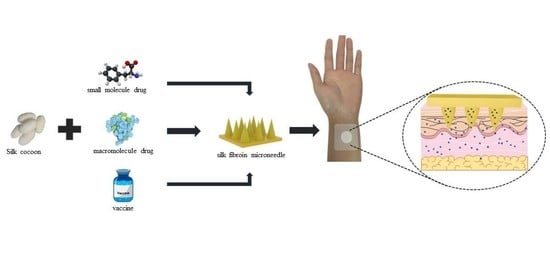
Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed?
Abstract
1. Introduction
2. Structure and Properties of Silk Fibroin
2.1. Structure of Silk Fibroin
2.2. Biocompatibility of Silk Fibroin
2.3. The Advantages and Disadvantages of Silk Fibroin for Drug Release
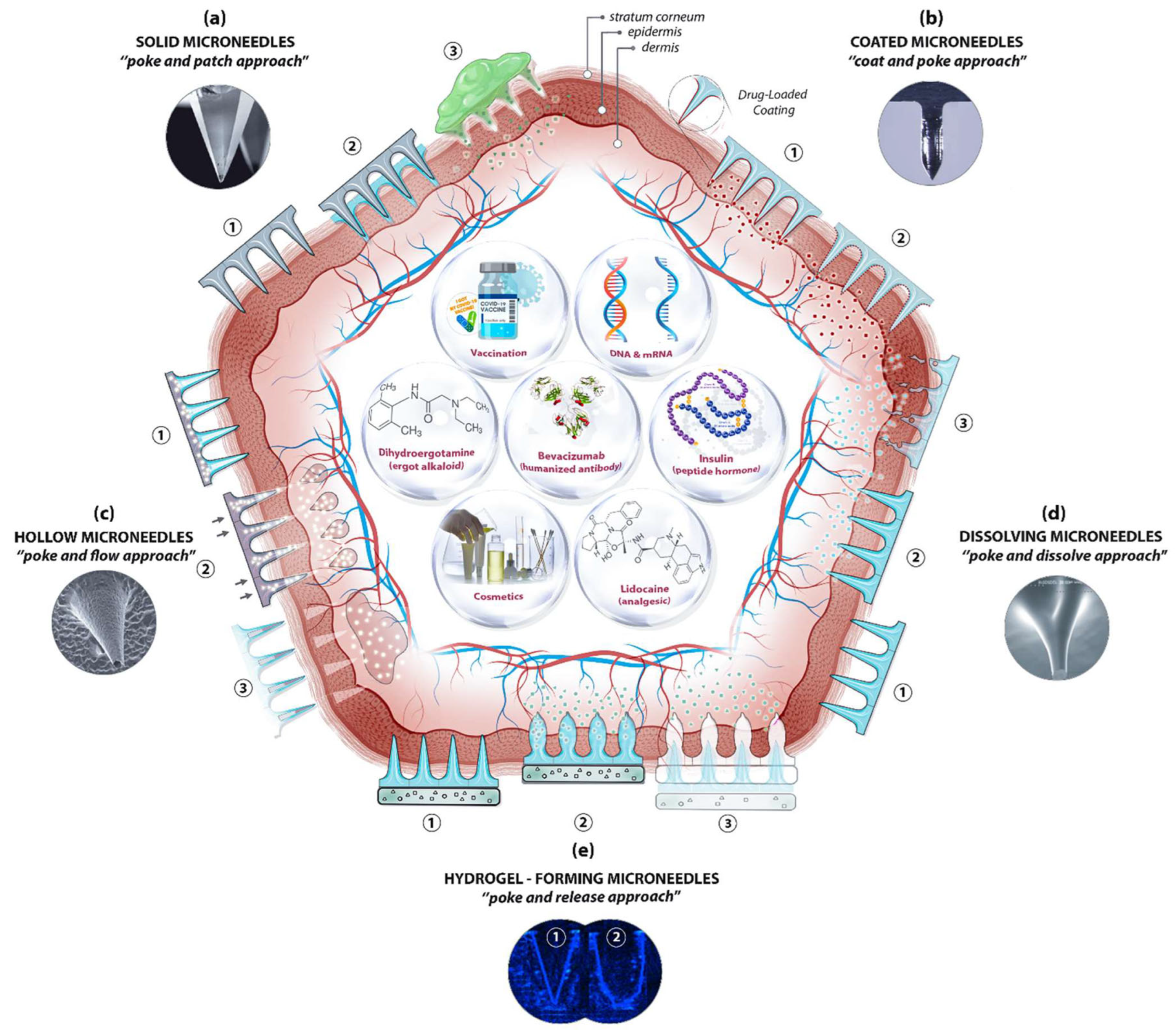
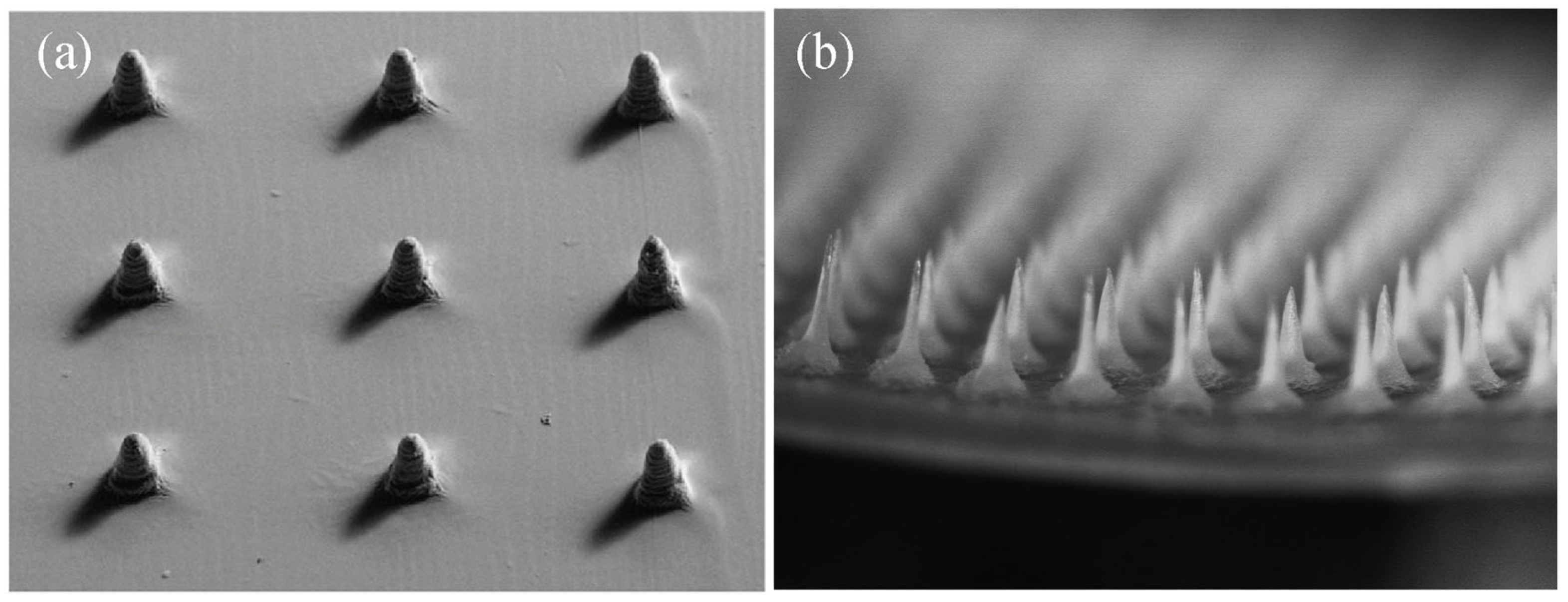
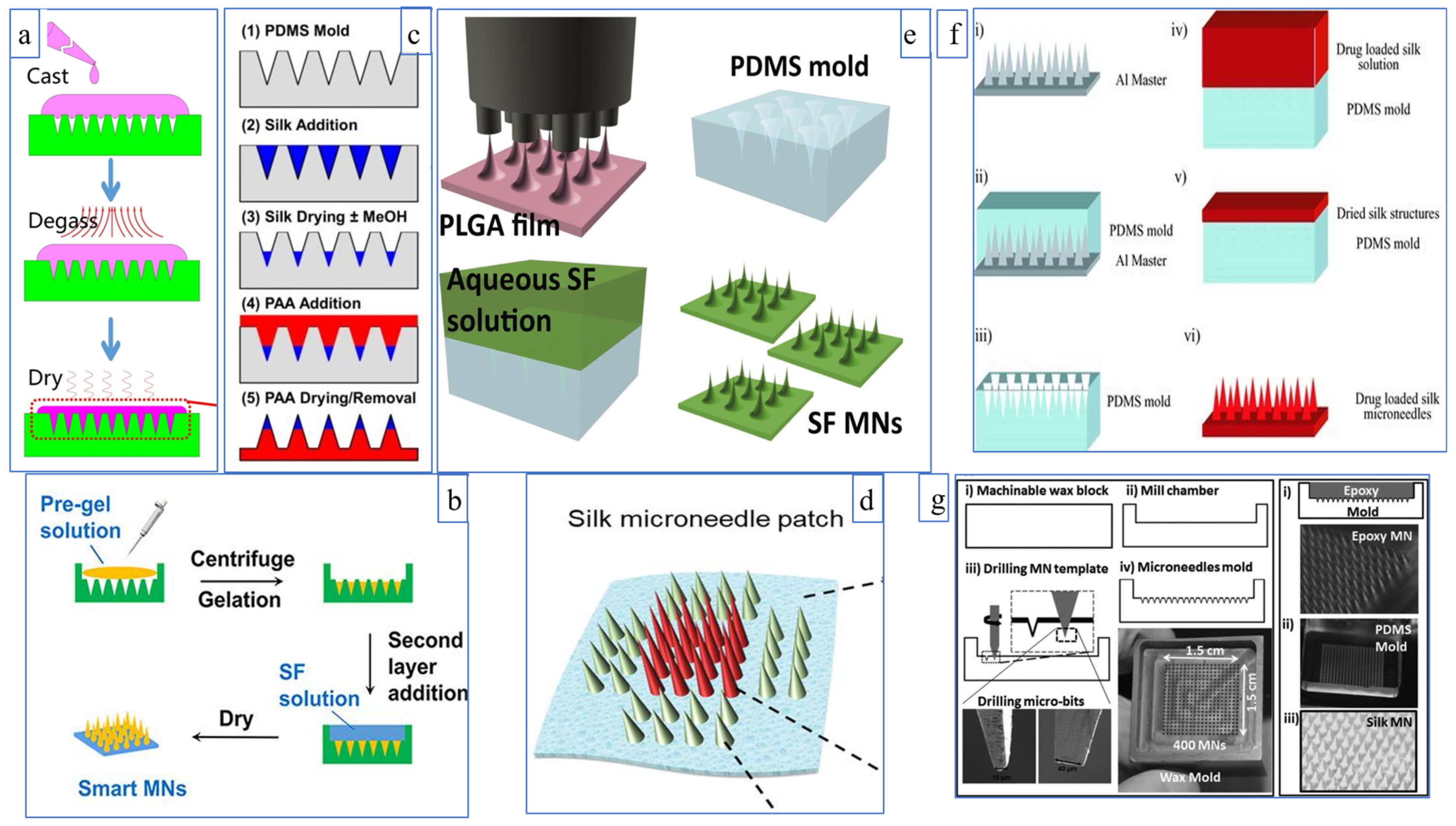
3. Types of Silk Fibroin Microneedles
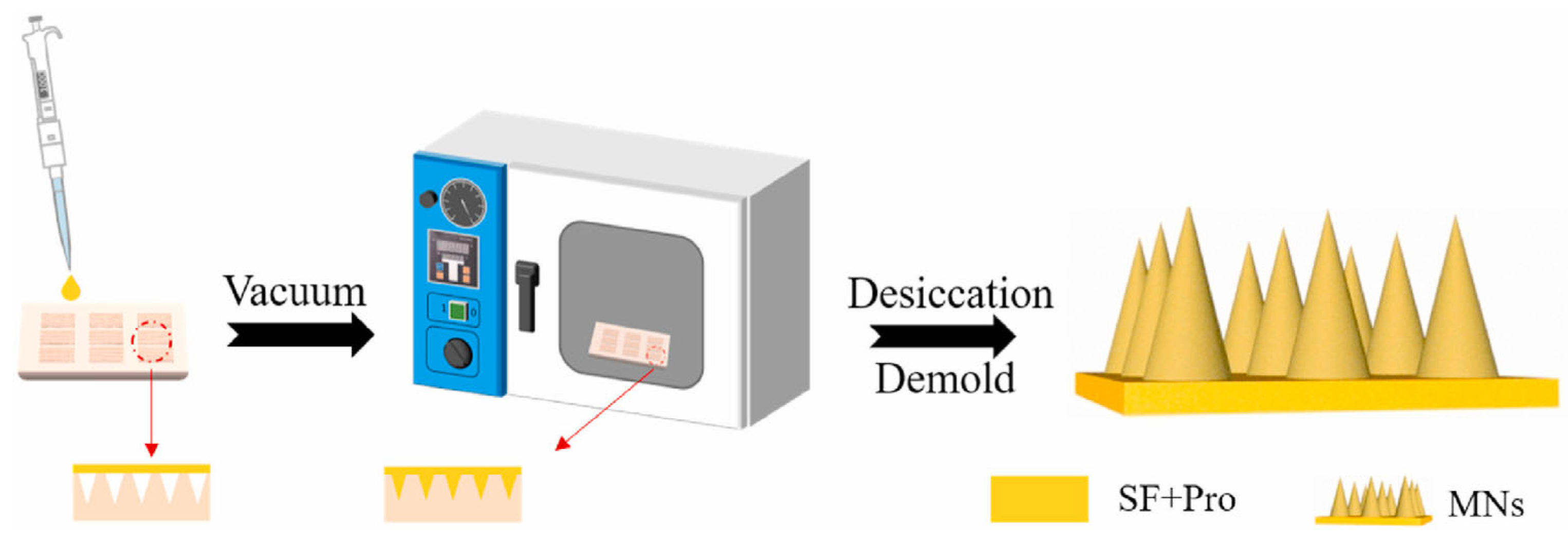
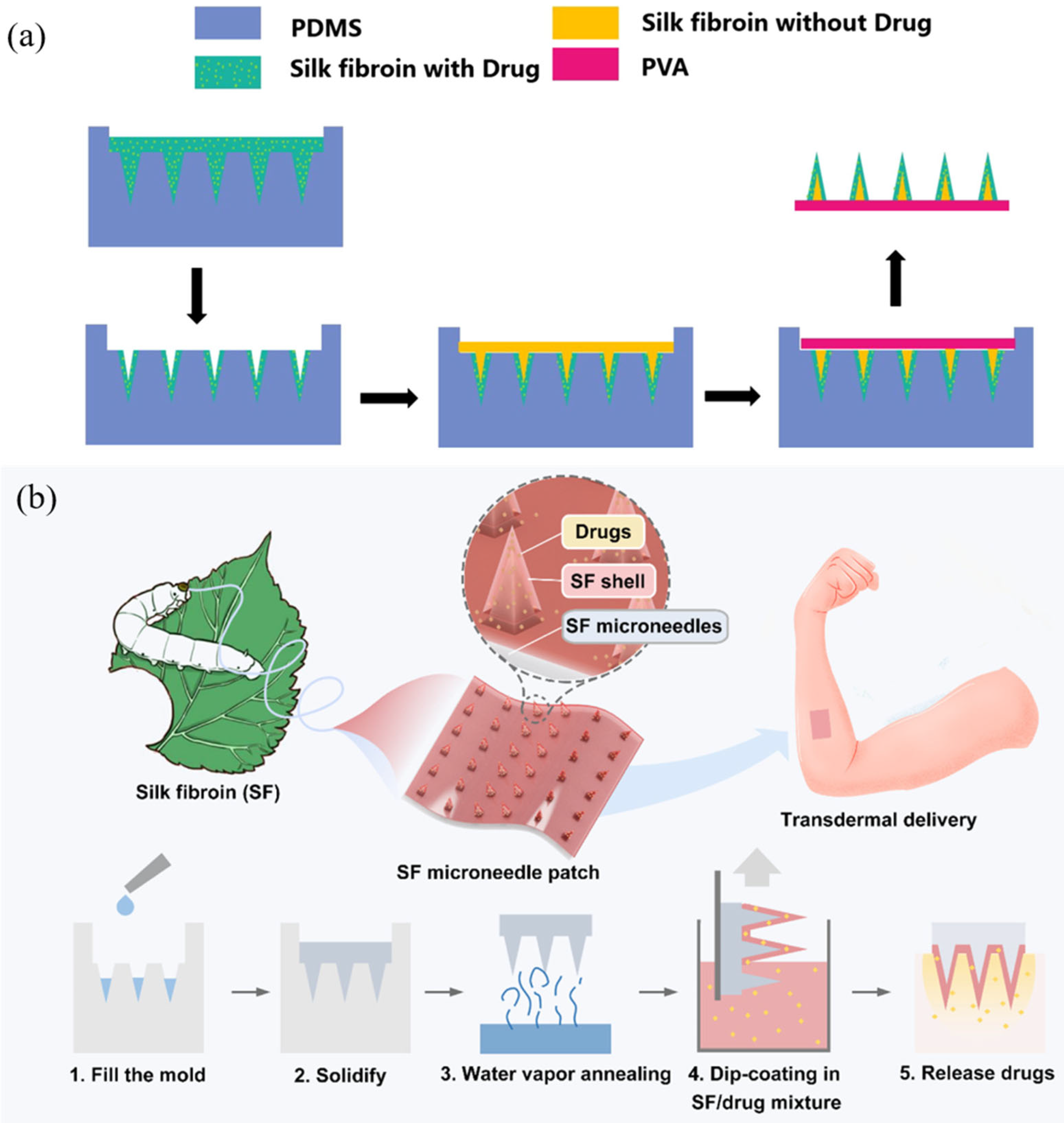
3.1. Dissolving Silk Fibroin Microneedles
3.2. Swelling Silk Fibroin Microneedles
3.3. Intelligently Responsive Silk Fibroin Microneedles
4. Silk Fibroin Microneedles for Drug Delivery
4.1. Transdermal Delivery of Small Molecule Drugs
4.2. Transdermal Delivery of Macromolecule Drugs
4.3. Silk Fibroin Microneedles for Transdermal Administration of Vaccines
5. Clinical Transformation of Microneedles and Silk Fibroin Materials
5.1. Commercial Application of Silk Fibroin Medical Apparatus
5.2. Clinical Application of Silk Fibroin Medical Apparatus
5.3. Clinical Application of Microneedle Devices
6. Future Development Trends
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adolphe, C.; Wainwright, B. pathways to improving skin regeneration. Expert Rev. Mol. Med. 2005, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Stratum Corneum Defensive Functions: An Integrated View. J. Gen. Intern. Med. 2005, 20, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Karwa, P.; Hiremath, S.R.R. Polymeric microneedles for transdermal drug delivery—A review of recent studies. J. Drug Deliv. Sci. Technol. 2022, 77, 103760. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Ruan, S.; Zhang, Y.; Feng, N. Microneedle-mediated transdermal nanodelivery systems: A review. Biomater. Sci. 2021, 9, 8065–8089. [Google Scholar] [CrossRef]
- Thomas, B.J.; Finnin, B.C. The transdermal revolution. Drug Discov. Today 2004, 9, 697–703. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- Mahmood, S.; Mandal, U.K.; Chatterjee, B. Transdermal delivery of raloxifene HCl via ethosomal system: Formulation, advanced characterizations and pharmacokinetic evaluation. Int. J. Pharm. 2018, 542, 36–46. [Google Scholar] [CrossRef]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef]
- Lim, D.-J.; Kim, H.-J. Microneedles in Action: Microneedling and Microneedles-Assisted Transdermal Delivery. Polymers 2022, 14, 1608. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Nayak, A.; Babla, H.; Han, T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016, 23, 658–669. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, M.; Zhao, L.; Kuang, D.; Kundu, S.C.; Lu, S. Insulin-Loaded Silk Fibroin Microneedles as Sustained Release System. ACS Biomater. Sci. Eng. 2019, 5, 1887–1894. [Google Scholar] [CrossRef]
- Yin, Z.P.; Kuang, D.J.; Wang, S.Y.; Zheng, Z.Z.; Yadavalli, V.K.; Lu, S.Z. Swellable silk fibroin microneedles for transdermal drug delivery. Int. J. Biol. Macromol. 2018, 106, 48–56. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, G.; Yao, X.; Zhou, H.; Lyu, B.; Pei, S.; Wen, P. Microneedle-based insulin transdermal delivery system: Current status and translation challenges. Drug Deliv. Transl. Res. 2022, 12, 2403–2427. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Zhang, M.; Ling, G.; Zhang, P. Research progress of microneedles in the treatment of melanoma. J. Control. Release 2022, 348, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and transdermal drug delivery using microneedles—Fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Freiherr, J.; Hallschmid, M.; Frey, W.H.; Brünner, Y.F.; Chapman, C.D.; Hölscher, C.; Craft, S.; De Felice, F.G.; Benedict, C. Intranasal Insulin as a Treatment for Alzheimer’s Disease: A Review of Basic Research and Clinical Evidence. CNS Drugs 2013, 27, 505–514. [Google Scholar] [CrossRef]
