomes could confer a significant neuroprotective role in combating AD, PD, ALS, stroke, TBI, and other neuronal disorders [117].
This is the first systematic review with a focus on the pivotal role of PI3K/Akt/mTOR pathway targeted by exosomes in NDDs. We critically highlighted the modulatory functions of exosomes in NDDs through PI3K/Akt/mTOR pathway. Future investigations should incorporate extensive in vitro and in vivo experimentations and well-designed randomized clinical trials on exosomes to clarify in more detail the crucial roles of the PI3K/Akt/mTOR pathway and the modulatory effects of exosomes in the context of NDDs.
Author Contributions
Conceptualization, S.F., A.I. and H.K.; drafting the manuscript, A.I., L.K. and S.Z.M.; software, S.F.; review and editing, L.S., S.F. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Student Research Committee at the Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant No. 50002205).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ou, G.-Y.; Lin, W.-W.; Zhao, W.-J. Neuregulins in neurodegenerative diseases. Front. Aging Neurosci. 2021, 13, 170. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Ayeni, E.A.; Aldossary, A.M.; Ayejoto, D.A.; Gbadegesin, L.A.; Alshehri, A.A.; Alfassam, H.A.; Afewerky, H.K.; Almughem, F.A.; Bello, S.M.; Tawfik, E.A. Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health 2022, 19, 12495. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted role of matrix metalloproteinases in neurodegenerative diseases: Pathophysiological and therapeutic perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Zheng, J.C.; Chen, S. Translational Neurodegeneration in the era of fast growing international brain research. Transl. Neurodegener. 2022, 11, 1. [Google Scholar] [CrossRef]
- Fakhri, S.; Abdian, S.; Zarneshan, S.N.; Moradi, S.Z.; Farzaei, M.H.; Abdollahi, M. Nanoparticles in Combating Neuronal Dysregulated Signaling Pathways: Recent Approaches to the Nanoformulations of Phytochemicals and Synthetic Drugs Against Neurodegenerative Diseases. Int. J. Nanomed. 2022, 17, 299–331. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxidative Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef]
- Fakhri, S.; Iranpanah, A.; Gravandi, M.M.; Moradi, S.Z.; Ranjbari, M.; Majnooni, M.B.; Echeverría, J.; Qi, Y.; Wang, M.; Liao, P. Natural products attenuate PI3K/Akt/mTOR signaling pathway: A promising strategy in regulating neurodegeneration. Phytomedicine 2021, 91, 153664. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Brockett, M.M.; Breyer, R.M.; Aschner, M.; Montine, T.J. Neuroinflammation and oxidative injury in developmental neurotoxicity. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1129–1140. [Google Scholar]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khanbabapour Sasi, A.; Hussen, B.M.; Shoorei, H.; Siddiq, A.; Taheri, M.; Ayatollahi, S.A. Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 2022, 49, 9767–9781. [Google Scholar] [CrossRef]
- Tokuhira, N.; Kitagishi, Y.; Suzuki, M.; Minami, A.; Nakanishi, A.; Ono, Y.; Kobayashi, K.; Matsuda, S.; Ogura, Y. PI3K/AKT/PTEN pathway as a target for Crohn’s disease therapy. Int. J. Mol. Med. 2015, 35, 10–16. [Google Scholar] [CrossRef]
- Follo, M.Y.; Manzoli, L.; Poli, A.; McCubrey, J.A.; Cocco, L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv. Biol. Regul. 2015, 57, 10–16. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. A critical kinase cascade in neurological disorders: PI3K, Akt and mTOR. Future Neurol. 2012, 7, 733–748. [Google Scholar] [CrossRef]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Pena-Leon, V.; Perez-Lois, R.; Seoane, L.M. mTOR pathway is involved in energy homeostasis regulation as a part of the gut–brain axis. Int. J. Mol. Sci. 2020, 21, 5715. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Pedraza-Chaverri, J. Novel compounds for the modulation of mTOR and autophagy to treat neurodegenerative diseases. Cell. Signal. 2020, 65, 109442. [Google Scholar] [CrossRef]
