Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiolabelling
2.1.1. Radiolabelling of [68Ga]Ga-DOTAGA-cKNGRE
2.1.2. Radiolabelling of [213Bi]Bi-DOTAGA-cKNGRE
2.2. LogP Measurement
2.2.1. Determination of LogP Value of [68Ga]Ga-DOTAGA-cKNGRE
2.2.2. Determination of LogP Value of [205/206Bi]Bi-DOTAGA-cKNGRE
2.3. Stability Studies
2.3.1. Serum Stability of [68Ga]Ga-DOTAGA-cKNGRE
2.3.2. Serum Stability of [205/206Bi]Bi-DOTAGA-cKNGRE
2.4. Cell Culturing
2.5. Animal Housing
2.6. HT1080 Tumour Generation
2.7. In Vivo MiniPET Imaging
2.8. Cancer Treatment Studies
2.9. Ex Vivo Biodistribution Studies
2.10. Statistical Analyses
3. Results and Discussion
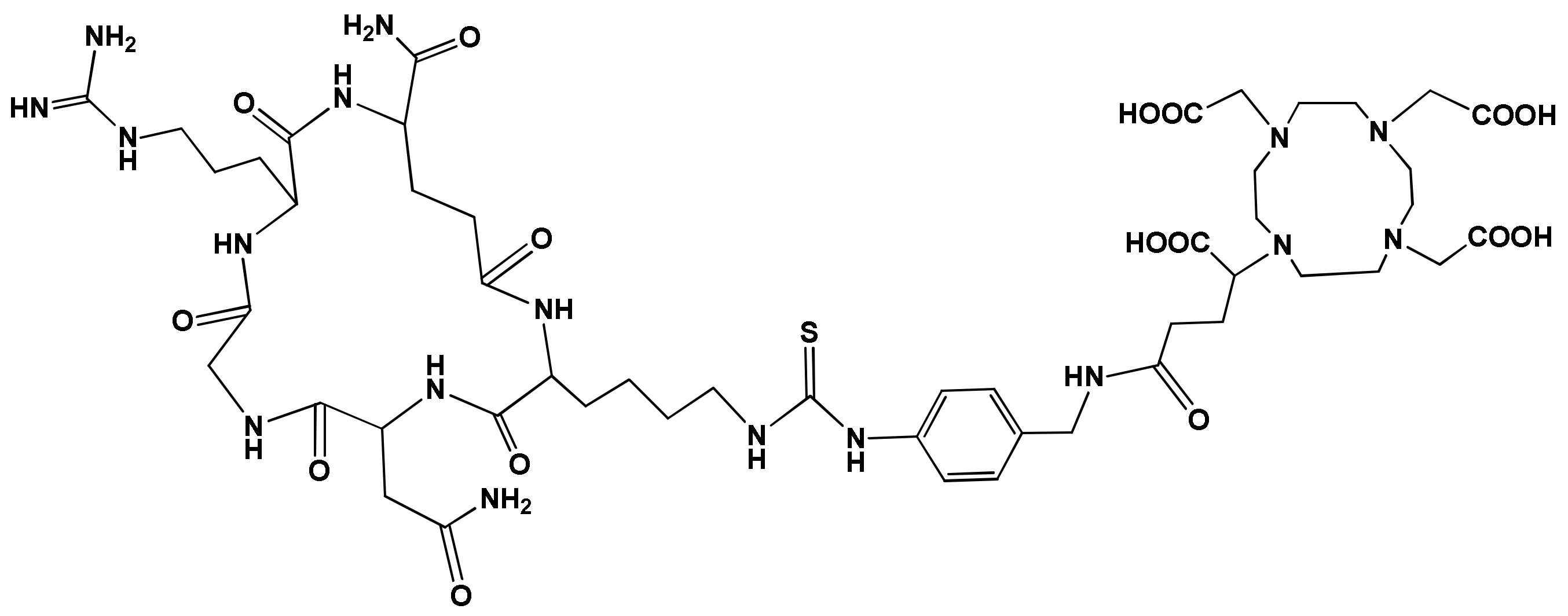
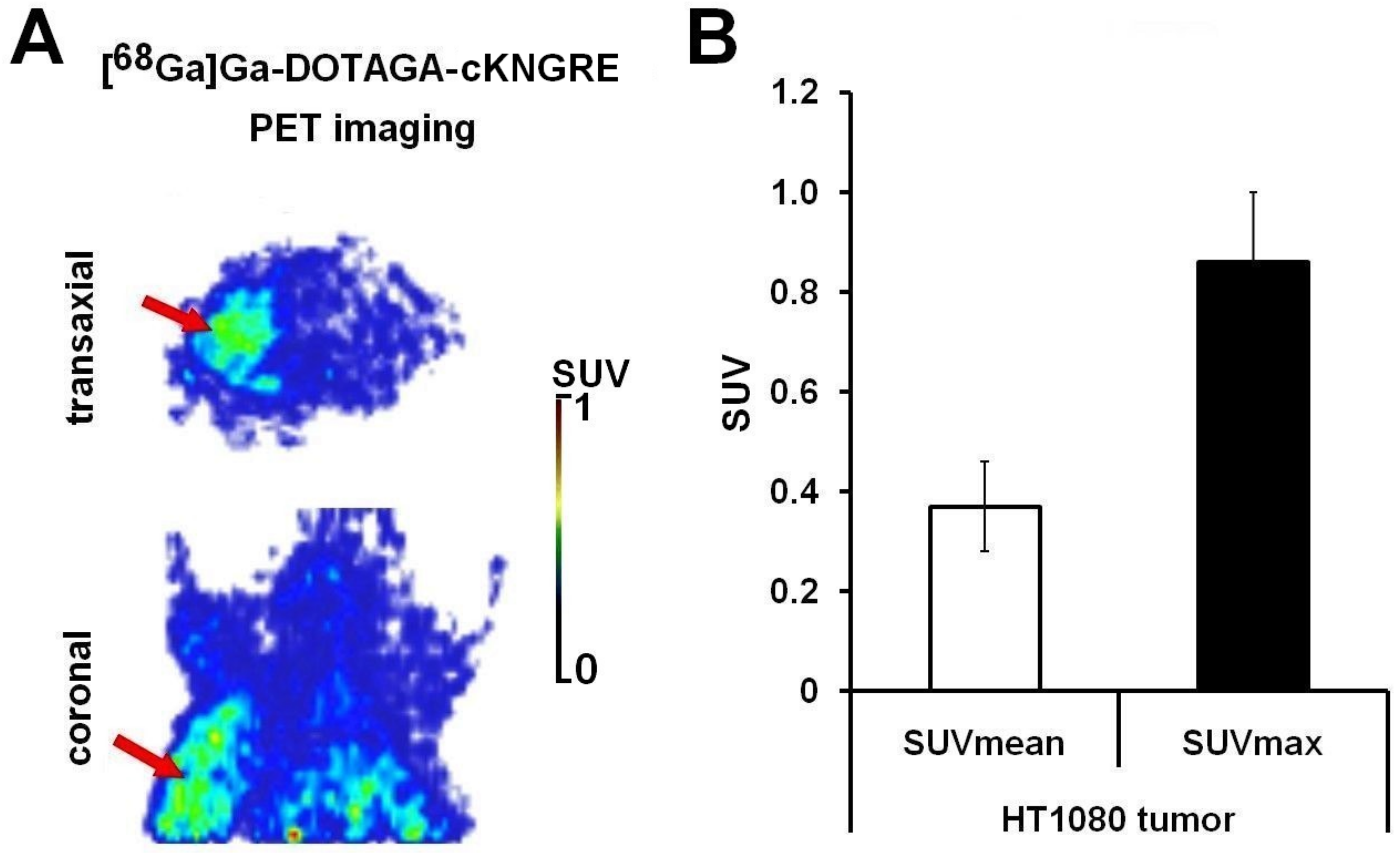
3.1. [68Ga]Ga-DOTAGA-cKNGRE MiniPET Imaging
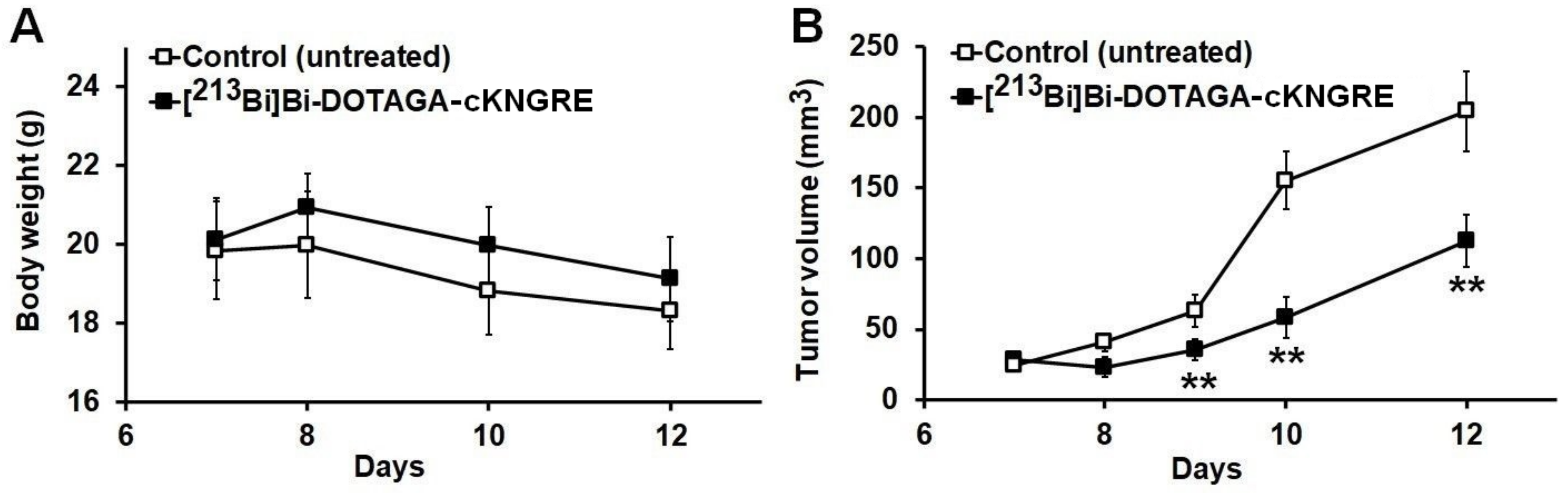
3.2. Performance Evaluation of [213Bi]Bi-DOTAGA-cKNGRE Treatment: Effects on Body Weight and Tumour Volume
3.2.1. Impact of [213Bi]Bi-DOTAGA-cKNGRE Treatment on Body Weight
3.2.2. Impact of [213Bi]Bi-DOTAGA-cKNGRE Treatment on Tumour Volume
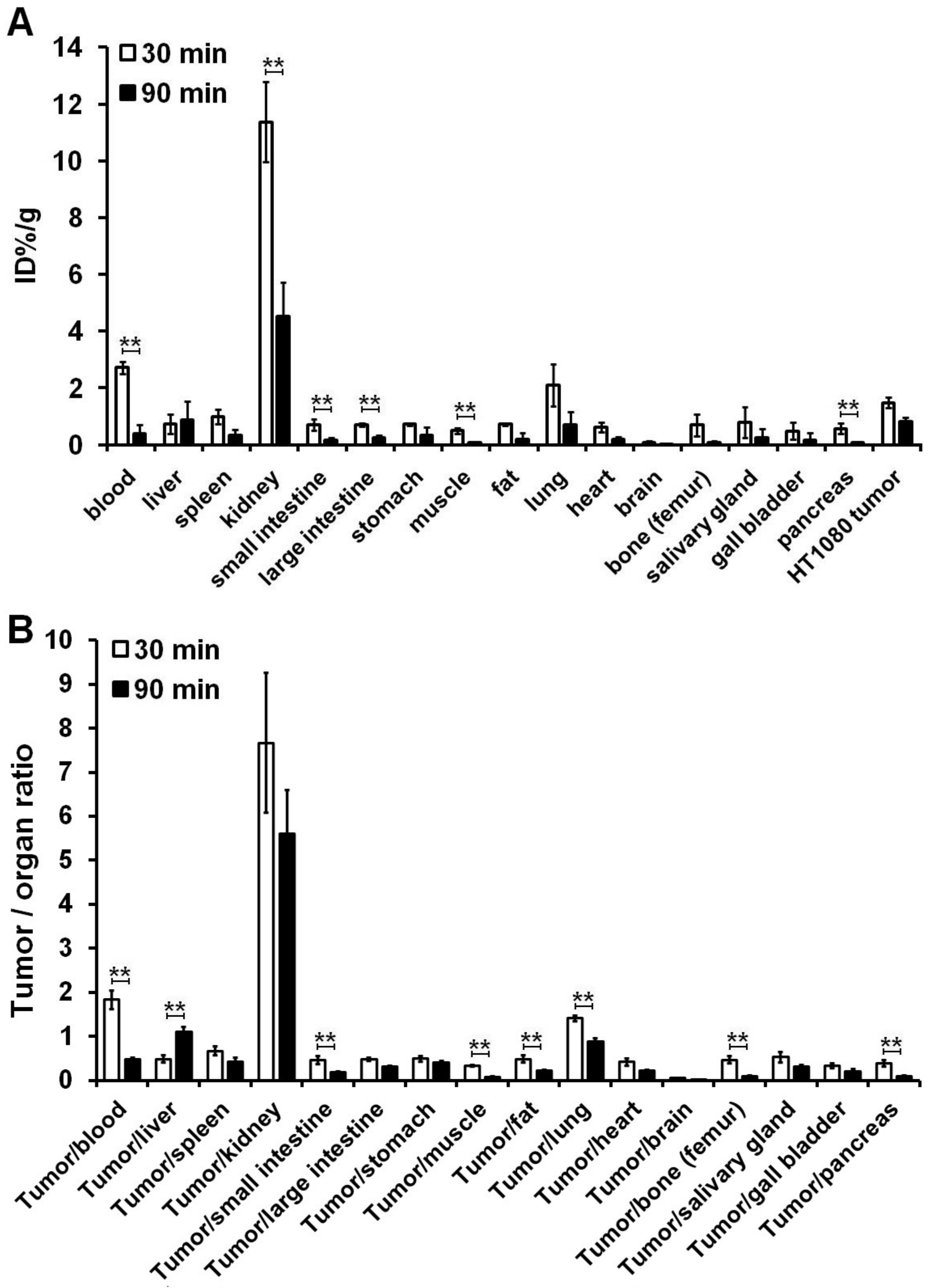
3.3. Ex Vivo Organ Distribution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Gai, Y.; Li, Z.; Li, H.; Li, J.; Muschler, J.; Kang, R.; Tang, D.; Zeng, D. Heterodimeric RGD-NGR PET Tracer for the Early Detection of Pancreatic Cancer. Mol. Imaging. Biol. 2022, 24, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Barnieh, F.M.; Loadman, P.M.; Falconer, R.A. Is tumour-expressed aminopeptidase N (APN/CD13) structurally and functionally unique? Biochim. Biophys. Acta. Rev. Cancer 2021, 1876, 188641. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends. Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef]
