Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment
Abstract
:1. Introduction
2. Material and Experimental
2.1. Material
2.2. Experimental
2.2.1. Optimization
2.2.2. Formulation of Miconazole Bilosomes (MZBSs)
2.2.3. Vesicle Characterization
2.2.4. Entrapment Efficiency (%)
2.2.5. Development of Miconazole Bilosome Gel (MZBS Gel)
2.2.6. Evaluation of MZBS Gel
2.2.7. Drug Content
2.2.8. In Vitro Drug Release
2.2.9. Permeation Study
2.2.10. In Vitro Irritation Study
2.2.11. In Vitro Antifungal Activity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Optimization
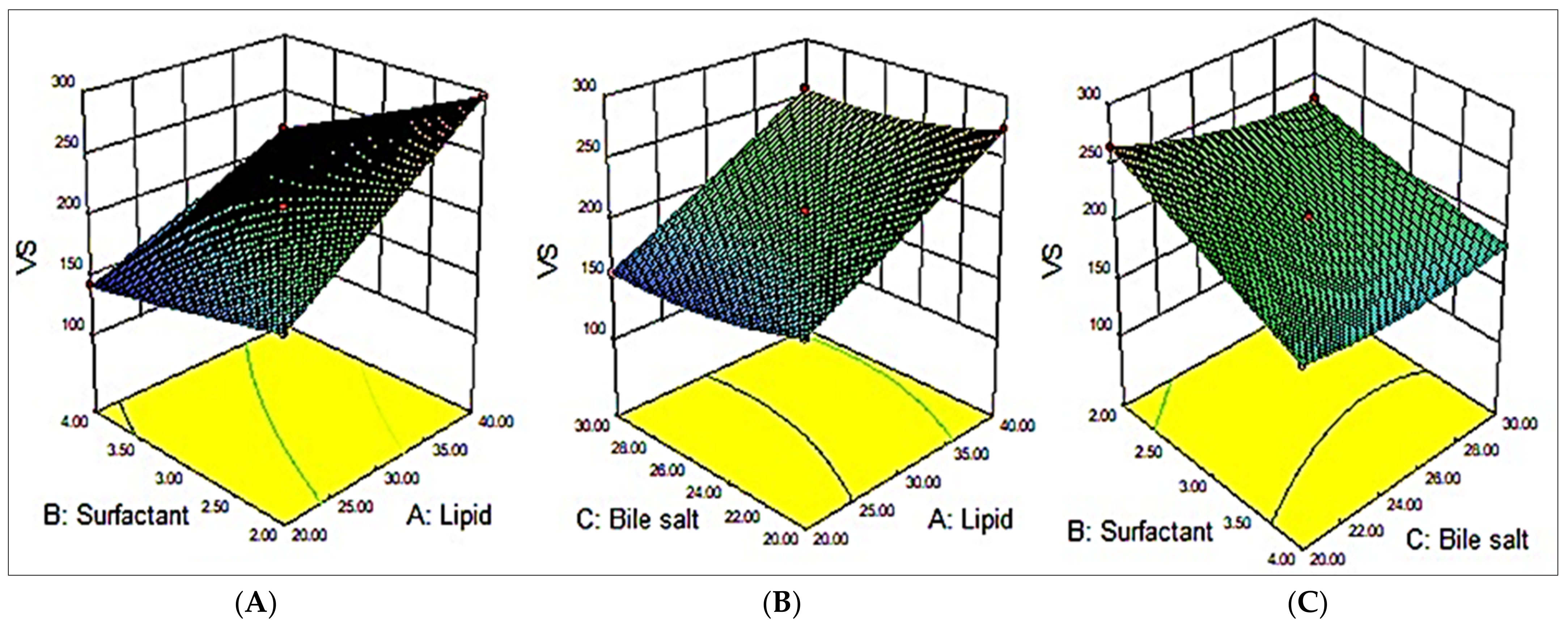
3.2. Effect of Variables on VS (Y1)
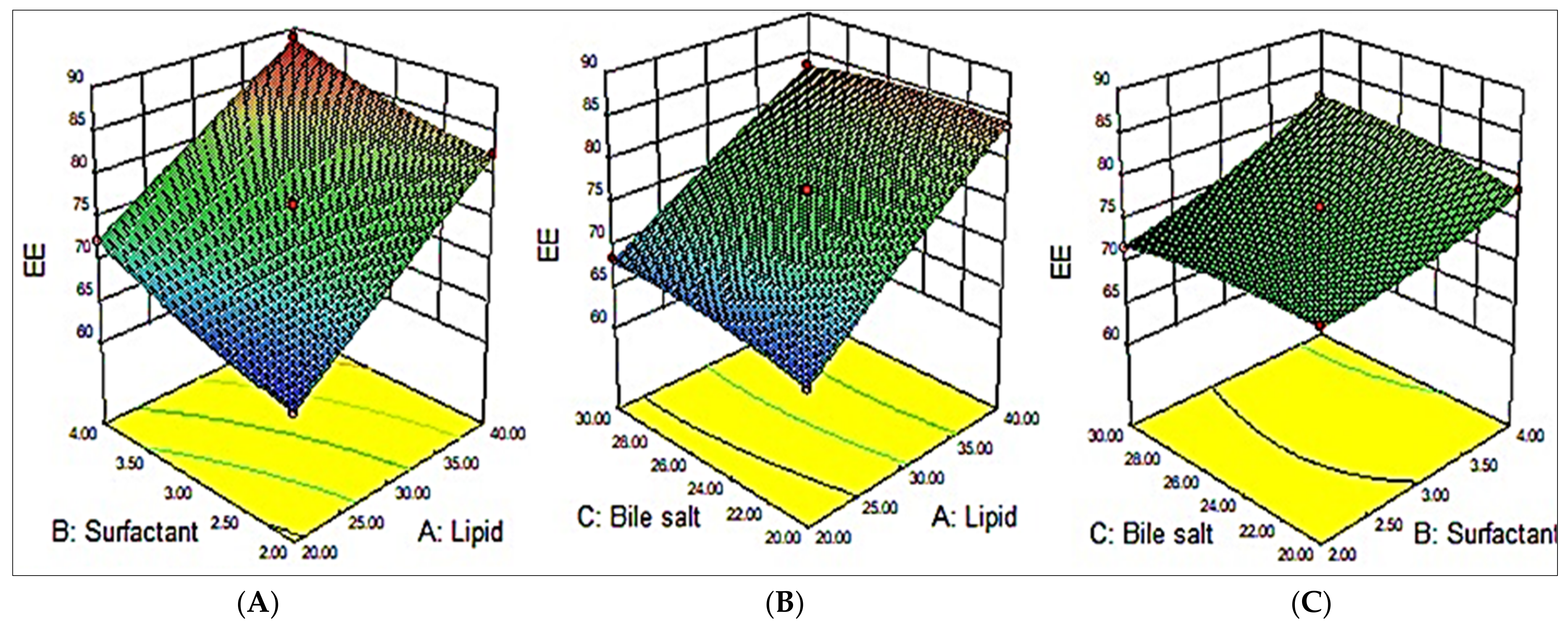
3.3. Effect of Variables on EE (Y2)
3.4. Optimized Formulation
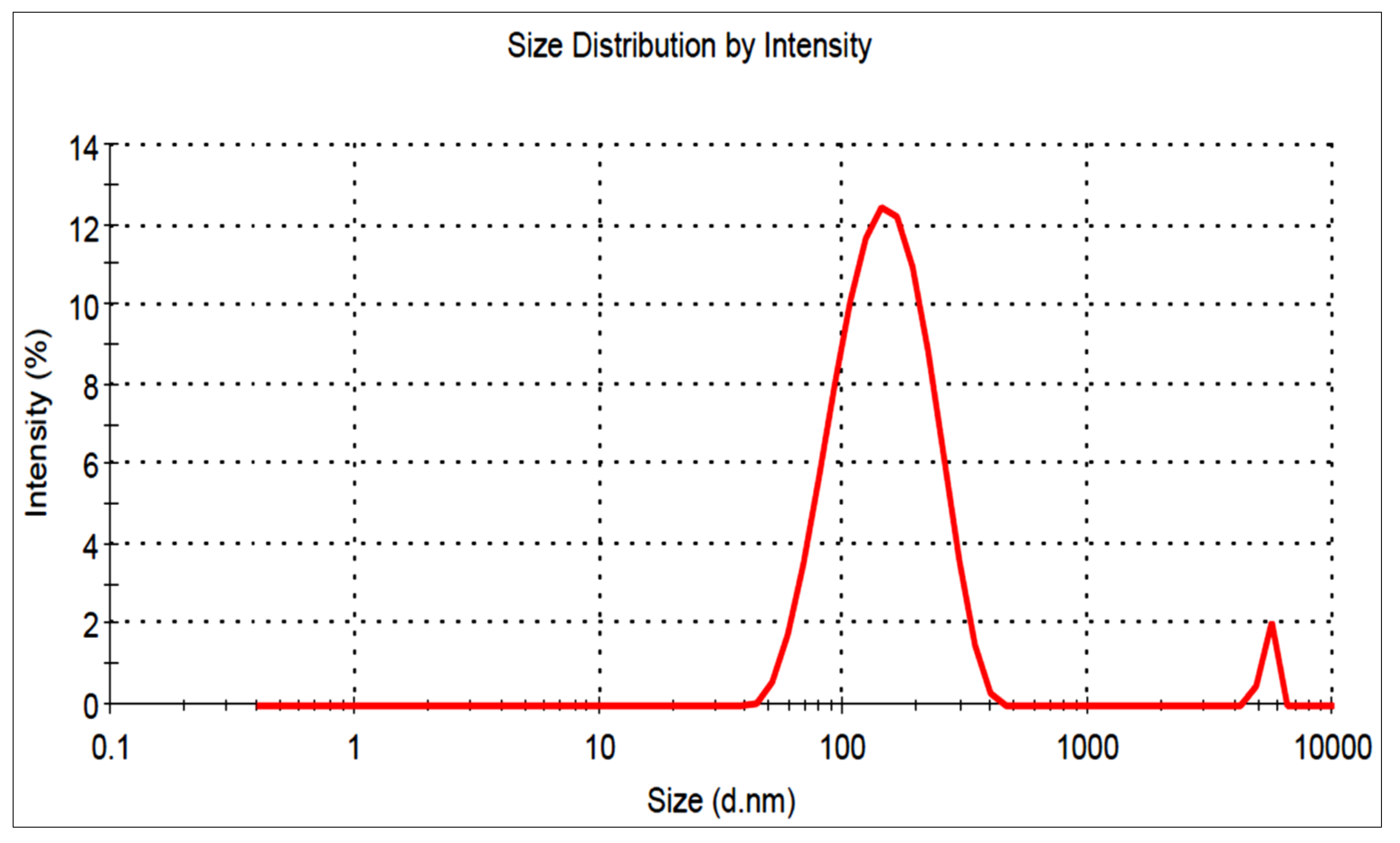
3.5. Vesicle Characterization
3.6. Formulation of Miconazole Bilosome Gel (MZBSoG)
3.7. Characterization
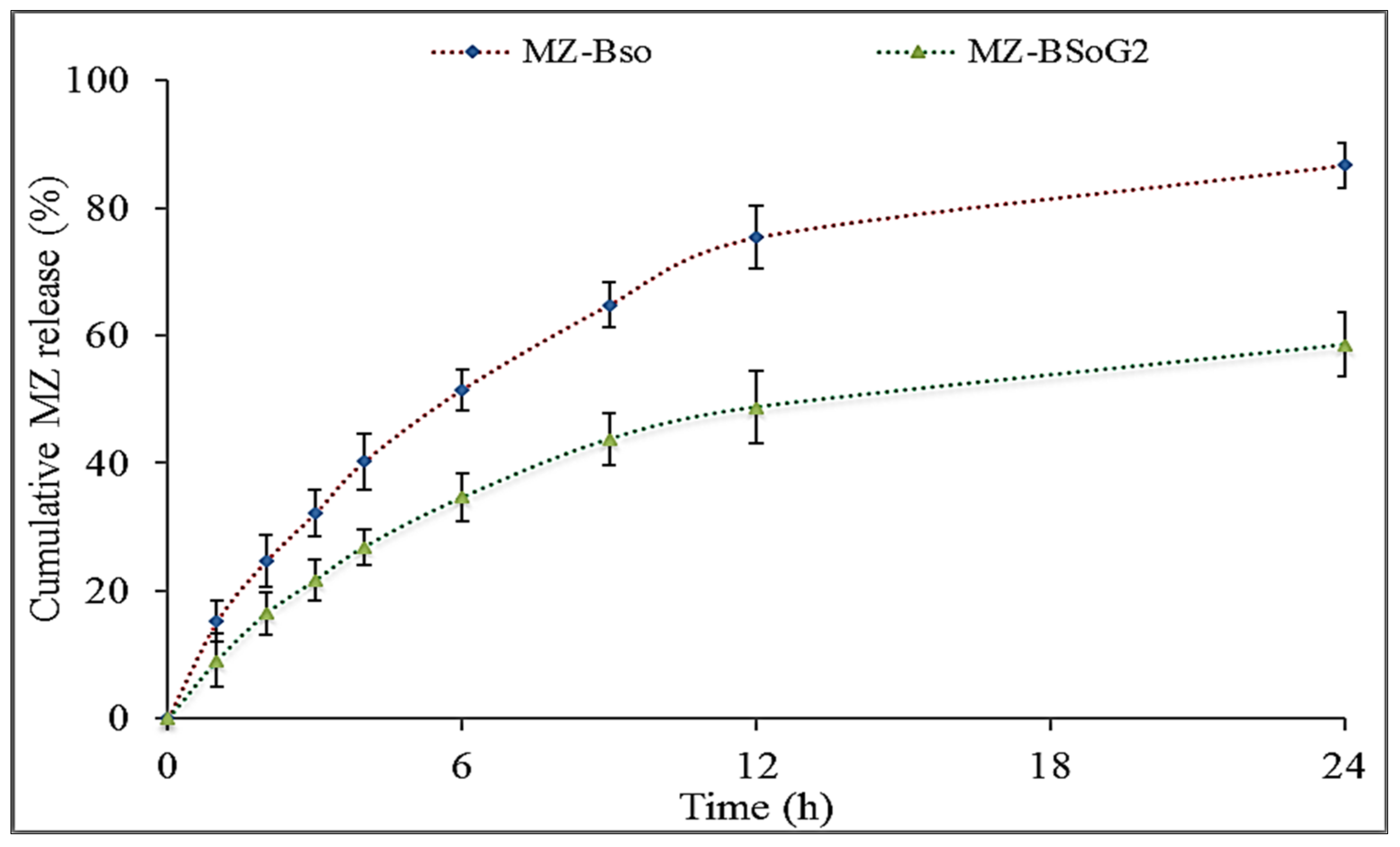
3.8. In Vitro Drug Release
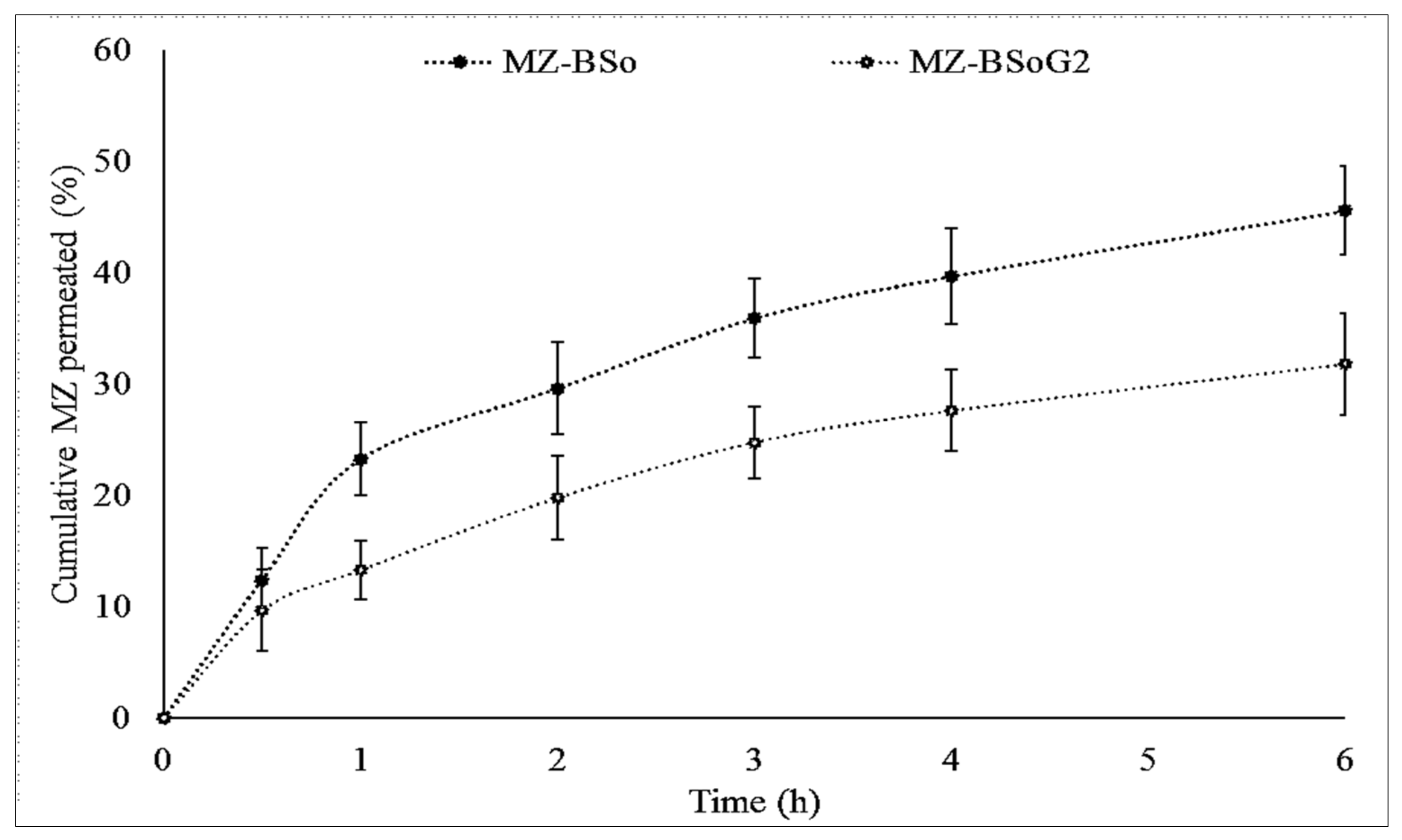
3.9. Permeation Study
3.10. In Vitro Irritation Test
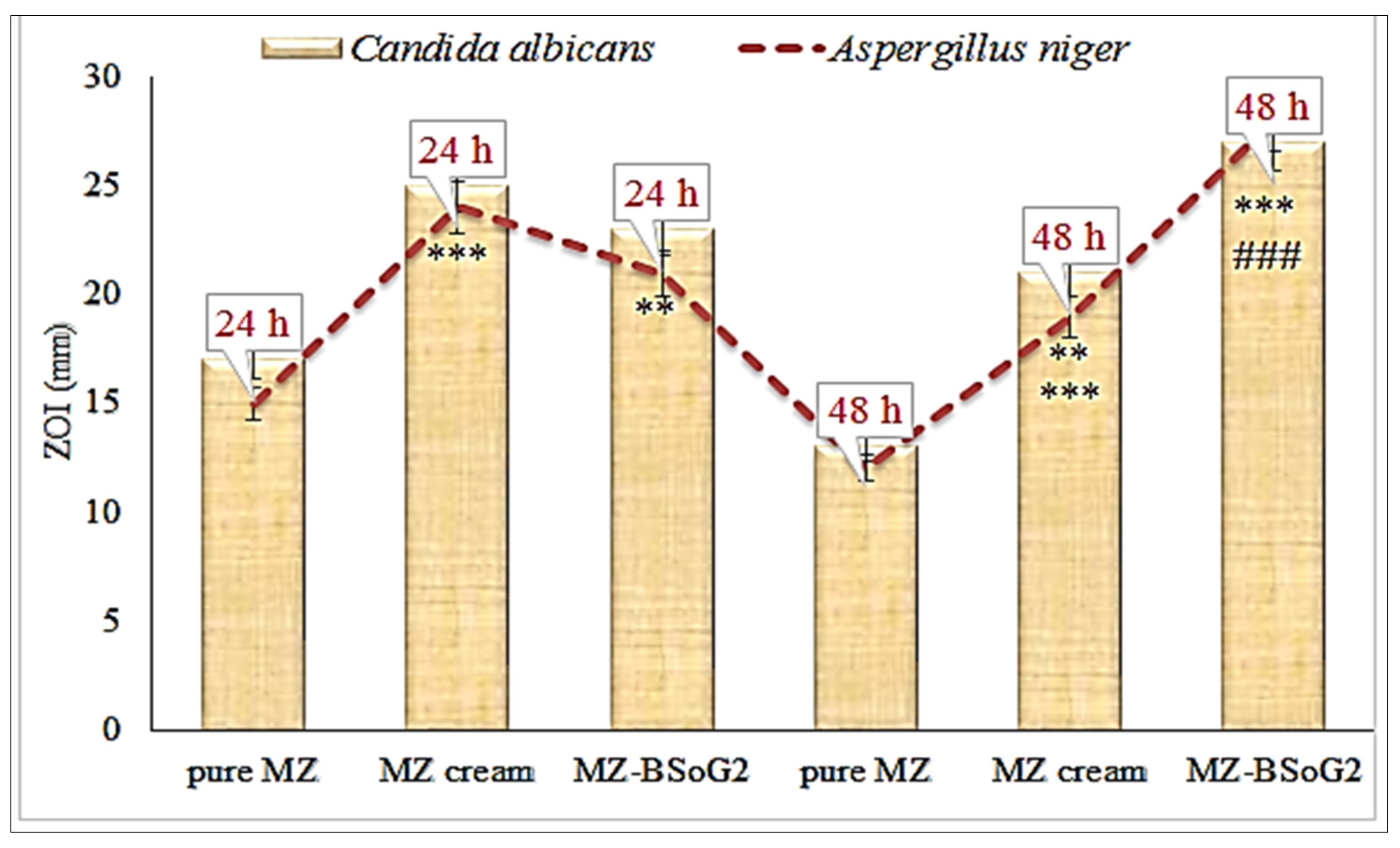