- Gera, A.K.; Burra, R.K. The Rise of Polymeric Microneedles: Recent Developments, Advances, Challenges, and Applications with Regard to Transdermal Drug Delivery. J. Funct. Biomater. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Khodaei, A.; Samadikuchaksaraei, A.; Reis, R.L.; Kundu, S.C.; Moroni, L. Ancient fibrous biomaterials from silkworm protein fibroin and spider silk blends: Biomechanical patterns. Acta Biomater. 2022, 153, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-z.; Wang, X.-q.; Uppal, N.; Kaplan, D.L.; Li, M.-z. Stabilization of horseradish peroxidase in silk materials. Front. Mater. Sci. China 2009, 3, 367–411. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Lu, Q.; Hu, X.; Uppal, N.; Omenetto, F.G.; Kaplan, D.L. Stabilization of Enzymes in Silk Films. Biomacromolecules 2009, 10, 1032–1042. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rivero, H.C.; Pérez Hernández, M.d.C.; Pagán, A.; Montalbán, M.G.; Víllora, G.; Cénis, J.L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharm. 2017, 518, 11–19. [Google Scholar] [CrossRef]
- Luo, T.; Yang, L.; Wu, J.; Zheng, Z.; Li, G.; Wang, X.; Kaplan, D.L. Stabilization of Natural Antioxidants by Silk Biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 13573–13582. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Kundu, S.C.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Kaplan, D.L. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin. Drug Deliv. 2011, 8, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, A.V.; Mandal, A.; Kulp, D.W.; Menis, S.; Bennett, N.R.; Watkins, H.C.; Wang, W.; Martin, J.T.; Thai, N.T.; He, Y.; et al. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. USA 2019, 116, 16473–16478. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Jiang, J.; Shi, Z.; Mao, Y.; Qin, N.; Tao, T.H. Silk Microneedle Patch Capable of On-Demand Multidrug Delivery to the Brain for Glioblastoma Treatment. Adv. Mater. 2022, 34, 2106606–2106688. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Cao, J.; Tao, X.; Wu, X.; Kundu, S.C.; Lu, S. Silk Fibroin Microneedle Patches for the Treatment of Insomnia. Pharmaceutics 2021, 13, 2198. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Guo, M.; Lyu, K.; Chu, T.; He, B. Intelligent Silk Fibroin Based Microneedle Dressing (i-SMD). Adv. Funct. Mater. 2021, 31, 2006839–2006897. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Hess, S.; van Beek, J.; Pannell, L.K. Acid hydrolysis of silk fibroins and determination of the enrichment of isotopically labeled amino acids using precolumn derivatization and high-performance liquid chromatography–electrospray ionization–mass spectrometry. Anal. Biochem. 2002, 311, 19–26. [Google Scholar] [CrossRef]
- Kurihara, H.; Sezutsu, H.; Tamura, T.; Yamada, K. Production of an active feline interferon in the cocoon of transgenic silkworms using the fibroin H-chain expression system. Biochem. Biophys. Res. Commun. 2007, 355, 976–980. [Google Scholar] [CrossRef]
