Oral Administration of TrkB Agonist, 7, 8–Dihydroxyflavone Regenerates Hair Cells and Restores Function after Gentamicin–Induced Vestibular Injury in Guinea Pig
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
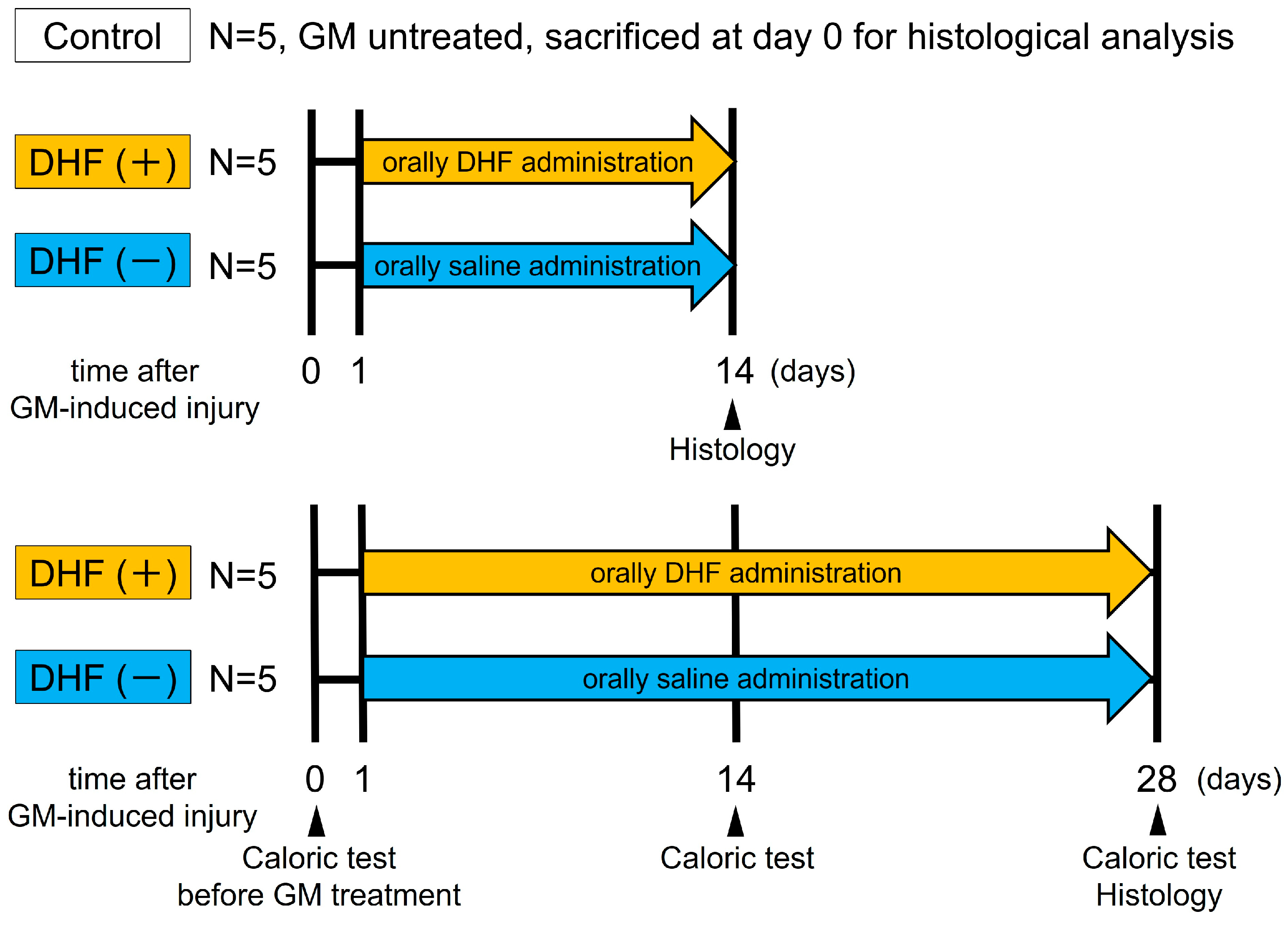
2.2. Experimental Protocol
2.3. Tissue Processing
2.4. Immunohistochemistry
2.5. Confocal Microscopy
2.6. HC Density
2.7. NF200 Positive Rate
2.8. Synaptic Analysis
2.9. Caloric Test
2.10. Statistical Analysis
3. Results
3.1. Increase in HC Densities by Oral Administration of DHF after GM Injury
3.2. Protection against Ampullary Nerve Damage by Oral Administration of DHF after GM Treatment
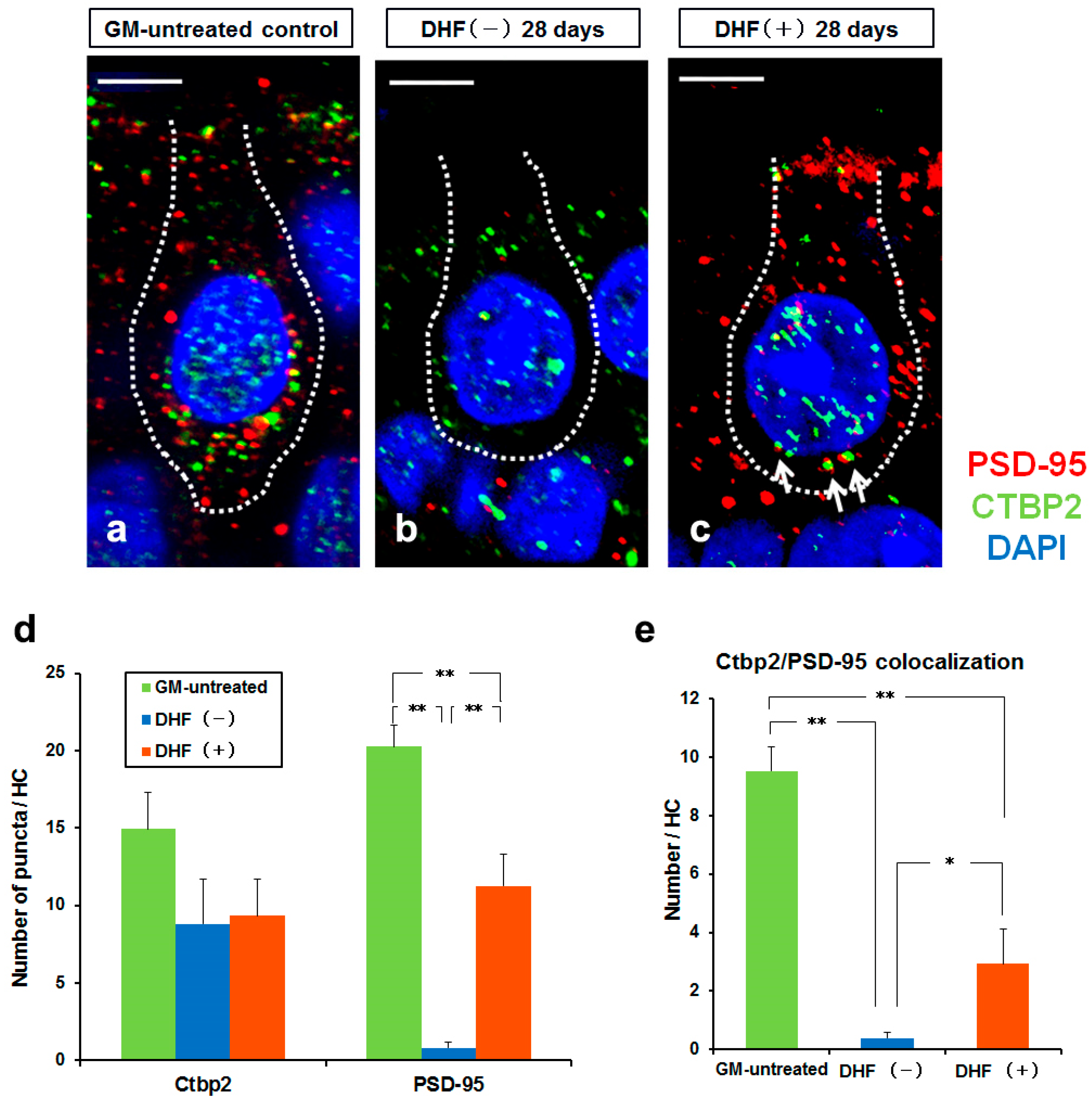
3.3. Synaptic Change in Type I HCs by Oral Administration of DHF after GM–Induced Injury
3.4. Recovery of Vestibular Function by Oral Administration of DHF after GM–Induced Injury
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neurootol. 2000, 5, 3–22. [Google Scholar] [CrossRef]
- Hodgkinson, L.; Prasher, D. Effects of industrial solvents on hearing and balance: A review. Noise Health 2006, 8, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Saldaña–Ruíz, S.; Hernández–Mir, G.; Sedó–Cabezón, L.; Cutillas, B.; Llorens, J. Vestibular toxicity of cis–2–pentenenitrile in the rat. Toxicol. Lett. 2012, 211, 281–288. [Google Scholar] [CrossRef]
- Saldaña–Ruíz, S.; Soler–Martín, C.; Llorens, J. Role of CYP2E1–mediated metabolism in the acute and vestibular toxicities of nineteen nitriles in the mouse. Toxicol. Lett. 2012, 208, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef]
- Sedó–Cabezón, L.; Boadas–Vaello, P.; Soler–Martín, C.; Llorens, J. Vestibular damage in chronic ototoxicity: A mini–review. Neurotoxicology 2014, 43, 21–27. [Google Scholar] [CrossRef]
- Sergi, B.; Ferraresi, A.; Troiani, D.; Paludetti, G.; Fetoni, A.R. Cisplatin ototoxicity in the guinea pig: Vestibular and cochlear damage. Hear Res. 2003, 182, 56–64. [Google Scholar] [CrossRef]
- Adler, H.J.; Komeda, M.; Raphael, Y. Further evidence for supporting cell conversion in the damaged avian basilar papilla. Int. J. Dev. Neurosci. 1997, 15, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.J.; Raphael, Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci. Lett. 1996, 205, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Cotanche, D.A. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987, 30, 181–195. [Google Scholar] [CrossRef]
