Impact of Omalizumab in Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Socio-Demographic and Clinical Variables
2.3. Statistical Analysis
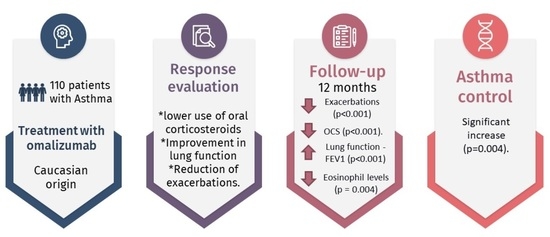
3. Results
3.1. Characteristics of the Patients Treated with Omalizumab
3.2. Clinical Effectiveness of Omalizumab
3.3. Comparison of Clinical Variables before and after Treatment
3.3.1. Daily Dose of ICS
3.3.2. OCS Cycles per Year
3.3.3. Lung Function
3.3.4. Asthma Control Test (ACT)
3.3.5. Frequency of Severe Exacerbations
3.3.6. Inflammatory Markers
3.3.7. Immunoglobulin E
3.4. Predictors of Response at 12 Months
3.4.1. Response to Reduction in Oral Corticosteroids (OCS)
3.4.2. Lung Function Response (FEV1)
3.4.3. Response to Reduction of Exacerbations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After Asthma: Redefining Airways Diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and Noneosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 22–37. [Google Scholar] [CrossRef]
- Vitale, C.; Maglio, A.; Pelaia, C.; Vatrella, A. Long-term treatment in pediatric asthma: An update on chemical pharmacotherapy. Expert Opin. Pharm. 2017, 18, 667–676. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K. New perspectives in bronchial asthma: Pathological, immunological alterations, biological targets, and pharmacotherapy. Immunopharmacol. Immunotoxicol. 2020, 42, 521–544. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2019. Available online: https://ginasthma.org/ (accessed on 3 May 2022).
- Luzán 5 Health Consulting S.A. Gema 5.2. Guía Española Para el Manejo del Asma; Luzán 5 Health Consulting S.A.: Madrid, Spain, 2022. [Google Scholar]
- Vatrella, A.; Maglio, A.; Pellegrino, S.; Pelaia, C.; Stellato, C.; Pelaia, G.; Vitale, C. Phenotyping severe asthma: A rationale for biologic therapy. Expert Rev. Precis. Med. Drug Dev. 2020, 5, 265–274. [Google Scholar] [CrossRef]
