Fighting Epilepsy with Nanomedicines—Is This the Right Weapon?
Abstract
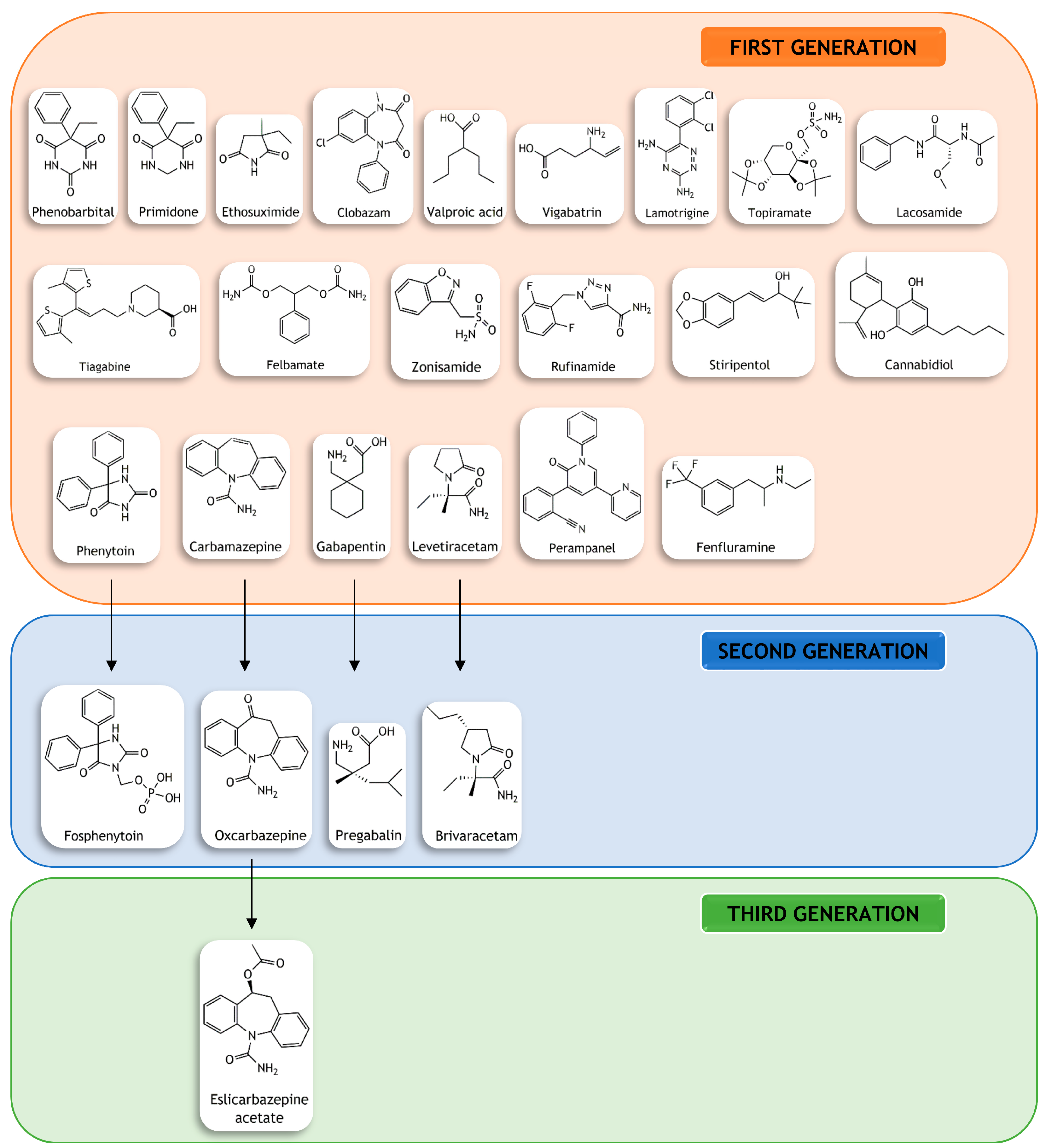
1. Introduction
2. Therapeutic Approaches
3. Barriers to the Development of New ASDs
3.1. Drug Resistance
3.2. Loss of Efficacy
3.3. Poor Safety Profile
3.4. Loss of Industry Enthusiasm
4. Are Nanomedicines the Solution?
4.1. Nanoformulated Antiseizure Drugs
4.1.1. Increase in Drug Brain Penetration
4.1.2. Reduction in Drug Adverse Effects
4.1.3. Overcoming P-gp-Mediated Drug Resistance
4.2. Nanoformulated Antiseizure Drug Candidates
4.2.1. Increase in Compound’s Brain Penetration
4.2.2. Overcoming Compound’s Poor Water Solubility
4.2.3. Reduction in Compound’s Toxicity
4.3. Intranasal Administration
5. Nanoparticles for Diagnosis and Theragnostics
6. Critical Overview
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yogarajah, M.; Mula, M. Social cognition, psychiatric comorbidities, and quality of life in adults with epilepsy. Epilepsy Behav. 2019, 100, 106321. [Google Scholar] [CrossRef]
- World Health Organization. Epilepsy. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 17 June 2022).