- Cruz, R.M.; Lambert, P.R.; Rubel, E.W. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; On, D.; Baker, W.; Collado, M.S.; Corwin, J.T. Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. J. Assoc. Res. Otolaryngol. 2012, 13, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Li, L.; Corwin, J.T.; Nevill, G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 1993, 259, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Tong, L.; Ngyuen, T.B.; Hume, C.R.; Palmiter, R.D.; Rubel, E.W.; Stone, J.S. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci. 2012, 32, 15093–15105. [Google Scholar] [CrossRef] [PubMed]
- Warchol, M.E.; Lambert, P.R.; Goldstein, B.J.; Forge, A.; Corwin, J.T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 1993, 259, 1619–1622. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin–regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Fritzsch, B.; Tessarollo, L.; Coppola, E.; Reichardt, L.F. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004, 146, 265–278. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef]
- Skaper, S.D. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol. Disord. Drug Targets 2008, 7, 46–62. [Google Scholar] [CrossRef]
- Ernfors, P.; Lee, K.F.; Jaenisch, R. Mice lacking brain–derived neurotrophic factor develop with sensory deficits. Nature 1994, 368, 147–150. [Google Scholar] [CrossRef]
- Ernfors, P.; Van De Water, T.; Loring, J.; Jaenisch, R. Complementary roles of BDNF and NT–3 in vestibular and auditory development. Neuron 1995, 14, 1153–1164. [Google Scholar] [CrossRef]
- Ylikoski, J.; Pirvola, U.; Moshnyakov, M.; Palgi, J.; Arumäe, U.; Saarma, M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993, 65, 69–78. [Google Scholar] [CrossRef]
- Pirvola, U.; Arumäe, U.; Moshnyakov, M.; Palgi, J.; Saarma, M.; Ylikoski, J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994, 75, 131–144. [Google Scholar] [CrossRef]
- Pirvola, U.; Ylikoski, J.; Palgi, J.; Lehtonen, E.; Arumäe, U.; Saarma, M. Brain–derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc. Natl. Acad. Sci. USA 1992, 89, 9915–9919. [Google Scholar] [CrossRef]
- Inoue, A.; Iwasaki, S.; Fujimoto, C.; Nakajima, T.; Yamasoba, T. Developmental changes in the protective effect of exogenous brain–derived neurotrophic factor and neurotrophin–3 against ototoxic drugs in cultured rat vestibular ganglion neurons. Cell Tissue Res. 2014, 356, 299–308. [Google Scholar] [CrossRef]