- Danielsen, S.A.; Eide, P.W.; Nesbakken, A.; Guren, T.; Leithe, E.; Lothe, R.A. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2015, 1855, 104–121. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, W.; Chen, J.; Wei, W. Upstream regulators of phosphoinositide 3-kinase and their role in diseases. J. Cell. Physiol. 2019, 234, 14460–14472. [Google Scholar] [CrossRef] [PubMed]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Säemann, M. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Sun, Y.; Tang, M.; Kong, L. Effect and mechanism of PI3K/AKT/mTOR signaling pathway in the apoptosis of GC-1 cells induced by nickel nanoparticles. Chemosphere 2020, 255, 126913. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Jalili, F.; Hoseinkhani, Z.; Mansouri, K. Regenerative Medicine and Angiogenesis; Focused on Cardiovascular Disease. Adv. Pharm. Bull. 2022, 12, 686–699. [Google Scholar] [CrossRef]
- O’Connor, D.M.; Boulis, N.M. Gene therapy for neurodegenerative diseases. Trends Mol. Med. 2015, 21, 504–512. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef]
- Tăuţan, A.-M.; Ionescu, B.; Santarnecchi, E. Artificial intelligence in neurodegenerative diseases: A review of available tools with a focus on machine learning techniques. Artif. Intell. Med. 2021, 117, 102081. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, S.; Guo, B.; Coleman, B.; Hill, A. Exosomes: Vehicles for the Transfer of Toxic Proteins Associated with Neurodegenerative Diseases? Front. Physiol. 2012, 3, 124. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.R.; Araldi, R.P.; Vigerelli, H.; D’Ámelio, F.; Mendes, T.B.; Gonzaga, V.; Policíquio, B.; Colozza-Gama, G.A.; Valverde, C.W.; Kerkis, I. Exosomes in the tumor microenvironment: From biology to clinical applications. Cells 2021, 10, 2617. [Google Scholar] [CrossRef] [PubMed]
- Jarmalavičiūtė, A.; Pivoriūnas, A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol. Res. 2016, 113, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Howitt, J.; Hill, A.F. Exosomes in the pathology of neurodegenerative diseases. J. Biol. Chem. 2016, 291, 26589–26597. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K.; de Leon, M. Alzheimer’s disease. Lancet 2006, 368, 387. [Google Scholar]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Y.; Zhu, Y.; Chen, X.; Lin, T.; Zhou, D. Adipose Mesenchymal Stem Cell-Derived Exosomes Enhance PC12 Cell Function through the Activation of the PI3K/AKT Pathway. Stem Cells Int. 2021, 2021, 2229477. [Google Scholar] [CrossRef]
- Xiong, W.-P.; Yao, W.-Q.; Wang, B.; Liu, K. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3β/β-catenin. Brain Res. Bull. 2021, 177, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Y.; Chen, Q.; Chen, H.; Zhu, X.; Li, Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020, 11, 290. [Google Scholar] [CrossRef]
- Spinelli, M.; Natale, F.; Rinaudo, M.; Leone, L.; Mezzogori, D.; Fusco, S.; Grassi, C. Neural Stem Cell-Derived Exosomes Revert HFD-Dependent Memory Impairment via CREB-BDNF Signalling. Int. J. Mol. Sci. 2020, 21, 8994. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, E.B.; Tzvetkov, N.T.; Jóźwik, M.S.; Strzałkowska, N.; Horbańczuk, A.G. Quercetin: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health–a review. Anim. Sci. Pap. Rep. 2021, 39, 199–212. [Google Scholar]