- Viswanathan, R.K.; Busse, W.W. Biologic Therapy and Asthma. Semin. Respir. Crit. Care Med. 2018, 39, 100–114. [Google Scholar] [CrossRef]
- Dullaers, M.; De Bruyne, R.; Ramadani, F.; Gould, H.J.; Gevaert, P.; Lambrecht, B.N. The Who, Where, and When of IgE in Allergic Airway Disease. J. Allergy Clin. Immunol. 2012, 129, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Boesel, K.M.; Griffith, D.T.; Prussin, C.; Foster, B.; Romero, F.; Townley, R.; Casale, T.B. Omalizumab Rapidly Decreases Nasal Allergic Response and FcεRI on Basophils. J. Allergy Clin. Immunol. 2004, 113, 297–302. [Google Scholar] [CrossRef]
- Loureiro, C.C.; Amaral, L.; Ferreira, J.A.; Lima, R.; Pardal, C.; Fernandes, I.; Semedo, L.; Arrobas, A. Omalizumab for Severe Asthma: Beyond Allergic Asthma. Biomed. Res. Int. 2018, 2018, 3254094. [Google Scholar] [CrossRef]
- Spector, S. Omalizumab: Efficacy in Allergic Disease. Panminerva Med. 2004, 46, 141–148. [Google Scholar]
- Domingo, C. Omalizumab for Severe Asthma: Efficacy beyond the Atopic Patient? Drugs 2014, 74, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.; Casale, T.; Wenzel, S.; Bousquet, J.; Deniz, Y.; Reisner, C. The Anti-Inflammatory Effects of Omalizumab Confirm the Central Role of IgE in Allergic Inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Flood-Page, P.; Sehmi, R.; Burman, J.; Hamid, Q.; Robinson, D.S.; Kay, A.B.; Denburg, J. Anti–IL-5 (Mepolizumab) Therapy Induces Bone Marrow Eosinophil Maturational Arrest and Decreases Eosinophil Progenitors in the Bronchial Mucosa of Atopic Asthmatics. J. Allergy Clin. Immunol. 2003, 111, 714–719. [Google Scholar] [CrossRef]
- Verma, P.; Randhawa, I.; Klaustermeyer, W.B. Clinical Efficacy of Omalizumab in an Elderly Veteran Population with Severe Asthma. Allergy Asthma Proc. 2011, 32, 346–350. [Google Scholar] [CrossRef]
