External Evaluation of Population Pharmacokinetic Models of Methotrexate for Model-Informed Precision Dosing in Pediatric Patients with Acute Lymphoid Leukemia
Abstract
1. Introduction
2. Methods
2.1. Literature Search for the PopPK Models of MTX in ALL Children
2.2. Dataset for External Evaluation
2.3. Evaluation of Predictive Performance
3. Results
3.1. Literature Search and Summary of Published PopPK Models
3.2. Characteristics of the Evaluation Dataset
3.3. Model Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazıcıoğlu, B.; Kaya, Z.; Güntekin Ergun, S.; Perçin, F.; Koçak, Ü.; Yenicesu, İ.; Gürsel, T. Influence of Folate-Related Gene Polymorphisms on High-Dose Methotrexate-Related Toxicity and Prognosis in Turkish Children with Acute Lymphoblastic Leukemia. Turk. J. Haematol. 2017, 34, 143–150. [Google Scholar] [PubMed]
- Jolivet, J.; Cowan, K.H.; Curt, G.A.; Clendeninn, N.J.; Chabner, B.A. The pharmacology and clinical use of methotrexate. N. Engl. J. Med. 1983, 309, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Childhood Cancer Collaborators. The global burden of childhood and adolescent cancer in 2017: An analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019, 20, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.F.; Yang, Y.; Liu, W.J. Progress in research on childhood T-cell acute lymphocytic leukemia, Notch1 signaling pathway, and its inhibitors: A review. Bosn. J. Basic Med. Sci. 2021, 21, 136–144. [Google Scholar]
- Mandal, P.; Samaddar, S.; Chandra, J.; Parakh, N.; Goel, M. Adverse effects with intravenous methotrexate in children with acute lymphoblastic leukemia/lymphoma: A retrospective study. Indian J. Hematol. Blood Transfus. 2020, 36, 498–504. [Google Scholar] [CrossRef]