- Zheng, J.L.; Stewart, R.R.; Gao, W.Q. Neurotrophin–4/5, brain–derived neurotrophic factor, and neurotrophin–3 promote survival of cultured vestibular ganglion neurons and protect them against neurotoxicity of ototoxins. J. Neurobiol. 1995, 28, 330–340. [Google Scholar] [CrossRef]
- Kopke, R.D.; Jackson, R.L.; Li, G.; Rasmussen, M.D.; Hoffer, M.E.; Frenz, D.A.; Costello, M.; Schultheiss, P.; Van De Water, T.R. Growth factor treatment enhances vestibular hair cell renewal and results in improved vestibular function. Proc. Natl. Acad. Sci. USA 2001, 98, 5886–5891. [Google Scholar] [CrossRef]
- Andero, R.; Heldt, S.A.; Ye, K.; Liu, X.; Armario, A.; Ressler, K.J. Effect of 7,8–dihydroxyflavone, a small–molecule TrkB agonist, on emotional learning. Am. J. Psychiatry. 2011, 168, 163–172. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8–dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef]
- Bollen, E.; Vanmierlo, T.; Akkerman, S.; Wouters, C.; Steinbusch, H.M.; Prickaerts, J. 7,8–Dihydroxyflavone improves memory consolidation processes in rats and mice. Behav. Brain Res. 2013, 257, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Andero, R.; Daviu, N.; Escorihuela, R.M.; Nadal, R.; Armario, A. 7,8–Dihydroxyflavone, a TrkB receptor agonist, blocks long–term spatial memory impairment caused by immobilization stress in rats. Hippocampus 2012, 22, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hung, T.H.; Chen, C.C.; Ke, C.H.; Lee, C.Y.; Wang, P.Y.; Chen, S.F. Post–injury treatment with 7,8–dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling. PLoS ONE 2014, 9, e113397. [Google Scholar] [CrossRef]
- Liu, X.; Chan, C.B.; Jang, S.W.; Pradoldej, S.; Huang, J.; He, K.; Phun, L.H.; France, S.; Xiao, G.; Jia, Y.; et al. A synthetic 7,8–dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J. Med. Chem. 2010, 53, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Selimoglu, E.; Kalkandelen, S.; Erdogan, F. Comparative vestibulotoxicity of different aminoglycosides in the Guinea pigs. Yonsei Med. J. 2003, 44, 517–522. [Google Scholar] [CrossRef]
- Haynes, D.S. Topical antibiotics: Strategies for avoiding ototoxicity. Ear Nose Throat J. 2004, 83, 12–14. [Google Scholar] [CrossRef]
- Ruedi, L.F.W.; Luthy, F.; Nager, G.; Tschirren, B. Further observations concerning the toxic effects of streptomycin and quinine on the auditory organ of guinea pigs. Laryngoscope 1952, 62, 333–351. [Google Scholar] [CrossRef]