- Soliman, H.M.; Ghonaim, G.A.; Gharib, S.M.; Chopra, H.; Farag, A.K.; Hassanin, M.H.; Nagah, A.; Emad-Eldin, M.; Hashem, N.E.; Yahya, G. Exosomes in Alzheimer’s disease: From being pathological players to potential diagnostics and therapeutics. Int. J. Mol. Sci. 2021, 22, 10794. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, Z.; Buller, B.; Li, Y.; Golembieski, W.; Gan, X.; Wang, F.; Lu, M.; Ali, M.M.; Zhang, Z.G. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J. Cereb. Blood Flow Metab. 2021, 41, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Song, J.-X.; Cai, H.; Wang, P.-P.; Yin, Q.-L.; Zhang, Y.-D.; Chen, J.; Li, M.; Song, J.-J.; Wang, Y.-L. Healthy Serum-Derived Exosomes Improve Neurological Outcomes and Protect Blood–Brain Barrier by Inhibiting Endothelial Cell Apoptosis and Reversing Autophagy-Mediated Tight Junction Protein Reduction in Rat Stroke Model. Front. Cell. Neurosci. 2022, 16, 841544. [Google Scholar] [CrossRef]
- Pan, Q.; Kuang, X.; Cai, S.; Wang, X.; Du, D.; Wang, J.; Wang, Y.; Chen, Y.; Bihl, J.; Chen, Y. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res. Ther. 2020, 11, 260. [Google Scholar] [CrossRef]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des. Dev. Ther. 2020, 14, 3143. [Google Scholar] [CrossRef]
- Shen, H.; Yao, X.; Li, H.; Li, X.; Zhang, T.; Sun, Q.; Ji, C.; Chen, G. Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J. Mol. Neurosci. 2018, 64, 421–430. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Zuo, Y.; Xue, Y.; Zhang, J.H.; Duan, J.; Liu, F.; Liu, A. Exosome-Encapsulated microRNA-140-5p Alleviates Neuronal Injury Following Subarachnoid Hemorrhage by Regulating IGFBP5-Mediated PI3K/AKT Signaling Pathway. Mol. Neurobiol. 2022, 59, 7212–7228. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Chen, L.; Shen, C.; Xiao, Z.; Xu, R.; Wang, J.; Luo, Y. HucMSCs-derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience 2019, 417, 11–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Liu, J.; Liu, H.; Li, J. Effects of stem cell-derived exosomes on neuronal apoptosis and inflammatory cytokines in rats with cerebral ischemia-reperfusion injury via PI3K/AKT pathway-mediated mitochondrial apoptosis. Immunopharmacol. Immunotoxicol. 2021, 43, 731–740. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res. Bull. 2020, 163, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, Y.; Liang, D.; He, H.; Liu, X.; Zhu, R.; Zhang, M.; Luo, X.; Wang, Y.; Huang, G. Exosomes secreted from bone marrow mesenchymal stem cells attenuate oxygen-glucose deprivation/reoxygenation-induced pyroptosis in PC12 cells by promoting AMPK-dependent autophagic flux. Front. Cell. Neurosci. 2020, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Li, D.; Wang, F.; Sun, Q.; Zhang, Z. Protective role of astrocyte-derived exosomal microRNA-361 in cerebral ischemic-reperfusion injury by regulating the AMPK/mTOR signaling pathway and targeting CTSB. Neuropsychiatr. Dis. Treat. 2020, 16, 1863. [Google Scholar] [CrossRef]
- Hou, K.; Li, G.; Zhao, J.; Xu, B.; Zhang, Y.; Yu, J.; Xu, K. Bone mesenchymal stem cell-derived exosomal microRNA-29b-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. J. Neuroinflamm. 2020, 17, 46. [Google Scholar] [CrossRef]
- Deng, M.; Xiao, H.; Peng, H.; Yuan, H.; Xu, Y.; Zhang, G.; Tang, J.; Hu, Z. Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions. Eur. J. Neurosci. 2018, 47, 150–157. [Google Scholar] [CrossRef]
- Halurkar, M.S.; Wang, J.; Chen, S.; Bihl, J.C. EPC-EXs improve astrocyte survival and oxidative stress through different uptaking pathways in diabetic hypoxia condition. Stem Cell Res. Ther. 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Martínez-Lara, E.; Siles, E.; Peinado, M.Á. New Strategies for Stroke Therapy: Nanoencapsulated Neuroglobin. Pharmaceutics 2022, 14, 1737. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, Y.; Li, Z.; Yong, V.W.; Xue, M. Extracellular matrix metalloproteinase inducer in brain ischemia and intracerebral hemorrhage. Front. Immunol. 2022, 13, 986469. [Google Scholar] [CrossRef]
- Mansfield, A.; Inness, E.L.; McIlroy, W.E. Stroke. Handb. Clin. Neurol. 2018, 159, 205–228. [Google Scholar] [CrossRef]