- Freeman, A.I.; Weinberg, V.; Brecher, M.L.; Jones, B.; Glicksman, A.S.; Sinks, L.F.; Weil, M.; Pleuss, H.; Hananian, J.; Burgert, E.O., Jr.; et al. Comparison of intermediate-dose methotrexate with cranial irradiation for the post-induction treatment of acute lymphocytic leukemia in children. N. Engl. J. Med. 1983, 308, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bührer, C.; Henze, G.; Hofmann, J.; Reiter, A.; Schellong, G.; Riehm, H. Central nervous system relapse prevention in 1165 standard-risk children with acute lymphoblastic leukemia in five BFM trials. Haematol. Blood Transfus. 1990, 33, 500–503. [Google Scholar]
- Pui, C.H.; Howard, S.C. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008, 9, 257–268. [Google Scholar]
- Delepine, N.; Delepine, G.; Cornille, H.; Brion, F.; Arnaud, P.; Desbois, J.C. Dose escalation with pharmacokinetics monitoring in methotrexate chemotherapy of osteosarcoma. Anticancer Res. 1995, 15, 489–494. [Google Scholar]
- Evans, W.E.; Relling, M.V.; Rodman, J.H.; Crom, W.R.; Boyett, J.M.; Pui, C.H. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N. Engl. J. Med. 1998, 338, 499–505. [Google Scholar] [PubMed]
- Medellin-Garibay, S.E.; Hernández-Villa, N.; Correa-González, L.C.; Morales-Barragán, M.N.; Valero-Rivera, K.P.; Reséndiz-Galván, J.E.; Ortiz-Zamudio, J.J.; Milán-Segovia, R.d.; Romano-Moreno, S. Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother. Pharmacol. 2020, 85, 21–31. [Google Scholar]
- Pui, C.H.; Relling, M.V.; Sandlund, J.T.; Downing, J.R.; Campana, D.; Evans, W.E. Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann. Hematol. 2004, 83 (Suppl. 1), S124–S126. [Google Scholar] [PubMed]
- Larsen, E.C.; Devidas, M.; Chen, S.; Salzer, W.L.; Raetz, E.A.; Loh, M.L.; Mattano, L.A., Jr.; Cole, C.; Eicher, A.; Haugan, M.; et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J. Clin. Oncol. 