2.1.2. Synthetic Procedures
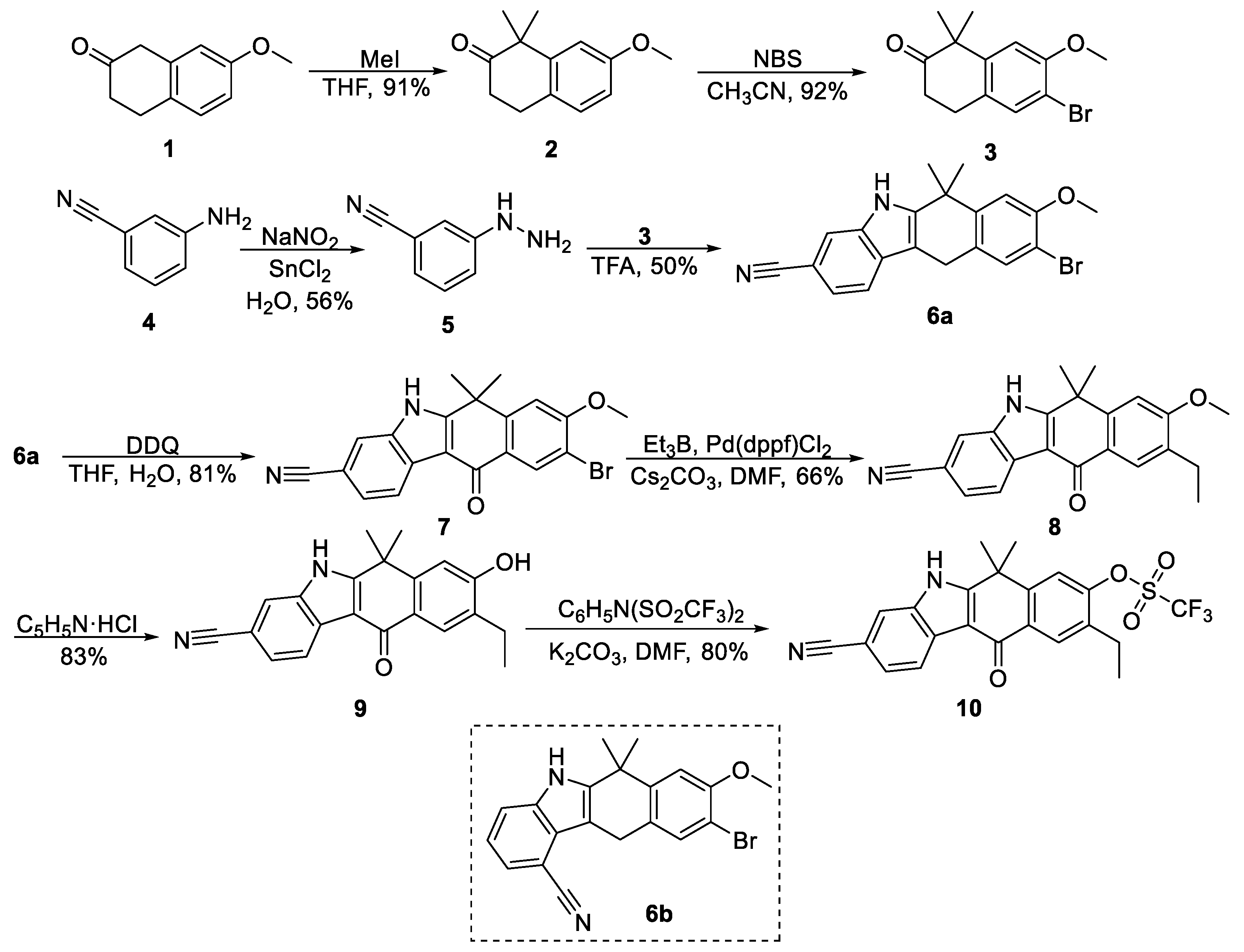
7-Methoxy-1,1-dimethyl-3,4-dihydronaphthalen-2(1
H)-one,
2. To a solution of 7-methoxy-2-tetralone (5 g, 28.5 mmol) in THF (20 mL) were added tetrabutylammonium bisulfate (1.53 g, 4.5 mmol), methyl iodide (5.25 mL, 85.5 mmol), and 50% aqueous KOH solution (32.5 mL) and stirred at room temperature for 2 h. The mixture was diluted with EA and washed with water and brine. The organic layer was dried over Na
2SO
4 and concentrated under reduced pressure. The residue was purified by flash column chromatography (gradient elution PS–EA, 15:1 to 10:1) to afford the desired product,
2 (5.3 g, 91%) as a white solid. The spectral data were in accordance with those reported in the literature [
29].
2:
1H NMR (500 MHz, CDCl
3) δ 7.09 (d,
J = 8.3 Hz, 1H), 6.88 (d,
J = 2.5 Hz, 1H), 6.74 (dd,
J = 8.3, 2.5 Hz, 1H), 3.81 (s, 3H), 3.03 (t,
J = 6.9 Hz, 2H), 2.66 (t,
J = 6.9 Hz, 2H), 1.42 (s, 6H).
13C NMR (126 MHz, CDCl
3) δ 214.7, 158.8, 145.0, 129.1, 127.5, 112.3, 111.4, 55.4, 48.0, 37.6, 27.8, 26.9. ESI-MS, positive mode:
m/z calcd mass for C
13H
16O
2Na [M + Na]
+ = 227.10, was found 227.05.
6-Bromo-7-methoxy-1,1-dimethyl-3,4-dihydronaphthalen-2(1
H)-one,
3. To a solution of
2 (2.00 g, 9.8 mmol) in CH
3CN (40 mL) was added
N-bromosuccinimide (1.92 g, 10.8 mmol). After stirring for 2.5 h at room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by column chromatography (gradient elution PS–EA, 15:1 to 13:1) to yield
3 (2.55 g, 92%) as a white solid.
1H-NMR data were in accordance with those reported in the literature [
30].
3:
1H NMR (500 MHz, CDCl
3) δ 7.35 (s, 1H), 6.83 (s, 1H), 3.90 (s, 3H), 3.01 (t,
J = 6.9 Hz, 2H), 2.65 (t,
J = 6.9 Hz, 2H), 1.43 (s, 6H).
13C NMR (126 MHz, CDCl
3) δ 213.8, 155.1, 144.2, 132.7, 129.0, 110.0, 109.7, 56.5, 48.0, 37.2, 27.5, 26.9.
3-Hydrazinylbenzonitrile,
5. 4-Cyanoaniline (472 mg, 4 mmol) was added to a round-bottomed flask containing 10 mL 6N HCl. The solution was vigorously stirred at 0 °C for 10 min. Sodium nitrite (280 mg, 4 mmol) dissolved in 2 mL water was added dropwise to the aniline solution, and the resulting mixture was stirred at 0 °C for an additional 1 h. To a beaker containing SnCl
2 (1.9 g, 10 mmol) was added 10 mL of concentrated HCl, and the solution was sonicated and cooled to 0 °C. The SnCl
2 solution was then added dropwise to the diazonium salt solution, and the mixture was stirred for additional 90 min at room temperature. Then, sodium hydroxide (7.2 g, 0.18 mol) dissolved in 20 mL water was added to quench the reaction. The solution was extracted with 20 mL of diethyl ether three times, and the organic layers were combined and concentrated under reduced pressure to give the crude product,
5 (300 mg, 56%), which was further purified by recrystallization in hot ethanol. The spectral data were in accordance with those reported in the literature [
31].
5:
1H NMR (500 MHz, DMSO-d
6) δ 7.27–7.20 (m, 2H), 7.08 (d,
J = 1.1 Hz, 1H), 7.01 (dd, J = 8.4, 1.1 Hz, 1H), 6.93 (d,
J = 7.4 Hz, 1H), 4.12 (s, 2H).
13C NMR (126 MHz, DMSO-
d6) δ 152.9, 129.7, 119.7, 119.4, 116.1, 113.2, 111.4.
9-Bromo-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, 6a. A mixture of 3 (2.8 g, 9.89 mmol), 5 (1.58 g, 11.9 mmol), and trifluoroacetic acid (48 mL) was refluxed at 80 °C for 2 h. The reaction mixture was cooled and neutralized by saturated aqueous Na2CO3, then by NaHCO3 and extracted with EA. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was resuspended in EA and precipitated solid (undesired regioisomer) was filtered off. The filtrate was evaporated under reduced pressure and purified by column chromatography (isocratic elution PS–EA 4:1) to yield 6a as an orange solid (1.9 g, 50%). 6a: 1H NMR (500 MHz, acetone-d6) δ 10.79 (s, 1H), 7.75 (s, 1H), 7.67 (d, J = 8.1 Hz, 1H), 7.55 (s, 1H), 7.34 (dd, J = 8.2, 1.2 Hz, 1H), 7.31 (s, 1H), 4.08 (s, 2H), 3.96 (s, 3H), 1.77 (s, 6H). 13C NMR (126 MHz, acetone-d6) δ 155.5, 145.4, 144.8, 136.6, 134.2, 131.2, 130.6, 127.7, 122.6, 119.8, 116.2, 111.1, 110.1, 106.8, 104.1, 56.7, 37.1, 31.1, 25.9. ESI-MS, negative mode: m/z calcd mass for C20H16BrN2O [M − H]− = 379.04, was found 380.85.
9-Bromo-8-methoxy-6,6-dimethyl-6,11-dihydro-5H-benzo[b]carbazole-1-carbonitrile, 6b: 1H NMR (500 MHz, DMSO) δ 11.68 (s, 1H), 7.68 (d, J = 7.9 Hz, 1H), 7.54 (s, 1H), 7.46 (d, J = 7.1 Hz, 1H), 7.26 (s, 1H), 7.21 (t, J = 7.6 Hz, 1H), 4.23 (s, 2H), 3.91 (s, 3H), 1.70 (s, 6H). 13C NMR (126 MHz, DMSO) δ 154.1, 143.7, 143.6, 136.4, 133.0, 126.3, 125.7, 124.7, 120.8, 119.4, 116.2, 110.4, 108.9, 103.2, 99.8, 56.4, 35.9, 30.8, 25.3.
9-Bromo-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5
H-benzo[
b]carbazole-3-carbonitrile,
7. To a solution of
6a (2.1 g, 4.96 mmol) in THF (21.4 mL) and water (2.14 mL) was gradually added DDQ (3.38 g, 14.9 mmol). After stirring for 3 h at 0 °C, the reaction mixture was evaporated under reduced pressure and the residue was dissolved in EA. The solution was washed with aqueous NaOH, water, and brine, dried over Na
2SO
4, filtered, and concentrated under reduced pressure. The residue was purified with column chromatography (gradient elution PS–EA 3:1 to 1:2) to afford
7 (1.58 g, 81%) as yellow solid.
1H-NMR data were in accordance with those reported in the literature [
31].
7:
1H NMR (500 MHz, acetone-
d6) δ 8.42 (s, 1H), 8.41 (s, 1H), 7.92 (d,
J = 0.6 Hz, 1H), 7.57 (dd,
J = 8.1, 1.4 Hz, 1H), 7.50 (s, 1H), 4.09 (s, 3H), 1.91 (s, 6H).
13C NMR (126 MHz, acetone-
d6) δ 178.6, 160.2, 159.9, 150.7, 136.9, 131.8, 128.9, 127.3, 125.7, 123.0, 120.4, 117.0, 111.4, 110.8, 110.6, 106.8, 57.2, 38.1, 30.4. ESI-MS, negative mode:
m/z calcd mass for C
20H
14BrN
2O
2 [M − H]
− = 393.02, was found 394.85.
9-Ethyl-8-methoxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, 8. To a degassed mixture of 7 (1.52 g, 3.85 mmol), Cs2CO3 (3.76 g, 11.5 mmol), Pd(dppf)Cl2 (57.7 mg, 0.08 mmol) and DMF (55.7 mL) in a Schlenk flask under argon atmosphere was added a solution of triethylborane in THF (11.5 mL, 1M solution, 11.5 mmol) and the reaction was stirred at 86 °C overnight. The reaction mixture was then cooled, diluted with EA, washed several times with water, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography (gradient elution PS–EA 2:1 to 1:2) to yield 8 (870 mg, 66%) as yellow crystalline solid. 8: 1H NMR (500 MHz, acetone-d6) δ 11.69 (s, 1H), 8.47 (d, J = 8.1 Hz, 1H), 8.12 (s, 1H), 7.90 (s, 1H), 7.57 (dd, J = 8.1, 0.5 Hz, 1H), 7.34 (s, 1H), 4.02 (s, 3H), 2.71 (q, J = 7.5 Hz, 2H), 1.88 (s, 6H), 1.24 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz, acetone-d6) δ 180.2, 161.9, 160.2, 149.1, 136.8, 132.2, 129.1, 127.4, 125.7, 125.5, 123.0, 120.6, 116.8, 111.1, 108.3, 106.4, 56.2, 37.8, 30.7, 23.6, 14.3. ESI-MS, positive mode: m/z calcd mass for C22H20N2O2 [M] + = 344.15, was found 344.95.