- Hashida, H.; Takabayashi, A.; Kanai, M.; Adachi, M.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. Aminopeptidase N is involved in cell motility and angiogenesis: Its clinical significance in human colon cancer. Gastroenterology 2002, 122, 376–386. [Google Scholar] [CrossRef]
- Ito, S.; Miyahara, R.; Takahashi, R.; Nagai, S.; Takenaka, K.; Wada, H.; Tanaka, F. Stromal aminopeptidase N expression: Correlation with angiogenesis in non-small-cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2009, 57, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Kajiyama, H.; Shibata, K.; Ino, K.; Nawa, A.; Mizutani, S.; Kikkawa, F. Inhibition of APN/CD13 leads to suppressed progressive potential in ovarian carcinoma cells. BMC Cancer 2007, 7, 140. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Z.; Jia, Y.; Cui, M.; Han, L.; Zhang, Y.; Liu, Z.; Bi, D.; Liu, S. Ubenimex inhibits cell proliferation, migration and invasion by inhibiting the expression of APN and inducing autophagic cell death in prostate cancer cells. Oncol. Rep. 2016, 35, 2121–2130. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Rugo, H.S.; Burstein, H.J. The role of angiogenesis inhibition in the treatment of breast cancer. Clin. Adv. Hematol. Oncol. 2006, 4, 1–10. [Google Scholar]
- Deshpande, N.; Ren, Y.; Foygel, K.; Rosenberg, J.; Willmann, J.K. Tumor angiogenic marker expression levels during tumor growth: Longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 2011, 258, 804–811. [Google Scholar] [CrossRef]
- Kessler, T.; Baumeier, A.; Brand, C.; Grau, M.; Angenendt, L.; Harrach, S.; Stalmann, U.; Schmidt, L.H.; Gosheger, G.; Hardes, J.; et al. Aminopeptidase N (CD13): Expression, Prognostic Impact, and Use as Therapeutic Target for Tissue Factor Induced Tumor Vascular Infarction in Soft Tissue Sarcoma. Transl. Oncol. 2018, 11, 1271–1282. [Google Scholar] [CrossRef]