2016, 34, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Sallan, S.; Relling, M.V.; Masera, G.; Evans, W.E. International Childhood Acute Lymphoblastic Leukemia Workshop: Sausalito, CA, 30 November–1 December 2000. Leukemia 2001, 15, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Y.; Zhang, L.; Guo, Y.; Chen, Y.; Yang, W.; Chen, X.; Wang, S.; Zhang, Y.; Ruan, M.; et al. A Novel Risk Defining System for Pediatric T-Cell Acute Lymphoblastic Leukemia From CCCG-ALL-2015 Group. Front. Oncol. 2022, 12, 841179. [Google Scholar] [CrossRef]
- Qin, F.L.; Sang, G.Y.; Zou, X.Q.; Cheng, D.H. Drug-Induced Liver Injury during Consolidation Therapy in Childhood Acute Lymphoblastic Leukemia as Assessed for Causality Using the Updated RUCAM. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5914593. [Google Scholar]
- Widemann, B.C.; Adamson, P.C. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar]
- Beechinor, R.J.; Thompson, P.A.; Hwang, M.F.; Vargo, R.C.; Bomgaars, L.R.; Gerhart, J.G.; Dreyer, Z.E.; Gonzalez, D. The Population Pharmacokinetics of High-Dose Methotrexate in Infants with Acute Lymphoblastic Leukemia Highlight the Need for Bedside Individualized Dose Adjustment: A Report from the Children’s Oncology Group. Clin. Pharmacokinet. 2019, 58, 899–910. [Google Scholar]
- Gao, X.; Qian, X.W.; Zhu, X.H.; Yu, Y.; Miao, H.; Meng, J.H.; Jiang, J.-Y.; Wang, H.-S.; Zhai, X.-W. Population Pharmacokinetics of High-Dose Methotrexate in Chinese Pediatric Patients With Acute Lymphoblastic Leukemia. Front. Pharmacol. 2021, 12, 701452. [Google Scholar] [CrossRef]
- Hui, K.H.; Chu, H.M.; Fong, P.S.; Cheng, W.T.F.; Lam, T.N. Population Pharmacokinetic Study and Individual Dose Adjustments of High-Dose Methotrexate in Chinese Pediatric Patients with Acute Lymphoblastic Leukemia or Osteosarcoma. J. Clin. Pharmacol. 2019, 59, 566–577. [Google Scholar] [CrossRef]
- Aumente, D.; Buelga, D.S.; Lukas, J.C.; Gomez, P.; Torres, A.; Garcia, M.J. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin. Pharmacokinet. 2006, 45, 1227–1238. [Google Scholar] [PubMed]
