Abstract
Apixaban and rivaroxaban have first-line use for many patients needing anticoagulation for venous thromboembolism (VTE). The pharmacokinetics of these drugs in non-obese subjects have been extensively studied, and, while changes in pharmacokinetics have been documented in obese patients, data remain scarce for these anticoagulants. The aim of this study was to perform an external validation of published population pharmacokinetic (PPK) models of apixaban and rivaroxaban in a cohort of obese patients with VTE. A literature search was conducted in the PubMed/MEDLINE, Scopus, and Embase databases following the PRISMA statement. External validation was performed using MonolixSuite software, using prediction-based and simulation-based diagnostics. An external validation dataset from the university hospitals of Brest and Rennes, France, included 116 apixaban pharmacokinetic samples from 69 patients and 121 rivaroxaban samples from 81 patients. Five PPK models of apixaban and 16 models of rivaroxaban were included, according to the inclusion criteria of the study. Two of the apixaban PPK models presented acceptable performances, whereas no rivaroxaban PPK model did. This study identified two published models of apixaban applicable to apixaban in obese patients with VTE. However, none of the rivaroxaban models evaluated were applicable. Dedicated studies appear necessary to elucidate rivaroxaban pharmacokinetics in this population.
1. Introduction
Overweight is a global health problem, and its worldwide prevalence is steadily increasing. In Europe, the prevalence of obesity, defined by a body mass index (BMI) ≥ 30 kg/m², has reached 20% in the most affected countries [1]. In the United States, the prevalence estimates were close to 40% of the adult population [2], and the recent data on children and adolescents suggested an acceleration of this trend during the COVID-19 pandemic [3]. Among other conditions, obesity is associated with an increased risk of cardiovascular morbidity and mortality, including venous thromboembolism (VTE) and a higher risk of recurrent VTE following the withdrawal of anticoagulation therapy [4]. Direct-acting oral anticoagulants (DOACs) have first-line use for many patients needing anticoagulation for VTE [5]. Compared to anti-vitamin K drugs, DOACs have a favorable benefit-risk profile with fewer drug interactions and a lower incidence of intracranial hemorrhage [6]. Their ease of use due to fixed-dose regimens and lack of routine monitoring makes DOACs an attractive therapeutic option for the management of VTE in people with high weight or BMI.
Although the PK of apixaban and rivaroxaban in non-obese subjects has been extensively studied [7,8], data on obese patients remain scarce. Yet, substantial changes in pharmacokinetics (PK) are observed in persons with obesity [9,10,11]; these effects are not consistent across drugs, and they have not been clearly characterized for DOACs. The two PK parameters most likely to be altered in this population are the volume of distribution, and drug elimination [9,11]. Increases in the volume of distribution are particularly seen for lipophilic drugs and are usually not proportional to body weight [10]. The impact of obesity on drug elimination clearance also depends on several drug- and patient-related factors, such as the cytochromes involved, variations in hepatic blood flow, and the duration of obesity. Therefore, it remains difficult to assess the impact of obesity on drug elimination. Although lean body weight is increasingly proposed as a measure of body mass in obesity pharmacology, particularly for renally eliminated drugs [12], there is still no consensus on the most appropriate descriptor of body weight in this population.
The initial recommendations published by the ISTH in 2016 for the use of DOACs in patients with obesity for the treatment and prevention of VTE were conservative [13]. The guidance suggested not using DOACs in patients with extreme obesity (BMI > 40 kg/m² or weight > 120 kg), and, if DOACs were nevertheless used in these patients, to monitor the peak and trough drug levels [13]. These recommendations were made in light of insufficient clinical evidence regarding efficacy and safety in patients with extreme obesity, as phase 3 clinical trials comparing DOACs with warfarin for the treatment of VTE included few patients with obesity and extreme obesity. Even though prospective and specific studies in this population are still lacking, especially in patients with morbid obesity, the recent update of these guidelines presented standard doses of rivaroxaban or apixaban as appropriate options for the treatment of deep vein thrombosis, regardless of patient weight or high BMI [14]. The monitoring of DOAC levels was no longer recommended because there were insufficient data to influence management decisions.
While there are no warning signs regarding the efficacy or safety of apixaban or rivaroxaban in patients with obesity, the ISTH guidelines are recent and have many limitations, and the question of the impact of obesity on the PK of DOACs remains unanswered. Several population PK (PPK) models were developed to characterize the complete PK of apixaban and rivaroxaban. A PPK analysis can identify and explain the determinants of inter-individual variability in drug exposure, and it is frequently used to guide drug development and inform recommendations on therapeutic individualization [15]. PPK models can be used to predict drug concentrations, either a priori or a posteriori, via Bayesian estimation procedures, given the measurement of one or more concentrations. However, using previously developed PPK models in the obese patient population requires validation of their predictive ability, as they were not initially developed in this population.
Therefore, we sought to determine whether current knowledge about the PK of apixaban and rivaroxaban, as formalized in published PPK models developed in obese and non-obese patient populations treated for various indications, was applicable to obese patients treated for VTE. To this end, we performed a systematic review of published apixaban and rivaroxaban PPK models and a subsequent external evaluation on an independent dataset to validate their predictive performance in the population of obese patients treated for VTE.