9-Ethyl-8-hydroxy-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, 9. Pyridine (141 μL, 1.74 mmol) was slowly added to a hydrochloric acid solution in 1,4-dioxane (436 μL, 4 M, 1.74 mmol) at 0 °C under argon. The mixture was stirred for 5 min and then concentrated under reduced pressure to form white highly hygroscopic pyridinium chloride salt. Both 8 (12 mg, 34.8 μmol) and pyridinium chloride (1.74 mmol) were quickly transferred to a sealed tube and stirred at 170 °C overnight. The reaction mixture was dissolved in water, neutralized by saturated aqueous NaHCO3, and extracted with EA three times. The combined organic layers were washed with aqueous NaHCO3 and brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (gradient elution of PS–EA 2:1 to 1:2) to yield 9 as a beige solid (9.5 mg, 83%). 9: 1H NMR (500 MHz, DMSO-d6) δ 12.67 (s, 1H), 10.20 (s, 1H), 8.32 (dd, J = 8.1, 0.4 Hz, 1H), 7.98 (d, J = 0.6 Hz, 1H), 7.94 (s, 1H), 7.59 (dd, J = 8.1, 1.4 Hz, 1H), 7.10 (s, 1H), 2.62 (q, J = 7.5 Hz, 2H), 1.70 (s, 6H), 1.19 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 179.2, 159.4, 159.3, 147.9, 135.6, 129.4, 127.8, 126.8, 124.7, 123.3, 121.7, 120.2, 116.4, 111.8, 109.4, 104.4, 36.2, 30.3, 22.5, 13.9. ESI-MS, positive mode: m/z calcd mass for C21H18N2O2 [M + H] + = 330.14, was found 330.90.
3-Cyano-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl trifluoromethanesulfonate, 10. A suspension of 9 (480 mg, 1.45 mmol) and K2CO3 (402 mg, 2.91 mmol) in DMF (13.7 mL) was slowly (portionwise) treated with N-phenyl-bis(trifluoromethanesulfonimide) (1.04 g, 2.91 mmol) at 0 °C. After 24 h, the reaction mixture was diluted with EA and poured into water. The layers were partitioned, and the organic phase washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel chromatography (gradient elution PS–EA 3:1 to 1:1) to afford 10 (540 mg, 80% yield) as a yellow solid. 10: 1H NMR (500 MHz, acetone-d6) δ 11.87 (s, 1H), 8.44 (d, J = 8.1 Hz, 1H), 8.37 (s, 1H), 7.94 (d, J = 1.3 Hz, 1H), 7.84 (s, 1H), 7.60 (dd, J = 8.1, 1.3 Hz, 1H), 2.89 (q, J = 7.6 Hz, 2H), 1.91 (s, 6H), 1.36 (t, J = 7.6 Hz, 3H). 13C NMR (126 MHz, acetone-d6) δ 178.8, 160.3, 151.5, 149.1, 137.1, 136.3, 132.9, 129.5, 128.9, 126.1, 123.2, 120.7, 120.4, 119.7, 117.2, 111.2, 107.2, 37.9, 30.4, 23.4, 14.2. ESI-MS, positive mode: m/z calcd mass for C22H17F3N2O4S [M] + = 462.08, was found 462.95.
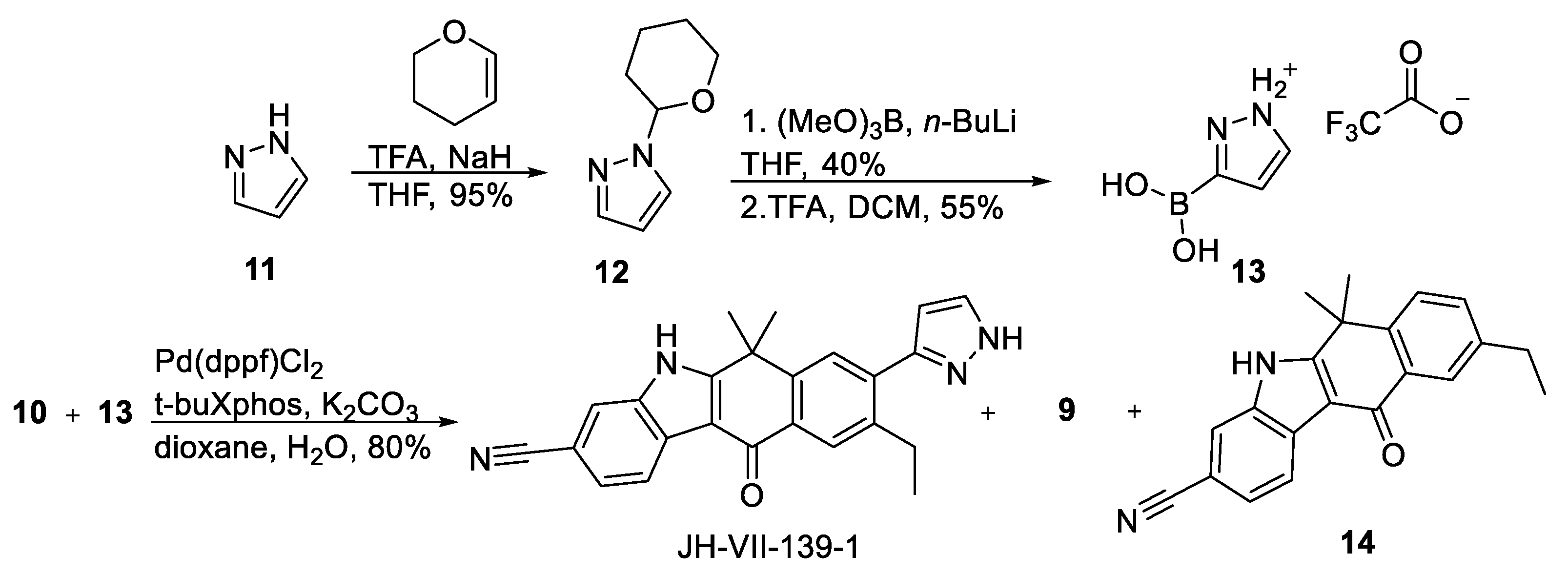
1-(Tetrahydro-2
H-pyran-2-yl)-1
H-pyrazole,
12. A mixture of pyrazole (1.43 g, 0.21 mol), 3,4-dihydro-2
H-pyran (29 mL, 0.32 mol), and trifluoroacetic acid (80 μL, 1 mmol) was refluxed for 2 h. After quenching with sodium hydride (30 μg, 1.26 mmol), the mixture was subjected to column chromatography (gradient elution PS–EA 20:1 to 5:1) to yield
12 (3.2 g, 95%) as a colorless oil. The spectral data were in accordance with those reported in the literature [
32].
12:
1H NMR (500 MHz, CDCl
3) δ 7.61 (d,
J = 2.4 Hz, 1H), 7.56 (d,
J = 1.4 Hz, 1H), 6.32–6.29 (m, 1H), 5.40 (dd,
J = 9.7, 2.5 Hz, 1H), 4.06 (dd,
J = 12.2, 2.6 Hz, 1H), 3.73–3.67 (m, 1H), 2.23–2.05 (m, 3H), 1.77–1.55 (m, 3H).
(1-(Tetrahydro-2H-pyran-2-yl)-1H-pyrazol-3-yl)boronic acid, S1. To a solution of 1-(tetrahydropyran-2-yl)-1H-pyrazole (7.64 g, 0.052 mol) in THF (50 mL), cooled to −78 °C, was added n-butyllithium (1.6 M in hexane, 32 mL, 0.052 mol) dropwise. Bulky precipitate formed and the mixture turned yellow. After 30 min, trimethyl borate (5.88 mL, 0.058 mol) was added over 10 min, and the reaction mixture was stirred at −78 °C for 1 h. The reaction was quenched with hydrochloric acid (2 M in water, 0.104 mol, 52 mL) and was left to return to ambient temperature in 1 h. The mixture was extracted with EA and the organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was crystallized with PS–EA (12:1) to yield S1 (3.94 g, 40%) as a white solid. S1 was used to the next step without further purification.
3-Borono-1H-pyrazol-1-ium trifluoroacetate,13. To a solution of
S1 (110 mg, 0.56 mmol) in dichloromethane (1 mL), trifluoroacetic acid (0.5 mL) was added at room temperature, and the reaction solution was stirred for 24 h. The reaction solution was concentrated, and the residue was crystallized with PS–DCM–dioxane (5:10:1) to afford
13 (70 mg 55%) as white powder. The spectral data were in accordance with those reported in the literature [
33].
13: 1H NMR (500 MHz, DMSO-d
6) δ 8.28 (s, 2H), 7.52 (d,
J = 1.6 Hz, 1H), 6.70 (d,
J = 1.7 Hz, 1H).
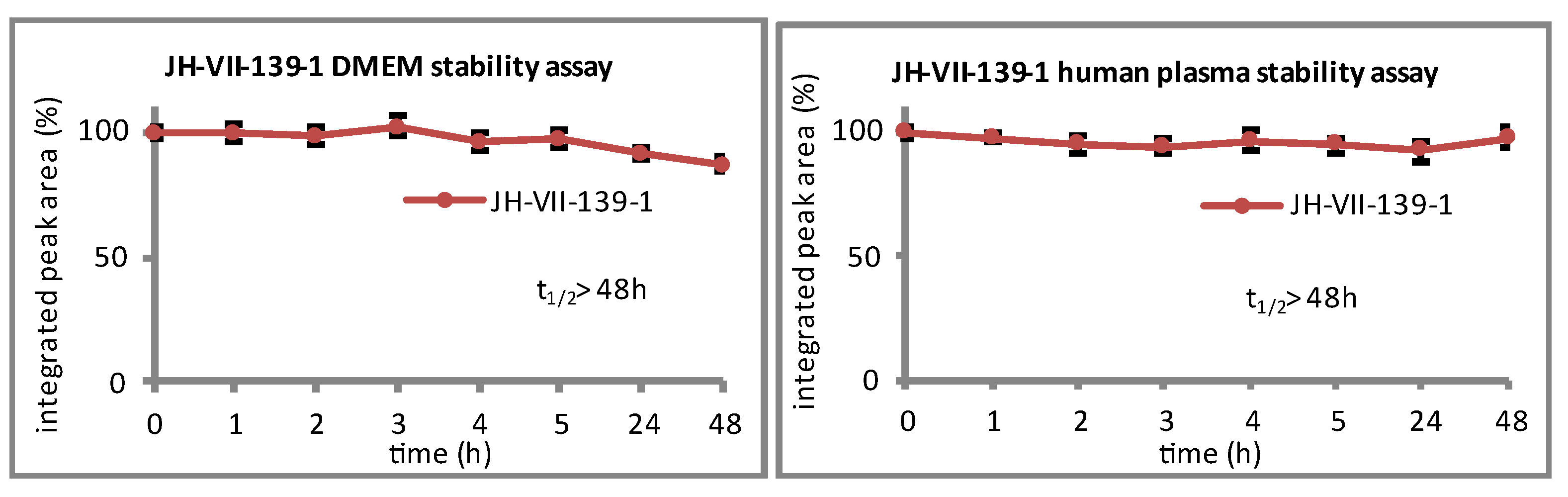
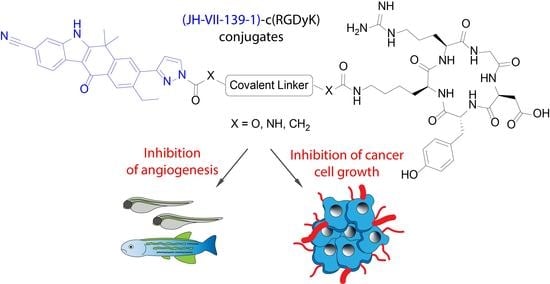
9-Ethyl-6,6-dimethyl-11-oxo-8-(1H-pyrazol-3-yl)-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile, JH-VII-139-1. A degassed mixture of 10 (76 mg, 0.164 mmol), K2CO3 (182 mg, 1.31 mmol), Pd(dppf)Cl2 (6 mg, 0.008 mmol), 13 (85 mg, 0.378 mmol), tBuXPhos (5.6 mg, 0.013 mmol), dioxane (5.06 mL) and H2O (1.26 mL) was stirred in a microwave tube at 70 °C in microwave for two hours. The reaction mixture was then cooled, diluted with EA, washed three times with water, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (gradient elution PS–EA 1:1, 1:2, 1:4) to yield JH-VII-139-1 (50 mg, 80%) as brown crystalline solid. JH-VII-139-1: 1H NMR (500 MHz, DMSO-d6) δ 13.08 (s, 1H), 8.35 (d, J = 8.1 Hz, 1H), 8.13 (s, 1H), 8.03 (s, 1H), 7.90 (s, 1H), 7.83 (s, 1H), 7.63 (d, J = 8.1 Hz, 1H), 6.65 (s, 1H), 2.94 (m, 2H), 1.80 (s, 6H), 1.19 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 180.3, 160.6, 149.2 146.2, 141.7, 137.5, 136.9, 132.4, 131.8, 129.1, 128.4, 127.6, 125.7, 123.1, 120.5, 117.0, 111.4, 106.7, 106.1, 37.4, 30.3, 27.2, 15.8. ESI-MS, negative mode: m/z calcd mass for C24H19N4O [M − H]− = 379.15, was found 379.00.