- Broeker, A.; Nardecchia, M.; Klinker, K.P.; Derendorf, H.; Day, R.O.; Marriott, D.J.; Carland, J.E.; Stocker, S.L.; Wicha, S.G. Towards precision dosing of vancomycin: A systematic evaluation of pharmacometric models for Bayesian forecasting. Clin. Microbiol. Infect. 2019, 25, 1286.e1–1286.e7. [Google Scholar]
- Zwart, T.C.; Guchelaar, H.J.; van der Boog, P.J.M.; Swen, J.J.; van Gelder, T.; de Fijter, J.W.; Moes, D.J.A.R. Model-informed precision dosing to optimise immunosuppressive therapy in renal transplantation. Drug Discov. Today 2021, 26, 2527–2546. [Google Scholar] [PubMed]
- Keizer, R.J.; Ter Heine, R.; Frymoyer, A.; Lesko, L.J.; Mangat, R.; Goswami, S. Model-Informed Precision Dosing at the Bedside: Scientific Challenges and Opportunities. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 785–787. [Google Scholar]
- Darwich, A.S.; Polasek, T.M.; Aronson, J.K.; Ogungbenro, K.; Wright, D.F.B.; Achour, B.; Reny, J.L.; Daali, Y.; Eiermann, B.; Cook, J.; et al. Model-Informed Precision Dosing: Background, Requirements, Validation, Implementation, and Forward Trajectory of Individualizing Drug Therapy. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 225–245. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, S.; Yang, L.; Wu, H.; Zhang, J.; Ke, X. Population pharmacokinetic study of methotrexate in children with acute lymphoblastic leukemia. Int J. Clin. Pharmacol. Ther. 2010, 48, 11–21. [Google Scholar] [CrossRef]
- Guo, T.; van Hest, R.M.; Roggeveen, L.F.; Fleuren, L.M.; Thoral, P.J.; Bosman, R.J.; van der Voort, P.H.J.; Girbes, A.R.J.; Mathot, R.A.A.; Elbers, P.W.G. External Evaluation of Population Pharmacokinetic Models of Vancomycin in Large Cohorts of Intensive Care Unit Patients. Antimicrob. Agents Chemother. 2019, 63, e02543-18. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Hara, M.; Masui, K.; Eleveld, D.J.; Struys, M.; Uchida, O. Predictive performance of eleven pharmacokinetic models for propofol infusion in children for long-duration anaesthesia. Br. J. Anaesth. 2017, 118, 415–423. [Google Scholar] [CrossRef]