- Paganin, F.; Mangiapan, G.; Proust, A.; Prudhomme, A.; Attia, J.; Marchand-Adam, S.; Pellet, F.; Milhe, F.; Melloni, B.; Bernady, A.; et al. Lung Function Parameters in Omalizumab Responder Patients: An Interesting Tool? Allergy 2017, 72, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kozawa, M.; Yoshisue, H.; Lee Milligan, K.; Nagasaki, M.; Sasajima, T.; Miyamoto, T.; Ohta, K. Real-World Safety and Efficacy of Omalizumab in Patients with Severe Allergic Asthma: A Long-Term Post-Marketing Study in Japan. Respir. Med. 2018, 141, 56–63. [Google Scholar] [CrossRef]
- Normansell, R.; Walker, S.; Milan, S.J.; Walters, E.H.; Nair, P. Omalizumab for Asthma in Adults and Children. Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar] [CrossRef]
- Yancey, S.W.; Keene, O.N.; Albers, F.C.; Ortega, H.; Bates, S.; Bleecker, E.R.; Pavord, I. Biomarkers for Severe Eosinophilic Asthma. J. Allergy Clin. Immunol. 2017, 140, 1509–1518. [Google Scholar] [CrossRef]
- Ortega, H.; Katz, L.; Gunsoy, N.; Keene, O.; Yancey, S. Blood Eosinophil Counts Predict Treatment Response in Patients with Severe Eosinophilic Asthma. J. Allergy Clin. Immunol. 2015, 136, 825–826. [Google Scholar] [CrossRef]
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of Effect and Impact on Health-Related Quality of Life, Exacerbation Rate, Lung Function, and Nasal Polyposis Symptoms for Patients with Severe Eosinophilic Asthma Treated with Benralizumab (ANDHI): A Randomised, Controlled, Phase 3b Trial. Lancet Respir. Med. 2021, 9, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Taillé, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M.; Agossou, M.; Appere De Vecchi, C.; Barbare, E.; Barbry, M.; et al. Omalizumab Effectiveness in Patients with Severe Allergic Asthma According to Blood Eosinophil Count: The STELLAIR Study. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and Safety of Omalizumab in Nasal Polyposis: 2 Randomized Phase 3 Trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of Omalizumab as Add-on Therapy in Patients with Severe Persistent Asthma Who Are Inadequately Controlled despite Best Available Therapy (GINA 2002 Step 4 Treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Cornelius, V.; Cro, S.; Harper, J.I.; Lack, G. Treatment Effect of Omalizumab on Severe Pediatric Atopic Dermatitis: The ADAPT Randomized Clinical Trial. JAMA Pediatr. 2020, 174, 29–37. [Google Scholar] [CrossRef]
- Hanania, N.A.; Wenzel, S.; Roseń, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the Effects of Omalizumab in Allergic Asthma: An Analysis of Biomarkers in the EXTRA Study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Damask, C.; Chen, M.; Holweg, C.T.J.; Yoo, B.; Millette, L.A.; Franzese, C. Defining the Efficacy of Omalizumab in Nasal Polyposis: A POLYP 1 and POLYP 2 Subgroup Analysis. Am. J. Rhinol. Allergy 2022, 36, 135–141. [Google Scholar] [CrossRef]
- Gevaert, P.; Saenz, R.; Corren, J.; Han, J.K.; Mullol, J.; Lee, S.E.; Ow, R.A.; Zhao, R.; Howard, M.; Wong, K.; et al. Long-Term Efficacy and Safety of Omalizumab for Nasal Polyposis in an Open-Label Extension Study. J. Allergy Clin. Immunol. 2022, 149, 957–965.e3. [Google Scholar] [CrossRef]
- Bousquet, J.; Humbert, M.; Gibson, P.G.; Kostikas, K.; Jaumont, X.; Pfister, P.; Nissen, F. Real-World Effectiveness of Omalizumab in Severe Allergic Asthma: A Meta-Analysis of Observational Studies. J. Allergy Clin. Immunol. Pract. 2021, 9, 2702–2714. [Google Scholar] [CrossRef]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 156–164. [Google Scholar] [CrossRef]