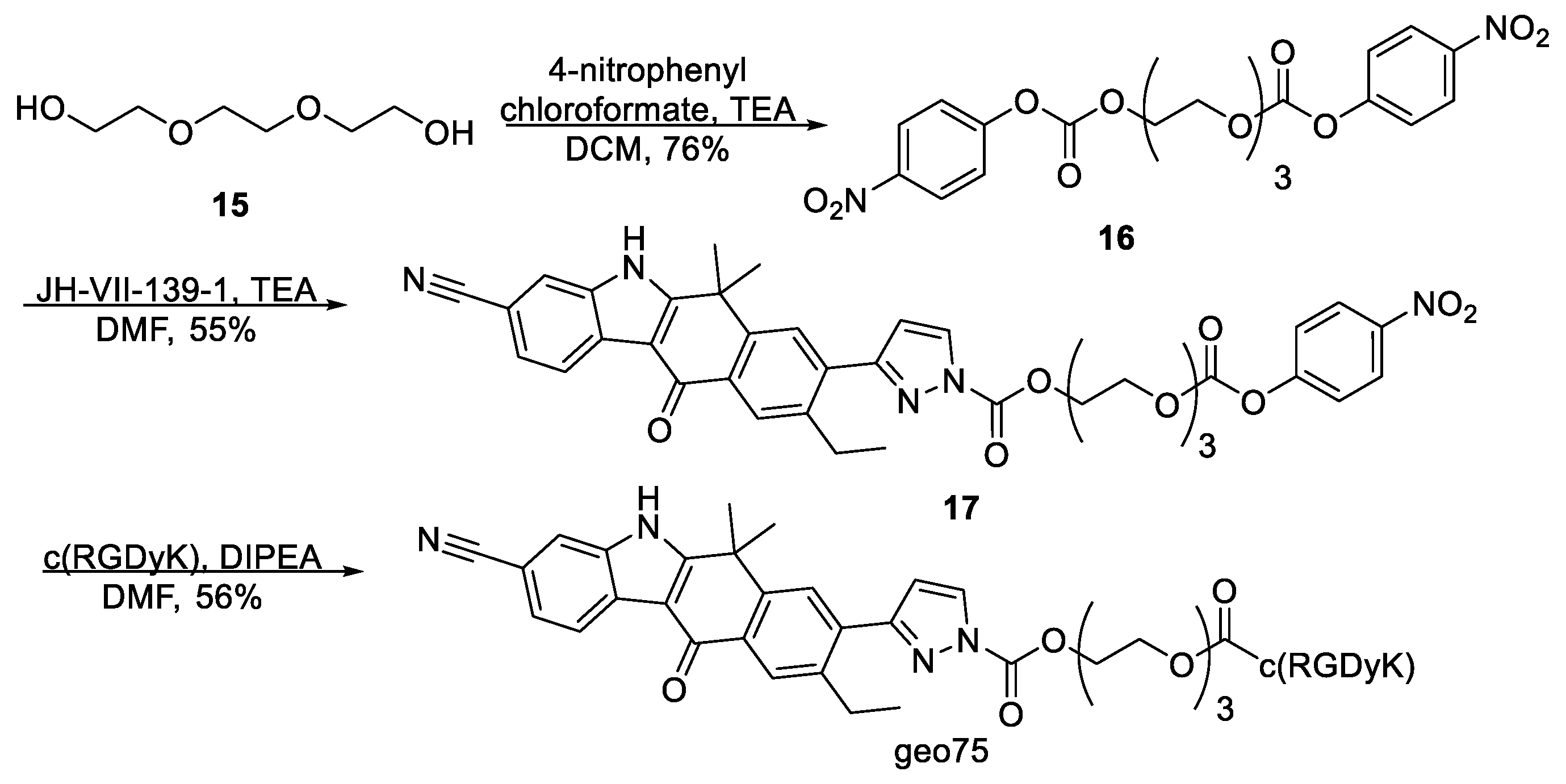
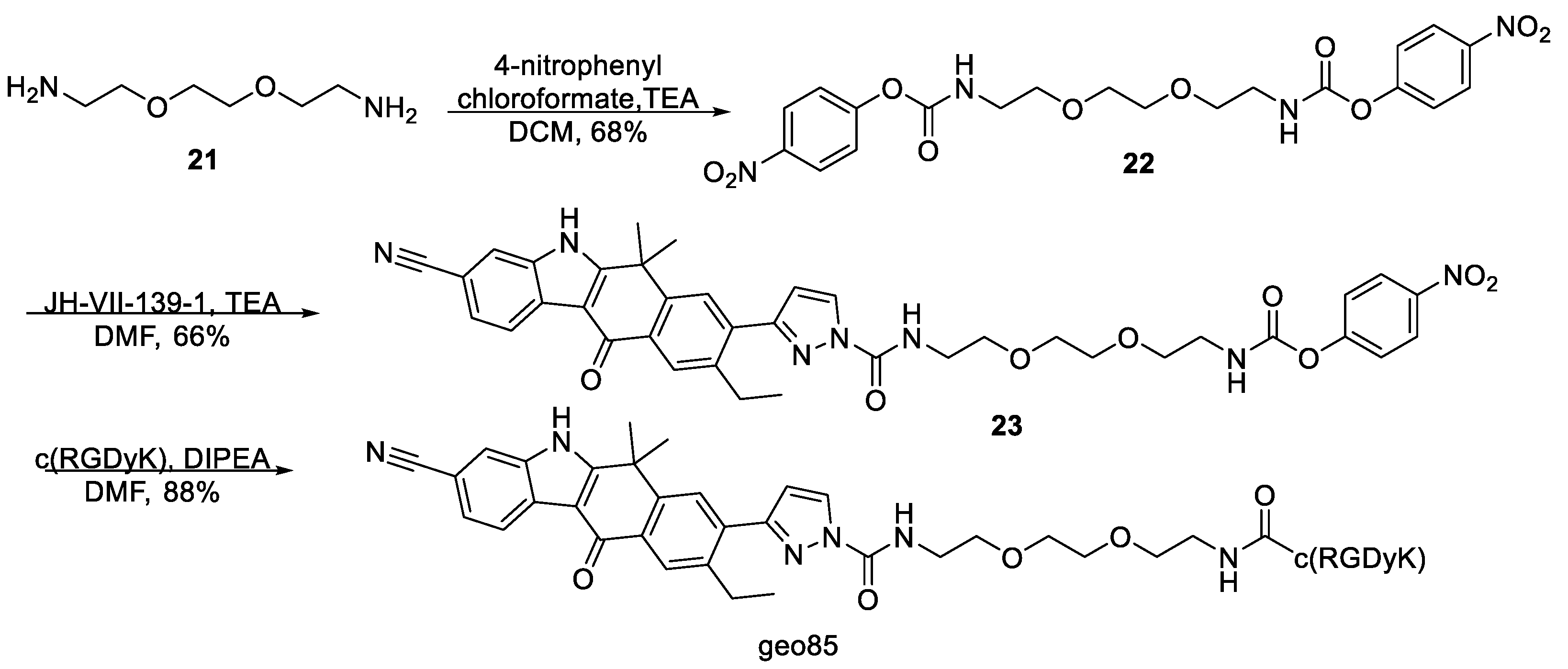
(Ethane-1,2-diylbis(oxy))bis(ethane-2,1-diyl) bis(4-nitrophenyl) dicarbonate,
16. To a stirred solution of triethyleneglycol (62 mg, 0.412 μmol) in DCM (1.3 mL) and triethylamine (344 μL, 2.47 mmol), at 0 °C under argon, was added 4-nitrophenyl chloroformate (250 mg, 1.23 mmol). As the solution turned yellow, the mixture was stirred at room temperature for 2 h. The solvent was removed under reduced pressure, and the residue was chromatographed on silica gel with PS–EA (4:1, 3:1, 2:1, 1:1, EA) to afford
15 (198 mg, 76%) as a colorless oil. The spectral data were in accordance with those reported in the literature [
34].
16: 1H NMR (500 MHz, CDCl
3) δ 8.27 (d,
J = 9.0 Hz, 4H), 7.38 (d,
J = 9.0 Hz, 4H), 4.45 (dd,
J = 5.4, 3.8 Hz, 4H), 3.83 (dd,
J = 5.3, 4.0 Hz, 4H), 3.74 (s, 4H).
2-(2-(2-(((4-Nitrophenoxy)carbonyl)oxy)ethoxy)ethoxy)ethyl 3-(3-cyano-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-1H-pyrazole-1-carboxylate, 17. To a stirred solution of (ethane-1,2-diylbis(oxy))bis(ethane-2,1-diyl) bis(4-nitrophenyl) dicarbonate 16 (53 mg, 0.11 mmol) and triethylamine (31 μL, 0.22 mmol) in dry DMF (1.2 mL) was added dropwise a solution of JH-VII-139-1 (12 mg, 31.5 μmol) in DMF (1.2 mL) at 0 °C under argon. The reaction mixture was stirred at room temperature for 24 h and then, the solvent was evaporated. The residue was chromatographed on silica gel with PS–EA (gradient elution 1:1, 1:2, 1:3, 1:4) to give 17 (22 mg, 55% yield) as a yellow solid. 17: 1H NMR (500 MHz, CDCl3) δ 9.52 (s, 1H), 8.54 (d, J = 8.1 Hz, 1H), 8.35 (s, 1H), 8.29–8.23 (m, 3H), 7.77 (s, 1H), 7.72 (s, 1H), 7.58 (dd, J = 8.2, 1.2 Hz, 1H), 7.40–7.35 (m, 2H), 6.65 (d, J = 2.8 Hz, 1H), 4.68–4.65 (m, 2H), 4.44–4.41 (m, 2H), 3.93–3.90 (m, 2H), 3.83–3.80 (m, 2H), 3.76–3.70 (m, 4H), 2.87 (q, J = 7.6 Hz, 2H), 1.75 (s, 6H), 1.24 (t, J = 7.6 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 180.4, 159.0, 156.2, 155.6, 152.6, 149.5, 145.6, 144.7, 142.0, 135.8, 135.5, 132.1, 131.9, 128.5, 127.7, 127.4, 125.9, 125.5, 123.1, 121.9, 120.2, 115.8, 111.5, 110.5, 106.5, 70.9, 68.9, 68.9, 68.4, 67.5, 36.5, 30.7, 26.5, 15.5. ESI-MS, negative mode: m/z calcd mass for C38H38N7O8 [M] + = 721.24, was found 719.95.