3.11. In Vitro Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulani, H.; Bhise, K. QbD Approach in the formulation and evaluation of Miconazole Nitrate loaded ethosomal cream-o-gel. Int. Res. J. Pharm. Sci. 2017, 8, 1–37. [Google Scholar]
- Pandit, J.; Garg, M.; Jain, N. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J. Liposome Res. 2014, 24, 163–3169. [Google Scholar] [CrossRef] [PubMed]
- Gungor, S.; Erdal, M.S.; Aksu, B. New formulation strategies in topical antifungal therapy. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 56–65. [Google Scholar]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, Optimization and Characterization of a Transfersomal Gel Using Miconazole Nitrate for the Treatment of Candida Skin Infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Mendes, A.; Silva, A.; Catita, J.; Cerqueira, F.; Gabriel, C.; Lopes, C. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointr. 2013, 111, 755–763. [Google Scholar] [CrossRef]
- Aljaeid, B.; Ibrahim, K.M.H. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef]
- Al-Maghrabi, P.M.; Khafagy, E.S.; Ghorab, M.M.; Gad, S. Influence of formulation variables on miconazole nitrate-loaded lipid based nanocarrier for topical delivery. Colloids Surf. B Biointer. 2020, 193, 111046. [Google Scholar] [CrossRef]