- Wickstrom, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Buehler, A.; van Zandvoort, M.A.; Stelt, B.J.; Hackeng, T.M.; Schrans-Stassen, B.H.; Bennaghmouch, A.; Hofstra, L.; Cleutjens, J.P.; Duijvestijn, A.; Smeets, M.B.; et al. cNGR: A novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2681–2687. [Google Scholar] [CrossRef]
- Oostendorp, M.; Douma, K.; Wagenaar, A.; Slenter, J.M.; Hackeng, T.M.; van Zandvoort, M.A.; Post, M.J.; Backes, W.H. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation 2010, 121, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Zong, S.; Wang, J.; Conti, P.S.; Chen, K. MicroPET imaging of CD13 expression using a (64)Cu-labeled dimeric NGR peptide based on sarcophagine cage. Mol. Pharm. 2014, 11, 3938–3946. [Google Scholar] [CrossRef] [PubMed]
- Máté, G.; Kertész, I.; Enyedi, K.N.; Mező, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabó, É.; Emri, M.; Bíró, T.; et al. In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer (68)Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef]
- Vats, K.; Satpati, A.K.; Sharma, R.; Sarma, H.D.; Satpati, D.; Dash, A. 177Lu-labeled cyclic Asn-Gly-Arg peptide tagged carbon nanospheres as tumor targeting radio-nanoprobes. J. Pharm. Biomed. Anal. 2018, 152, 173–178. [Google Scholar] [CrossRef]
- Ma, W.; Shao, Y.; Yang, W.; Li, G.; Zhang, Y.; Zhang, M.; Zuo, C.; Chen, K.; Wang, J. Evaluation of (188)Re-labeled NGR-VEGI protein for radioimaging and radiotherapy in mice bearing human fibrosarcoma HT-1080 xenografts. Tumour. Biol. 2016, 37, 121–129. [Google Scholar] [CrossRef]
- Chen, K.; Ma, W.; Li, G.; Wang, J.; Yang, W.; Yap, L.P.; Hughes, L.D.; Park, R.; Conti, P.S. Synthesis and evaluation of 64Cu-labeled monomeric and dimeric NGR peptides for MicroPET imaging of CD13 receptor expression. Mol. Pharm. 2013, 10, 417–427. [Google Scholar] [CrossRef]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircso, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef]