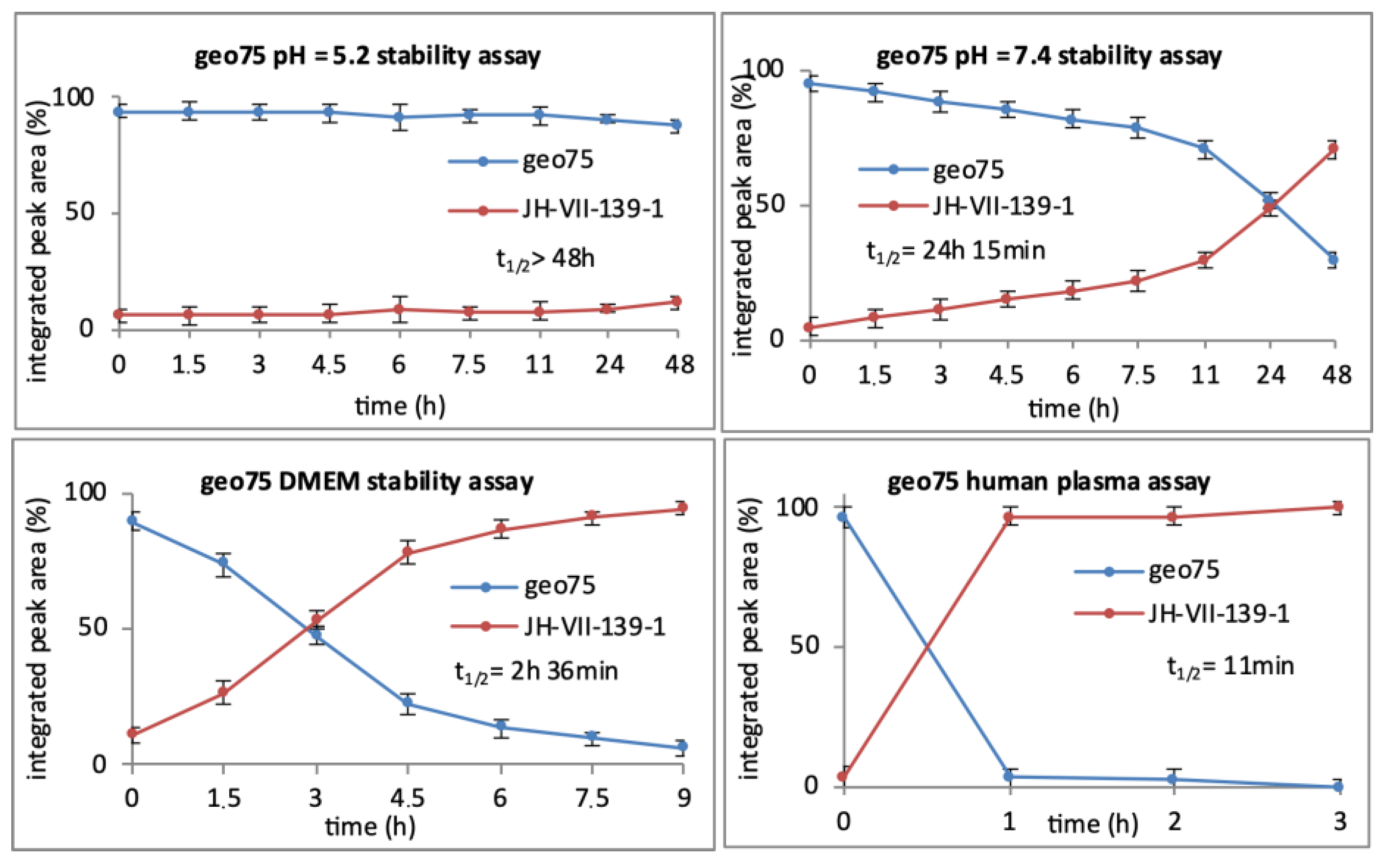
Conjugate
geo75. To a stirred solution of
17 (3.48 mg, 4.83 μmol) and c(RGDyK) (2.3 mg, 3.71 μmol) in dry DMF (1.15 mL), under argon, was added DIPEA (2.59 μL, 14.8 μmol), and the mixture was stirred at room temperature for 24 h. The solvent was evaporated, and the product was purified by HPLC (method 1,
Supplementary Materials) to yield
geo75 as white solid (2.5 mg, 56%).
geo75:
1H NMR (500 MHz, DMSO-
d6) δ 13.03 (s, 1H), 9.09 (s, 1H), 8.47 (d,
J = 2.7 Hz, 1H), 8.33 (d,
J = 8.1 Hz, 2H), 8.27 (s, 1H), 8.20 (d,
J = 9.3 Hz, 2H), 8.16 (s, 2H), 8.12 (d,
J = 9.2 Hz, 1H), 8.01 (s, 1H), 7.91 (s, 1H), 7.66–7.59 (m, 1H), 7.13 (s, 3H), 7.01 (d,
J = 2.7 Hz, 1H), 6.92 (d,
J = 8.4 Hz, 2H), 6.58 (d,
J = 8.4 Hz, 2H), 4.54 (m, 2H), 4.47 (s, 1H), 4.23 (m, 1H), 4.13 (m, 1H), 3.98 (s, 2H), 3.80–3.76 (m, 2H), 3.62–3.57 (m, 2H), 3.53 (s, 4H), 3.16 (m, 3H), 3.04 (dd,
J = 12.3, 6.2 Hz, 2H), 2.95–2.85 (m, 4H), 2.70 (m, 1H), 2.50 (m, 2H)2.11 (m, 1H), 1.78 (s, 6H), 1.62 (m, 3H), 1.43 (s, 4H), 1.30 (d,
J = 7.1 Hz, 2H), 1.16 (t,
J = 7.5 Hz, 3H), 1.10 (m, 1H).
13C NMR (126 MHz, DMSO-d
6) δ 178.9, 173.5, 172.0, 170.9, 170.8, 170.6, 167.8, 160.4, 156.8, 156.1, 155.5, 155.0, 148.8, 145.6, 140.9, 135.9, 135.0, 132.4, 131.3, 129.8, 128.0, 127.9, 127.7, 127.4, 126.3, 125.0, 121.6, 120.0, 116.6, 114.8, 110.5, 109.5, 104.8, 69.9, 69.7, 69.6, 68.9, 68.0, 67.4, 63.0, 54.9, 53.3, 50.8, 48.6, 40.6, 38.9, 36.4, 31.8, 29.9, 29.0, 28.8, 27.3, 26.0, 24.5, 22.9, 15.3. ESI-MS, positive mode:
m/z calcd mass for C
59H
71N
13O
15 [M + H]
+ = 1202.52, was found 1202.30.
Bis(2,5-dioxopyrrolidin-1-yl) glutarate,
19 was synthesized and characterized by our group in a previous work [
26].
2,5-Dioxopyrrolidin-1-yl 5-(3-(3-cyano-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-1H-pyrazol-1-yl)-5-oxopentanoate, 20. To a stirred solution of bis(2,5-dioxopyrrolidin-1-yl) glutarate 19 (18.9 mg, 57.8 μmol) and JH-VII-139-1 (11 mg, 28.9 μmol) in DMF (0.5 mL) was added triethylamine (16.6 μL, 116 μL) under argon. The reaction was then stirred at room temperature for 48 h. The solvent was removed under vacuum and the residue was chromatographed on silica gel with DCM–Acetone (gradient elution 4:0.1 to 4:0.3) to give 20 (8 mg, 47%) as white solid. 20: 1H NMR (500 MHz, DMSO-d6) δ 12.84 (s, 1H), 8.57 (d, J = 2.8 Hz, 1H), 8.34 (d, J = 8.1 Hz, 1H), 8.17 (s, 1H), 8.02 (s, 2H), 7.62 (d, J = 8.2 Hz, 1H), 7.14 (d, J = 2.8 Hz, 1H), 3.32–3.36 (m, 2H), 3.00 (q, J = 7.4 Hz, 2H), 2.87 (t, J = 7.4 Hz, 2H), 2.82 (s, 4H), 2.11–2.04 (m, 2H), 1.81 (s, 6H), 1.23 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 178.7, 171.1, 170.2, 168.7, 154.6, 145.7, 140.9, 134.5, 131.4, 129.4, 128.0, 126.6, 124.8, 121.6, 120.1, 119.5, 118.1, 117.9, 116.7, 110.9, 109.5, 104.5, 36.5, 32.2, 29.9, 29.4, 26.4, 25.4, 19.1, 15.4. ESI-MS, positive mode: m/z calcd mass for C33H30N5O6 [M + H] + = 592.21, was found 592.00.
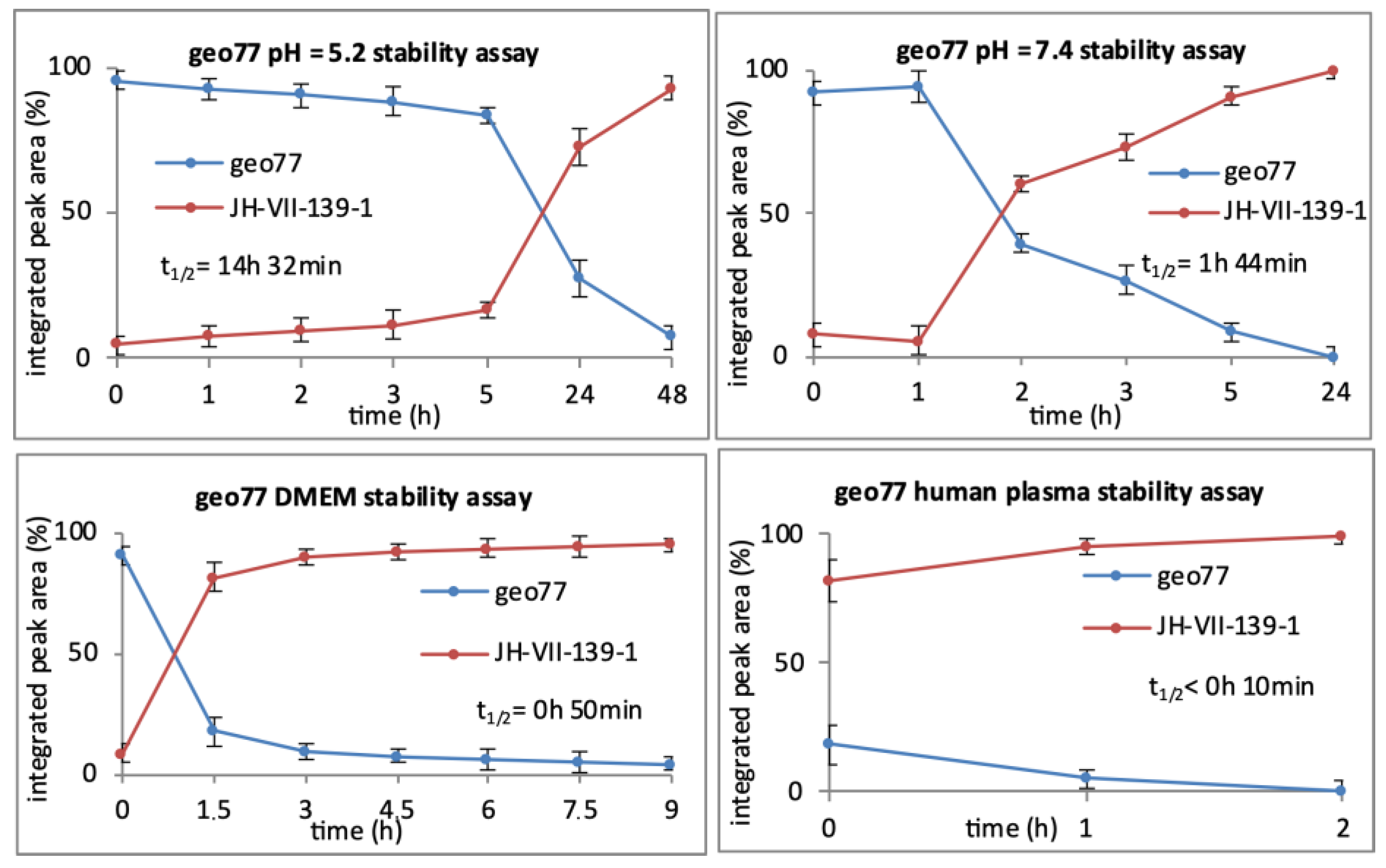
Conjugate
geo77. To a stirred solution of
20 (3.82 mg, 6.46 μmol) and c(RGDyK) (4 mg, 6.46 μmol) in DMF (2 mL), under argon, was added DIPEA (4.5 μL, 25.8 μmol) and the mixture was stirred at room temperature for 24 h. The solvent was evaporated and
geo77 was purified by HPLC (method 1,
Supplementary Materials) to yield
geo77 as a white solid (3.2 mg, 45%).
geo77:
1H NMR (500 MHz, DMSO-
d6) δ 12.97 (s, 1H), 9.11 (s, 1H), 8.54 (d,
J = 2.8 Hz, 1H), 8.40 (d,
J = 8.7 Hz, 1H), 8.35 (d,
J = 8.1 Hz, 1H), 8.20–8.30 (m, 3H), 8.21–8.12 (m, 3H), 8.03 (s, 1H), 8.01 (s, 1H), 7.91 (t,
J = 5.6 Hz, 1H), 7.68–7.59 (m, 2H), 7.00–7.25 (m, 3H), 6.93 (d,
J = 8.5 Hz, 2H), 6.59 (d,
J = 8.4 Hz, 2H), 4.56–4.46 (m, 2H), 4.33–4.26 (m, 1H), 4.23–4.11 (m, 2H), 3.20–3.16 (m, 4H), 3.09–3.03 (m, 2H), 2.95–3.42 (m, 4H), 2.75–2.69 (m, 1H), 2.21 (t,
J = 7.4 Hz, 2H), 2.06–2.13 (m, 1H), 1.98–1.90 (m, 2H), 1.81 (d,
J = 1.7 Hz, 6H), 1.54–1.70 (m, 3H), 1.53–1.42 (m, 2H), 1.41–1.33 (m, 2H), 1.22 (t,
J = 7.5 Hz, 3H), 1.20–1.11 (m, 2H).