- Shah, R.M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Microwave-assisted microemulsion technique for production of Miconazole nitrate- and Econazole nitrate-loaded solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 141–150. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Alaa-Eldin, A.A.; Abdel-Monem, R. Polymeric versus lipid nanocapsules for miconazole nitrate enhanced topical delivery: In vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 294–304. [Google Scholar] [CrossRef]
- Ambreen, Z.; Faran, S.A.; Daniel, A.; Khalid, S.H.; Khan, I.U.; Asif, M.; Rehman, A.; Mehmood, H.Q.; Asghar, S. Physicochemical, rheological and antifungal evaluation of miconazole nitrate organogels for topical delivery. Pak. J. Pharm. Sci. 2022, 35, 1215–1221. [Google Scholar]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylinska, U. Novel surface-modified bilosomes as functional and biocompatible nanocarriers of hybrid compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Mahmoud, T.M.; Nafady, M.M.; Farouk, H.O.; Mahmoud, D.M.; Ahmed, Y.M.; Zaki, R.M.; Hamad, D.S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. Pharmaceuticals 2022, 15, 1106. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Cienc. 2016, 88, 2303–2317. [Google Scholar] [CrossRef]
- Jana, S.; Manna, S.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Carbopol gel containing chitosan-egg albumin nanoparticles for transdermal aceclofenac delivery. Colloids Surf. B Biointerfaces 2014, 114, 36–44. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, L.; Dai, J.; Yi, X.; He, J.; Li, H. N, O-carboxymethyl chitosan/oxidized cellulose composite sponge containing ε-poly-l-lysine as a potential wound dressing for the prevention and treatment of postoperative adhesion. Int. J. Biol. Macromol. 2022, 209 Pt B, 2151–2164. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. , Mat. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, H.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS PharmSciTech 2012, 13, 184–192. [Google Scholar] [CrossRef]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Jain, S. A Rapid and Validated RP-HPLC Method for the Simultaneous Quantification of Benzoic Acid, Metronidazole and Miconazole Nitrate in Vaginal Formulations. J. Chromatogr. Sci. 2016, 54, 1613–1618. [Google Scholar] [CrossRef]