- Franchi, S.; Di Marco, V.; Tosato, M. Bismuth chelation for targeted alpha therapy: Current state of the art. Nucl. Med. Biol. 2022, 114–115, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Nayak, T.K.; Norenberg, J.P.; Anderson, T.L.; Prossnitz, E.R.; Stabin, M.G.; Atcher, R.W. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(-)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007, 34, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma therapy via peptide-targeted {alpha}-radiation. Clin. Cancer. Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef]
- Silindir-Gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted Alpha Therapy and Nanocarrier Approach. Cancer Biother. Radiopharm. 2020, 35, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Pillai, M.R. Options to meet the future global demand of radionuclides for radionuclide therapy. Nucl. Med. Biol. 2013, 40, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bé, M.M.; Chisté, V.; Dulieu, C.; Kellett, M.; Mougeot, X.; Chechev, V.P.; Kuzmenko, N.K.; Kondev, F.G.; Luca, A.; Galán, M.; et al. Table of radionuclides. Bur. Int. Des. Poids. Mes. 2008, 6, 1–6. [Google Scholar]
- Ballangrud, A.M.; Yang, W.H.; Charlton, D.E.; McDevitt, M.R.; Hamacher, K.A.; Panageas, K.S.; Ma, D.; Bander, N.H.; Scheinberg, D.A.; Sgouros, G. Response of LNCaP spheroids after treatment with an alpha-particle emitter (213Bi)-labeled anti-prostate-specific membrane antigen antibody (J591). Cancer Res. 2001, 61, 2008–2014. [Google Scholar]