13C NMR (126 MHz, DMSO-
d6) δ 178.9, 173.5, 172.0, 171.5, 171.4, 170.9, 170.8, 170.6, 167.7, 160.4, 156.7, 155.5, 154.4, 145.6, 140.9, 135.8, 134.7, 131.2, 129.8, 129.4, 128.3 127.9, 127.6, 126.7, 125.0, 123.9, 121.7, 120.0, 116.5, 114.8, 110.8, 109.5, 104.8, 54.9, 53.3, 50.8, 48.6, 42.9, 40.6, 38.3, 36.4, 35.9, 34.3, 32.9, 31.8, 29.8, 29.0, 28.5, 27.2, 26.5, 24.5, 23.0, 20.2, 15.5. ESI-MS, positive mode:
m/z calcd mass for C
56H
66N
13O
11 [M + H]
+ = 1096.49, was found 1096.00.
Bis(4-nitrophenyl) ((ethane-1,2-diylbis(oxy))bis(ethane-2,1-diyl))dicarbamate, 22. 2,2′-(Ethylenedioxy)bis(ethylamine) (1.00 g, 6.75 mmol) and pyridine (2.18 mL, 27.0 mmol) were dissolved in CH2Cl2 (250 mL). The reaction flask was cooled to 0 °C and then p-nitrophenyl chloroformate (5.44 g, 27.0 mmol) was added. The solution was warmed to room temperature while stirring for 24 h. The reaction was filtered, and the solvent was evaporated leaving a white solid. The crude product was dissolved in CH2Cl2 (150 mL) and washed with aqueous 1 M NaHSO4 (3 × 30 mL). The organic layer was dried with Na2SO4, and the solvent was evaporated. The resulting solid was crystallized twice from CH2Cl2–hexanes (1:1) providing 22 (2.19 g, 68%) as a white solid. 22: 1H NMR (500 MHz, acetone-d6, DMSO-d6) δ 8.27 (d, J = 9.1 Hz, 4H), 7.91 (t, J = 5.3 Hz, 2H), 7.43 (d, J = 9.2 Hz, 4H), 3.63 (s, 4H), 3.60 (t, J = 5.8 Hz, 4H), 3.34 (q, J = 5.8 Hz, 4H). 13C NMR (126 MHz, acetone-d6, DMSO-d6) δ 157.12, 153.93, 144.86, 125.54, 122.86, 70.48, 69.66, 41.28.
4-Nitrophenyl (2-(2-(2-(3-(3-cyano-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-1H-pyrazole-1-carboxamido)ethoxy)ethoxy)ethyl)carbamate, 23. To a stirred solution of bis(4-nitrophenyl) ((ethane-1,2-diylbis(oxy))bis(ethane-2,1-diyl))dicarbamate 22 (30.2 mg, 63.1 μmol) and triethylamine (17.6 μL, 126 μmol) in dry DMF (0.8 mL) was added dropwise a solution of JH-VII-139-1 (1eq) in dry DMF (0.8 μL) at 0 °C under Ar. The reaction was stirred at 25 °C overnight. Subsequently, the solvent was evaporated, and the residue was chromatographed on silica gel with DCM–Acetone (gradient elution 4:0.1, 4:0.2, 4:0.3, 4:0.4, 4:0.6) to give 23 (15 mg, 66%) as a beige solid. 23: 1H NMR (500 MHz, acetone-d6) δ 11.79 (s, 1H), 8.49 (d, J = 8.1 Hz, 1H), 8.39 (d, J = 2.6 Hz, 1H), 8.29 (s, 1H), 8.22 (d, J = 9.1 Hz, 2H), 8.01 (s, 1H), 7.93 (s, 1H), 7.90 (s, 1H), 7.61 (d, J = 8.1 Hz, 1H), 7.37 (d, J = 9.1 Hz, 2H), 6.99 (s, 1H), 6.88 (d, J = 2.6 Hz, 1H), 3.74 (t, J = 5.5 Hz, 2H), 3.69–3.60 (m, 8H), 3.35 (q, J = 5.6 Hz, 2H), 3.02 (q, J = 7.5 Hz, 2H), 1.91 (s, 6H), 1.26 (d, J = 7.5 Hz, 3H). 13C NMR (126 MHz, acetone-d6) δ 178.9, 159.4, 156.3, 153.2, 152.9, 149.2, 145.2, 141.0, 135.8, 135.4, 131.5, 128.9, 127.9, 127.7, 127.6, 126.5, 124.6, 124.5, 121.9, 121.7, 119.3, 115.8, 110.2, 108.7, 105.6, 69.9, 69.0, 40.6, 39.7, 36.3, 29.4, 26.1, 14.6. ESI-MS, positive mode: m/z calcd mass for C38H38N7O8 [M + H] + = 720.27, was found 720.00.
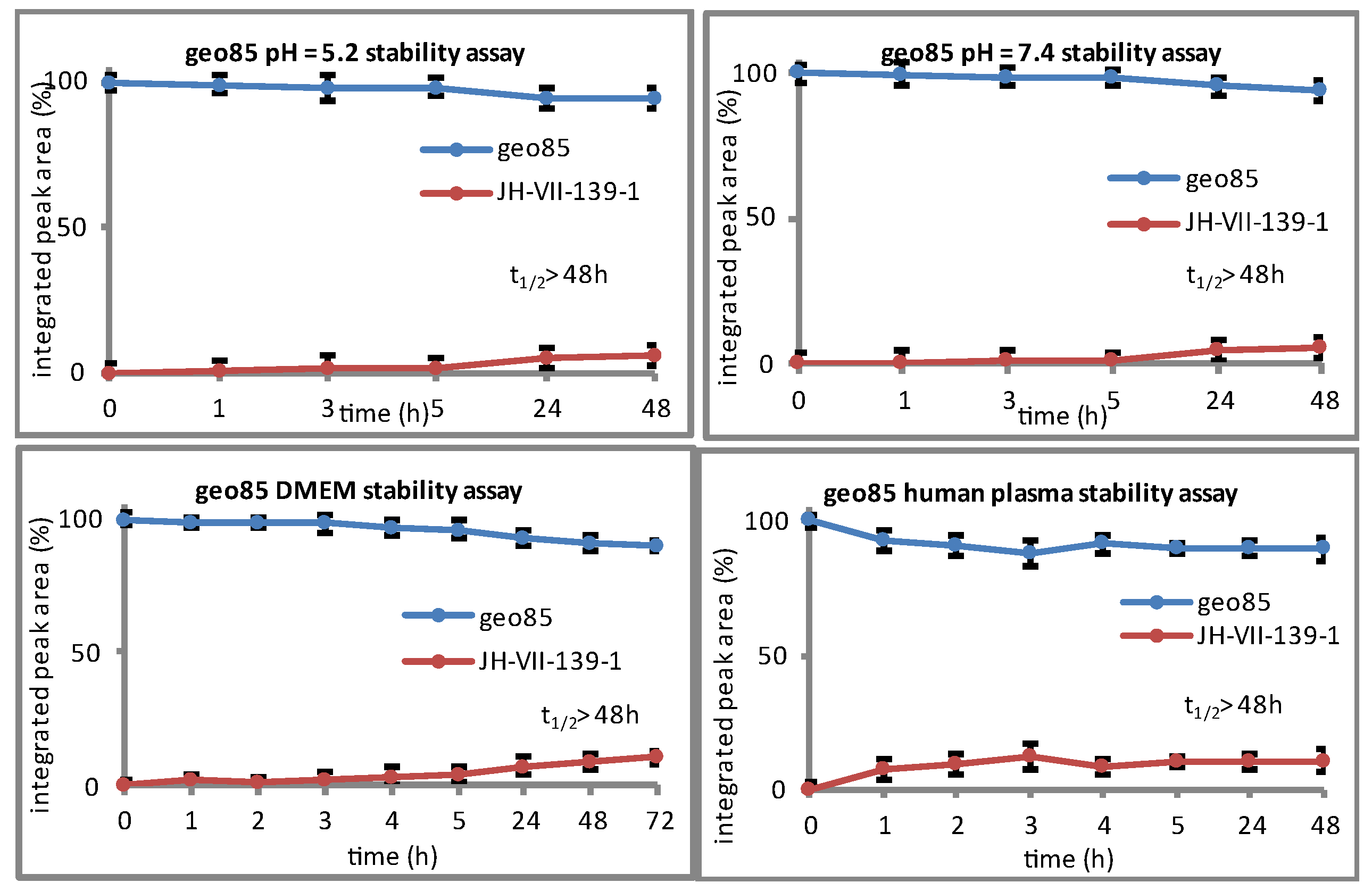
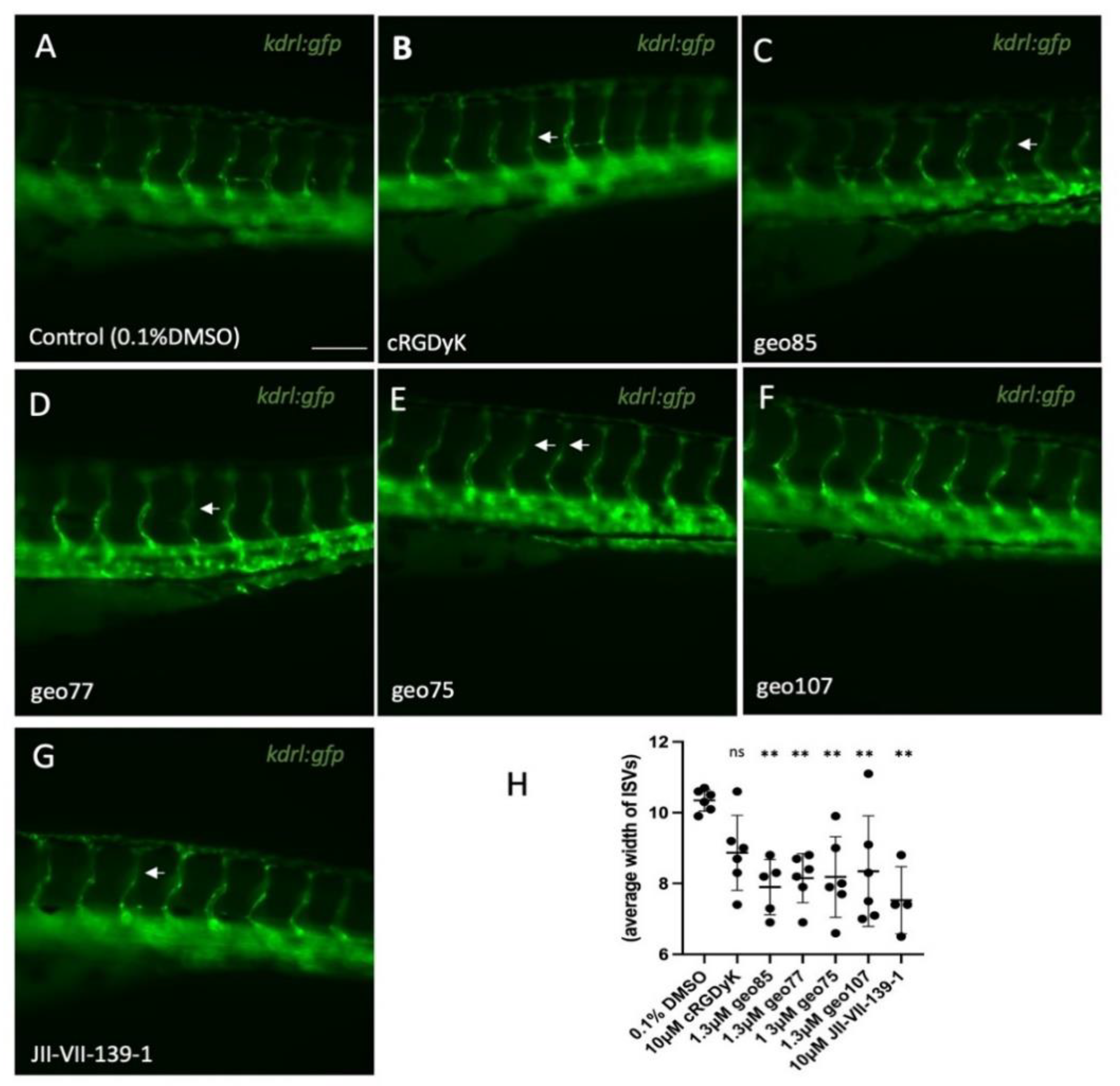
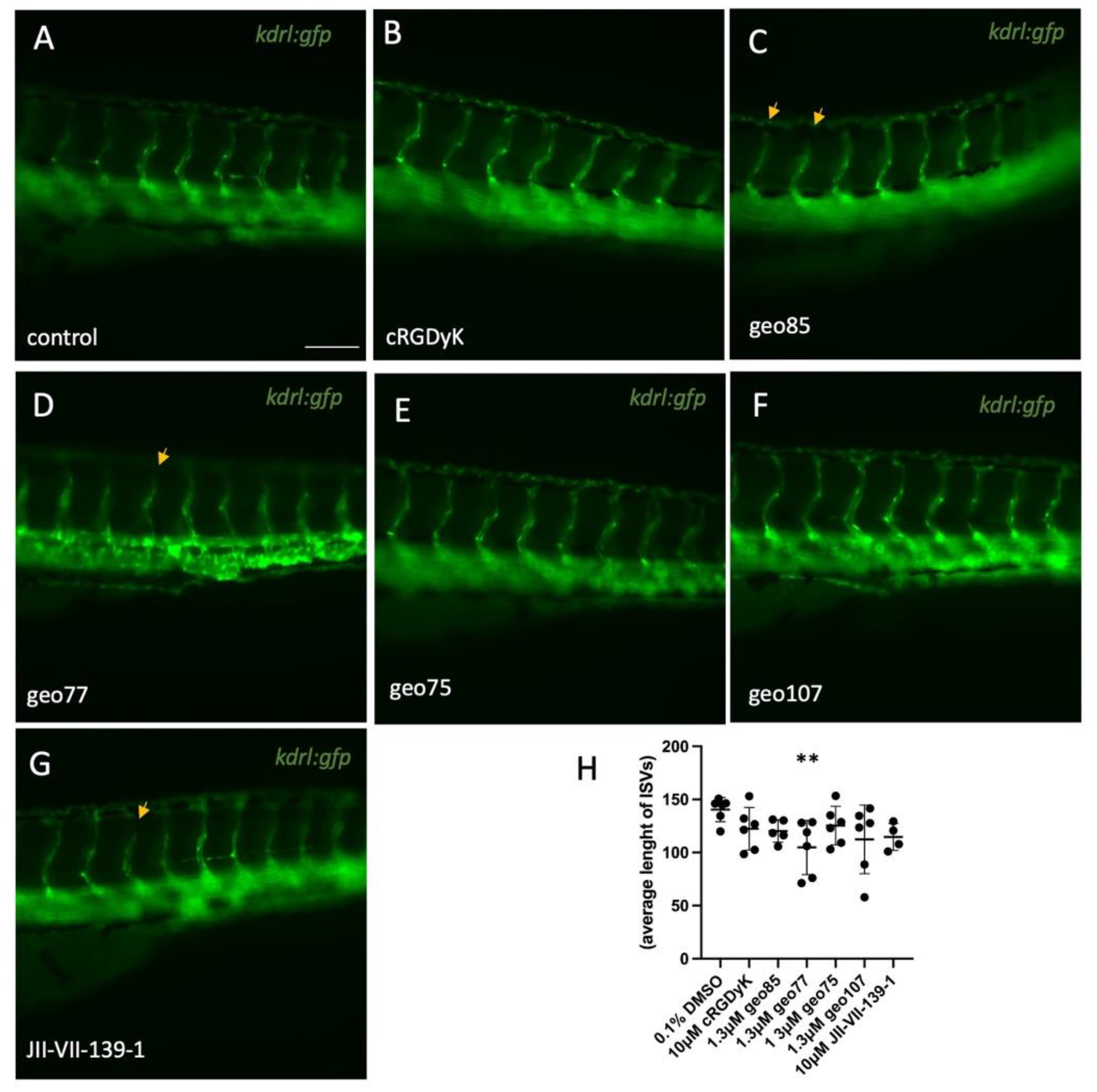
Conjugate
geo85. To a stirred solution of
23 (3 mg, 4.2 μmol) and c(RGDyK) (2 mg, 3.23 μmol) in dry DMF (1 mL), under argon, was added DIPEA (2.25 uL, 12.9 μmol) and the mixture was stirred at room temperature for 24 h. The solvent was evaporated and