- Zhou, C.-Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.-G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins Struct. Funct. Bioinform. 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Zhou, C.-Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lu, Q.; Sheng, W.; Liu, L.; Dong, B.; Kaplan, D.L.; Zhu, H. Reversible Hydrogel–Solution System of Silk with High Beta-Sheet Content. Biomacromolecules 2014, 15, 3044–3051. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-chain, L-chain, and P25, with a 6:6:1 Molar Ratio*. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Z.; Chen, X.; Zhou, P. Optical Spectroscopy to Investigate the Structure of Regenerated Bombyx mori Silk Fibroin in Solution. Biomacromolecules 2004, 5, 773–779. [Google Scholar] [CrossRef]
- Koide, S.; Huang, X.; Link, K.; Koide, A.; Bu, Z.; Engelman, D.M. Design of single-layer β-sheets without a hydrophobic core. Nature 2000, 403, 456–460. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Nova, A.; Keten, S.; Pugno, N.M.; Redaelli, A.; Buehler, M.J. Molecular and Nanostructural Mechanisms of Deformation, Strength and Toughness of Spider Silk Fibrils. Nano Lett. 2010, 10, 2626–2634. [Google Scholar] [CrossRef]
- Marsh, R.E.; Corey, R.B.; Pauling, L. An investigation of the structure of silk fibroin. Biochim. Et Biophys. Acta 1955, 16, 1–34. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wu, F.; Xing, T.; Yadavalli, V.K.; Kundu, S.C.; Lu, S. A silk fibroin hydrogel with reversible sol–gel transition. RSC Adv. 2017, 7, 24085–24096. [Google Scholar] [CrossRef]
- Rusa, C.C.; Bridges, C.; Ha, S.-W.; Tonelli, A.E. Conformational Changes Induced in Bombyx mori Silk Fibroin by Cyclodextrin Inclusion Complexation. Macromolecules 2005, 38, 5640–5646. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99B, 89–101. [Google Scholar] [CrossRef]
- Liu, X.; Xia, Q.; Zhou, J.; Zhang, Y.; Ju, H.; Deng, Z. Chemical Modification of Silk Fibroin through Serine Amino Acid Residues. Materials 2022, 15, 4399. [Google Scholar] [CrossRef]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Chen, S.; Zhang, J.; Li, S.; Li, X.; Ikoma, T.; Chen, W. In situ decoration of TiO2 nanowire microspheres with silk fibroin for enhanced biocompatibility. Mater. Lett. 2022, 324, 132688. [Google Scholar] [CrossRef]
- Hofmann, S.; Wong Po Foo, C.T.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef]
- Sakunpongpitiporn, P.; Naeowong, W.; Sirivat, A. Enhanced transdermal insulin basal release from silk fibroin (SF) hydrogels via iontophoresis. Drug Deliv. 2022, 29, 2234–2244. [Google Scholar] [CrossRef]
- Vepari, C.P.; Kaplan, D.L. Covalently immobilized enzyme gradients within three-dimensional porous scaffolds. Biotechnol. Bioeng. 2006, 93, 1130–1137. [Google Scholar] [CrossRef]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Yavuz, B.; Chambre, L.; Kaplan, D.L. Extended release formulations using silk proteins for controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2019, 16, 741–756. [Google Scholar] [CrossRef]
- Choi, M.; Choi, D.; Hong, J. Correction to “Multilayered Controlled Drug Release Silk Fibroin Nanofilm by Manipulating Secondary Structure”. Biomacromolecules 2018, 19, 3902–3903. [Google Scholar] [CrossRef]
- Coburn, J.M.; Na, E.; Kaplan, D.L. Modulation of vincristine and doxorubicin binding and release from silk films. J. Control. Release 2015, 220, 229–238. [Google Scholar] [CrossRef]