- Gyuricza, B.; Szabó, J.P.; Arató, V.; Dénes, N.; Szűcs, Á.; Berta, K.; Kis, A.; Szücs, D.; Forgács, V.; Szikra, D.; et al. Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging. Pharmaceutics 2021, 13, 2103. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Sharma, R.; Kumar, C.; Sarma, H.D.; Dash, A. 68Ga-Chelation and comparative evaluation of N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. Medchemcomm 2017, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Shinto, A.; Kamaleshwaran, K.K.; Sarma, H.D.; Dash, A. Preliminary PET/CT Imaging with Somatostatin Analogs [68Ga]DOTAGA-TATE and [68Ga]DOTAGA-TOC. Mol. Imaging. Biol. 2017, 19, 878–884. [Google Scholar] [CrossRef]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rösch, F. Processing of generator-produced 68Ga for medical application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef]
- Lagunas-Solar, M.C.; Carvacho, O.F.; Nagahara, L.; Mishra, A.; Parks, N.J. Cyclotron Production of No-Carrier-Added 206Bi (6.24 d) and 205Bi (15.31 d) as Tracers for Biological Studies and for the Development of Alpha-Emitting Radiotherapeutic Agents. Int. J. Rad. Appl. Instrum. A 1987, 38, 129–137. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zou, H.; Shen, Y.; Deng, S.; Wu, Y. Synthesis and evaluation of 68Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft. J. Cancer 2021, 12, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Szabo, J.P.; Denes, N.; Arato, V.; Racz, S.; Kis, A.; Opposits, G.; Kepes, Z.; Hajdu, I.; Joszai, I.; Emri, M.; et al. In Vivo Imaging of Neo-angiogenesis of Transplanted Metastases in Subrenal Capsule Assay Induced Rat Model. In Vivo 2022, 36, 1667–1675. [Google Scholar] [CrossRef]
- Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Borghi, E.; Levy, L. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future. Oncol. 2012, 8, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liang, W.; Kang, F.; Yang, W.; Ma, X.; Li, G.; Zong, S.; Chen, K.; Wang, J. 68Ga-labeled cyclic NGR peptide for microPET imaging of CD13 receptor expression. Molecules 2014, 19, 11600–11612. [Google Scholar] [CrossRef]