- Aran, J.M.; Erre, J.P.; Guilhaume, A.; Aurousseau, C. The comparative ototoxicities of gentamicin, tobramycin and dibekacin in the guinea pig. A functional and morphological cochlear and vestibular study. Acta Otolaryngol. Suppl. 1982, 390, 1–30. [Google Scholar] [CrossRef]
- Kitasato, I.; Yokota, M.; Inouye, S.; Igarashi, M. Comparative ototoxicity of ribostamycin, dactimicin, dibekacin, kanamycin, amikacin, tobramycin, gentamicin, sisomicin and netilmicin in the inner ear of guinea pigs. Chemotherapy 1990, 36, 155–168. [Google Scholar] [CrossRef]
- Kinoshita, M.; Fujimoto, C.; Iwasaki, S.; Kashio, A.; Kikkawa, Y.S.; Kondo, K.; Okano, H.; Yamasoba, T. Alteration of Musashi1 intra–cellular distribution during regeneration following gentamicin–induced hair cell loss in the guinea pig crista ampullaris. Front. Cell Neurosci. 2019, 13, 481. [Google Scholar] [CrossRef]
- Kanda, S.; Akazome, Y.; Matsunaga, T.; Yamamoto, N.; Yamada, S.; Tsukamura, H.; Maeda, K.; Oka, Y. Identification of KiSS–1 product kisspeptin and steroid–sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes). Endocrinology 2008, 149, 2467–2476. [Google Scholar] [CrossRef]
- Lysakowski, A.; Goldberg, J.M. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J. Comp. Neurol. 1997, 389, 419–443. [Google Scholar] [CrossRef]
- Simmons, D.D.; Tong, B.; Schrader, A.D.; Hornak, A.J. Oncomodulin identifies different hair cell types in the mammalian inner ear. J. Comp. Neurol. 2010, 518, 3785–3802. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Gao, W.Q. Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J. Neurosci. 1997, 17, 8270–8282. [Google Scholar] [CrossRef]
- Sedó–Cabezón, L.; Jedynak, P.; Boadas–Vaello, P.; Llorens, J. Transient alteration of the vestibular calyceal junction and synapse in response to chronic ototoxic insult in rats. Dis. Models Mech. 2016, 9, 821. [Google Scholar] [CrossRef]
- Kristensen, H.K. Caloric reactions in guinea pig and rabbit. Acta Oto–Laryngol. 1954, 44, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Schmäl, F.; Lübben, B.; Weiberg, K.; Stoll, W. The minimal ice water caloric test compared with established vestibular caloric test procedures. J. Vestib. Res. 2005, 15, 215–224. [Google Scholar] [CrossRef]
- Ramekers, D.; Versnel, H.; Grolman, W.; Klis, S.F. Neurotrophins and their role in the cochlea. Hear Res. 2012, 288, 19–33. [Google Scholar] [CrossRef]
- Burns, J.C.; Cox, B.C.; Thiede, B.R.; Zuo, J.; Corwin, J.T. In vivo proliferative regeneration of balance hair cells in newborn mice. J. Neurosci. 2012, 32, 6570–6577. [Google Scholar] [CrossRef]