- Pandey, V.; Haider, T.; Jain, P.; Gupta, P.N.; Soni, V. Silk as a leading-edge biological macromolecule for improved drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101294. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Cui, Y.; Cheng, K.; Dong, M.; Liu, L. Effect of molecular weight of regenerated silk fibroin on silk-based spheres for drug delivery. Korean J. Chem. Eng. 2020, 37, 1732–1742. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, R.; Wang, S.; Yang, X.; Ling, G.; Zhang, P. Fabrication, evaluation and applications of dissolving microneedles. Int. J. Pharm. 2021, 604, 120749. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Ling, P.; Hashmi, G.; Reed, M.; Gaugler, R.; Trimmer, W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotechniques 1995, 19, 766–770. [Google Scholar] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid Hormone (1-34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Scoutaris, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv. Transl. Res. 2015, 5, 451–461. [Google Scholar] [CrossRef]
- Meyer, B.K.; Kendall, M.A.F.; Williams, D.M.; Bett, A.J.; Dubey, S.; Gentzel, R.C.; Casimiro, D.; Forster, A.; Corbett, H.; Crichton, M.; et al. Immune response and reactogenicity of an unadjuvanted intradermally delivered human papillomavirus vaccine using a first generation Nanopatch™ in rhesus macaques: An exploratory, pre-clinical feasibility assessment. Vaccine X 2019, 2, 100030–100083. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef]
- Nicholas, D.; Logan, K.A.; Sheng, Y.; Gao, J.; Farrell, S.; Dixon, D.; Callan, B.; McHale, A.P.; Callan, J.F. Rapid paper based colorimetric detection of glucose using a hollow microneedle device. Int. J. Pharm. 2018, 547, 244–249. [Google Scholar] [CrossRef]
- Lim, D.-J.; Vines, J.B.; Park, H.; Lee, S.-H. Microneedles: A versatile strategy for transdermal delivery of biological molecules. Int. J. Biol. Macromol. 2018, 110, 30–38. [Google Scholar] [CrossRef]
- Ita, K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Control. Release 2017, 265, 113–119. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Lai, L.; Tandon, S.; McCullough, M.P.; Kong, Y.; Kabbani, S.; Natrajan, M.S.; Xu, Y.; Zhu, Y.; Wang, D.; et al. Immunologic mechanisms of seasonal influenza vaccination administered by microneedle patch from a randomized phase I trial. NPJ Vaccines 2021, 6, 89–139. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J.; Chang, J.H. Rapidly dissolving silk protein microneedles for transdermal drug delivery. In Proceedings of the 2010 IEEE International Conference on Nano/Molecular Medicine and Engineering, Hung Hom, China, 5–9 December 2010; pp. 144–147. [Google Scholar]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Qi, Z.; Tao, X.; Kundu, S.C.; Lu, S. Sustained release of insulin from silk microneedles. J. Drug Deliv. Sci. Technol. 2022, 74, 103611. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Meng, G.; Hu, X.; Zeng, Z.; Zhao, B.; Lin, N.; Liu, X.Y. Reinforcement of Silk Microneedle Patches for Accurate Transdermal Delivery. Biomacromolecules 2021, 22, 5319–5326. [Google Scholar] [CrossRef]
- Shin, D.; Hyun, J. Silk fibroin microneedles fabricated by digital light processing 3D printing. J. Ind. Eng. Chem. 2021, 95, 126–133. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.C.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.-C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Ranjan Yadav, P.; Iqbal Nasiri, M.; Vora, L.K.; Larrañeta, E.; Donnelly, R.F.; Pattanayek, S.K.; Bhusan Das, D. Super-swelling hydrogel-forming microneedle based transdermal drug delivery: Mathematical modelling, simulation and experimental validation. Int. J. Pharm. 2022, 622, 121835. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Microneedle-Array Patch Fabricated with Enzyme-Free Polymeric Components Capable of On-Demand Insulin Delivery. Adv. Funct. Mater. 2019, 29, 1807369. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H.; Seo, I.H.; Lee, K.J.; Ryu, W. Rapid and repeatable fabrication of high A/R silk fibroin microneedles using thermally-drawn micromolds. Eur. J. Pharm. Biopharm. 2015, 94, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of Silk Microneedles for Controlled-Release Drug Delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Raja, W.K.; MacCorkle, S.; Diwan, I.M.; Abdurrob, A.; Lu, J.; Omenetto, F.G.; Kaplan, D.L. Transdermal Delivery Devices: Fabrication, Mechanics and Drug Release from Silk. Small 2013, 9, 3704–3713. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Min, Y.; Irvine, D.J.; Hammond, P.T. Implantable Silk Composite Microneedles for Programmable Vaccine Release Kinetics and Enhanced Immunogenicity in Transcutaneous Immunization. Adv. Healthc. Mater. 2014, 3, 47–58. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Lim, K.H.; Gonuguntla, S.; Lim, K.W.; Pranantyo, D.; Yong, W.P.; Yam, W.J.T.; Low, Z.; Teo, W.J.; et al. The Pathway to Intelligence: Using Stimuli-Responsive Materials as Building Blocks for Constructing Smart and Functional Systems. Adv. Mater. 2019, 31, 1804540. [Google Scholar] [CrossRef]
- Klajn, R. Immobilized azobenzenes for the construction of photoresponsive materials. Pure Appl. Chem. 2010, 82, 2247–2279. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Su, B.; Wen, Y.; Li, C.; Zhou, Y.; Li, M.; Shi, X.; Du, H.; Song, Y.; et al. A Light-Responsive Release Platform by Controlling the Wetting Behavior of Hydrophobic Surface. ACS Nano 2014, 8, 744–751. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Patra, D.; Sengupta, S.; Duan, W.; Zhang, H.; Pavlick, R.; Sen, A. Intelligent, self-powered, drug delivery systems. Nanoscale 2013, 5, 1273–1283. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Di, J.; Yu, J.; Wang, Q.; Yao, S.; Suo, D.; Ye, Y.; Pless, M.; Zhu, Y.; Jing, Y.; Gu, Z. Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Res. 2017, 10, 1393–1402. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Highly Porous Silk Fibroin Scaffold Packed in PEGDA/Sucrose Microneedles for Controllable Transdermal Drug Delivery. Biomacromolecules 2019, 20, 1334–1345. [Google Scholar] [CrossRef]
- Chen, J.; Ren, H.; Zhou, P.; Zheng, S.; Du, B.; Liu, X.; Xiao, F. Microneedle-Mediat. Drug Deliv. Cutan. Dis. 2022, 10, 1032041. [Google Scholar]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control. Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.-H.; Youn, Y.-N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Release 2021, 340, 125–135. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Alpert, J.S. An Amazing Story: The Discovery of Insulin. Am. J. Med. 2016, 129, 231–232. [Google Scholar] [CrossRef]
- Heinemann, L.; Braune, K.; Carter, A.; Zayani, A.; Krämer, L.A. Insulin Storage: A Critical Reappraisal. J. Diabetes Sci. Technol. 2020, 15, 147–159. [Google Scholar] [CrossRef]
- Mishra, R.; Maiti, T.K.; Bhattacharyya, T.K. Feasibility Studies on Nafion Membrane Actuated Micropump Integrated with Hollow Microneedles for Insulin Delivery Device. J. Microelectromechanical Syst. 2019, 28, 987–996. [Google Scholar] [CrossRef]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.C.; Podoleanu, A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit. Manuf. 2021, 38, 101815. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Seo, M.-S.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef]