- Caminati, M.; Vianello, A.; Chieco Bianchi, F.; Festi, G.; Guarnieri, G.; Marchi, M.R.; Micheletto, C.; Olivieri, M.; Tognella, S.; Guerriero, M.; et al. Relevance of TH2 Markers in the Assessment and Therapeutic Management of Severe Allergic Asthma: A Real-Life Perspective. J. Investig. Allergol. Clin. Immunol. 2020, 30, 35–41. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Fierro, M.T.; Dapavo, P.; Crimi, N.; Ferrucci, S.; Pepe, P.; Liberati, S.; Pigatto, P.D.; et al. Predictors of Response to Omalizumab and Relapse in Chronic Spontaneous Urticaria: A Study of 470 Patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, W.J.; Krouse, R.Z.; Calatroni, A.; Gergen, P.J.; Gern, J.E.; Gill, M.A.; Gruchalla, R.S.; Khurana Hershey, G.K.; Kattan, M.; Kercsmar, C.M.; et al. Aeroallergen Sensitization, Serum IgE, and Eosinophilia as Predictors of Response to Omalizumab Therapy During the Fall Season Among Children with Persistent Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3021–3028.e2. [Google Scholar] [CrossRef] [PubMed]
- Kallieri, M.; Papaioannou, A.I.; Papathanasiou, E.; Ntontsi, P.; Papiris, S.; Loukides, S. Predictors of Response to Therapy with Omalizumab in Patients with Severe Allergic Asthma-A Real Life Study. Postgrad. Med. 2017, 129, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española del Medicamento y Productos Sanitarios Ficha Tecnica Xolair 150 Mg Solucion Inyectable. Available online: https://cima.aemps.es/cima/dochtml/ft/05319008/FT_05319008.html#10-fecha-de-la-revisi-n-del-texto (accessed on 5 April 2022).
- Asthma Control Test (ACT). Available online: https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/act.php (accessed on 4 May 2022).
| N | % | Mean ± SD/p50 (p25, p75) | |
|---|---|---|---|
| Age | 110 | 48.48 ± 16.08 | |
| Sex | |||
| Women | 68 | 61.82 | |
| Men | 42 | 38.18 | |
| BMI | |||
| Underweight | 1 | 0.91 | |