geo85 was isolated by HPLC (method 1,
Supplementary Materials) to yield
geo85 (3.4 mg, 88%) as a white solid.
geo85:
1H NMR (500 MHz, DMSO-
d6) δ 13.05 (s, 1H), 9.12 (s, 1H), 8.44 (d,
J = 2.7 Hz, 1H), 8.40–8.30 (m, 3H), 8.27 (s, 1H), 8.23 (d,
J = 9.2 Hz, 2H), 8.17 (s, 1H), 8.13–8.08 (m, 3H), 8.02 (s, 1H), 7.95 (s, 1H), 7.65 (d,
J = 9.9 Hz, 1H), 7.63 (dd,
J = 8.2, 1.3 Hz, 1H), 7.30–6.98 (br, 3H) 6.94 (d,
J = 8.5 Hz, 2H), 6.91 (d,
J = 2.7 Hz, 1H), 6.60 (d,
J = 8.4 Hz, 2H), 6.00 (t,
J = 5.5 Hz, 1H), 5.86 (t,
J = 5.7 Hz, 1H), 4.60–4.53 (m, 1H), 4.52–4.47 (m, 1H), 4.32–4.25 (m, 1H), 4.23–4.19 (m, 1H), 4.19–4.13 (m, 1H), 3.62–3.54 (m, 4H), 3.52–3.46 (m, 4H), 3.20 (dd,
J = 14.2, 4.8 Hz, 2H), 3.12–3.05 (m, 4H), 2.97–2.92 (m, 2H), 2.91–2.86 (m, 2H), 2.74 (dd,
J = 16.9, 2.8 Hz, 1H), 2.53–2.51 (m, 2H), 2.10 (d,
J = 14.2 Hz, 1H), 1.80 (d,
J = 2.5 Hz, 6H), 1.73–1.66 (m, 2H), 1.65–162 (m, 1H), 1.57–1.49 (m, 2H), 1.48–1.41 (m, 2H), 1.35–1.30 (m, 2H), 1.17 (t,
J = 7.5 Hz, 3H), 1.15–1.10 (m, 1H).
13C NMR (126 MHz, DMSO-
d6) δ 179.0, 173.7, 172.0, 171.0, 170.7, 170.5, 167.6, 160.4, 158.1, 156.7, 155.5, 153.0, 149.4, 145.5, 140.8, 135.9, 135.5, 131.1, 129.8, 129.6, 128.3, 128.0, 127.6, 126.0, 124.9, 121.6, 120.0, 116.6, 114.8, 109.5, 109.3, 104.8, 70.1, 69.5, 55.0, 53.2, 50.7, 48.6, 42.9, 40.6, 36.4, 35.8, 31.9, 29.9, 29.8, 29.4, 27.3, 26.0, 24.6, 23.0, 15.19; 4 peaks are missing due to overlapping. ESI-MS, positive mode:
m/z calcd mass for C
59H
74N
15O
13 [M + H]
+ = 1200.55, was found 1199.80.
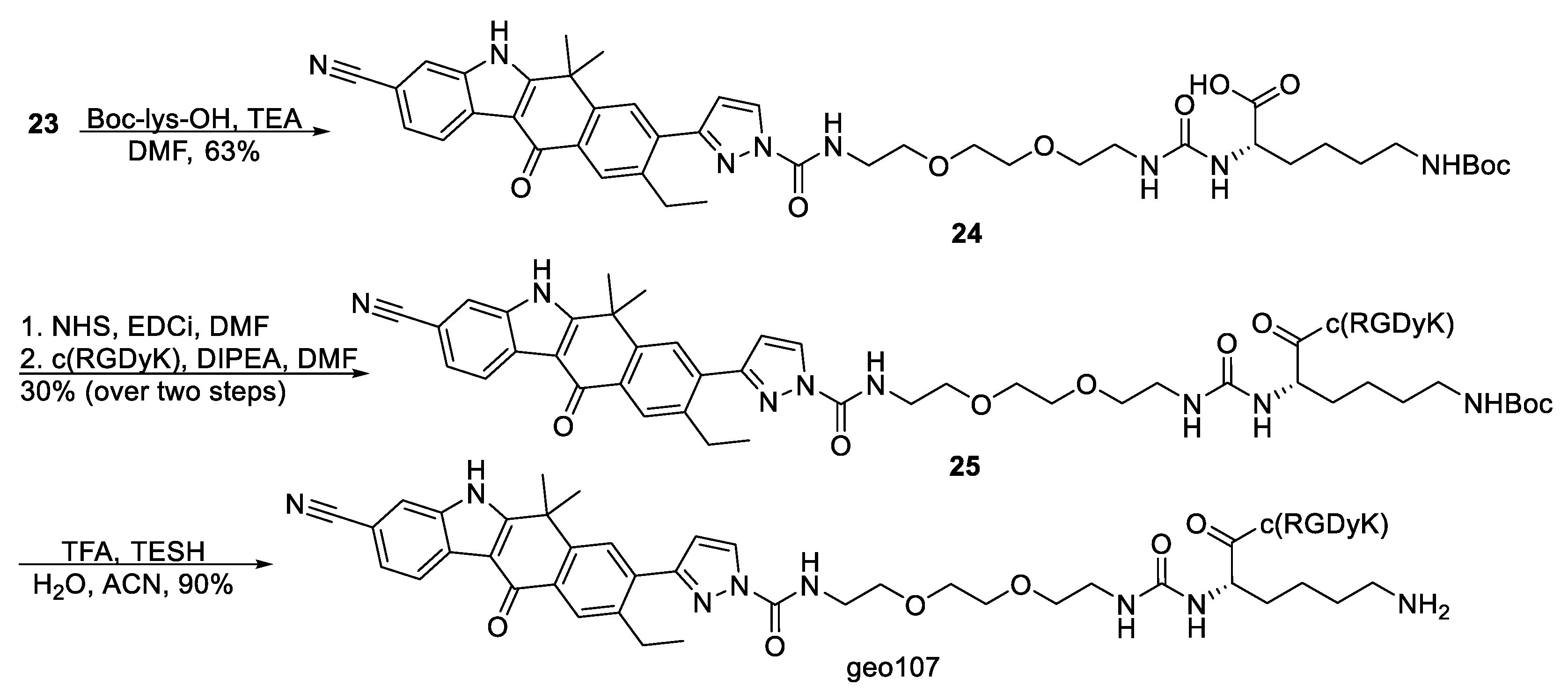
(S)-14-(4-((tert-butoxycarbonyl)amino)butyl)-1-(3-(3-cyano-9-ethyl-6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazol-8-yl)-1H-pyrazol-1-yl)-1,12-dioxo-5,8-dioxa-2,11,13-triazapentadecan-15-oic acid, 24. To a stirred solution of 23 (11 mg, 15.3 μmol) and Nε-Boc-L-lysine (3.8 mg, 15.3 μmol) in DMF (0.8 mL) was added triethylamine (8.5 μL, 61 μmol) at ambient temperature, under Ar. The mixture was stirred at room temperature for 24 h. Subsequently, the solvent was evaporated, and the product was purified with flash column chromatography (gradient elution DCM–MeOH 10:1, 10:2, 10:3) to yield 24 (8 mg, 63%) as white solid. 24: 1H NMR (500 MHz, DMSO-d6) δ 8.40 (d, J = 2.7 Hz, 1H), 8.33 (d, J = 8.1 Hz, 1H), 8.23 (t, J = 4.9 Hz, 1H), 8.17 (s, 1H), 8.10 (s, 1H), 7.94 (s, 1H), 7.58 (dd, J = 8.1, 1.3 Hz, 1H), 6.87 (d, J = 2.7 Hz, 1H), 6.57 (s, 1H), 6.07–6.01 (m, 2H), 3.95 (dd, J = 12.6, 7.3 Hz, 1H), 3.61 (t, J = 5.7 Hz, 2H), 3.58 (dd, J = 5.7, 4.0 Hz, 2H), 3.53 (dd, J = 6.1, 4.1 Hz, 2H), 3.51–3.46 (m, 3H), 3.38 (t, J = 5.7 Hz, 2H), 3.13–3.07 (m, 4H), 2.96–2.91 (m, 2H), 2.87 (dd, J = 12.9, 6.8 Hz, 2H), 1.80 (d, J = 3.7 Hz, 6H), 1.66–1.59 (m, 2H), 1.53–1.46 (m, 2H), 1.37–1.31 (m, 11H), 1.18 (t, J = 7.6 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 178.7, 174.8, 160.4, 157.6, 155.3, 152.7, 149.2, 145.4, 140.4, 136.1, 135.2, 131.1, 129.4, 127.7, 127.6, 125.9, 124.5, 121.4, 119.8, 116.6, 109.3, 109.0, 104.4, 77.1, 70.0, 69.4, 68.5, 53.1, 36.3, 32.2, 31.0, 29.8, 29.7, 28.7, 28.1, 25.7, 22.5, 21.8, 14.9. ESI-MS, positive mode: m/z calcd mass for C43H55N8O9 [M + H] + = 827.40, was found 826.95.