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Moshé, S.L. The evolution of the concepts of seizures and epilepsy: What’s in a name? Epilepsia Open 2020, 5, 22–35. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel Jr., J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T. New classification efforts in epilepsy: Opportunities for clinical neurosciences. Epilepsy Behav. 2016, 64, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Santulli, L.; Coppola, A.; Balestrini, S.; Striano, S. The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol. Res. 2016, 107, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Raut, D.; Bhatt, L.K. Evolving targets for anti-epileptic drug discovery. Eur. J. Pharmacol. 2020, 887, 173582. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, K.; Gryta, P.; Łuszczki, J.; Czuczwar, S.J. Exploring the latest avenues for antiepileptic drug discovery and development. Expert Opin. Drug Discov. 2016, 11, 369–382. [Google Scholar] [CrossRef]
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 2021, 265, 118826. [Google Scholar] [CrossRef]
- Gross, C.; Tiwari, D. Regulation of ion channels by microRNAs and the implication for epilepsy. Curr. Neurol. Neurosci. Rep. 2018, 18, 60. [Google Scholar] [CrossRef]
- Wells, B.G.; Dipiro, J.T.; Schwinghammer, T.L.; Dipiro, C.V. Pharmacotherapy Handbook; McGraw-Hill Medical: New York, NY, USA, 2009; ISBN 9780071643269. [Google Scholar]
- Beghi, E. The epidemiology of epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.P. The impact of epilepsy on patients’ lives. Acta Neurol. Scand. 2012, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nilo, A.; Gelisse, P.; Crespel, A. Genetic/idiopathic generalized epilepsies: Not so good as that! Rev. Neurol. 2020, 176, 427–438. [Google Scholar] [CrossRef]
- Tolchin, B.; Hirsch, L.J.; LaFrance, W.C. Neuropsychiatric aspects of epilepsy. Psychiatr. Clin. North Am. 2020, 43, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Brown, C. Pharmacological management of epilepsy. Prog. Neurol. Psychiatry 2016, 20, 27–34. [Google Scholar] [CrossRef]
- Johannessen Landmark, C.; Johannessen, S.I.; Patsalos, P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Odi, R.; Bibi, D.; Wager, T.; Bialer, M. A perspective on the physicochemical and biopharmaceutic properties of marketed antiseizure drugs—From phenobarbital to cenobamate and beyond. Epilepsia 2020, 61, 1543–1552. [Google Scholar] [CrossRef]
- Matias, M.; Campos, G.; Silvestre, S.; Falcão, A.; Alves, G. Early preclinical evaluation of dihydropyrimidin(thi)ones as potential anticonvulsant drug candidates. Eur. J. Pharm. Sci. 2017, 102, 264–274. [Google Scholar] [CrossRef]
- Dalkara, S.; Karakurt, A. Recent progress in anticonvulsant drug research: Strategies for anticonvulsant drug development and applications of antiepileptic drugs for non-epileptic central nervous system disorders. Curr. Top. Med. Chem. 2012, 12, 1033–1071. [Google Scholar] [CrossRef]
- French, J.A.; Perucca, E. Time to Start Calling Things by Their Own Names? The Case for Antiseizure Medicines. Epilepsy Curr. 2020, 20, 69–72. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.W.; Lee, S.T.; Byun, J.I.; Seo, J.G.; No, Y.J.; Kang, K.W.; Kim, D.; Kim, K.T.; Cho, Y.W.; et al. Antiepileptic drug selection according to seizure type in adult patients with epilepsy. J. Clin. Neurol. 2020, 16, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Park, K.M.; Kim, S.E.; Heo, K. Clinical opinion: Earlier employment of polytherapy in sequential pharmacotherapy of epilepsy. Epilepsy Res. 2019, 156, 106165. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Kim, D.W.; Yang, K.I.; Lee, S.T.; Byun, J.I.; Seo, J.G.; No, Y.J.; Kang, K.W.; Kim, D.; Cho, Y.W. Refining general principles of antiepileptic drug treatments for epilepsy. J. Clin. Neurol. 2020, 16, 383–389. [Google Scholar] [CrossRef]
- Verrotti, A.; Lattanzi, S.; Brigo, F.; Zaccara, G. Pharmacodynamic interactions of antiepileptic drugs: From bench to clinical practice. Epilepsy Behav. 2020, 104, 106939. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E. Medical management of refractory epilepsy-Practical treatment with novel antiepileptic drugs. Epilepsia 2014, 55, 3–8. [Google Scholar] [CrossRef]