- Bloomfield, C.; Staatz, C.E.; Unwin, S.; Hennig, S. Assessing Predictive Performance of Published Population Pharmacokinetic Models of Intravenous Tobramycin in Pediatric Patients. Antimicrob. Agents Chemother. 2016, 60, 3407–3414. [Google Scholar]
- Zhan, M.; Sun, Y.; Zhou, F.; Wang, H.; Chen, Z.; Yan, L.; Li, X. Population pharmacokinetics of methotrexate in paediatric patients with acute lymphoblastic leukaemia and malignant lymphoma. Xenobiotica 2022, 52, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.C.; Roberts, J.K.; Huang, J.; Lin, T.; Daryani, V.M.; Harstead, K.E.; Patel, Y.T.; Onar-Thomas, A.; Campagne, O.; Ward, D.A.; et al. Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br. J. Clin. Pharmacol. 2020, 86, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, S.; Nikanjam, M.; Lin, M.; Le, S.; Saunders, I.; Kuo, D.J.; Capparelli, E.V. Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother. Pharmacol. 2019, 84, 1339–1348. [Google Scholar] [PubMed]
- Lui, G.; Treluyer, J.M.; Fresneau, B.; Piperno-Neumann, S.; Gaspar, N.; Corradini, N.; Gentet, J.-C.; Berard, P.M.; Laurence, V.; Schneider, P.; et al. A Pharmacokinetic and Pharmacogenetic Analysis of Osteosarcoma Patients Treated With High-Dose Methotrexate: Data from the OS2006/Sarcoma-09 Trial. J. Clin. Pharmacol. 2018, 58, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Tian, X.; Zhao, H.; Lu, W.; Zhen, J.; Niu, X. Population pharmacokinetics of high-dose methotrexate after intravenous administration in Chinese osteosarcoma patients from a single institution. Chin. Med. J. (Engl.). 2015, 128, 111–118. [Google Scholar]