- Vinardell, M.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar] [CrossRef]
- Budai, P.; Lehel, J.; Tavaszi, J.; Kormos, E. HET-CAM test for determining the possible eye irritancy of pesticides. Acta Vet. Hung. 2010, 58, 369–377. [Google Scholar] [CrossRef]
- Mohsen, M.A.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Ameeduzzafar; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alhakamy, N.A.; Alharbi, K.S.; Alshehri, S.; Afzal, M.; Alenezi, S.K.; Bukhari, S.N.A. Formulation of Chitosan Polymeric Vesicles of Ciprofloxacin for Ocular Delivery: Box-Behnken Optimization, In Vitro Characterization, HET-CAM Irritation, and Antimicrobial Assessment. AAPS PharmSciTech 2020, 21, 167. [Google Scholar]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Liposome Res. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Marasini, N.; Yan, Y.D.; Poudel, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development and optimization of self-nanoemulsifying drug delivery system with enhanced bioavailability by Box-Behnken design and desirability function. J. Pharm. Sci. 2012, 101, 4584–4596. [Google Scholar] [CrossRef]
- Chaudhary, H.; Rohilla, A.; Rathee, P.; Kumar, V. Optimization and formulation design of carbopol loaded Piroxicam gel using novel penetration enhancers. Int. J. Biol. Macromol. 2013, 55, 246–253. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- Uprit, S.; Kumar Sahu, R.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. 2019, 21, 379–385. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
| Independent Variables | Code | Low (–) | High (+) | Dependent Variables |
|---|---|---|---|---|
| Cholesterol (mg) | A | 20 | 40 | Vesicle size (nm; Y1) |
| Surfactant (%) | B | 2 | 4 | Entrapment efficiency (%, Y2) |
| Bile salt (mg) | C | 20 | 30 | - |
| Formulations | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| Cholesterol (mg; A) | Surfactant (%; B) | Bile Salt (mg; C) | Vesicle Size (nm; Y1) | Entrapment Efficiency (%; Y2) | |
| MZBS 1 | - | + | 0 | 182 ± 4.9 | 79 ± 4.7 |
| MZBS 2 | + | - | 0 | 295 ± 9.4 | 71 ± 5.3 |
| MZBS 3 | + | 0 | - | 272 ± 12.5 | 81 ± 4.1 |
| MZBS 4 | 0 | - | - | 265 ± 11.6 | 79 ± 4.1 |
| MZBS 5 | - | 0 | - | 188 ± 8.2 | 74 ± 3.5 |