- Brodie, M.J. Pharmacological treatment of drug-resistant epilepsy in adults: A practical guide. Curr. Neurol. Neurosci. Rep. 2016, 16, 82. [Google Scholar] [CrossRef]
- Perucca, E.; French, J.; Bialer, M. Development of new antiepileptic drugs: Challenges, incentives, and recent advances. Lancet Neurol. 2007, 6, 793–804. [Google Scholar] [CrossRef]
- Bialer, M. New antiepileptic drugs that are second generation to existing antiepileptic drugs. Expert Opin. Investig. Drugs 2006, 15, 637–647. [Google Scholar] [CrossRef]
- Alqahtani, F.; Imran, I.; Pervaiz, H.; Ashraf, W.; Perveen, N.; Rasool, M.F.; Alasmari, A.F.; Alharbi, M.; Samad, N.; Alqarni, S.A.; et al. Non-pharmacological interventions for intractable epilepsy. Saudi Pharm. J. 2020, 28, 951–962. [Google Scholar] [CrossRef]
- Gschwind, M.; Seeck, M. Modern management of seizures and epilepsy. Swiss Med. Wkly 2016, 146, w14310. [Google Scholar] [CrossRef]
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117, 114–125. [Google Scholar] [CrossRef] [PubMed]
- McGovern, R.A.; Banks, G.P.; McKhann, G.M. New Techniques and Progress in Epilepsy Surgery. Curr. Neurol. Neurosci. Rep. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wu, D.; Li, X.; Ren, L.; Wang, Y. Towards precision medicine in epilepsy surgery. Ann. Transl. Med. 2016, 4, 24. [Google Scholar] [CrossRef]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T.; Neuroscience, L. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Aznarez, P.B.; Cabeza, M.P.; Quintana, A.S.A.; Lara-Almunia, M.; Sanchez, J.A. Evolution of patients with surgically treated drug-resistant occipital lobe epilepsy. Surg. Neurol. Int. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.W.; McGovern, R.A.; Wang, S.G.; Chen, C.C.; Park, M.C. Resective epilepsy surgery: Assessment of randomized controlled trials. Neurosurg. Rev. 2021, 44, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Matricardi, S.; Pastorino, G.M.G.; Verrotti, A.; Coppola, G. The ketogenic diet for the treatment of mood disorders in comorbidity with epilepsy in children and adolescents. Front. Pharmacol. 2020, 11, 578396. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Shi, R.; Song, Z.; Wu, J. Metabolic control of epilepsy: A promising therapeutic target for epilepsy. Front. Neurol. 2020, 11, 592514. [Google Scholar] [CrossRef]
- Merlotti, D.; Cosso, R.; Eller-Vainicher, C.; Vescini, F.; Chiodini, I.; Gennari, L.; Falchetti, A. Energy metabolism and ketogenic diets: What about the skeletal health? a narrative review and a prospective vision for planning clinical trials on this issue. Int. J. Mol. Sci. 2021, 22, 435. [Google Scholar] [CrossRef]
- Löscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 2013, 12, 757–776. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Janmohamed, M.; Brodie, M.J.; Kwan, P. Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020, 168, 107790. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 3, 18024. [Google Scholar] [CrossRef]
- Méreaux, J.L.; Gilard, V.; Le Goff, F.; Chastan, N.; Magne, N.; Gerardin, E.; Maltête, D.; Lebas, A.; Derrey, S. Practice of stereoelectroencephalography (sEEG) in drug-resistant epilepsy: Retrospective series with surgery and thermocoagulation outcomes. Neurochirurgie 2020, 66, 139–143. [Google Scholar] [CrossRef]
- Miziak, B.; Chroscinska-Krawczyk, M.; Błaszczyk, B.; Radzik, I.; Czuczwar, S.J. Novel approaches to anticonvulsant drug discovery. Expert Opin. Drug Discov. 2012, 7, 417–428. [Google Scholar] [CrossRef]
- Franco, V.; French, J.A.; Perucca, E. Challenges in the clinical development of new antiepileptic drugs. Pharmacol. Res. 2016, 103, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs a 30-year longitudinal cohort study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]