| Normal weight | 27 | 24.55 | |
| Overweight | 44 | 40 | |
| Obesity | 38 | 34.55 | |
| Tobacco consumption | |||
| Non-smoker | 77 | 70 | |
| Former smoker | 28 | 25.45 | |
| Current smoker | 5 | 4.55 | |
| Previous respiratory disease | |||
| Yes | 23 | 20.91 | |
| No | 87 | 79.09 | |
| Polyps | |||
| Yes | 21 | 19.09 | |
| No | 89 | 80.91 | |
| Allergies | |||
| Yes | 83 | 75.45 | |
| No | 27 | 24.55 | |
| GERD | |||
| Yes | 30 | 27.27 | |
| No | 80 | 72.73 | |
| SAHS | |||
| Yes | 20 | 18.18 | |
| No | 90 | 81.82 | |
| COPD | |||
| Yes | 16 | 14.55 | |
| No | 94 | 85.45 | |
| Years with EA | 110 | 8.5 [5–13.75] | |
| ICS (mg/day) | 110 | 320 [184–640] | |
| OCS cycles per year | 110 | 2 [1–3] | |
| Yes | 85 | 77.27 | |
| No | 25 | 22.73 | |
| Baseline %FEV1 | 106 | 76.12 ± 21.68 | |
| <80 | 62 | 58.49 | - |
| >80 | 44 | 41.51 | - |
| Baseline ACT | 25 | 12 [10–16] | |
| Exacerbation in previous year | 110 | 1 [0–2] | |
| Yes | 68 | 61.82 | |
| No | 42 | 38.18 | |
| Baseline blood eosinophil count (cells/μL) | 100 | 250 [127.5–485] | |
| Baseline IgE (IU/mL) | 96 | 310.45 [142.5–716.25] | |
| Years with omalizumab | 110 | 2.5 [1–5] | |
| Omalizumab dose (mg/4 weeks) | 110 | 450 [300–600] | |
| Change of BT | |||
| Yes | 47 | 42.73 | |
| No | 63 | 57.27 |
| Response Definition | N | % |
|---|---|---|
| OCS reduction ≥ 50% | ||
| Yes | 56 | 50.91 |
| No | 37 | 33.63 |
| No OCS | 17 | 15.45 |
| OCS reduction ≥ 50% or absence | ||
| Yes | 71 | 64.55 |
| No | 39 | 35.45 |
| FEV1 increase ≥ 10% | ||
| Yes | 42 | 46.15 |
| No | 49 | 53.85 |
| FEV1 increase ≥ 10% or FEV1 ≥ 80% | ||
| Yes | 71 | 75.53 |
| No | 23 | 24.47 |
| Exacerbation reduction ≥ 50% | ||
| Yes | 54 | 49.09 |
| No | 18 | 16.36 |
| No exacerbations | 38 | 34.55 |
| Exacerbation reduction ≥ 50% or absence | ||
| Yes | 90 | 81.82 |