| MZBS 6 | 0 | - | + | 227 ± 9.8 | 71 ± 4.1 |
| MZBS 7 | - | 0 | + | 156 ± 6.9 | 68 ± 5.6 |
| MZBS 8 | 0 | 0 | 0 | 176 ± 8.4 | 76 ± 4.3 |
| MZBS 9 | 0 | + | + | 184 ± 9 | 83 ± 2.5 |
| MZBS 10 | 0 | + | - | 188 ± 8.3 | 82 ± 2.5 |
| MZBS 11 | - | - | 0 | 163 ± 6.3 | 64 ± 3.2 |
| MZBS 12 | + | 0 | + | 256 ± 5.7 | 73 ± 3.9 |
| MZBS 13 | + | + | 0 | 246 ± 10.3 | 87 ± 6.2 |
| Responses | Regression Parameters | Models | Model | ||
|---|---|---|---|---|---|
| Linear | 2FI | Quadratic | |||
| Vesicle size(Y1) | SD | 9.16 | 5.74 | 0.82 | Quadratic |
| R² | 0.9598 | 0.9879 | 0.9998 | ||
| Adjusted R² | 0.9505 | 0.9806 | 0.9996 | ||
| Predicted R² | 0.9209 | 0.9547 | 0.9989 | ||
| % CV | - | - | 0.39 | ||
| Adeq precision | 2172.51 | 1230.13 | 30.55 | ||
| p-value | <0.0001 | 0.0059 | <0.0001 | ||
| Entrapment efficiency(Y2) | SD | 1.14 | 0.81 | 0.37 | Quadratic |
| R² | 0.9760 | 0.9907 | 0.9987 | ||
| Adjusted R² | 0.9705 | 0.9851 | 0.9970 | ||
| Predicted R² | 0.9506 | 0.9555 | 0.9927 | ||
| % CV | 0.48 | ||||
| Adeq precision | 34.72 | 31.31 | 7.02 | ||
| p-value | <0.0001 | 0.0199 | <0.0004 | ||