- de Almeida Campos, M.S.; Ayres, L.R.; Morelo, M.R.S.; Carizio, F.A.M.; Pereira, L.R.L. Comparative efficacy of antiepileptic drugs for patients with generalized epileptic seizures: Systematic review and network meta-analyses. Int. J. Clin. Pharm. 2018, 40, 589–598. [Google Scholar] [CrossRef]
- Angelova, V.; Karabeliov, V.; Andreeva-Gateva, P.A.; Tchekalarova, J. Recent developments of hydrazide/hydrazone derivatives and their analogs as anticonvulsant agents in animal models. Drug Dev. Res. 2016, 77, 379–392. [Google Scholar] [CrossRef]
- Kamiński, K.; Socała, K.; Zagaja, M.; Andres-Mach, M.; Abram, M.; Jakubiec, M.; Pieróg, M.; Nieoczym, D.; Rapacz, A.; Gawel, K.; et al. N-Benzyl-(2,5-dioxopyrrolidin-1-yl)propanamide (AS-1) with hybrid structure as a candidate for a broad-spectrum antiepileptic drug. Neurotherapeutics 2020, 17, 309–328. [Google Scholar] [CrossRef]
- Golyala, A.; Kwan, P. Drug development for refractory epilepsy: The past 25 years and beyond. Seizure 2017, 44, 147–156. [Google Scholar] [CrossRef]
- Simonato, M.; Brooks-Kayal, A.R.; Engel, J., Jr.; Galanopoulou, A.S.; Jensen, F.E.; Moshé, S.L.; O’Brien, T.J.; Pitkanen, A.; Wilcox, K.S.; French, J.A. The challenge and promise of anti-epileptic therapy development in animal models. Lancet Neurol. 2014, 13, 949–960. [Google Scholar] [CrossRef]
- Perucca, E. Antiepileptic drugs: Evolution of our knowledge and changes in drug trials. Epileptic Disord. 2019, 21, 319–329. [Google Scholar] [CrossRef]
- Loscher, W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016, 126, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshé, S.L.; Perucca, E.; Engel, J.; Löscher, W.; Noebels, J.L.; Pitkänen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Kowski, A.B.; Weissinger, F.; Gaus, V.; Fidzinski, P.; Losch, F.; Holtkamp, M. Specific adverse effects of antiepileptic drugs—A true-to-life monotherapy study. Epilepsy Behav. 2016, 54, 150–157. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Mula, M.; Cock, H.R. More than seizures: Improving the lives of people with refractory epilepsy. Eur. J. Neurol. 2015, 22, 24–30. [Google Scholar] [CrossRef]
- Singh, K.P.; Verma, N. Teratogenic potential of third-generation antiepileptic drugs: Current status and research needs. Pharmacol. Reports 2019, 71, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Mostacci, B.; Ranzato, F.; Giuliano, L.; La Neve, A.; Aguglia, U.; Bilo, L.; Durante, V.; Ermio, C.; Monti, G.; Zambrelli, E.; et al. Alternatives to valproate in girls and women of childbearing potential with Idiopathic Generalized Epilepsies: State of the art and guidance for the clinician proposed by the Epilepsy and Gender Commission of the Italian League Against Epilepsy (LICE). Seizure Eur. J. Epilepsy 2021, 85, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2021, 18, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef] [PubMed]
- Anoop, V.; Cutinho, L.I.; Mourya, P.; Maxwell, A.; Thomas, G.; Rajput, B.S. Approaches for encephalic drug delivery using nanomaterials: The current status. Brain Res. Bull. 2020, 155, 184–190. [Google Scholar] [CrossRef]
- Farinha, P.; Pinho, J.O.; Matias, M.; Gaspar, M.M. Nanomedicines in the treatment of colon cancer: A focus on metallodrugs. Drug Deliv. Transl. Res. 2022, 12, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric nanoparticles properties and brain delivery. Pharmaceutics 2021, 13, 2045. [Google Scholar] [CrossRef]
- Rosillo-de la Torre, A.; Luna-Bárcenas, G.; Orozco-Suárez, S.; Salgado-Ceballos, H.; García, P.; Lazarowski, A.; Rocha, L. Pharmacoresistant epilepsy and nanotechnology. Front. Biosci. 2014, 6, 329–340. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Darłak, P.; Markiewicz, A.; Sikora, J.; Kumar Adla, S.; Bagina, S.; Huttunen, K.M. Current approaches to facilitate improved drug delivery to the central nervous system. Eur. J. Pharm. Biopharm. 2022, 181, 249–262. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.O.; Matias, M.; Gaspar, M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials 2019, 9, 1455. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Nguyễn, H.; Hsieh, P.C.H. Emerging nano-carrier strategies for brain tumor drug delivery and considerations for clinical translation. Pharmaceutics 2021, 13, 1193. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid lipid nanoparticles (SLNs): An advanced drug delivery system targeting brain through bbb. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In vitro studies on nasal formulations of nanostructured lipid carriers (NLC) and solid lipid nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.M.; Islam, F.; Emran, T.B.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles—Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef]