Compound
25. In a stirred solution of
24 (7 mg, 8.32 μmol) and NHS (1.44 mg, 12.5 μmol) in dry DMF (0.5 mL), was added EDCi (4.8 mg, 25 μmol) under argon at room temperature. After 3 h and upon starting material consumption (TLC monitoring), c(RGDyK) (3.6 mg, 5.83 μmol) was added, and the reaction was stirred for 48 h at room temperature. The mixture was then concentrated under reduced pressure and the residue purified by HPLC (method 1,
Supplementary Materials) to afford conjugate
25 (3.5 mg, 30%) as white solid.
25:
1H NMR (500 MHz, DMSO) δ 13.10 (s, 1H), 9.15 (s, 1H), 8.49–8.31 (m, 6H), 8.24–8.16 (m, 6H), 8.04 (s, 1H), 7.96 (s, 1H), 7.91 (s, 2H), 7.70–7.62 (m, 2H), 7.27 (s, 2H), 6.98–6.85 (m, 3H), 6.77–6.70 (m, 1H), 6.60 (d,
J = 8.2 Hz, 2H), 6.16 (d,
J = 8.4 Hz, 2H), 6.10 (s, 1H), 4.59–4.46 (m, 2H), 4.32–4.26 (m, 1H), 4.22–4.10 (m, 2H), 4.07–4.00 (m, 1H), 3.62–3.54 (m, 4H), 3.53–3.45 (m, 4H), 3.20 (d,
J = 9.3 Hz, 3H), 3.14–3.04 (m, 6H), 3.00–2.91 (m, 4H), 2.89–2.83 (m, 2H), 2.73 (d,
J = 15.5 Hz, 1H), 2.57–2.53 (m, 2H), 2.12–2.06 (m, 1H), 1.80 (s, 6 H), 1.72–1.58 (m, 3H), 1.57–1.44 (m, 6H), 1.40–1.29 (m, 13H), 1.23–1.10 (m, 6H).
13C NMR (126 MHz, DMSO) δ 179.1, 173.7, 172.5, 172.0, 171.1, 170.1, 170.6, 167.7, 160.5, 157.6, 156.8, 155.5, 153.0, 149.5, 145.5, 140.8, 135.9, 135.5, 131.2, 129.9, 129.7, 128.4, 128.1, 127.7, 126.1, 125.0, 121.7, 120.1, 118.2, 116.7, 114.8, 109.5, 109.4, 104.8, 77.3, 70.1, 69.6, 69.6, 68.7, 55.0, 52.9, 50.8, 48.6, 40.4, 38.2, 36.4, 33.2, 29.9, 29.9, 29.3, 28.5, 28.3, 26.0, 24.6, 23.0, 22.6, 15.2; 3 peaks are missing due to overlapping. ESI-MS, negative mode:
m/z calcd mass for C
70H
93N
17O
16 [M − H]
− = 1426.70, was found 1426.10.
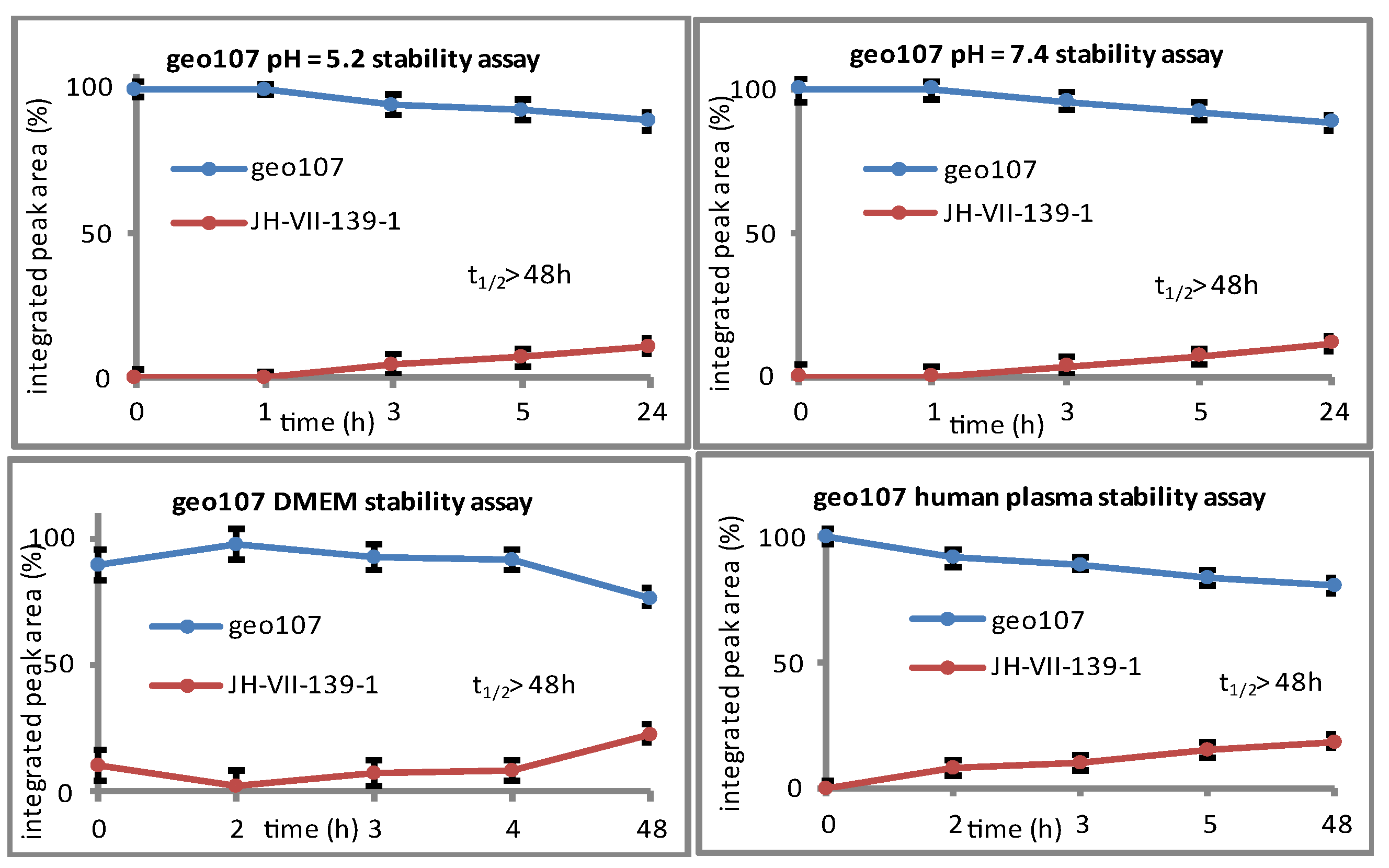
Conjugate
geo107. Compound
25 (1.8 mg, 1.26 μmol) was dissolved in a H
2O/ACN/TESH/TFA (0.36 mL, 80/180/20/80) mixture and stirred for 2 h at room temperature. The mixture was then freeze dried and subjected to HPLC (method 1,
Supplementary Materials) to yield
geo107 (1.5mg, 90%) as beige solid.
geo107:
1H NMR (500 MHz, DMSO-
d6) δ
1H NMR (500 MHz, DMSO-
d6) δ 9.02 (s, 1H), 8.46–8.36 (m, 4H), 8.34 (d,
J = 8.1 Hz, 1H), 8.28 (d,
J = 8.4 Hz, 1H), 8.20 (d,
J = 9.7 Hz, 1H), 8.17 (s, 1H), 8.10 (d,
J = 9.2 Hz, 1H), 8.07–7.89 (m, 5H), 7.66 (d,
J = 9.1 Hz, 1H), 7.63 (d,
J = 8.7 Hz, 1H), 7.49–7.24 (br, 3H), 6.96–6.90 (m, 3H), 6.86 (d,
J = 8.4 Hz, 1H), 6.60 (d,
J = 8.3 Hz, 2H), 6.26–6.14 (m, 2H), 4.58–4.46 (m, 2H), 4.35–4.28 (m, 1H), 4.20–4.11 (m, 2H) 4.08–0.2 (m, 1H), 3.60–3.54 (m, 4H), 3.53–3.44 (m, 4H), 3.26–3.21 (m, 3H), 3.14–3.03 (m, 6H), 2.98–2.80 (m, 4H), 2.74–2.68 (m, 3H), 2.10–2.04 (m, 1H), 1.80 (s, 6H), 1.62–1.55 (m, 3H), 1.54–1.42 (m, 6H), 1.27–1.20 (m, 4H), 1.19–1.13 (m, 6H).
13C NMR (126 MHz, DMSO) δ 179.0, 173.9, 172.3, 170.8, 166.6, 166.4, 166.3, 160.6, 157.6, 157.1, 157.0, 155.8, 155.5, 153.0, 149.5, 145.6, 140.8, 136.1, 135.5, 131.2, 129.9, 129.8, 129.7, 128.1, 127.7, 126.1, 125.0, 121.6, 120.1, 116.7, 115.1, 114.9, 109.5, 109.3, 104.8, 70.1, 69.6, 68.7, 55.8, 52.7, 49.8, 46.0, 40.5, 38.9, 36.5, 32.9, 29.9, 28.5, 27.6, 26.0, 25.2, 25.1, 22.2, 22.1, 15.2; 3 peaks are missing due to overlapping. ESI-MS, positive mode:
m/z calcd mass for C
65H
86N
17O
14 [M + H]
+ = 1328.65, was found 1327.9.