| No | 20 | 18.18 |
| Independent Variable | Biological Therapy with Omalizumab |
|---|---|
| ICS dose (mg/day) | |
| Baseline, p50 (p25, p75) | 320 [184–640] |
| Follow-up, p50 (p25, p75) | 184 [92.75–400] |
| Change from baseline | p < 0.001 |
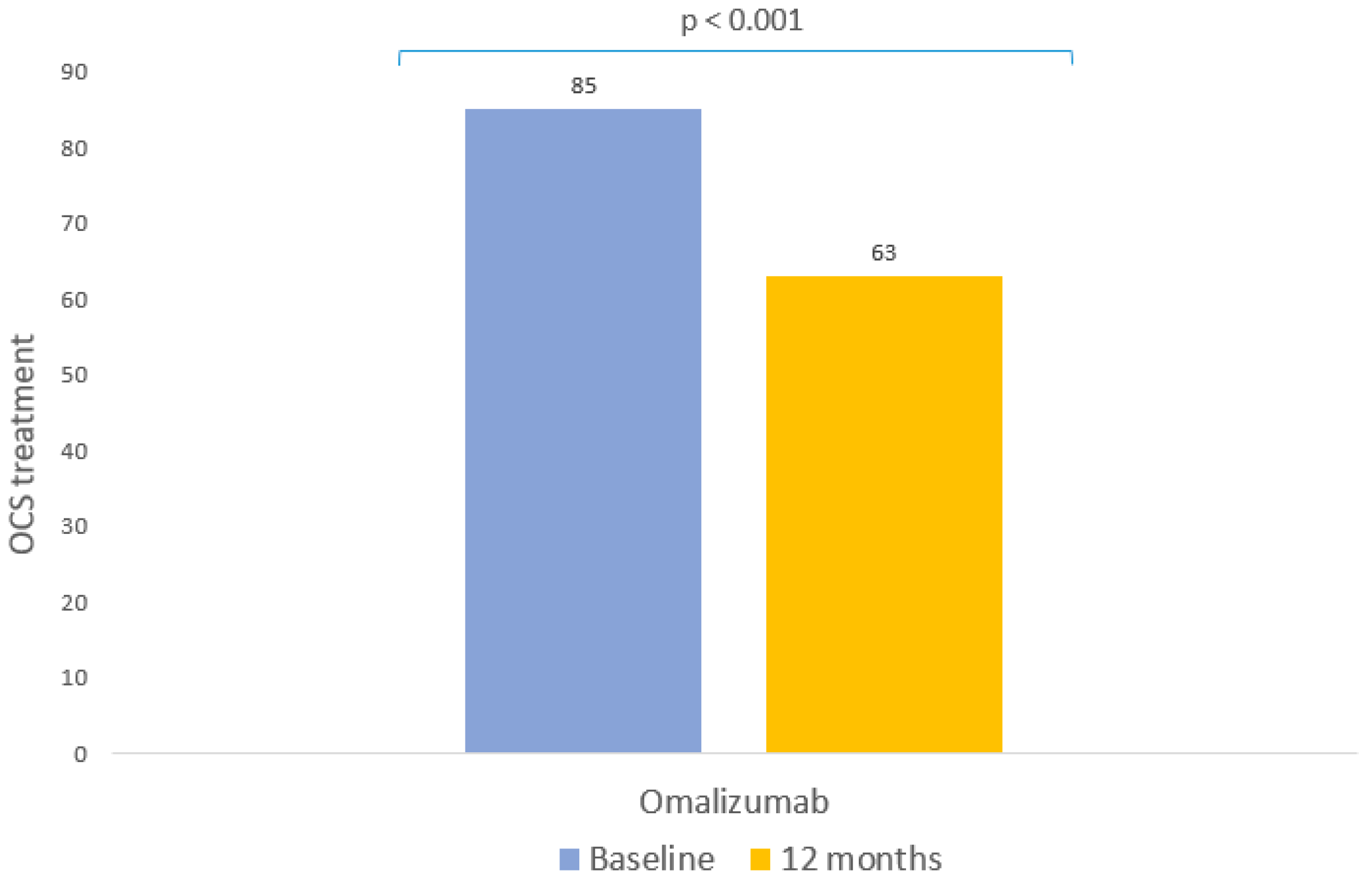
| Use of OCS (Yes/No) | |
| Baseline, n (%) | 85 (77.27) |
| Follow-up, n (%) | 63 (57.27) |
| Change from baseline | p < 0.001 |
| Cycles of OCS per year | |
| Baseline, p50 (p25, p75) | 2 [1–3] |
| Follow-up, p50 (p25, p75) | 1 [0–2] |
| Change from baseline | p < 0.001 |
| Patients with FEV1 >80% | |
| Baseline, n (%) | 44 (41.51) |
| Follow-up, n (%) | 53 (56.38) |
| Change from baseline | p = 0.745 |
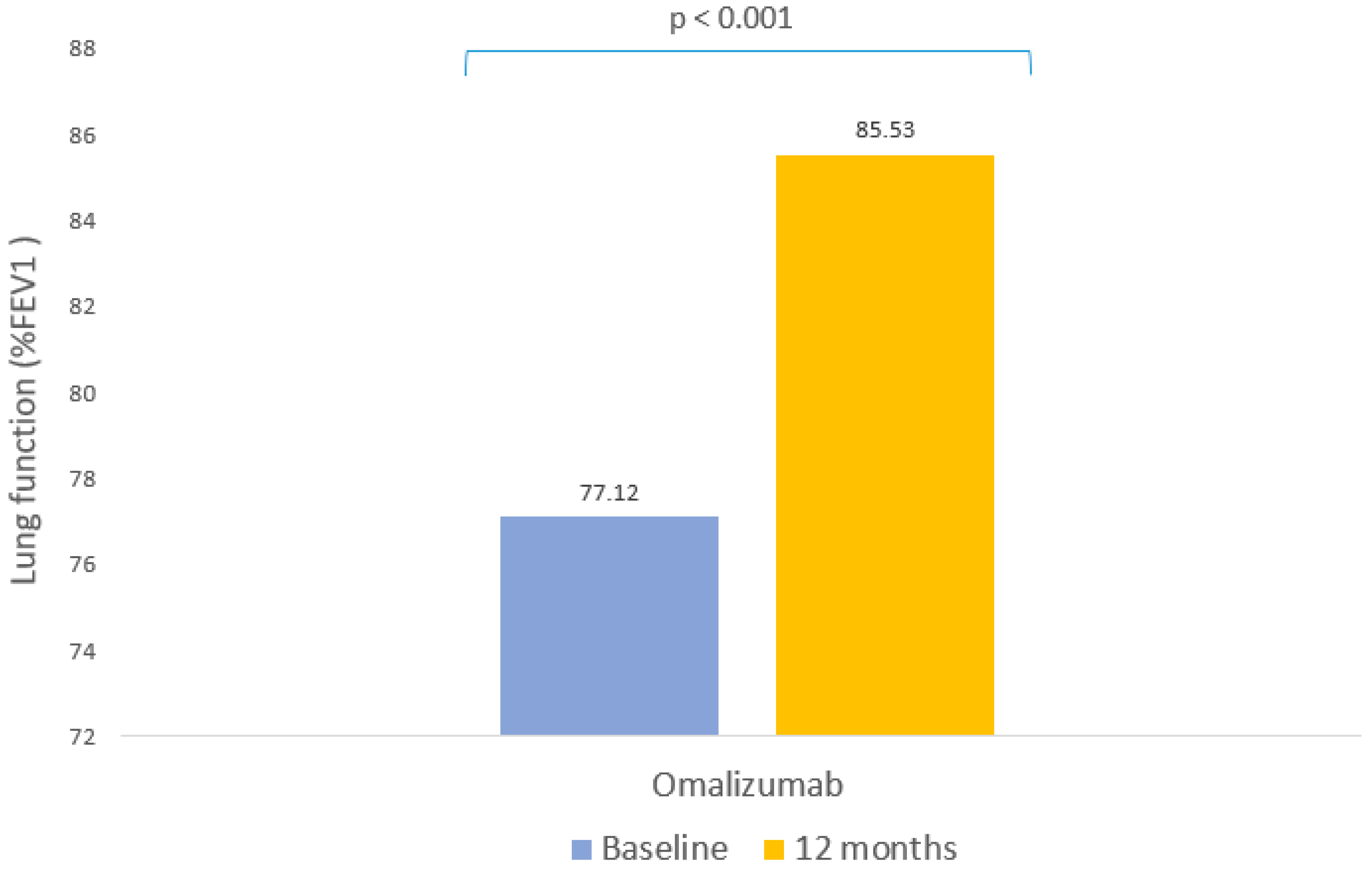
| %FEV1 | |
| Baseline, mean (SD) | 76.12 ± 21.68 |
| Follow-up (SD) | 85.53 ± 20.10 |
| Change from baseline | p < 0.001 |
| ACT | |
| Baseline, p50 (p25, p75) | 12 [10–16] |
| Follow-up, p50 (p25, p75) | 20.5 [16–23] |
| Change from baseline | p = 0.004 |
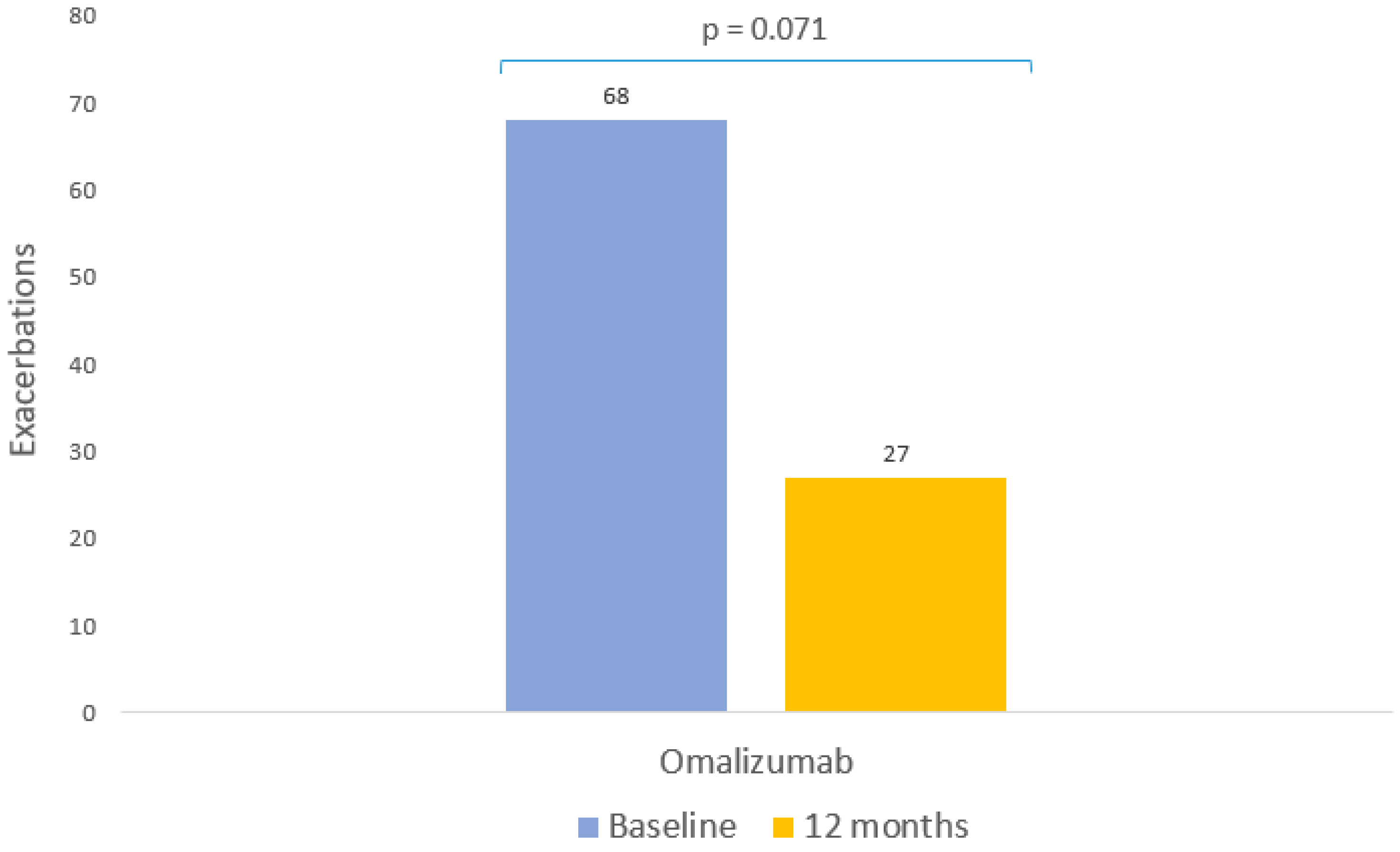
| Presence of exacerbations (Yes/No) | |
| Baseline, n (%) | 68 (61.82) |
| Follow-up, n (%) | 27 (24.55) |
| Change from baseline | p = 0.071 |
| Exacerbations per year | |
| Baseline, p50 (p25, p75) | 1 [0–2] |
| Follow-up, p50 (p25, p75) | 0 [0–0] |
| Change from baseline | p < 0.001 |
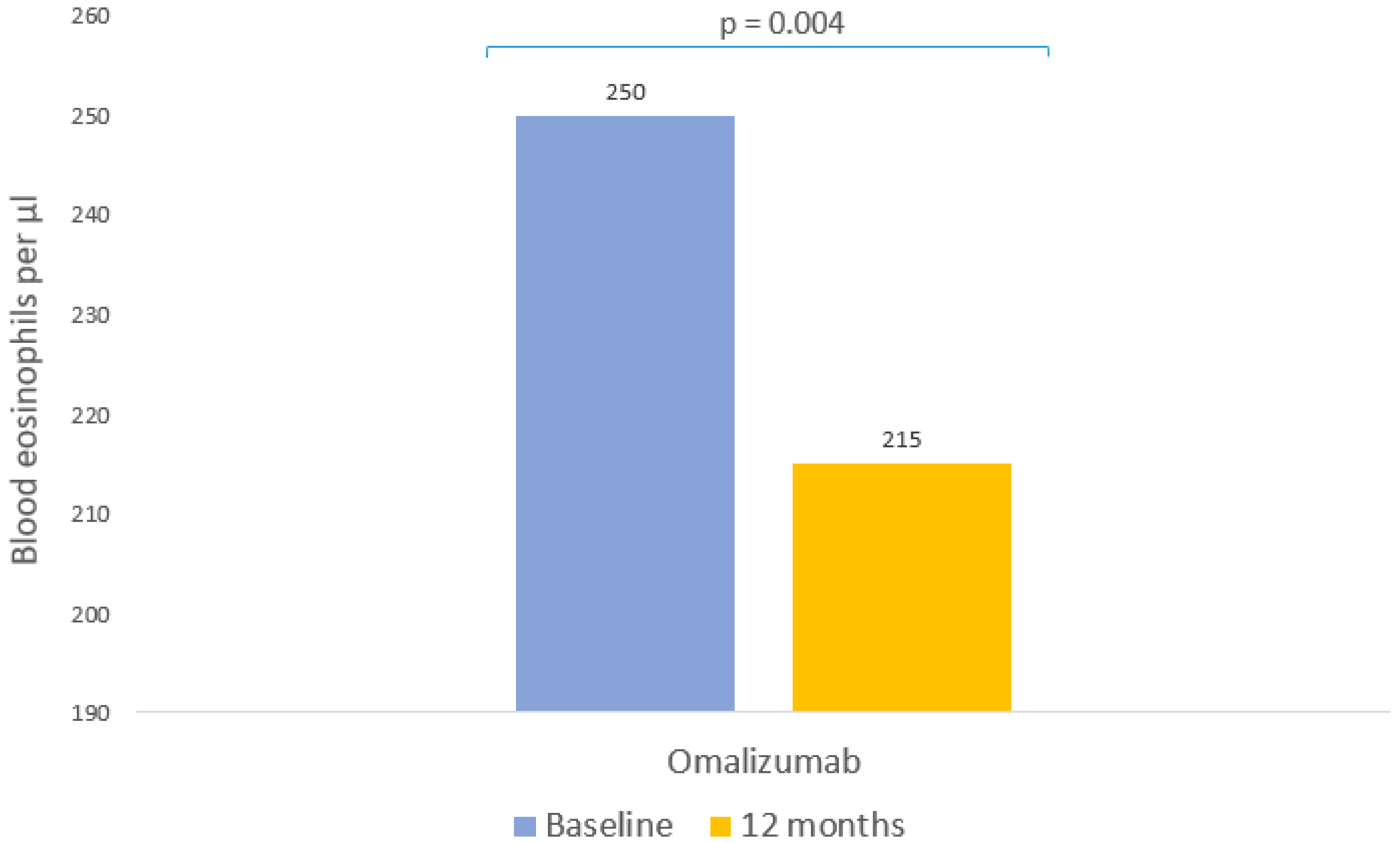
| Blood eosinophils (cell/μL) | |
| Baseline, p50 (p25, p75) | 250 [127.5–485] |
| Follow-up, p50 (p25, p75) | 215 [120–327.5] |
| Change from baseline | p = 0.004 |
| IgE (IU/mL) | |
| Baseline, p50 (p25, p75) | 310.45 [142.5–716.25] |
| Follow-up, p50 (p25, p75) | 496 [191–852] |
| Change from baseline | p = 0.888 |
| B | Odds Ratio | p-Value | 95% CI | |
|---|---|---|---|---|
| OCS reduction predictors | ||||
| ACT | −0.2994 | 0.74 | 0.045 | 0.53–0.97 |