- Shende, P.; Trivedi, R. Nanotheranostics in epilepsy: A perspective for multimodal diagnosis and strategic management. Nano Sel. 2021, 2, 1277–1290. [Google Scholar] [CrossRef]
- Long, Q.; Li, J.; Luo, Q.; Hei, Y.; Wang, K.; Tian, Y.; Yang, J.; Lei, H.; Qiu, B.; Liu, W. MRI tracking of bone marrow mesenchymal stem cells labeled with ultra-small superparamagnetic iron oxide nanoparticles in a rat model of temporal lobe epilepsy. Neurosci. Lett. 2015, 606, 30–35. [Google Scholar] [CrossRef]
- Zingale, E.; Bonaccorso, A.; Carbone, C.; Musumeci, T.; Pignatello, R. Drug Nanocrystals: Focus on Brain Delivery from Therapeutic to Diagnostic Applications. Pharmaceutics 2022, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Higazy, I.M. Lamotrigine loaded poly-ε-(d,l-lactide-co-caprolactone) nanoparticles as brain delivery system. Eur. J. Pharm. Sci. 2018, 115, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wang, Y.; Liang, J.; Yue, J.; Xu, C.; Lu, L.; Xu, Z.; Gao, J.; Du, Y.; Chen, Z. Angiopep-conjugated electro-responsive hydrogel nanoparticles: Therapeutic potential for epilepsy. Angew. Chemie—Int. Ed. 2014, 53, 12436–12440. [Google Scholar] [CrossRef]
- Wilson, B.; Lavanya, Y.; Priyadarshini, S.R.B.; Ramasamy, M.; Jenita, J.L. Albumin nanoparticles for the delivery of gabapentin: Preparation, characterization and pharmacodynamic studies. Int. J. Pharm. 2014, 473, 73–79. [Google Scholar] [CrossRef]
- Qushawy, M.; Prabahar, K.; Abd-Alhaseeb, M.; Swidan, S.; Nasr, A. Preparation and evaluation of carbamazepine solid lipid nanoparticle for alleviating seizure activity in pentylenetetrazole-kindled mice. Molecules 2019, 24, 3971. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Lv, Y.; Xiao, Q.; Ye, L.; Cai, B.; Qin, C.; Han, X.; Cai, T.; Yin, L. Denatured protein stabilized drug nanoparticles: Tunable drug state and penetration across the intestinal barrier. J. Mater. Chem. B 2017, 5, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Ugur Yilmaz, C.; Emik, S.; Orhan, N.; Temizyurek, A.; Atis, M.; Akcan, U.; Khodadust, R.; Arican, N.; Kucuk, M.; Gurses, C.; et al. Targeted delivery of lacosamide-conjugated gold nanoparticles into the brain in temporal lobe epilepsy in rats. Life Sci. 2020, 257, 118081. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; del V. Alonso, S.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, S.; Qin, J.; Chen, B.; Ni, G.; Chen, Z.; Zhou, J.; Li, Z.; Ning, Y.; Wu, C.; et al. Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials 2016, 97, 110–121. [Google Scholar] [CrossRef]
- Rosillo-de la Torre, A.; Zurita-Olvera, L.; Orozco-Suárez, S.; Garcia Casillas, P.E.; Salgado-Ceballos, H.; Luna-Bárcenas, G.; Rocha, L. Phenytoin carried by silica core iron oxide nanoparticles reduces the expression of pharmacoresistant seizures in rats. Nanomedicine 2015, 10, 3563–3577. [Google Scholar] [CrossRef]
- Zybina, A.; Anshakova, A.; Malinovskaya, J.; Melnikov, P.; Baklaushev, V.; Chekhonin, V.; Maksimenko, O.; Titov, S.; Balabanyan, V.; Kreuter, J.; et al. Nanoparticle-based delivery of carbamazepine: A promising approach for the treatment of refractory epilepsy. Int. J. Pharm. 