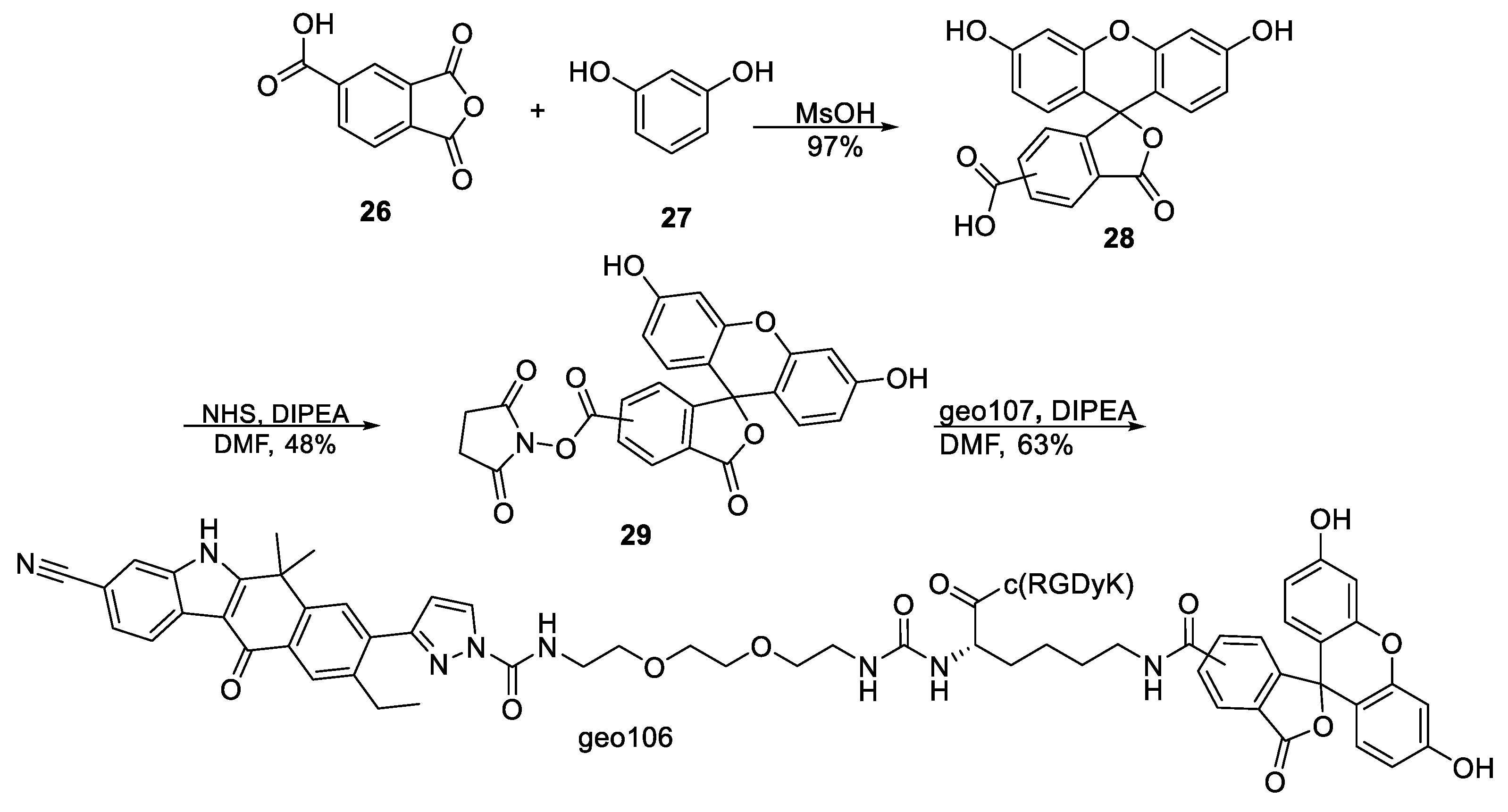
3′,6′-Dihydroxy-3-oxo-3
H-spiro[isobenzofuran-1,9′-xanthene]-5(6)-carboxylic acid,
28. 1,3-Dioxo-1,3-dihydroisobenzofuran-5-carboxylic acid
26 (1 g, 5.1 mmol) and resorcinol
27 (1.15 g, 10.4 mmol) in methanesulfonic acid (5 mL) were placed in a pressure tube equipped with a magnetic stirrer bar. The reaction mixture was stirred at 80 °C overnight and then poured to cold water (500 mL) with stirring and filtered. The solid residue was heated at reflux in EtOH (200 mL), H
2O was added until precipitation, and the mixture cooled to room temperature, filtered, and freeze-dried for 24 h to yield 1.9 g (97%) of crude 5(6)-carboxyfluorescein as an orange powder. The crude compound was used without further purification. The spectral data were in accordance with those reported in the literature [
35].
28:
1H NMR (500 MHz, DMSO-
d6) δ 10.14 (s, 2H), 8.40 (s, 1H), 8.29 (dd,
J = 8.0, 1.4 Hz, 1H), 8.22 (d,
J = 8.0 Hz, 0.7H), 8.11 (d,
J = 8.0 Hz, 0.7H), 7.65 (s, 0.7H), 7.39 (d,
J = 8.0 Hz, 1H), 6.70–6.65 (m, 2.2H), 6.66–6.58 (m, 3.2H), 6.56–6.52 (m, 4.8H), regioisomer ratio present: 60–40 (5/6).
2,5-Dioxopyrrolidin-1-yl 3′,6′-dihydroxy-3-oxo-3
H-spiro[isobenzofuran-1,9′-xanthene]-5(6)-carboxylate,
29. 5(6)-Carboxyfluorescein
28 (200 mg, 0.531 mmol) was dissolved in dry DMF (2.5 mL). EDCi (306 mg, 1.59 mmol) and
N-hydroxysuccinimide (122 mg, 1.06 mmol) were added to the mixture. The reaction was stirred under argon for 2 h and after completion, was washed with aqueous KH
2PO
4 three times (EA was used in moderation as additional organic solvent, since EA does not dissolve fluorescein derivatives). Organic layers were dried with Na
2SO
4 and concentrated under reduced pressure. The residue was then subjected to column chromatography (gradient elution EA, EA–MeOH, 10–1, 10–2) and yielded
29 as dark brown solid (120 mg, 48%). The spectral data were in accordance with those reported in the literature [
35].
29:
1H NMR (500 MHz, DMSO-
d6) δ 10.18 (s, 2H), 8.54 (s, 1H), 8.43 (dd,
J = 8.1, 1.6 Hz, 1H), 7.56 (d,
J = 8.1 Hz, 1H), 6.72–6.65 (m, 4H), 6.57–6.53 (m, 2H), 2.93 (s, 4H), regioisomer ratio present: 72–28 (5/6). ESI-MS, positive mode:
m/z calcd mass for C
25H
15NO
9 [M]
+ = 473.07 was found 473.85.
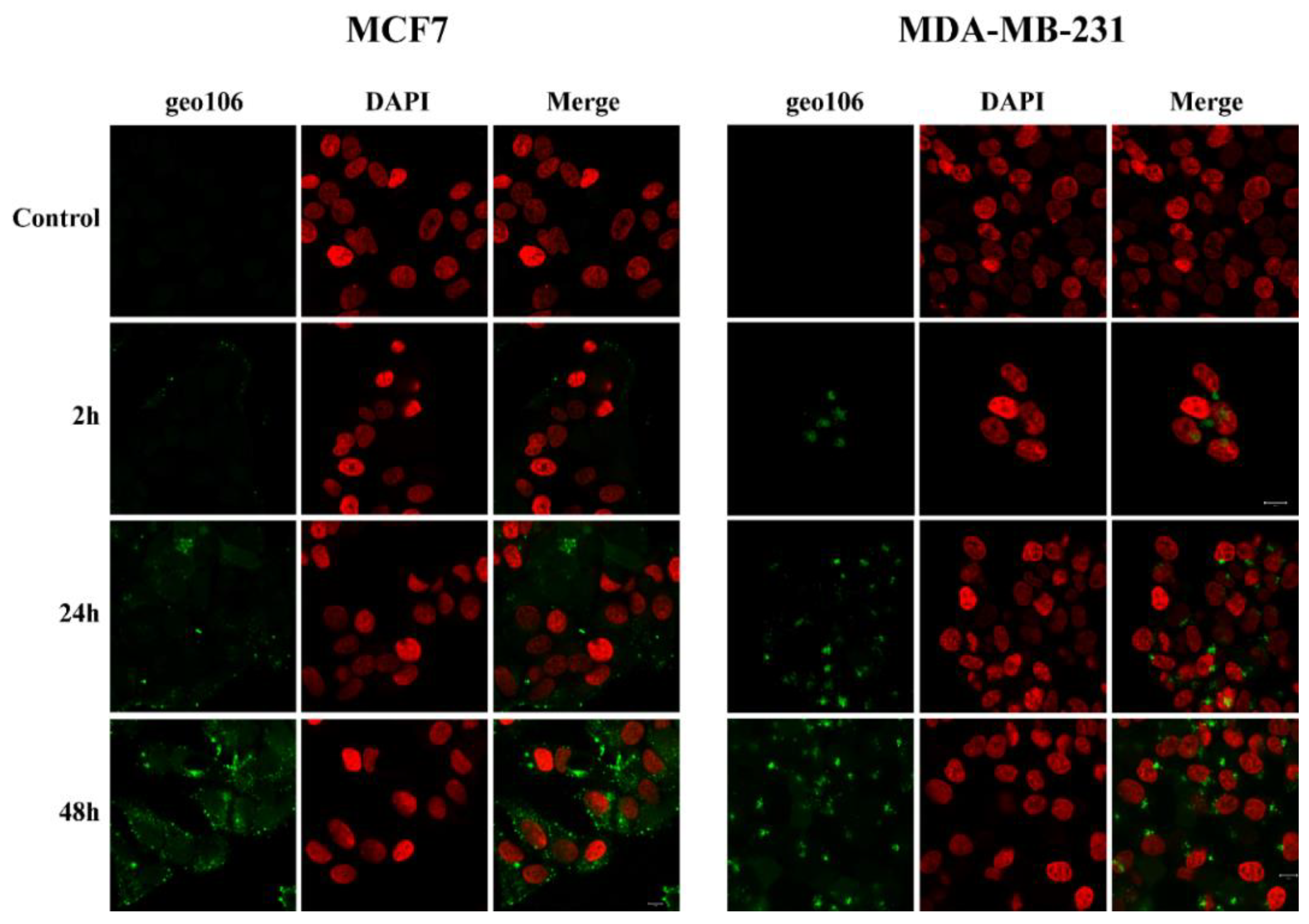
5(6)-Carboxyfluorescein tagged conjugates
geo106. To a stirred solution of
geo107 (2 mg, 1.51 μmol) and
geo98 (0.7 mg, 1.51 μmol) in dry DMF (1.33 mL), under argon, was added DIPEA (2.62 μL, 15.1 μmol), and the mixture was stirred at room temperature for 48 h. The solvent was evaporated, and the product was purified by HPLC (method 5,
Supplementary Materials) to yield
geo106 (1.6 mg, 63%) as a yellow solid.
geo106:
1H NMR (500 MHz, DMSO-
d6) δ 9.10 (s, 1H), 8.83 (s, 1H), 8.72 (s, 1H), 8.63 (s, 1H), 8.45 (d,
J = 12.9 Hz, 1H), 8.39 (s, 1H), 8.34 (d,
J = 8.0 Hz, 1H), 8.29 (s, 1H), 8.23–8.15 (m, 2H), 8.13 (d,
J = 8.1 Hz, 1H), 8.04–8.02 (m, 2H), 7.96 (s, 1H), 7.88 (s, 1H), 7.83 (s, 1H), 7.63 (t,
J = 6.9 Hz, 2H), 7.35–7.30 (m, 1H), 7.19 (s, 2H), 6.96–6.90 (m, 2H), 6.86 (d, J = 8.2 Hz, 1H), 6.69–6.56 (m, 4H), 6.55–6.51 (m, 1H), 6.17 (d,
J = 8.0 Hz, 1H), 6.16–6.04 (m, 1H), 4.92–4.79 (m, 26H), 4.69–4.63 (m, 1H), 4.60–4.53 (m, 1H), 4.49 (dd,
J = 13.8, 7.3 Hz, 1H), 4.38–4.25 (m, 2H), 4.23–4.16 (m, 1H), 4.14 (d,
J = 9.3 Hz, 1H), 4.12–4.04 (m, 2H), 3.63–3.54 (m, 4H), 3.52–3.46 (m, 4H), 3.17 (s, 1H), 3.14–3.10 (m, 2H), 3.09–3.03 (m, 2H), 2.94 (q,
J = 15.0, 2H), 2.80–2.73 (s, 3H), 2.09 (d,
J = 9.3 Hz, 1H), 2.04–1.96 (m, 2H), 1.80 (s, 6H), 1.59–1.51 (m, 3H), 1.50–1.43 (m, 3H), 1.40–1.36 (m, 2H), 1.31–1.22 (m, 7H), 1.17 (t,
J = 7.4 Hz, 4H), 1.16–1.07 (m, 2H). ESI-MS, negative mode:
m/z calcd mass for C
86H
95N
17O
20 [M/2-H]
− = 841.84, was found 841.60.