- Hawkins, J.E., Jr.; Johnsson, L.G.; Stebbins, W.C.; Moody, D.B.; Coombs, S.L. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Oto–Laryngol. 1976, 81, 337–343. [Google Scholar] [CrossRef]
- Oesterle, E.C.; Campbell, S.; Taylor, R.R.; Forge, A.; Hume, C.R. Sox2 and JAGGED1 expression in normal and drug–damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol. 2008, 9, 65–89. [Google Scholar] [CrossRef]
- Kawamoto, K.; Izumikawa, M.; Beyer, L.A.; Atkin, G.M.; Raphael, Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009, 247, 17–26. [Google Scholar] [CrossRef]
- Bremer, H.G.; Versnel, H.; Hendriksen, F.G.; Topsakal, V.; Grolman, W.; Klis, S.F. Does vestibular end–organ function recover after gentamicin–induced trauma in Guinea pigs? Audiol. Neurootol. 2014, 19, 135–150. [Google Scholar] [CrossRef]
- Kuntz, A.L.; Oesterle, E.C. Transforming growth factor alpha with insulin stimulates cell proliferation in vivo in adult rat vestibular sensory epithelium. J. Comp. Neurol. 1998, 399, 413–423. [Google Scholar] [CrossRef]
- Lambert, P.R. Inner ear hair cell regeneration in a mammal: Identification of a triggering factor. Laryngoscope 1994, 104, 701–718. [Google Scholar] [CrossRef]
- Lopez, I.; Honrubia, V.; Lee, S.C.; Chung, W.H.; Li, G.; Beykirch, K.; Micevych, P. The protective effect of brain–derived neurotrophic factor after gentamicin ototoxicity. Am. J. Otol. 1999, 20, 317–324. [Google Scholar]
- Oesterle, E.C.; Tsue, T.T.; Rubel, E.W. Induction of cell proliferation in avian inner ear sensory epithelia by insulin–like growth factor–I and insulin. J. Comp. Neurol. 1997, 380, 262–274. [Google Scholar] [CrossRef]
- Yamashita, H.; Oesterle, E.C. Induction of cell proliferation in mammalian inner–ear sensory epithelia by transforming growth factor alpha and epidermal growth factor. Proc. Natl. Acad. Sci. USA 1995, 92, 3152–3155. [Google Scholar] [CrossRef]
- Zheng, J.L.; Frantz, G.; Lewis, A.K.; Sliwkowski, M.; Gao, W.Q. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J. Neurocytol. 1999, 28, 901–912. [Google Scholar] [CrossRef]
- Chen, J.; Chua, K.W.; Chua, C.C.; Yu, H.; Pei, A.; Chua, B.H.; Hamdy, R.C.; Xu, X.; Liu, C.F. Antioxidant activity of 7,8–dihydroxyflavone provides neuroprotection against glutamate–induced toxicity. Neurosci. Lett. 2011, 499, 181–185. [Google Scholar] [CrossRef]