2018, 547, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Y.; Zhang, J.; Li, J.; Yu, X.; Cao, Z.; Meng, F.; Zhao, Y.; Wu, X.; Shen, T.; et al. Functionalized nanocarrier combined seizure-specific vector with P-glycoprotein modulation property for antiepileptic drug delivery. Biomaterials 2016, 74, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, X.; Chen, L.; Liu, Y.; Wang, Y.; Liang, J.; Xu, C.; Guo, Y.; Wang, S.; Hu, W.; et al. Electroresponsive nanoparticles improve antiseizure effect of phenytoin in generalized tonic-clonic seizures. Neurotherapeutics 2016, 13, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Sbaraglini, M.L.; Talevi, A.; Couyoupetrou, M.; Di Ianni, M.; Pesce, G.O.; Alvarez, V.A.; Bruno-Blanch, L.E.; Castro, G.R.; Ruiz, M.E.; et al. Carbamazepine-loaded solid lipid nanoparticles and nanostructured lipid carriers: Physicochemical characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 167, 73–81. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Calpena, A.C.; Folch, J.; Barenys, M.; Sánchez-López, E.; Camins, A.; García, M.L. Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1073–1085. [Google Scholar] [CrossRef]
- Ren, T.; Hu, M.; Cheng, Y.; Shek, T.L.; Xiao, M.; Ho, N.J.; Zhang, C.; Leung, S.S.Y.; Zuo, Z. Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control. Eur. J. Pharm. Sci. 2019, 137, 104988. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Y.; Lin, L.; Song, S.; Cheng, L.; Zhu, R. Solid lipid nanoparticles enhanced the neuroprotective role of curcumin against epilepsy through activation of Bcl-2 family and P38 MAPK pathways. ACS Chem. Neurosci. 2020, 11, 1985–1995. [Google Scholar] [CrossRef]
- Yurtdaş Kırımlıoğlu, G.; Menceloğlu, Y.; Erol, K.; Yazan, Y. In vitro/in vivo evaluation of gamma-aminobutyric acid-loaded N,N-dimethylacrylamide-based pegylated polymeric nanoparticles for brain delivery to treat epilepsy. J. Microencapsul. 2016, 33, 625–635. [Google Scholar] [CrossRef]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef]
- Kohane, D.S.; Holmes, G.L.; Chau, Y.; Zurakowski, D.; Langer, R.; Cha, B.H. Effectiveness of muscimol-containing microparticles against pilocarpine-induced focal seizures. Epilepsia 2002, 43, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Chorilli, M. Characteristics, biological properties and analytical methods of piperine: A review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Recent Highlights on Molecular Hybrids Potentially Useful in Central Nervous System Disorders. Mini Rev. Med. Chem. 2017, 17, 486–517. [Google Scholar] [CrossRef] [PubMed]
- Loeb, C.; Besio, G.; Mainardi, P.; Scotto, P.; Benassi, E.; Bo, G.P. Liposome-entrapped γ-aminobutyric acid inhibits isoniazid-induced epileptogenic activity in rats. Epilepsia 1986, 27, 98–102. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Considerations and pitfalls in selecting the drug vehicles for evaluation of new drug candidates: Focus on in vivo pharmaco-toxicological assays based on the rotarod performance test. J. Pharm. Pharm. Sci. 2018, 21, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.R.; Hashemian, M.; Khalili-Fomeshi, M.; Ashrafpour, M.; Moghadamnia, A.A.; Ghasemi-Kasman, M. Upregulation of klotho and erythropoietin contributes to the neuroprotection induced by curcumin-loaded nanoparticles in experimental model of chronic epilepsy. Brain Res. Bull. 2018, 142, 281–288. [Google Scholar] [CrossRef]
- Heiss, J.D.; Argersinger, D.P.; Theodore, W.H.; Butman, J.A.; Sato, S.; Khan, O.I. Convection-enhanced delivery of muscimol in patients with drug-resistant epilepsy. Clin. Neurosurg. 2019, 85, E4–E15. [Google Scholar] [CrossRef]
- Mareš, P.; Tichá, K.; Mikulecká, A. Anticonvulsant and behavioral effects of muscimol in immature rats. Brain Res. 2014, 1582, 227–236. [Google Scholar] [CrossRef]
- Musumeci, T.; Serapide, M.F.; Pellitteri, R.; Dalpiaz, A.; Ferraro, L.; Dal Magro, R.; Bonaccorso, A.; Carbone, C.; Veiga, F.; Sancini, G.; et al. Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur. J. Pharm. Biopharm. 2018, 133, 309–320. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N. Superparamagnetic iron oxide–loaded lipid nanocarriers incorporated in thermosensitive in situ gel for magnetic brain targeting of clonazepam. J. Pharm. Sci. 2018, 107, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Fazendeiro, A.