- Jönsson, P.; Skärby, T.; Heldrup, J.; Schrøder, H.; Höglund, P. High dose methotrexate treatment in children with acute lymphoblastic leukaemia may be optimised by a weight-based dose calculation. Pediatr. Blood Cancer 2011, 57, 41–46. [Google Scholar]
- Colom, H.; Farre, R.; Soy, D.; Peraire, C.; Cendros, J.M.; Pardo, N.; Torrent, M.; Domenech, J.; Mangues, M.-A. Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther. Drug Monit. 2009, 31, 76–85. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Liu, Y.O.; Gu, H.Y.; Xu, X.Q.; Yan, C.; Yang, X.Y.; Yan, D. Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with medulloblastoma. Biopharm. Drug Dispos. 2020, 41, 101–110. [Google Scholar]
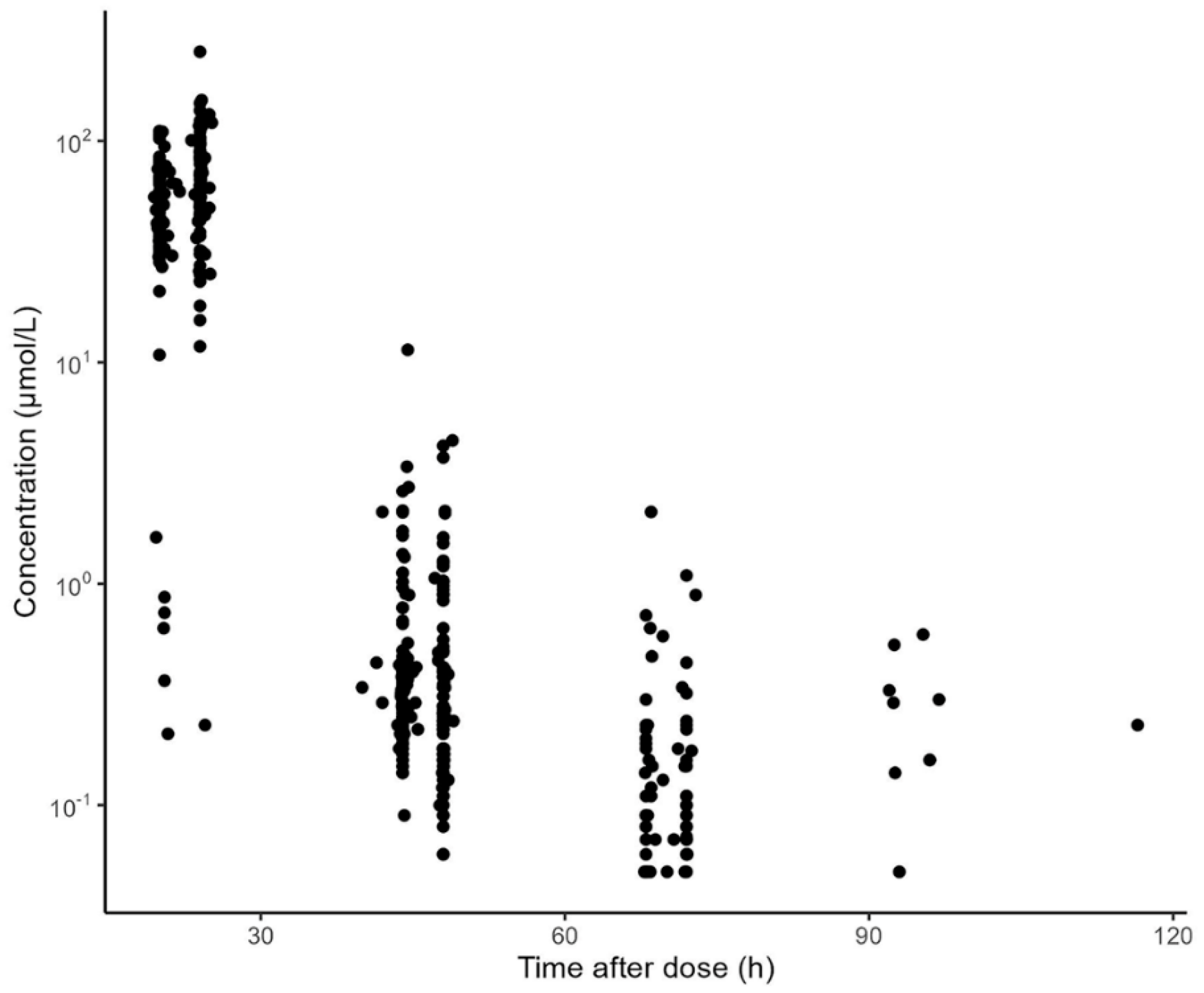
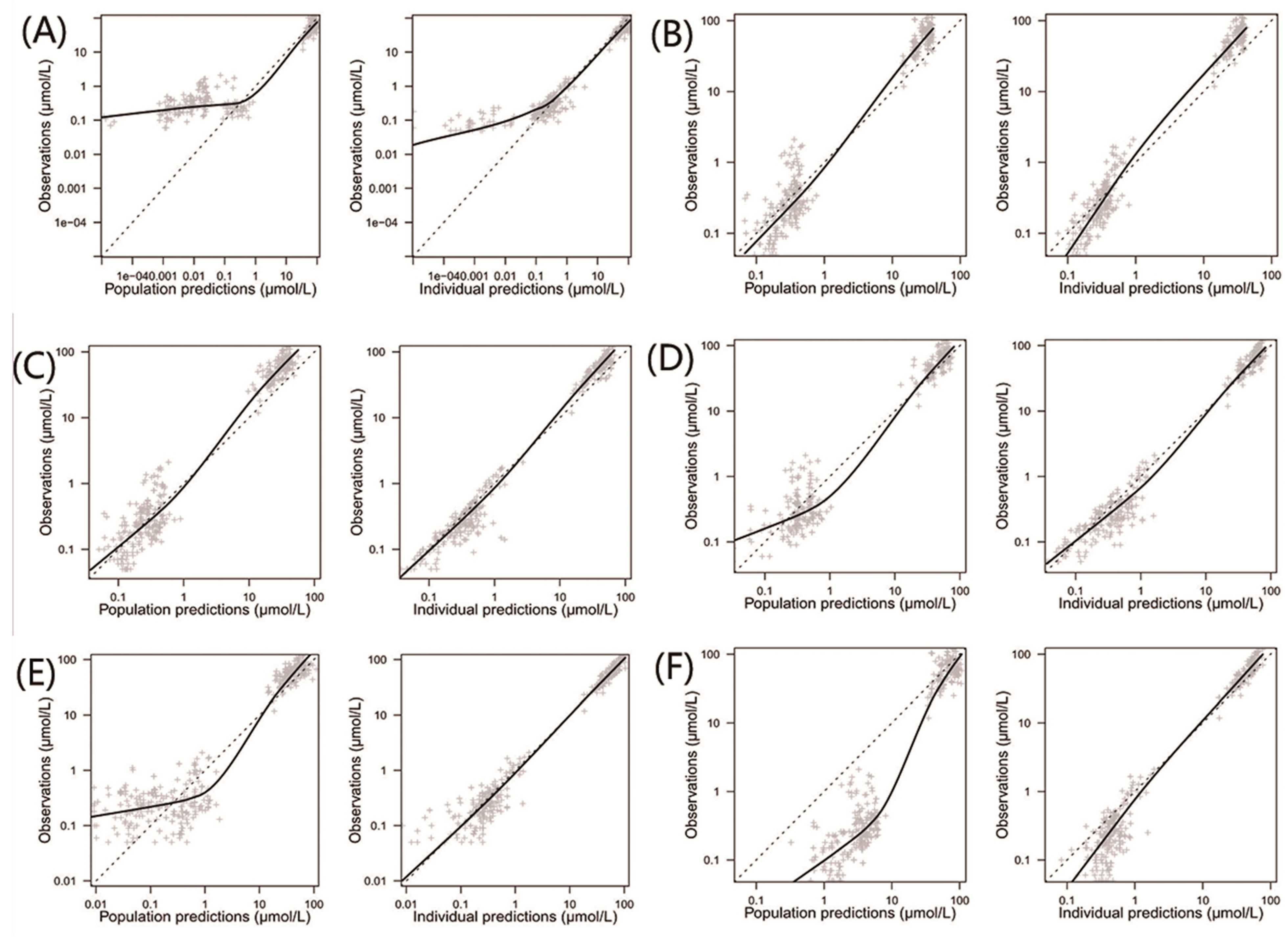


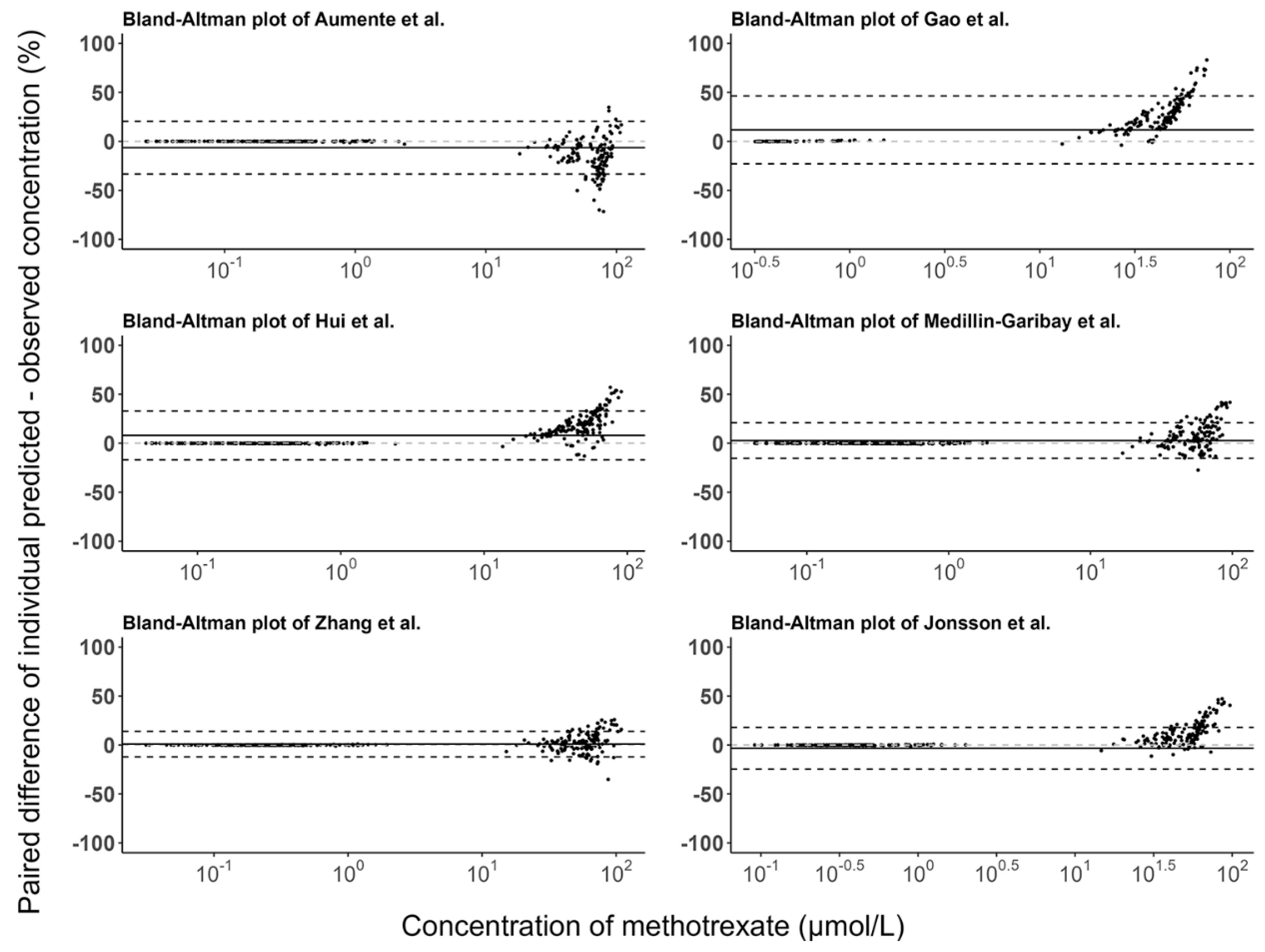
| Characteristics | Aumente et al. [21] | Gao et al. [19] | Hui et al. [20] | Medellin-Garibay et al. [11] | Zhang et al. [26] | Jonsson et al. [36] | Evaluation Dataset |
|---|---|---|---|---|---|---|---|
| Tumor Type | ALL | ALL | ALL | ALL | ALL | ALL | ALL |
| N | 37 | 311 | 36 | 41 | 96 | 304 | 51 |
| M/F | 17/20 | 197/114 | 23/13 | NA | 66/30 | NA | 40/11 |
| Age (years) | 5.0 (0.5–17.0) | 5.0 (0.75–15.2) | 5.3 (1.3–15.8) | 5.0 (1.0–15.0) | 7.4 ± 3.1 | 5.0 (0.4–17.8) | 5.5 (1.0–13.0) |
| Weight (kg) | 24.2 (7.5–80.0) | 19.0 (4.5–113.0) | 18.4 (10.4–57.8) | 21.2 (8.0–57.3) | 26.1 ± 10.4 | 19.0 (5.8–93.3) | 19.0 (9.5–62.0) |
| Height (cm) | 115 (69–174) | 112 (67–175) | 107 (77–176) | 115 ± 24 | 120 ± 21 | 110 (63–192) | 113 (73–168) |
| BSA (m2) | 1.07 (0.30–1.90) | NA | 0.74 (0.47–1.64) | 0.79 (0.41–1.6) | 0.97 ± 0.29 | 0.76 (0.31–2.19) | 0.77 (0.41–1.60) |
| MTX dose (g/m2) | 1.23–5.23 | 1–5 | 2–5 | 2.89 ± 0.9 | 2–3.5 | 5–8 | 1–5 |
| ALT (IU/L) | NA | 16.0 (2.0–390.0) | 16.5 (5.0–247.0) | 19.1 (7.2–98.2) | 32.7 ± 44.5 | 31.2 (6.0–228.6) | 32.5 (4.0–250.0) |