- Wang, B.; Wu, N.; Liang, F.; Zhang, S.; Ni, W.; Cao, Y.; Xia, D.; Xi, H. 7,8–Dihydroxyflavone, a small–molecule tropomyosin–related kinase B (TrkB) agonist, attenuates cerebral ischemia and reperfusion injury in rats. J. Mol. Histol. 2014, 45, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. 7,8–Dihydroxyflavone, a small–molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2012, 37, 434–444. [Google Scholar] [CrossRef]
- Yu, Q.; Chang, Q.; Liu, X.; Wang, Y.; Li, H.; Gong, S.; Ye, K.; Lin, X. Protection of spiral ganglion neurons from degeneration using small–molecule TrkB receptor agonists. J. Neurosci. 2013, 33, 13042–13052. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Li, L.; Nevill, G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J. Comp. Neurol. 1998, 397, 69–88. [Google Scholar] [CrossRef]
- López, I.; Honrubia, V.; Lee, S.C.; Li, G.; Beykirch, K. Hair cell recovery in the chinchilla crista ampullaris after gentamicin treatment: A quantitative approach. Otolaryngol. Head Neck Surg. 1998, 119, 255–262. [Google Scholar] [CrossRef]
- López, I.; Honrubia, V.; Lee, S.C.; Schoeman, G.; Beykirch, K. Quantification of the process of hair cell loss and recovery in the chinchilla crista ampullaris after gentamicin treatment. Int. J. Dev. Neurosci. 1997, 15, 447–461. [Google Scholar] [CrossRef]
- Ard, M.D.; Morest, D.K.; Hauger, S.H. Trophic interactions between the cochleovestibular ganglion of the chick embryo and its synaptic targets in culture. Neuroscience 1985, 16, 151–170. [Google Scholar] [CrossRef]
- Johnson, E.M., Jr.; Yip, H.K. Central nervous system and peripheral nerve growth factor provide trophic support critical to mature sensory neuronal survival. Nature 1985, 314, 751–752. [Google Scholar] [CrossRef]
- Al–Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.S.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef]
- English, A.W.; Liu, K.; Nicolini, J.M.; Mulligan, A.M.; Ye, K. Small–molecule trkB agonists promote axon regeneration in cut peripheral nerves. Proc. Natl. Acad. Sci. USA 2013, 110, 16217–16222. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K. The challenge of hair cell regeneration. Exp. Biol. Med. 2010, 235, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Rubel, E.W.; Furrer, S.A.; Stone, J.S. A brief history of hair cell regeneration research and speculations on the future. Hear Res. 2013, 297, 42–51. [Google Scholar] [CrossRef]