2. Materials and Methods
2.1. Review of Published PPK Models
The Scopus, MEDLINE, and Embase databases were searched to identify published PPK analyses of rivaroxaban or apixaban using the following keywords: “rivaroxaban” [AND] “pharmacokinetics” for rivaroxaban, and “apixaban” [AND] “pharmacokinetics” for apixaban. The Cochrane database was searched less stringently, using only the keywords “rivaroxaban” or “apixaban”. The reference lists of identified articles were manually screened for additional relevant studies. The search included studies published in English or French between the inception of the databases and 1 November 2020. The included PPK models were those developed using the following: (1) human DOAC data from adult patients; (2) a compartmental, parametric modeling approach. The PPK models were excluded if (1) the model description was insufficient/inadequate to fully reproduce the model, or (2) the model was a physiological-based pharmacokinetic (PBPK) model.
2.2. Independent External Validation Data Set
DOAC PK and demographic data were collected from adult patients with obesity (BMI ≥ 30 kg/m²) receiving apixaban or rivaroxaban for VTE treatment and enrolled in a prospective multicenter observational study [16], conducted in the outpatients’ thrombosis clinics of Rennes and Brest University Hospitals between August 2017 and January 2019. Any patient with obesity with VTE followed by the thrombosis center could be included in the study, whatever the time since the initiation of anticoagulation. Apixaban or rivaroxaban plasma concentrations were measured just after the inclusion visit, whatever the time since the last intake, as part of routine care. The exact time between the DOAC intake and blood sampling was strictly recorded, as well as the DOAC dose. No patient in this study was analyzed in previously published DOAC PPK studies. The patients’ age, weight, BMI, gender, and creatinine clearance (calculated using the Cockcroft and Gault formula) were recorded. The plasma apixaban and rivaroxaban concentrations were measured using the commercial assay STA-Liquid-anti-Xa, with specific controls and calibrators on a STA-R Evolution analyzer from STAGO Diagnostica (Asnières sur Seine, France) in each center. The lower limit of quantification (LLOQ) was 20 ng/mL. The study was approved by the local ethics committee. All the patients were informed and did not object to the inclusion.
2.3. External Predictive Performance Evaluation of Apixaban and Rivaroxaban PPK Models
The MonolixSuite 2020R1 (Lixoft SAS, Antony, France) was used for external evaluation. The R language and environment (version 4.1.3, R Foundation for Statistical Computing) was used to postprocess the Monolix output and generate graphics. The published PPK models were employed using reported model equations, parameter values, covariate relationships, interpatient variability, parameter covariance, intra-patient variability, and unexplained residual variability. For each model, the apixaban and rivaroxaban concentrations, respectively, were simulated using dosing regimens, sampling times, and covariate information from the external validation dataset (EVD). To assess steady-state concentrations, ten doses were simulated before the first observation. Plasma concentrations below the LLOQ were left censored, according to the M3 method described by Beal [17]. For the models with study-dependent parameter values, an external evaluation was performed for each set of values.
2.4. Prediction-Based Diagnostics
Based on the observed concentration (Cobs) and population prediction (Cpred), the prediction error percentage (PE%) and absolute prediction error percentage (APE%) were calculated using the following equations:
The median prediction error (MDPE) and the median absolute prediction error (MDAE) were used to evaluate the accuracy and precision of the predictive performance, respectively. The PE% within ±20% (F20) and the PE% within ±30% (F30) were calculated as joint predictors of accuracy and precision. The predictive performance of the candidate models was considered satisfactory if |MDPE| ≤ 20%, MDAE ≤ 30%, F_20 ≥ 35%, and F_30 ≥ 50% [18,19].
2.5. Simulation-Based Diagnostics
The predictive performance of each PPK model was evaluated by performing Monte Carlo simulations in Monolix (n = 5000) using patient characteristics, dosing, and the sampling scheme from the EVD. The prediction-corrected visual predictive checks (pcVPC) were computed and plotted using the vpc R package, to visually assess if the prediction-corrected simulations generated by a candidate model deviated from the prediction-corrected observed data. The VPC diagnoses both the fixed and random effects in mixed-effects models, helping to determine if the intrapatient and interpatient variability were adequately specified in each model to reproduce the central trend and variability in the EVD. Prediction correction accounts for the differences in dosing and influent patient covariates [20].
The normalized prediction distribution errors (NPDE) were computed with Monolix. Under the null hypothesis that the model under scrutiny describes the EVD, the NPDE should follow the standard normal distribution [21]. Histograms and quantile–quantile plots (QQ plots) of the NPDE were visually inspected for each model.
3. Results
3.1. Review of Published PPK Studies
The details of the literature search are provided in Figure 1. The dosing information, patient characteristics, and the intended application of the PPK model for each study are described in Table 1.
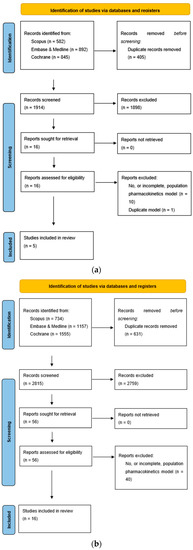
Figure 1.
(a) PRISMA flowchart for apixaban population pharmacokinetic studies; (b) PRISMA flowchart for rivaroxaban pharmacokinetic studies.

Table 1.
Design of the selected population pharmacokinetic studies.