- Fakhraei Lahiji, S.; Jang, Y.; Ma, Y.; Dangol, M.; Yang, H.; Jang, M.; Jung, H. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme. Eur. J. Pharm. Sci. 2018, 117, 290–296. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Martanto, W.; Moore, J.S.; Couse, T.; Prausnitz, M.R. Mechanism of fluid infusion during microneedle insertion and retraction. J. Control. Release 2006, 112, 357–361. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Sheng, T.; Luo, B.; Zhang, W.; Ge, X.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-Mediated Vaccination: Innovation and Translation. Adv. Drug Deliv. Rev. 2021, 179, 113919. [Google Scholar] [CrossRef]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Stinson, J.A.; Raja, W.K.; Lee, S.; Kim, H.B.; Diwan, I.; Tutunjian, S.; Panilaitis, B.; Omenetto, F.G.; Tzipori, S.; Kaplan, D.L. Silk Fibroin Microneedles for Transdermal Vaccine Delivery. ACS Biomater. Sci. Eng. 2017, 3, 360–369. [Google Scholar] [CrossRef]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’Souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Shin, C.I.; Jeong, S.D.; Rejinold, N.S.; Kim, Y.-C. Microneedles for vaccine delivery: Challenges and future perspectives. Ther. Deliv. 2017, 8, 447–460. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of drugs with microneedles: Strategies and outcomes. J. Drug Deliv. Sci. Technol. 2015, 29, 16–23. [Google Scholar] [CrossRef]
- Kornstein, A.N. SERI Surgical Scaffold as an Adjunct to Conventional Brachioplasty. Plast. Reconstr. Surg. Glob. Open 2014, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.P.; Collin, T. Re: ‘Seri™: A surgical scaffold for breast reconstruction or for bacterial ingrowth?’. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 1629. [Google Scholar]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Wound Healing: Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized Silk Biomaterials for Wound Healing. Adv. Healthcare Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef]
- Smith, F.; Sabri, A.H.; Heppel, M.; Fonseca, I.; Chowdhury, F.; Cheung, K.; Willmor, S.; Rawson, F.; Marlow, M. The clinical and translational prospects of microneedle devices, with a focus on insulin therapy for diabetes mellitus as a case study. Int. J. Pharm. 2022, 628, 122234. [Google Scholar] [CrossRef]
- Wu, J.; Sahoo, J.K.; Li, Y.; Xu, Q.; Kaplan, D.L. Challenges in delivering therapeutic peptides and proteins: A silk-based solution. J. Control. Release 2022, 345, 176–189. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Kaplan, D.L. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020, 21, 1678–1686. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, X.; Hu, X.; Cebe, P.; Omenetto, F.; Kaplan, D.L. Stabilization and Release of Enzymes from Silk Films. Macromol. Biosci. 2010, 10, 359–368. [Google Scholar] [CrossRef]
- Kuzuhara, A.; Asakura, T.; Tomoda, R.; Matsunaga, T. Use of silk fibroin for enzyme membrane. J. Biotechnol. 1987, 5, 199–207. [Google Scholar] [CrossRef]
- Zhang, J.; Pritchard, E.; Hu, X.; Valentin, T.; Panilaitis, B.; Omenetto, F.G.; Kaplan, D.L. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc. Natl. Acad. Sci. USA 2012, 109, 11981–11986. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; Alkilani, A.Z.; Courtenay, A.J.; McCrudden, C.M.; McCloskey, B.; Walker, C.; Alshraiedeh, N.; Lutton, R.E.M.; Gilmore, B.F.; Woolfson, A.D.; et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 3–14. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef]