- Sadeghi, S.G.; Pyott, S.J.; Yu, Z.; Glowatzki, E. Glutamatergic signaling at the vestibular hair cell calyx synapse. J. Neurosci. 2014, 34, 14536–14550. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Valat, J.; Travo, C.; Sans, A. A role for BDNF in early postnatal rat vestibular epithelia maturation: Implication of supporting cells. Eur. J. Neurosci. 1998, 10, 598–606. [Google Scholar] [CrossRef]
- Gao, L.; Tian, M.; Zhao, H.Y.; Xu, Q.Q.; Huang, Y.M.; Si, Q.C.; Tian, Q.; Wu, Q.M.; Hu, X.M.; Sun, L.B.; et al. TrkB activation by 7, 8–dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2016, 136, 620–636. [Google Scholar] [CrossRef]
- Tian, M.; Zeng, Y.; Hu, Y.; Yuan, X.; Liu, S.; Li, J.; Lu, P.; Sun, Y.; Gao, L.; Fu, D.; et al. 7, 8–dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology 2015, 89, 43–53. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, Y.; Wu, M.; Liu, J.; Hu, Q. Activation of TrkB by 7,8–dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J. Alzheimers Dis. 2012, 31, 765–778. [Google Scholar] [CrossRef]
- Zeng, Y.; Lv, F.; Li, L.; Yu, H.; Dong, M.; Fu, Q. 7,8–Dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J. Neurochem. 2012, 122, 800–811. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, M.; Fujimoto, C.; Iwasaki, S.; Kondo, K.; Yamasoba, T. Oral Administration of TrkB Agonist, 7, 8–Dihydroxyflavone Regenerates Hair Cells and Restores Function after Gentamicin–Induced Vestibular Injury in Guinea Pig. Pharmaceutics 2023, 15, 493. https://doi.org/10.3390/pharmaceutics15020493
Kinoshita M, Fujimoto C, Iwasaki S, Kondo K, Yamasoba T. Oral Administration of TrkB Agonist, 7, 8–Dihydroxyflavone Regenerates Hair Cells and Restores Function after Gentamicin–Induced Vestibular Injury in Guinea Pig. Pharmaceutics. 2023; 15(2):493. https://doi.org/10.3390/pharmaceutics15020493
Chicago/Turabian StyleKinoshita, Makoto, Chisato Fujimoto, Shinichi Iwasaki, Kenji Kondo, and Tatsuya Yamasoba. 2023. "Oral Administration of TrkB Agonist, 7, 8–Dihydroxyflavone Regenerates Hair Cells and Restores Function after Gentamicin–Induced Vestibular Injury in Guinea Pig" Pharmaceutics 15, no. 2: 493. https://doi.org/10.3390/pharmaceutics15020493
APA StyleKinoshita, M., Fujimoto, C., Iwasaki, S., Kondo, K., & Yamasoba, T. (2023). Oral Administration of TrkB Agonist, 7, 8–Dihydroxyflavone Regenerates Hair Cells and Restores Function after Gentamicin–Induced Vestibular Injury in Guinea Pig. Pharmaceutics, 15(2), 493. https://doi.org/10.3390/pharmaceutics15020493