- Dixon, J.; Kaklamanis, L.; Turley, H.; Hickson, I.D.; Leek, R.D.; Harris, A.L.; Gatter, K.C. Expression of aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J. Clin. Pathol. 1994, 47, 43–47. [Google Scholar] [CrossRef]
- Hogg, N.; Horton, M.A. Myeloid antigens: New and previously defined clusters. In Leukocyte Typing III. Proceedings of the Third International Workshop on Human Leukocyte Differentiation Antigens; McMichael, A.J., Ed.; Oxford University Press: Oxford, UK, 1987; pp. 576–621. [Google Scholar]
- Israel, I.; Elflein, K.; Schirbel, A.; Chen, K.; Samnick, S. A comparison of the monomeric [68Ga]NODAGA-NGR and dimeric [68Ga]NOTA-(NGR)2 as aminopeptidase N ligand for positron emission tomography imaging in tumor-bearing mice. Eur. J. Pharm. Sci. 2021, 166, 105964. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, I.; Angyal, J.; Szikra, D.; Kertész, I.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vecsernyés, M.; Bácskay, I.; Váradi, J.; et al. Radiochemical synthesis and preclinical evaluation of 68Ga-labeled NODAGA-hydroxypropyl-beta-cyclodextrin (68Ga-NODAGA-HPBCD). Eur. J. Pharm. Sci. 2019, 128, 202–208. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Ma, X.; Ma, W.; Zhao, M.; Fu, T.; Li, G.; Wang, S.; Wang, Z.; Yang, W.; et al. The uptake exploration of 68Ga-labeled NGR in well-differentiated hepatocellular carcinoma xenografts: Indication for the new clinical translational of a tracer based on NGR. Oncol. Rep. 2017, 38, 2859–2866. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Wan, N.; Hua, Z.; Wang, Z.; Huang, H.; Yang, M.; Wang, F. 68Ga-DOTA-NGR as a novel molecular probe for APN-positive tumor imaging using MicroPET. Nucl. Med. Biol. 2014, 41, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Sharma, R.; Sarma, H.D.; Dash, A. Comparative evaluation of 68 Ga-labeled NODAGA, DOTAGA, and HBED-CC-conjugated cNGR peptide chelates as tumor-targeted molecular imaging probes. Chem. Biol. Drug. Des. 2018, 91, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, W.; Zhang, M.; Li, G.; Wang, S.; Wang, Z.; Ma, X.; Kang, F.; Wang, J. Evaluation of 68Ga-labeled iNGR peptide with tumor-penetrating motif for microPET imaging of CD13-positive tumor xenografts. Tumour. Biol. 2016, 37, 12123–12131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Képes, Z.; Arató, V.; Szabó, J.P.; Gyuricza, B.; Szücs, D.; Hajdu, I.; Fekete, A.; Bruchertseifer, F.; Szikra, D.; Trencsényi, G. Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology. Pharmaceutics 2023, 15, 491. https://doi.org/10.3390/pharmaceutics15020491
Képes Z, Arató V, Szabó JP, Gyuricza B, Szücs D, Hajdu I, Fekete A, Bruchertseifer F, Szikra D, Trencsényi G. Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology. Pharmaceutics. 2023; 15(2):491. https://doi.org/10.3390/pharmaceutics15020491
Chicago/Turabian StyleKépes, Zita, Viktória Arató, Judit P. Szabó, Barbara Gyuricza, Dániel Szücs, István Hajdu, Anikó Fekete, Frank Bruchertseifer, Dezső Szikra, and György Trencsényi. 2023. "Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology" Pharmaceutics 15, no. 2: 491. https://doi.org/10.3390/pharmaceutics15020491
APA StyleKépes, Z., Arató, V., Szabó, J. P., Gyuricza, B., Szücs, D., Hajdu, I., Fekete, A., Bruchertseifer, F., Szikra, D., & Trencsényi, G. (2023). Therapeutic Performance Evaluation of 213Bi-Labelled Aminopeptidase N (APN/CD13)-Affine NGR-Motif ([213Bi]Bi-DOTAGA-cKNGRE) in Experimental Tumour Model: A Treasured Tailor for Oncology. Pharmaceutics, 15(2), 491. https://doi.org/10.3390/pharmaceutics15020491