| AST (IU/L) | NA | 26.0 (8.0–135.0) | NA | 25.8 (13.9–112.7) | 45.3 ± 34.5 | NA | 36.0 (17.0–131.0) |
| Scr (mg/dL) | 0.5 (0.3–0.8) | 0.3 (0.1–1.5) | 0.362 (0.11–1.07) | 0.37 ± 0.11 | 0.36 ± 0.10 | NA | 0.31 (0.16–0.71) |
| Studies | Structural Model | Fixed Parameters and Covariate Models | IIV (%) (IOV (%)) | RUV |
|---|---|---|---|---|
| Aumente et al. [21] | 2 CMT | K12 (1/h) = 0.0155 K21 (1/h) = 0.0724 CL (L/h, age > 10 years) = 0.149 × Weight CL (L/h, age < 10 years) = 0.287 × Weight0.876 V1 (L, age > 10 years) = 0.437 × Weight V1 (L, age < 10 years) = 0.465 × Weight | K12 = 20.8 K21 = 35.2 CL = 41.7 V1 = 41.6 | Prop = 16.2% Add = 0.0035 μmol/L |
| Gao et al. [19] | 3 CMT | CL (L/h) = 6.9 × (Weight/19 kg)0.75 × (1 + (Scr-26) × (−0.0097)) × eηCL V1 (L) = 20.7 × (Weight/19 kg) V2 (L) = 41.0 × (Weight/19 kg) × eηV2 Q1 (L/h) = 0.255 × (Weight/19 kg)0.75 V3 (L) = 3.17 × (Weight/19 kg) Q2 (L/h) = 0.217 × (Weight/19 kg)0.75 | CL = 17.9 V1 = 26.2 | Prop * = 35.4% |
| Hui et al. [20] | 2 CMT | CL (L/h) = 7.73 × (BSA/0.735)0.721 × (eGFR × 1.73/192 × BSA)0.256 × eηCL+IOV V1 (L) = 19.0 × (BSA/0.735)0.985 Q (L/h) = 0.283 × (Age/5.29)0.278 V2 (L) = 6.63 × eηV2 | CL = 14.3 (14.9) V2 = 34.6 | Prop = 30.2% |
| Medellin-Garibay et al. [11] | 2 CMT | CL (L/h) = 6.5 × BSA0.62 × eηCL V1 (L) = 0.36 × Weight × eηV1 Q (L/h) = 0.41 V2 (L) = 3.2 × eηV2 | CL = 8.2 V1 = 25.9 V2 = 26.7 | Prop = 20.1% |
| Zhang et al. [26] | 2 CMT | CL (L/h) = (5.04 × (1 − 0.278 × Gender) × BSA0.777 + (OH/100)0.514) × eηCL V1 (L) = 16.1 × eηV1 Q (L/h) = 0.203 × (Age/10)1.56 × eηQ V2 (L) = 7.05 × (Age/10)1.76 × eηV2 | CL = 49.6 V1 = 29.4 Q = 137.6 V2 = 107.7 | Prop = 19.0% Add = 0.0872 μmol/L |
| Jonsson et al. [36] | 2 CMT | CL (L/h) = Weight × 0.185 × eηCL V1 (L) = Weight × 1.27 × eηV1 Q (L/h) = Weight × 0.017 × eηQ V2 = Weight × 1.02 × eηV2 | CL = 109.0 V1 = 26.0 Q = 22.0 V2 = 44.0 | NR |