3.1.1. Apixaban
A total of five PPK models of apixaban were included for external evaluation after the literature retrieval [22,23,24,25,26]. Among them, four were multicenter studies (A1–A4), and one was a single-center study (A5). The sample size of subjects administered apixaban was >100 in all the multicenter studies. Liquid chromatography coupled with mass spectrometry (LC–MS/MS) methods were used to measure the apixaban plasma concentrations in all but one study (A3), which used an anti-Xa chromogenic assay (Table 1). Both intensive and sparse samples were collected in the three studies sponsored by pharmaceutical companies (A1, A2, A4), and only sparse samples were obtained in the other two studies (A3, A5). Table 2 details the models.

Table 2.
Details of the published population pharmacokinetic models.
3.1.2. Rivaroxaban
A total of sixteen PPK models of rivaroxaban were included for external evaluation after the literature retrieval [8,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Among them, eleven were multicenter studies (R2–R4, R6–R8, R10, R12–R14, R16), and five were single-center studies (R1, R5, R9, R11, R15). The sample size of the subjects administered rivaroxaban was >100 in all the multicenter studies, and in one single-center study (R1). LC–MS/MS methods were used to measure the rivaroxaban plasma concentrations in twelve studies, and an anti-Xa chromogenic assay was used in four studies (R1, R3, R10, R15). Most of the studies used sparse sampling to collect the PK samples (R1–R4, R8, R10–R16); intensive sampling was used in two studies on healthy subjects (R5, R9); and one study used both sparse and intensive sampling for a subset of patients (R6). Table 2 details the rivaroxaban PPK models. With the exception of one model (R5), all the structural models were one-compartment models. The relative bioavailability was modelled as dose-independent with intersubject variability in four models (R3, R4, R9, R12), as dose-dependent with discrete values according to rivaroxaban intake in four models (R6, R7, R8, R14), and a non-linear dose-dependent relative bioavailability was included in one model (R13). Bodyweight was included as a covariate influencing rivaroxaban PK in ten studies (R1–R4, R8, R10, R11, R13, R14, R16). Total bodyweight was incorporated as a predictor of both rivaroxaban clearance and of the volume of the central compartment in one study (R13). More often, lean body mass (LBM) was used to include the subjects’ weight in the models (R2, R8, R10, R12, R14, R16), although the formulas differed between the studies. Body mass was included indirectly via the estimation of creatinine clearance, using various adaptations of the Cockcroft and Gault formula, in six studies (R1, R3, R4, R10, R13). The other covariates identified were rivaroxaban dose, gender, age, serum creatinine, comedications, HCT, pharmacogenomics, serum alanine aminotransferase (ALT), and blood urea nitrogen (BUN).
3.2. External Validation Dataset Cohort
The EVD included 116 PK samples from 69 patients with obesity taking apixaban for VTE treatment, and 121 PK samples from 81 patients with obesity taking rivaroxaban [16]. The median and range of the age and weight of each subpopulation are presented in Table 1. All the patients were sampled after at least one month of treatment. Since the genotypes, HCT, comedications, ALT, and BUN were not collected in our dataset, the values of the parameters affected were arbitrarily set either to median population values to cancel the effect of the covariate, or to credible values for the population (that is ALT = 22 UI/L, HCT = 0.4 for females, HCT = 0.45 for males).
3.3. External Predictability Evaluation
3.3.1. Prediction-Based Diagnostics
The accuracy and precision measures generated for each model are provided in Table 3.

Table 3.
Bias and imprecision of the published of the published population pharmacokinetic models applied to the EVD.
Apixaban
All two-compartment models and one of the mono-compartment models met the precision and accuracy objectives (A1, A2, A4, A5), regardless of the subpopulation selected for the study-dependent parameter values for A2 and A4 (Figure 2).
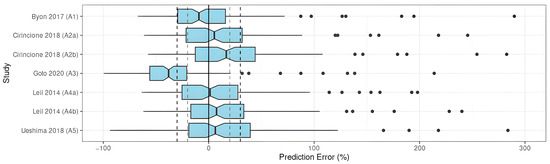
Figure 2.
PE% of apixaban population pharmacokinetic studies applied to the external validation dataset [22,23,24,25,26].
The remaining model did not meet any of the objectives, showing insufficient precision and accuracy of this model when applied to the EVD (Table 3).
Rivaroxaban
The results indicated an unsatisfactory predictive performance in the prediction-based diagnostics (Table 3). None of the investigated models met all the aforementioned criteria (|MDPE| ≤ 20%, MDAE ≤ 30%, F_20 ≥ 35%, and F_30 ≥ 50%). The values of MDPE, an indicator of predictive accuracy, were within ±20% in ten studies (R2–R4, R6, R7, R10, R13 (with SUB = NVAF), R14–R16) (Figure 3).
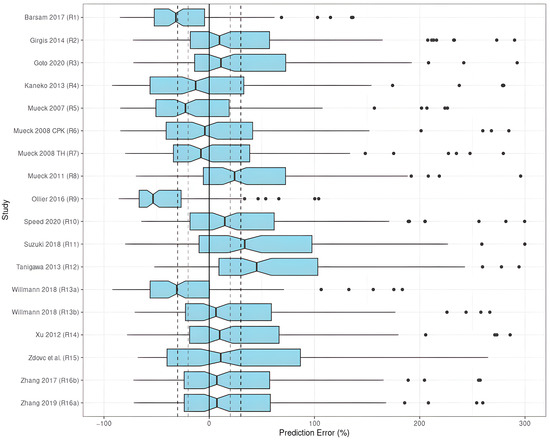
Figure 3.