- Correa-Paz, C.; da Silva-Candal, A.; Polo, E.; Parcq, J.; Vivien, D.; Maysinger, D.; Pelaz, B.; Campos, F. New Approaches in Nanomedicine for Ischemic Stroke. Pharmaceutics 2021, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Eskey, C.J. Hemorrhagic stroke. Radiol. Clin. North Am. 2011, 49, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, L.-Y.; Hong, R.; Song, J.-X.; Guo, X.-J.; Zhou, W.; Hu, Z.-L.; Wang, W.; Wang, Y.-L.; Shen, J.-G. Momordica charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharmacol. 2022, 13, 908830. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, C.Z.; Liu, J.; Anastasio, N.C.; Johnson, K.M. Brain-derived neurotrophic factor prevents phencyclidine-induced apoptosis in developing brain by parallel activation of both the ERK and PI-3K/Akt pathways. Neuropharmacology 2010, 58, 330–336. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.G.; Li, C.; Zhang, G.Y. Subtoxic N-methyl-d-aspartate delayed neuronal death in ischemic brain injury through TrkB receptor-and calmodulin-mediated PI-3K/Akt pathway activation. Hippocampus 2007, 17, 525–537. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Yang, G.-Y. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc. Neurol. 2021, 6, 483–495. [Google Scholar] [CrossRef]
- Seyedaghamiri, F.; Salimi, L.; Ghaznavi, D.; Sokullu, E.; Rahbarghazi, R. Exosomes-based therapy of stroke, an emerging approach toward recovery. Cell Commun. Signal. 2022, 20, 110. [Google Scholar] [CrossRef]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112. [Google Scholar] [CrossRef]
- Flack, J.A.; Sharma, K.D.; Xie, J.Y. Delving into the recent advancements of spinal cord injury treatment: A review of recent progress. Neural Regen. Res. 2022, 17, 283. [Google Scholar]
- Alcántar-Garibay, O.V.; Incontri-Abraham, D.; Ibarra, A. Spinal cord injury-induced cognitive impairment: A narrative review. Neural Regen. Res. 2022, 17, 2649. [Google Scholar] [PubMed]
- Ding, Y.; Chen, Q. mTOR pathway: A potential therapeutic target for spinal cord injury. Biomed. Pharmacother. 2022, 145, 112430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, Z.; He, L.; Liu, C.; Wang, N.; Rong, L.; Liu, B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res. Ther. 2021, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bai, Z.; Yi, W.; Hu, Z.; Hao, J. Overexpression of miR-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci. Lett. 2021, 761, 136124. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, T.; Liu, W.; Rong, Y.; Wang, J.; Fan, J.; Yin, G.; Cai, W. Exosomes derived from GIT1-overexpressing bone marrow mesenchymal stem cells promote traumatic spinal cord injury recovery in a rat model. Int. J. Neurosci. 2021, 131, 170–182. [Google Scholar] [CrossRef]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell. Physiol. Biochem. 2018, 50, 1535–1559. [Google Scholar] [CrossRef]
- Huang, J.-H.; Xu, Y.; Yin, X.-M.; Lin, F.-Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience 2020, 424, 133–145. [Google Scholar] [CrossRef]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 105. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Li, S.; Li, Z.; Lai, X.; Xia, Q. Exosomes derived from nerve stem cells loaded with FTY720 promote the recovery after spinal cord injury in rats by PTEN/AKT signal pathway. J. Immunol. Res. 2021, 2021, 8100298. [Google Scholar] [CrossRef]