| Official Title | Type | Company | Application | Announced Date | Registration Certificate Number |
|---|---|---|---|---|---|
| AST-1 Silk Fibroin Wound Protective Dressing | Single-layer dressing | Soochow University (Suzhou Institute of Silk Technology) | It is used to prevent bacterial infection of the wound. It presents no irritation to the skin, can promote the growth of wound cells, and has good permeable steam. | 1995 | Su1995-95264098 |
| Silk Protein Wound Dressing | Double-layer dressing | Suzhou Soho Biomaterial Science and Technology Co., Ltd. | It is used for clinical second-degree wound healing; is suitable for mild and severe acne, skin allergies and laser photon treatment of early postoperative pigmentation; and reduces the formation of fatigue marks. | 2012 | Su2012-2640182 |
| SILK VOICE® Injectable Implant | Injectable implant | SOFREGEN MEDICAL Inc. | It is used for clinical second-degree wound healing; is suitable for mild and severe acne, skin allergies and laser photon treatment of early postoperative pigmentation; and reduces the formation of fatigue marks. | 2012 | - |
| Silk fibroin membrane dressing | Single-layer dressing | Zhejiang Xingyue Biotechnology Co., Ltd. | It covers the skin wound, blocks the external bacteria, and prevents the granulation tissue from growing into the dressing. At the same time, the water in the wound blood and exudate is discharged as water vapor to provide a healing environment for the wound, and the blood cells and other visible components left behind form a scab. | 2020 | SFDA2020-3140593 |
| SERI® Surgical Scaffold | Scaffold | SOFREGEN MEDICAL Inc. | A temporary brace used for soft tissue support and repair to strengthen defects where weaknesses or gaps exist. This includes soft tissue reinforcement in plastic and reconstructive surgery, as well as soft tissue reconstruction in general. | 2021 | - |
| Official Title | Type | Condition | Primary Purpose | Status | Start Date /Completion Date | Trial ID |
|---|---|---|---|---|---|---|
| A Multi-Center Open Study to Evaluate the SeriACL™ Device for Primary Anterior Cruciate Ligament Repair | ACL Reconstruction (SeriACL™ Device) | Phase 1 | Treatment | Unknown | 2007.6/ 2008.10 | NCT00490594 |
| Randomized, Active-controlled, Single-blind, Parallel Two-group Trial of HQ® Matrix Medical Wound Dressing and Sidaiyi® Wound Dressing for the Treatment of Donor Site Wounds | HQ® Matrix Medical Wound Dressing | Not Applicable | Treatment | Completed | 2013.8/ 2015.6 | NCT01993030 CQZ1800141 |
| Efficacy and Safety of Wound Dressing Containing Silk Fibroin with Bioactive Coating Layer Versus Medicated Paraffin Gauze Dressing in the Treatment of Split-thickness Skin Graft Donor Sites | Silk fibroin with bioactive coating layer dressing | Phase 1/2 | Treatment | Completed | 2014.3/ 2015.5 | NCT02091076 |
| A Prospective Open-Label Study to Evaluate the Safety of the Meniscal Repair Scaffold, FibroFix™ Meniscus, in the Treatment of Meniscal Defects | FibroFix™ Meniscus scaffold | Not Applicable | Treatment | Terminated (Safety devices explanted. 12 m post-explant safety f/u as agreed with UK MHRA) | 2015.4/ 2017.10 | NCT02205645 |
| Multi-center, Randomized, Active-controlled, Single-blind, Parallel Two-group Trial of HQ® Matrix Soft Tissue Mesh and ULTRAPRO® Partially Absorbable Lightweight Mesh for the Treatment of Inguinal Hernia | HQ® Matrix Soft Tissue Mesh | Not Applicable | Treatment | Unknown | 2015.7/ 2016.12 (estimated) | NCT02487628 |