PE% of rivaroxaban population pharmacokinetic studies applied to the external validation dataset [8,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
The MDAE, an indicator of predictive precision, was less than 30% in two studies (R2, R13 with SUB = NVAF). As a combined predictor of both accuracy and precision, F_20 was over 35% in only one study (R3), and F_30was over 50% in two studies (R2, R13 with SUB = NVAF). Taking both accuracy and precision into account, the studies by Girgis et al. (R2) [28], and Willmann et al. (R13) [8] with SUB = NVAF showed preferable predictive performances compared to the others when applied to the EVD, in which three out of four criteria were met with |MDPE| ≤ 20%, MDAE ≤ 30%, and F_30 ≥ 50%. When selecting SUB = VTE, the model by Willmann et al. (R13) [8] did not perform well.
3.3.2. Simulation-Based Diagnostics
Apixaban
The pcVPC of the prediction-corrected plasma apixaban concentrations versus time since the last apixaban dose showed a substantial discrepancy between the observations and simulations (Figure 4), except in two studies (A2, A4).
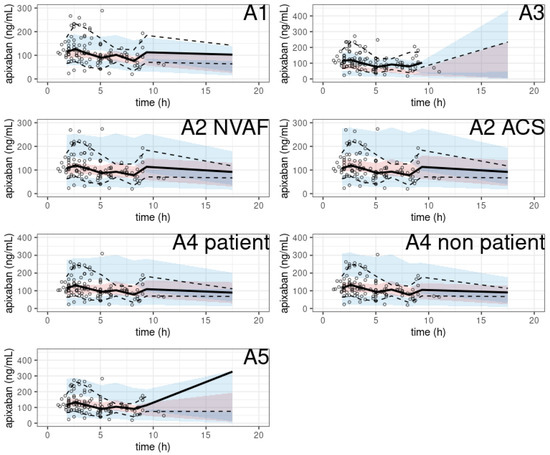
Figure 4.
pcVPC of apixaban population pharmacokinetic studies applied to the external validation dataset.
In particular, two models showed no obvious misspecification (A2 with SUB = ACS, A4 with SUB = non-patient). The NPDE histograms and QQ plots are shown in Figure 5.
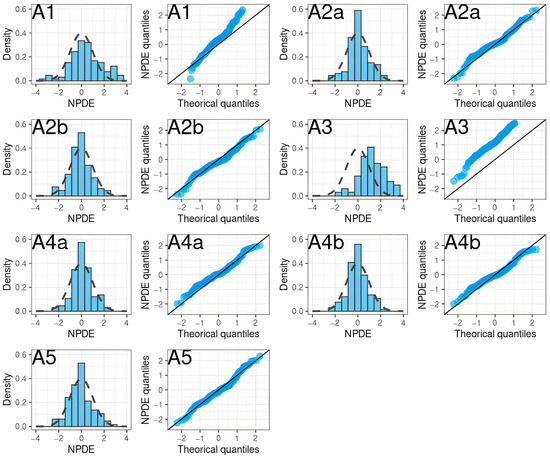
Figure 5.
NPDE histograms and QQ plots of apixaban population pharmacokinetic studies applied to the external validation dataset.
Rivaroxaban
The pcVPC showed a large discrepancy between the prediction-corrected observations and the simulations in most of the models (Figure 6).
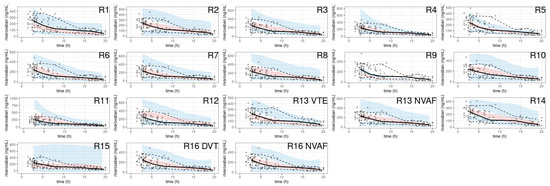
Figure 6.
pcVPC of rivaroxaban population pharmacokinetic studies applied to the external validation dataset.
A noticeable trend of over- or under-prediction was observed, except in four models (R2, R13 with SUB = NVAF, R16) which showed slight misspecifications in the early concentrations post-dose. The NPDE histograms and QQ plots confirmed the poor characterization of the rivaroxaban PK of the EVD (Figure 7).
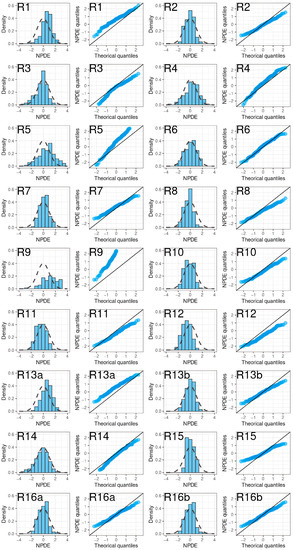
Figure 7.
NPDE histograms and QQ plots of rivaroxaban population pharmacokinetic studies applied to the external validation dataset.
4. Discussion
This is the first study to systematically evaluate the predictive performance of published PPK models for apixaban and rivaroxaban by external validation in a population of obese patients treated for VTE. This analysis highlights the value of some models to explore hypothetical scenarios to determine apixaban and rivaroxaban dosing in patients with high body weight or BMI. Based on our analysis, two of the published apixaban PPK models evaluated adequately described the observed anticoagulant PK in the EVD, whereas no published rivaroxaban PPK model did. The PPK models based on studies with relatively large patient populations and an intensive sampling strategy better characterized the DOAC PK of the EVD overall. The sampling including the distribution phase—and not just sampling around the peak and trough—improved the assessment of the number of compartments needed to effectively characterize the DOAC PK.