| Lung improvement predictors | ||||
| FEV1 | −0.0719 | 0.93 | <0.001 | 0.90–0.96 |
| COPD | 3.5341 | 34.26 | 0.001 | 5.20–395.88 |
| Exacerbation reduction predictors | ||||
| GERD | 1.1238 | 3.08 | 0.033 | 0.11–0.92 |
| FEV1 (>80) | 1.1759 | 3.24 | 0.054 | 1.06–12.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Tolosa, S.; González-Gutiérrez, M.V.; Sánchez-Martínez, J.A.; Jiménez-Gálvez, G.; Pineda-Lancheros, L.E.; Gálvez-Navas, J.M.; Jiménez-Morales, A.; Pérez-Ramírez, C.; Morales-García, C. Impact of Omalizumab in Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study. Pharmaceutics 2023, 15, 523. https://doi.org/10.3390/pharmaceutics15020523
Rojo-Tolosa S, González-Gutiérrez MV, Sánchez-Martínez JA, Jiménez-Gálvez G, Pineda-Lancheros LE, Gálvez-Navas JM, Jiménez-Morales A, Pérez-Ramírez C, Morales-García C. Impact of Omalizumab in Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study. Pharmaceutics. 2023; 15(2):523. https://doi.org/10.3390/pharmaceutics15020523
Chicago/Turabian StyleRojo-Tolosa, Susana, María Victoria González-Gutiérrez, José Antonio Sánchez-Martínez, Gonzalo Jiménez-Gálvez, Laura Elena Pineda-Lancheros, José María Gálvez-Navas, Alberto Jiménez-Morales, Cristina Pérez-Ramírez, and Concepción Morales-García. 2023. "Impact of Omalizumab in Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study" Pharmaceutics 15, no. 2: 523. https://doi.org/10.3390/pharmaceutics15020523
APA StyleRojo-Tolosa, S., González-Gutiérrez, M. V., Sánchez-Martínez, J. A., Jiménez-Gálvez, G., Pineda-Lancheros, L. E., Gálvez-Navas, J. M., Jiménez-Morales, A., Pérez-Ramírez, C., & Morales-García, C. (2023). Impact of Omalizumab in Patients with Severe Uncontrolled Asthma and Possible Predictive Biomarkers of Response: A Real-Life Study. Pharmaceutics, 15(2), 523. https://doi.org/10.3390/pharmaceutics15020523