| Comparison of Microbial Adherence to Various Sutures in Patients Undergoing Oral Surgery | Silk suture | Not Applicable | Treatment | Unknown | 2016.1/ 2017.1 (estimated) | NCT02653924 |
| A Pilot Feasibility Randomized Controlled Trial to Assess the Clinical and Cost Effectiveness of Dialkylcarbamoylchloride (DACC)-Coated Postoperative Dressings Versus Standard Care in the Prevention of Surgical Site Infection in Clean or Clean-contaminated Vascular and Cardiothoracic Surgery | DACC-Coated Post-Operative Dressing | Not Applicable | Treatment | Recruiting | 2017.1/ 2025.1 (estimated) | NCT02992951 |
| Silk Scaffold Surgical Incision Dressing Interventional Study | Experimental silk/adhesive prototype | Phase 1 | Assessment | Recruiting | 2022.8/ 2023.5 (estimated) | NCT05508945 |
| Safety and efficacy of absorbable silk fibroin film for alveolar ridge preservation after extraction | Absorbable silk fibroin film | Phase 3/4 | Assessment | Completed | - | CQZ1900597 ChiCTR-IOR-17025031 |
| Official Title | Type | Phase | Primary Purpose | Status | Start Date /Completion Date | Trial ID |
|---|---|---|---|---|---|---|
| Insulin Delivery Using Microneedles in Type 1 Diabetes | Hollow | Phase 2/3 | Treatment | Completed | 2009.2/ 2014.1 | NCT00837512 |
| Clinical Assessment of a Novel Microprobe Array Continuous Glucose Monitor for Type 1 Diabetes | Microprobe glucose sensor | Phase 1/2/3/4 | Diagnostic | Completed | 2013.11/ 2018.6 | NCT01908530 |
| A Phase I Study of the Safety, Reactogenicity, Acceptability and Immunogenicity of Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle | Dissolving microneedle | Phase 1 | Prevention | Completed | 2015.5/ 2019.7 | NCT02438423 |
| Microneedle Sensing of Beta-lactam Antibiotic Concentrations in Human Interstitial Fluid | Biosensors | Phase 1 | Device Feasibility | Completed | 2019.2/ 2020.12 | NCT03847610 |
| A clinical trial of dose-response using a microneedle array containing Japanese encephalitis vaccine in healthy adult individuals | Dissolving microneedle | Phase 1/2 | Prevention | Completed | 2019.7/ 2020.2 | jRCTs011190004 |
| A Phase I/II, Double-blind, Randomized, Active-controlled, Age De-escalation Trial to Assess the safety and Immunogenicity of a Measles Rubella Vaccine (MRV) Microneedle Patch (MRV-MNP) in Adults, MRV-primed Toddlers, and MRV-naïve Infants | Dissolving microneedle | Phase 1/2 | Assessment | Recruiting | 2021.5/ 2022.6 (estimated) | NCT04394689 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Yan, Z.; Tan, G.; Jia, T.; Geng, Y.; Shao, H.; Kundu, S.C.; Lu, S. Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed? Pharmaceutics 2023, 15, 355. https://doi.org/10.3390/pharmaceutics15020355
Qi Z, Yan Z, Tan G, Jia T, Geng Y, Shao H, Kundu SC, Lu S. Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed? Pharmaceutics. 2023; 15(2):355. https://doi.org/10.3390/pharmaceutics15020355
Chicago/Turabian StyleQi, Zhenzhen, Zheng Yan, Guohongfang Tan, Tianshuo Jia, Yiyu Geng, Huiyan Shao, Subhas C. Kundu, and Shenzhou Lu. 2023. "Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed?" Pharmaceutics 15, no. 2: 355. https://doi.org/10.3390/pharmaceutics15020355
APA StyleQi, Z., Yan, Z., Tan, G., Jia, T., Geng, Y., Shao, H., Kundu, S. C., & Lu, S. (2023). Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed? Pharmaceutics, 15(2), 355. https://doi.org/10.3390/pharmaceutics15020355