The two-compartment models best described the pharmacokinetics of apixaban in the external validation dataset. Models A2 and A4 were based on studies that used both intensive and sparse sampling and described the PK of apixaban in the EVD satisfactorily. The mass descriptor included in models A2 and A4 was Total Body Weight (TBW), similar to the all-apixaban PPK models analyzed, and the influence of renal function on apixaban PK was included using the Cockcroft formula (model A4), or the Cockcroft formula limited to 150 mL/min (model A2). Because these approaches were common to all the apixaban models analyzed, it was not possible to conclude whether other descriptors of weight (e.g., Lean Body Weight (LBW), adjusted weight) or renal function (e.g., serum creatinine, CKD-epi) would be of any value. The choice of method for estimating renal function has been cited as a cause of dosing discrepancies; however, the ELIQUIS package insert refers to renal function only in the context of renal failure; hence, no impact is expected with a cutoff of 150 mL/min on the Cockcroft estimate.
Although similarly bi-compartmental, and developed from rich data, the A1 model did not satisfactorily describe the EVD, as revealed by the pcVPC and the NPDE residuals inspection. The A1 model differed mainly from the other two-compartment apixaban models (A2, A4) by the absence of a dose-dependent bioavailability model. Not unexpectedly, although the interindividual variability associated with the absorption constant, Ka, was comparable between these three models, the discrepancies between the A1 model simulations and observed concentrations objectified by the pcVPCs were mainly observed soon after the apixaban dosing.
Notwithstanding their large number, no published rivaroxaban PPK models characterized the EVD adequately. Two models performed better than the others, without meeting all the validity criteria (model R2, R13). Both were one-compartment models, derived from sparse sampling studies. Otherwise, the models were different. Model R13 included a dose-dependent bioavailability model with interindividual variability in the absorption constant, Ka. The influence of weight was integrated with TBW, and the influence of renal function on rivaroxaban clearance was modeled by a modified version of Cockcroft clearance (referred to as “Tietz-truncated clearance” by the authors). The R2 model described absorption with an absorption constant, Ka, without interindividual variability. The effect of body mass on rivaroxaban PK was included via LBW, and the effect of renal function on anticoagulant clearance was included using serum creatinine. The different approaches of the two best-performing rivaroxaban PPK models did not allow for a conclusion as to which strategy was best overall. There was no trend toward better model performance with respect to the number of subjects included in the studies, the number of samples collected, the study population (e.g., healthy volunteers, orthopedic surgery patients, patients with NVAF), or the magnitude of the residual error (although nine models had a proportional error greater than 40%).
Of the papers reviewed, two studies were notable for their common objective: to identify the influence of body mass on rivaroxaban PK (R1, R10). The study by Speed et al. (R10) [35] was a large study with a sparse sampling strategy. The influence of body mass on PK was included with LBM, and the impact of renal function on rivaroxaban clearance was incorporated by Cockcroft creatinine clearance calculated with LBM instead of TBW. In the model developed by Barsam et al. (R1) [27], the only covariate retained was the influence of Cockcroft creatinine clearance, calculated with TBW, on rivaroxaban clearance. However, the performance of neither model was satisfactory when applied to EVD. The underestimation (R1) or overestimation (R10) of the simulations compared with the observed concentrations, along with the high bias and imprecision identified through our analysis, did not allow us to confirm the limited impact of weight on rivaroxaban PK suggested by the authors.
The appropriateness of the external data set to assess each model needs consideration in light of the high degree of bias and imprecision found across most of the published models. The dosing in the observational prospective study [16] used to generate the EVD was standard, comparable with the dosing described in the published PPK models and is an unlikely source of bias. An additional limitation was the lack of information in the dataset regarding some of the covariates in the published models. The introduction of bias due to the imputation of missing variables (HCT for models A4 and R4, BUN for model R12, pharmacogenomics for models A5 and R15) cannot be excluded. Among these covariates, race is a special case (models A1, A2). Firstly, the collection of ethnic statistics is not allowed in France, and, secondly, the concept of race is questionable and an inadequate descriptor of the distribution of genetic variability in our species [41]. In the specific context of PK, a considerable amount of literature comparing PK between ethnic groups has been produced, with more papers reporting similarities than differences, and a decrease in the proportion of papers showing ethnic differences over time [42]. Overall, these data suggest disregarding racial covariates. The EVD anticoagulant assay could also be questioned, but the correlation between drug-calibrated anti-FXa methods and LC–MS/MS has been demonstrated for routine concentrations [43], and the satisfactory performance of some apixaban PPK models developed from LC–MS/MS data (A2, A4) is reassuring. Finally, as all the models were implemented in Monolix software, the implementation of the residual error models might have introduced additional imprecision in the model evaluations because of its difference with the implementation of NONMEM [44].
In light of the reassuring clinical data, the latest ISTH guidance suggested using standard doses of apixaban or rivaroxaban in the treatment of VTE, regardless of patient weight or high BMI [14]. Nevertheless, appropriate PPK models for this population of patients could be invaluable in bridging the gap between analytical chemistry and patient clinical data.