- Pan, D.; Li, Y.; Yang, F.; Lv, Z.; Zhu, S.; Shao, Y.; Huang, Y.; Ning, G.; Feng, S. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J. Neuroinflammation 2021, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhu, S.; Zhang, W.; Wei, Z.; Yang, F.; Guo, Z.; Ning, G.; Feng, S. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol. Lett. 2022, 44, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, Y.; Chen, C.; Xie, H.; Lu, H.; Hu, J. Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. Stem Cell Res. Ther. 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lai, X.; Wu, D.; Liu, B.; Wang, N.; Rong, L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res. Ther. 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lin, F.; Dong, J.; Liu, J.; Ding, Z.; Xu, J. Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int. J. Biol. Sci. 2021, 17, 1339. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, Y.; Huang, S.; Xu, H.; Li, H.; Liu, B. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci. Lett. 2020, 736, 135262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes derived from pericytes improve microcirculation and protect blood–spinal cord barrier after spinal cord injury in mice. Front. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef]
- Quillinan, N.; Herson, P.S.; Traystman, R.J. Neuropathophysiology of Brain Injury. Anesthesiol. Clin. 2016, 34, 453–464. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.-C.; Ledreux, A. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019, 33, 5082. [Google Scholar] [CrossRef]
- Gao, W.; Li, F.; Liu, L.; Xu, X.; Zhang, B.; Wu, Y.; Yin, D.; Zhou, S.; Sun, D.; Huang, Y. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp. Neurol. 2018, 307, 99–108. [Google Scholar] [CrossRef]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, H.; Yang, Y.; Ma, Y. Exosomal microRNAs have great potential in the neurorestorative therapy for traumatic brain injury. Exp. Neurol. 2022, 352, 114026. [Google Scholar] [CrossRef] [PubMed]
- Guedes, V.A.; Devoto, C.; Leete, J.; Sass, D.; Acott, J.D.; Mithani, S.; Gill, J.M. Extracellular vesicle proteins and microRNAs as biomarkers for traumatic brain injury. Front. Neurol. 2020, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Y.; Zhu, Z.; Gu, C.; Waqas, A.; Chen, L. Emerging exosomes and exosomal MiRNAs in spinal cord injury. Front. Cell Dev. Biol. 2021, 9, 703989. [Google Scholar] [CrossRef]
- Harman, D. Aging: Overview. Ann. New York Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Brandi, J.; Manfredi, M.; Scambi, I.; Schiaffino, L.; Merigo, F.; Turano, E.; Bonetti, B.; Marengo, E.; Cecconi, D. The anti-apoptotic effect of ASC-exosomes in an in vitro ALS model and their proteomic analysis. Cells 2019, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Tassew, N.G.; Charish, J.; Shabanzadeh, A.P.; Luga, V.; Harada, H.; Farhani, N.; D’Onofrio, P.; Choi, B.; Ellabban, A.; Nickerson, P.E. Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 2017, 20, 99–111. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Xu, Z.; Liu, J.; Liu, X.; Ma, J.; Sun, C.; Yang, Y. Exosomal miR-673-5p from fibroblasts promotes Schwann cell-mediated peripheral neuron myelination by targeting the TSC2/mTORC1/SREBP2 axis. J. Biol. Chem. 2022, 298, 101718. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef]
- Feng, N.; Jia, Y.; Huang, X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-kB and MAPK pathway. J. Neuroimmunol. 2019, 334, 576996. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, Y.; Xue, C.; Ma, B.; Shen, Y.; Guan, J.; Bao, X.; Wu, H.; Han, Q.; Wang, R. Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad. Med. Sin. 2016, 38, 33–36. [Google Scholar]
- Cui, Y.; Liu, C.; Huang, L.; Chen, J.; Xu, N. Protective effects of intravitreal administration of mesenchymal stem cell-derived exosomes in an experimental model of optic nerve injury. Exp. Cell Res. 2021, 407, 112792. [Google Scholar] [CrossRef]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and therapeutic potential for neurodegenerative disease. Front. Neurosci. 2017, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, W.; Ye, J.; Wang, Y. Potential role of exosomes in ischemic stroke treatment. Biomolecules 2022, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).