| Model | IPRED | PRED | ||||
|---|---|---|---|---|---|---|
| Median PE (%) | MPE (%) | RMSE (%) | Median PE (%) | MPE (%) | RMSE (%) | |
| Aumente et al. [21] | 6.52 | 12.06 | 76.09 | −31.70 | −25.51 | 81.15 |
| Gao et al. [19] | −25.20 | 1.33 | 63.96 | −26.76 | 3.22 | 72.39 |
| Hui et al. [20] | −10.43 | 8.78 | 82.96 | −33.23 | −8.24 | 62.88 |
| Medellin-Garibay et al. [11] | −0.75 | 22.71 | 75.55 | −7.56 | 6.39 | 68.33 |
| Zhang et al. [26] | 1.16 | 16.00 | 63.39 | −29.92 | 15.62 | 145.92 |
| Jonsson et al. [36] | 5.25 | 64.44 | 152.25 | 442.04 | 780.87 | 1182.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yin, Q.; Yang, M.; Cheng, Z.; Xie, F. External Evaluation of Population Pharmacokinetic Models of Methotrexate for Model-Informed Precision Dosing in Pediatric Patients with Acute Lymphoid Leukemia. Pharmaceutics 2023, 15, 569. https://doi.org/10.3390/pharmaceutics15020569
Wang S, Yin Q, Yang M, Cheng Z, Xie F. External Evaluation of Population Pharmacokinetic Models of Methotrexate for Model-Informed Precision Dosing in Pediatric Patients with Acute Lymphoid Leukemia. Pharmaceutics. 2023; 15(2):569. https://doi.org/10.3390/pharmaceutics15020569
Chicago/Turabian StyleWang, Shengfeng, Qiufen Yin, Minghua Yang, Zeneng Cheng, and Feifan Xie. 2023. "External Evaluation of Population Pharmacokinetic Models of Methotrexate for Model-Informed Precision Dosing in Pediatric Patients with Acute Lymphoid Leukemia" Pharmaceutics 15, no. 2: 569. https://doi.org/10.3390/pharmaceutics15020569
APA StyleWang, S., Yin, Q., Yang, M., Cheng, Z., & Xie, F. (2023). External Evaluation of Population Pharmacokinetic Models of Methotrexate for Model-Informed Precision Dosing in Pediatric Patients with Acute Lymphoid Leukemia. Pharmaceutics, 15(2), 569. https://doi.org/10.3390/pharmaceutics15020569