For apixaban, the results of systematic external evaluations supported a satisfactory description of its PK in the obese patient population treated for VTE by two published PPK models, using TBW as a descriptor of body mass (A2, A4). In contrast, for rivaroxaban, the lack of alignment of the published PPK analyses with the aim of adequately describing rivaroxaban PK in the obese patient population, combined with their poor predictive performance in this population, emphasized the need to develop such a PPK model. The development of this PPK model should be based on a well-powered study in the population of interest, with an intensive PK sampling strategy to identify clinically relevant covariates that can adequately characterize rivaroxaban PK. Since our last literature search, new pharmacokinetic studies have been conducted or are underway. For example, a Pubmed/MEDLINE search in February 2023 using the keywords “rivaroxaban population pharmacokinetics” for 2021–2023 identified five studies [45,46,47,48,49] that would have met our systematic review inclusion criteria by title and abstract. All were single-compartment models with first-order uptake, and none were specifically designed for the obese patient population. The present study did not evaluate these models from studies conducted after our last literature search, but an ongoing phase 1 study aiming to assess the PK of rivaroxaban used as a therapeutic anticoagulant dose in patients with previous bariatric surgery, and in morbidly obese subjects (NCT04180436) [50], will provide the data necessary to develop an adequate PPK model in this population.
5. Conclusions
The study was not designed to draw clinical conclusions about the management of obese patients with VTE. However, the analysis provided information on the pharmacokinetics of the direct oral anticoagulants studied. Several population pharmacokinetic models of apixaban were applicable to the population of obese patients receiving curative apixaban treatment for VTE, suggesting that the results of these models developed in the general population are relevant to this specific population. In contrast, none of the models evaluated for rivaroxaban were applicable to obese patients treated for VTE. Extrapolations from these models, their parameter values, or their simulation results should not be applied to obese patients treated with rivaroxaban for VTE.
Author Contributions
Conceptualization, C.L. and K.L.; methodology, C.L. and E.O.; investigation, C.L., P.M., I.G.-T., A.B. and K.L.; data curation, C.L.; writing—original draft preparation, C.L. and P.M.; writing—review and editing, C.L., P.M., I.G.-T., A.B., K.L., E.O. and J.T.; supervision, K.L. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallus, S.; Lugo, A.; Murisic, B.; Bosetti, C.; Boffetta, P.; La Vecchia, C. Overweight and Obesity in 16 European Countries. Eur. J. Nutr. 2015, 54, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Ihaddadene, R.; Carrier, M. The Use of Anticoagulants for the Treatment and Prevention of Venous Thromboembolism in Obese Patients: Implications for Safety. Expert Opin. Drug Saf. 2016, 15, 65–74. [Google Scholar] [CrossRef]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.-J.; Kyrle, P.A. The Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease Categorization of Patients as Having Provoked or Unprovoked Venous Thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef]
- van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Buller, H.R. Direct Oral Anticoagulants Compared with Vitamin K Antagonists for Acute Venous Thromboembolism: Evidence from Phase 3 Trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef]
- Byon, W.; Garonzik, S.; Boyd, R.A.; Frost, C.E. Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review. Clin. Pharmacokinet. 2019, 58, 1265–1279. [Google Scholar] [CrossRef]
- Willmann, S.; Zhang, L.; Frede, M.; Kubitza, D.; Mueck, W.; Schmidt, S.; Solms, A.; Yan, X.; Garmann, D. Integrated Population Pharmacokinetic Analysis of Rivaroxaban Across Multiple Patient Populations. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 309–320. [Google Scholar] [CrossRef]
- Jain, R.; Chung, S.M.; Jain, L.; Khurana, M.; Lau, S.W.J.; Lee, J.E.; Vaidyanathan, J.; Zadezensky, I.; Choe, S.; Sahajwalla, C.G. Implications of Obesity for Drug Therapy: Limitations and Challenges. Clin. Pharmacol. Ther. 2011, 90, 77–89. [Google Scholar] [CrossRef]
- Morrish, G.A.; Pai, M.P.; Green, B. The Effects of Obesity on Drug Pharmacokinetics in Humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 697–706. [Google Scholar] [CrossRef]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and Drug Pharmacology: A Review of the Influence of Obesity on Pharmacokinetic and Pharmacodynamic Parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Han, P.Y.; Duffull, S.B.; Kirkpatrick, C.M.J.; Green, B. Dosing in Obesity: A Simple Solution to a Big Problem. Clin. Pharmacol. Ther. 2007, 82, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of the Direct Oral Anticoagulants in Obese Patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of Direct Oral Anticoagulants in Patients with Obesity for Treatment and Prevention of Venous Thromboembolism: Updated Communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J. Thromb. Haemost. 2021, 19, 1874–1882. [Google Scholar] [CrossRef]