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Nose-to-brain delivery of phenytoin and its hydrophilic prodrug fosphenytoin combined in a microemulsion—Formulation development and in vivo pharmacokinetics. Eur. J. Pharm. Sci. 2021, 164, 105918. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J.; Amir, M. Impact of ultrasonication techniques on the preparation of novel Amiloride-nanoemulsion used for intranasal delivery in the treatment of epilepsy. Artif. Cells Nanomed. Biotechnol. 2018, 46, S192–S207. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Amir, M.; Sarafroz, M. Quantification and Evaluations of Catechin Hydrate Polymeric Nanoparticles Used in Brain Targeting for the Treatment of Epilepsy. Pharmaceutics. 2020, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al Qatifi, S.; Alessa, M.; Al Hajji, H.; Sarafroz, M. A bioanalytical UHPLC based method used for the quantification of thymoquinone-loaded-PLGA-nanoparticles in the treatment of epilepsy. BMC Chem. 2020, 14, 10. [Google Scholar] [CrossRef]
- Velly, J.; Grima, M.; Decker, N.; Cragoe, E.J.; Schwartz, J. Effects of amiloride and its analogues on [3H]batrachotoxinin-A 20-α benzoate binding, [3H]tetracaine binding and 22Na influx. Eur. J. Pharmacol. 1988, 149, 97–105. [Google Scholar] [CrossRef]
- Liang, J.-J.; Huang, L.-F.; Chen, X.-M.; Pan, S.-Q.; Lu, Z.-N.; Xiao, Z.-M. Amiloride suppresses pilocarpine-induced seizures via ASICs other than NHE in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 14507–14513. [Google Scholar]
- Hosseinzadeh, H.; Parvardeh, S.; Nassiri-Asl, M.; Mansouri, M.-T. Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2005, 11, BR106–BR110. [Google Scholar]
- Akhtari, M.; Bragin, A.; Cohen, M.; Moats, R.; Brenker, F.; Lynch, M.D.; Vinters, H.V.; Engel, J. Functionalized magnetonanoparticles for MRI diagnosis and localization in epilepsy. Epilepsia 2008, 49, 1419–1430. [Google Scholar] [CrossRef]
- Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-zadeh, E. MRI—Guided epilepsy detection. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 4001–4004. [Google Scholar]
- Portnoy, E.; Polyak, B.; Inbar, D.; Kenan, G.; Rai, A.; Wehrli, S.L.; Roberts, T.P.L.; Bishara, A.; Mann, A.; Shmuel, M.; et al. Tracking inflammation in the epileptic rat brain by bi-functional fluorescent and magnetic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Kong, Q.; Sheng, H.; Gao, L. Value of functionalized superparamagnetic iron oxide nanoparticles in the diagnosis and treatment of acute temporal lobe epilepsy on MRI. Neural Plast. 2016, 2016, 2412958. [Google Scholar] [CrossRef] [PubMed]
- Kandilli, B.; Ugur Kaplan, A.B.; Cetin, M.; Taspinar, N.; Ertugrul, M.S.; Aydin, I.C.; Hacimuftuoglu, A. Carbamazepine and levetiracetam-loaded PLGA nanoparticles prepared by nanoprecipitation method: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2020, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- So, E.L.; Ruggles, K.H.; Cascino, G.D.; Ahmann, P.A.; Weatherford, K.W. Seizure exacerbation and status epileptics related to carbamazepine-10,11-epoxide. Ann. Neurol. 1994, 35, 743–746. [Google Scholar] [CrossRef]
- Simper, G.S.; Hò, G.-G.T.; Celik, A.A.; Huyton, T.; Kuhn, J.; Kunze-Schumacher, H.; Blasczyk, R.; Bade-Döding, C. Carbamazepine-Mediated Adverse Drug Reactions: CBZ-10,11-epoxide but Not Carbamazepine Induces the Alteration of Peptides Presented by HLA-B∗15:02. J. Immunol. Res. 2018, 2018, 5086503. [Google Scholar] [CrossRef]
- Meenu, M.; Reeta, K.H.; Dinda, A.K.; Kottarath, S.K.; Gupta, Y.K. Evaluation of sodium valproate loaded nanoparticles in acute and chronic pentylenetetrazole induced seizure models. Epilepsy Res. 2019, 158, 106219. [Google Scholar] [CrossRef]