| Formulations | Viscosity (cP) | pH | Drug Content (%) | Spreadability (cm2) |
|---|---|---|---|---|
| MZBSoG1 | 1631 ± 32 | 6.5 ± 0.2 | 99.34 ± 3.13 | 7.92 ± 0.14 |
| MZBSoG2 | 1856 ± 21 | 6.4 ± 0.1 | 99.75 ± 2.72 | 6.65 ± 0.21 |
| MZBSoG3 | 2376 ± 18 | 6.5 ± 0.3 | 99.86 ± 3.32 | 5.21 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, S.S.; Gilani, S.J.; Zafar, A.; Jumah, M.N.B.; Alshehri, S. Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. https://doi.org/10.3390/pharmaceutics15020581
Imam SS, Gilani SJ, Zafar A, Jumah MNB, Alshehri S. Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics. 2023; 15(2):581. https://doi.org/10.3390/pharmaceutics15020581
Chicago/Turabian StyleImam, Syed Sarim, Sadaf Jamal Gilani, Ameeduzzafar Zafar, May Nasser Bin Jumah, and Sultan Alshehri. 2023. "Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment" Pharmaceutics 15, no. 2: 581. https://doi.org/10.3390/pharmaceutics15020581
APA StyleImam, S. S., Gilani, S. J., Zafar, A., Jumah, M. N. B., & Alshehri, S. (2023). Formulation of Miconazole-Loaded Chitosan–Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics, 15(2), 581. https://doi.org/10.3390/pharmaceutics15020581