- Brum, L. Population Pharmacokinetics; FDA Guidance; Food and Drug Administration: Rockville, MD, USA, 2022; p. 27. [Google Scholar]
- Ballerie, A.; Nguyen Van, R.; Lacut, K.; Galinat, H.; Rousseau, C.; Pontis, A.; Nédelec-Gac, F.; Lescoat, A.; Belhomme, N.; Guéret, P.; et al. Apixaban and Rivaroxaban in Obese Patients Treated for Venous Thromboembolism: Drug Levels and Clinical Outcomes. Thromb. Res. 2021, 208, 39–44. [Google Scholar] [CrossRef]
- Beal, S.L. Ways to Fit a PK Model with Some Data Below the Quantification Limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef]
- Hanafin, P.O.; Nation, R.L.; Scheetz, M.H.; Zavascki, A.P.; Sandri, A.M.; Kwa, A.L.; Cherng, B.P.Z.; Kubin, C.J.; Yin, M.T.; Wang, J.; et al. Assessing the Predictive Performance of Population Pharmacokinetic Models for Intravenous Polymyxin B in Critically Ill Patients. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1525–1537. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, C.; Liu, L.; Luo, B.; Fu, Q.; Zhao, Q.; Li, J.; Liu, Y.; Deng, R.; Jiao, Z.; et al. Systematic External Evaluation of Published Population Pharmacokinetic Models of Mycophenolate Mofetil in Adult Kidney Transplant Recipients Co-administered with Tacrolimus. Br. J. Clin. Pharmacol. 2019, 85, 746–761. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentré, F. Computing Normalised Prediction Distribution Errors to Evaluate Nonlinear Mixed-Effect Models: The Npde Add-on Package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef]
- Byon, W.; Sweeney, K.; Frost, C.; Boyd, R. Population Pharmacokinetics, Pharmacodynamics, and Exploratory Exposure-Response Analyses of Apixaban in Subjects Treated for Venous Thromboembolism: Subjects Treated for Venous Thromboembolism. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Cirincione, B.; Kowalski, K.; Nielsen, J.; Roy, A.; Thanneer, N.; Byon, W.; Boyd, R.; Wang, X.; Leil, T.; LaCreta, F.; et al. Population Pharmacokinetics of Apixaban in Subjects with Nonvalvular Atrial Fibrillation. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Horinaka, S.; Ishimitsu, T.; Kato, T. Factor Xa Inhibitors in Clinical Practice: Comparison of Pharmacokinetic Profiles. Drug Metab. Pharmacokinet. 2020, 35, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Leil, T.A.; Frost, C.; Wang, X.; Pfister, M.; LaCreta, F. Model-Based Exposure–Response Analysis of Apixaban to Quantify Bleeding Risk in Special Populations of Subjects Undergoing Orthopedic Surgery. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, 136. [Google Scholar] [CrossRef]
- Ueshima, S.; Hira, D.; Kimura, Y.; Fujii, R.; Tomitsuka, C.; Yamane, T.; Tabuchi, Y.; Ozawa, T.; Itoh, H.; Ohno, S.; et al. Population Pharmacokinetics and Pharmacogenomics of Apixaban in Japanese Adult Patients with Atrial Fibrillation. Br. J. Clin. Pharmacol. 2018, 84, 1301–1312. [Google Scholar] [CrossRef]
- Barsam, S.J.; Patel, J.P.; Roberts, L.N.; Kavarthapu, V.; Patel, R.K.; Green, B.; Arya, R. The Impact of Body Weight on Rivaroxaban Pharmacokinetics. Res. Pract. Thromb. Haemost. 2017, 1, 180–187. [Google Scholar] [CrossRef]
- Girgis, I.G.; Patel, M.R.; Peters, G.R.; Moore, K.T.; Mahaffey, K.W.; Nessel, C.C.; Halperin, J.L.; Califf, R.M.; Fox, K.A.A.; Becker, R.C. Population Pharmacokinetics and Pharmacodynamics of Rivaroxaban in Patients with Non-Valvular Atrial Fibrillation: Results from ROCKET AF. J. Clin. Pharmacol. 2014, 54, 917–927. [Google Scholar] [CrossRef]
- Kaneko, M.; Tanigawa, T.; Hashizume, K.; Kajikawa, M.; Tajiri, M.; Mueck, W. Confirmation of Model-Based Dose Selection for a Japanese Phase III Study of Rivaroxaban in Non-Valvular Atrial Fibrillation Patients. Drug Metab. Pharmacokinet. 2013, 28, 321–331. [Google Scholar] [CrossRef]
- Mueck, W.; Becka, M.; Kubitza, D.; Voith, B.; Zuehlsdorf, M. Population Model of the Pharmacokinetics and Pharmacodynamics of Rivaroxaban--an Oral, Direct Factor Xa Inhibitor--in Healthy Subjects. Int. J. Clin. Pharmacol. Ther. 2007, 45, 335–344. [Google Scholar] [CrossRef]
- Mueck, W.; Eriksson, B.I.; Bauer, K.A.; Borris, L.; Dahl, O.E.; Fisher, W.D.; Gent, M.; Haas, S.; Huisman, M.V.; Kakkar, A.K.; et al. Population Pharmacokinetics and Pharmacodynamics of Rivaroxaban—an Oral, Direct Factor Xa Inhibitor—in Patients Undergoing Major Orthopaedic Surgery: Clin. Pharmacokinet. 2008, 47, 203–216. [Google Scholar] [CrossRef]
- Mueck, W.; Borris, L.C.; Dahl, O.E.; Haas, S.; Huisman, M.V.; Kakkar, A.K.; Kälebo, P.; Muelhofer, E.; Misselwitz, F.; Eriksson, B.I. Population Pharmacokinetics and Pharmacodynamics of Once and Twice-Daily Rivaroxaban for the Prevention of Venous Thromboembolism in Patients Undergoing Total Hip Replacement. Thromb. Haemost. 2008, 100, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Lensing, A.W.A.; Agnelli, G.; Decousus, H.; Prandoni, P.; Misselwitz, F. Rivaroxaban: Population Pharmacokinetic Analyses in Patients Treated for Acute Deep-Vein Thrombosis and Exposure Simulations in Patients with Atrial Fibrillation Treated for Stroke Prevention. Clin. Pharmacokinet. 2011, 50, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Ollier, E.; Hodin, S.; Lanoiselée, J.; Escal, J.; Accassat, S.; De Magalhaes, E.; Basset, T.; Bertoletti, L.; Mismetti, P.; Delavenne, X. Effect of Activated Charcoal on Rivaroxaban Complex Absorption. Clin. Pharmacokinet. 2017, 56, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Speed, V.; Green, B.; Roberts, L.N.; Woolcombe, S.; Bartoli-Abdou, J.; Barsam, S.; Byrne, R.; Gee, E.; Czuprynska, J.; Brown, A.; et al. Fixed Dose Rivaroxaban Can Be Used in Extremes of Bodyweight: A Population Pharmacokinetic Analysis. J. Thromb. Haemost. 2020, 18, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yamashita, T.; Kasai, H.; Otsuka, T.; Sagara, K. An Analysis on Distribution and Inter-Relationships of Biomarkers under Rivaroxaban in Japanese Patients with Non-Valvular Atrial Fibrillation (CVI ARO 1). Drug Metab. Pharmacokinet. 2018, 33, 188–193. [Google Scholar] [CrossRef]
- Tanigawa, T.; Kaneko, M.; Hashizume, K.; Kajikawa, M.; Ueda, H.; Tajiri, M.; Paolini, J.F.; Mueck, W. Model-Based Dose Selection for Phase III Rivaroxaban Study in Japanese Patients with Non-Valvular Atrial Fibrillation. Drug Metab. Pharmacokinet. 2013, 28, 59–70. [Google Scholar] [CrossRef]
- Xu, X.S.; Moore, K.; Burton, P.; Stuyckens, K.; Mueck, W.; Rossenu, S.; Plotnikov, A.; Gibson, M.; Vermeulen, A. Population Pharmacokinetics and Pharmacodynamics of Rivaroxaban in Patients with Acute Coronary Syndromes: Pharmacokinetics and Pharmacodynamics of Rivaroxaban. Br. J. Clin. Pharmacol. 2012, 74, 86–97. [Google Scholar] [CrossRef]
- Zdovc, J.; Petre, M.; Pišlar, M.; Repnik, K.; Mrhar, A.; Vogrin, M.; Potočnik, U.; Grabnar, I. Downregulation of ABCB1 Gene in Patients with Total Hip or Knee Arthroplasty Influences Pharmacokinetics of Rivaroxaban: A Population Pharmacokinetic-Pharmacodynamic Study. Eur. J. Clin. Pharmacol. 2019, 75, 817–824. [Google Scholar] [CrossRef]
- Zhang, L.; Peters, G.; Haskell, L.; Patel, P.; Nandy, P.; Moore, K.T. A Cross-Study Analysis Evaluating the Effects of Food on the Pharmacokinetics of Rivaroxaban in Clinical Studies. J. Clin. Pharmacol. 2017, 57, 1607–1615. [Google Scholar] [CrossRef]
- Tishkoff, S.A.; Kidd, K.K. Implications of Biogeography of Human Populations for “race” and Medicine. Nat. Genet. 2004, 36, S21–S27. [Google Scholar] [CrossRef]
- Olafuyi, O.; Parekh, N.; Wright, J.; Koenig, J. Inter-Ethnic Differences in Pharmacokinetics—Is There More That Unites than Divides? Pharmacol. Res. Perspect. 2021, 9, e00890. [Google Scholar] [CrossRef]
- Gosselin, R.C.; Adcock, D.M.; Bates, S.M.; Douxfils, J.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2018, 118, 437–450. [Google Scholar] [CrossRef]
- Leven, C.; Coste, A.; Mané, C. Free and Open-Source Posologyr Software for Bayesian Dose Individualization: An Extensive Validation on Simulated Data. Pharmaceutics 2022, 14, 442. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, W.; Qin, W.; Du, W.; Wang, X.; Zuo, X.; Li, P. Population Pharmacokinetics and Hemorrhagic Risk Analysis of Rivaroxaban in Elderly Chinese Patients with Nonvalvular Atrial Fibrillation. J. Clin. Pharmacol. 2023, 63, 66–76. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, T.; Huang, N.; Li, L.; Yuan, D.; Xiang, J.; Wang, N.; Chen, W.; Zhang, J. Population Pharmacokinetics of Rivaroxaban in Chinese Patients with Non-Valvular Atrial Fibrillation: A Prospective Multicenter Study. Clin. Pharmacokinet. 2022, 61, 881–893. [Google Scholar] [CrossRef]
- Singkham, N.; Phrommintikul, A.; Pacharasupa, P.; Norasetthada, L.; Gunaparn, S.; Prasertwitayakij, N.; Wongcharoen, W.; Punyawudho, B. Population Pharmacokinetics and Dose Optimization Based on Renal Function of Rivaroxaban in Thai Patients with Non-Valvular Atrial Fibrillation. Pharmaceutics 2022, 14, 1744. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Ding, H.; Yan, M.; Jiao, Z.; Zhong, M.; Ma, C. Population Pharmacokinetic and Pharmacodynamic Analysis of Rivaroxaban in Chinese Patients with Non-Valvular Atrial Fibrillation. Acta Pharmacol. Sin. 2022, 43, 2723–2734. [Google Scholar] [CrossRef]
- Esmaeili, T.; Rezaee, M.; Abdar Esfahani, M.; Davoudian, A.; Omidfar, D.; Rezaee, S. Rivaroxaban Population Pharmacokinetic and Pharmacodynamic Modeling in Iranian Patients. J. Clin. Pharm. Ther. 2022, 47, 1284–1292. [Google Scholar] [CrossRef]
- University Hospital. Brest Pharmacokinetics and Pharmacodynamics of RivAroxaban After Bariatric Surgery and in MORBid Obesity; clinicaltrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).