- Bonilla, L.; Esteruelas, G.; Ettcheto, M.; Espina, M.; García, M.L.; Camins, A.; Souto, E.B.; Cano, A.; Sánchez-López, E. Biodegradable nanoparticles for the treatment of epilepsy: From current advances to future challenges. Epilepsia Open 2022, 7, S121–S132. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Page, K.M. Validation of Early Human Dose Prediction: A Key Metric for Compound Progression in Drug Discovery. Mol. Pharm. 2016, 13, 609–620. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Margineanu, D.G. Systems biology, complexity, and the impact on antiepileptic drug discovery. Epilepsy Behav. 2014, 38, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-resistant epilepsy: Multiple hypotheses, few answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.E.; Mirza, N.; Yip, V.L.M.; Marson, A.G.; Pirmohamed, M. Personalized medicine approaches in epilepsy. J. Intern. Med. 2015, 277, 218–234. [Google Scholar] [CrossRef]
- Wolking, S.; Schulz, H.; Nies, A.T.; Mccormack, M.; Schaeffeler, E.; Auce, P.; Avbersek, A.; Becker, F.; Klein, K.M.; Krenn, M.; et al. Pharmacoresponse in genetic generalized epilepsy: A genome-wide association study. Pharmacogenomics 2020, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.; Fortuna, A.; Falcão, A.; Alves, G. In vitro and in vivo experimental models employed in the discovery and development of antiepileptic drugs for pharmacoresistant epilepsy. Epilepsy Res. 2018, 146, 63–86. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Pawlik, M.J.; Miziak, B.; Walczak, A.; Konarzewska, A.; Chrościńska-Krawczyk, M.; Albrecht, J.; Czuczwar, S.J. Selected molecular targets for antiepileptogenesis. Int. J. Mol. Sci. 2021, 22, 9737. [Google Scholar] [CrossRef]
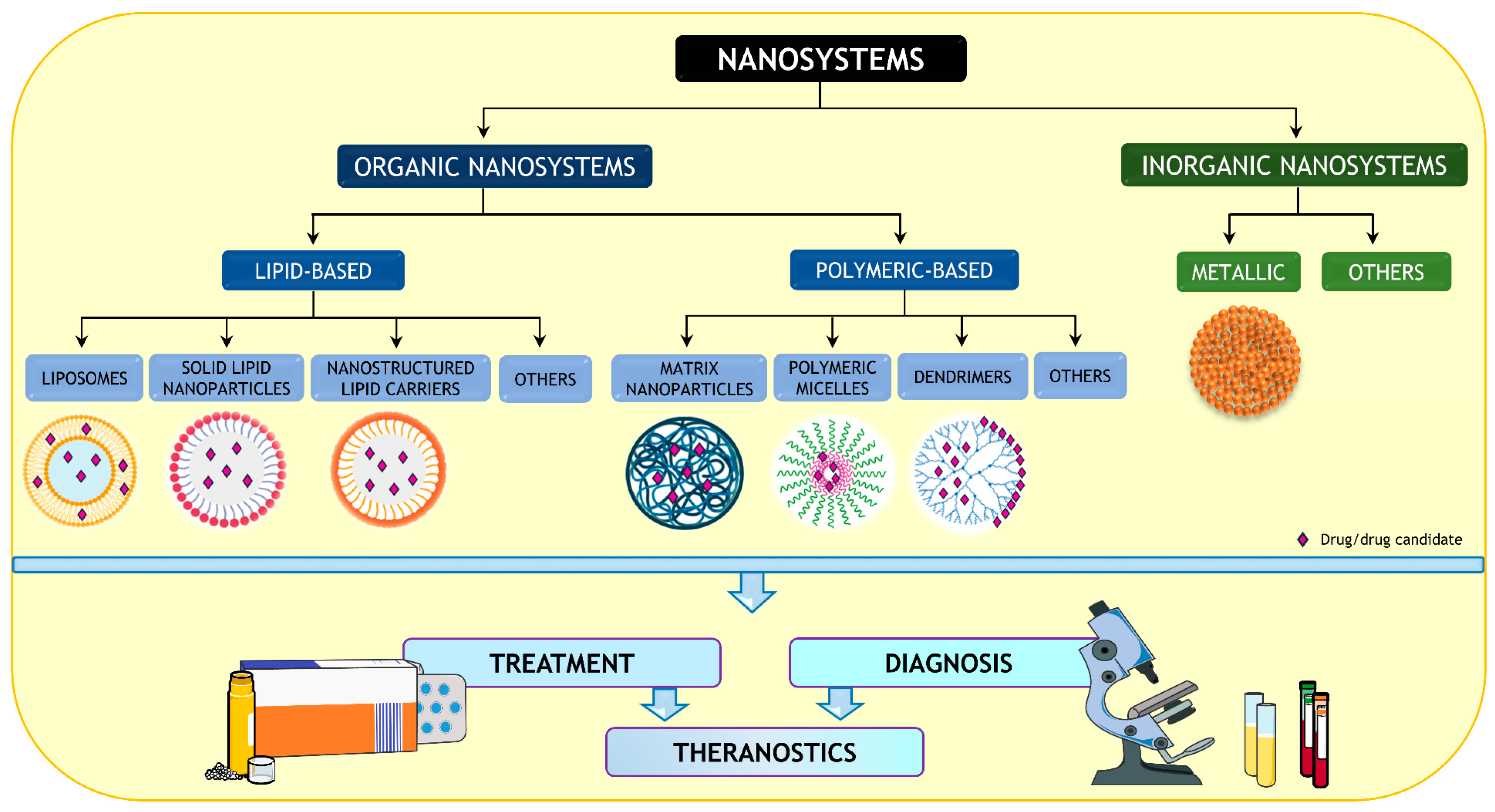
| Entry | Bioactive Compound | Main NP Composition (Size [nm], PdI) | Type of Study: Animal Model; Protocol (Positive Control) | PD Effect | Advantages of NPs | Reference |
|---|---|---|---|---|---|---|
| 1 | Lamotrigine | PLCL:Poloxamer 407 * (125, 0.184) | PK: Wistar Albino rats; i.v. single dose (oral lamotrigine tablet) | _ | Increased brain penetration | [84] |
| 2 | Phenytoin | ANG-DMAEMA: NaSS:ST:ACLT-PEG-NHS:MBA (130.8 ± 22.4) | PD: Sprague-Dawley rats, amygdala kindling (chronic model); i.p., 10, 20 and 50 mg/kg, single dose (free phenytoin) | NPs lowered seizure stages and the severity of the seizure behaviour, in comparison with free phenytoin | Increased brain penetration | [85] |
| 3 | Gabapentin | Albumin:NaCl solution:Glutaraldehyde solution (141.9) | PD: Wistar rats, MES, PTZ (acute models); i.p., 50 mg/kg, single dose (free gabapentin) | Reduced the duration and average time of all phases of convulsion by polysorbate 80 coated NPs, compared with other formulations | Increased brain penetration | [86] |
| 4 | CBZ | GMS:poloxamer 188 (45.1 ± 6.7, 0.277 ± 0.03) | PD: Albino mice, PTZ, PTZ-induced kindling (chronic model); p.o., 50 mg/kg, single dose (free CBZ) | Prolonged time to death after a lethal dose of PTZ (3720 ± 245 s) compared with the free drug (2340 ± 141 s); reduced seizure score (15 and 35%, respectively) | Improved absorption profile and penetration | [87] |
| 5 | Stiripentol | Nanosuspensions stabilized with denatured soybean protein isolate | PK: Sprague-Dawley rats; p.o., single dose | _ | Increased bioavailability; penetration across the intestinal barrier (in vitro and ex vivo) | [88] |
| 6 | Lacosamide | Gold-NPs:glucose (1.1, 0.252) | PD: Wistar rats, KA (chronic model); i.v., 62.5 μg/mL, single dose | Decreased amplitude and frequency of EEG-waves in both ictal and interictal stages Decreased number of seizures (not statistically significant) | Increased brain penetration | [89] |
| 7 | CBZ | PAMAM dendrimers DG4.5 | Zebrafish larvae; 0.3–30 μM (free CBZ) | _ | Reduced side effects Increased water solubility | [90] |
| 8 | Phenytoin | Poloxamer 235 *-PBCA (268.0 ± 2.5, 0.09 ± 0.01) | PD: Sprague-Dawley rats, lithium-pilocarpine, phenytoin-resistance (chronic models); i.p., 75 mg/kg, followed by twice daily 50 mg/kg (free phenytoin, free phenytoin + tariquidar) | Reduced seizure frequency, similar to phenytoin + tariquidar group | Reduced drug resistance Increased brain penetration | [91] |
| 9 | Phenytoin | Iron oxide NPs:silica (24.3 ± 9.93) | PD: Wistar rats, 3-MPA resistant model (P-gp overexpression) (chronic model); i.p., 120 mg/kg (free phenytoin, 75 mg/kg) | Significant reduced prevalence of clonic (40%) and tonic–clonic seizures (20%) No observed significant changes in myoclonic seizures | Reduced drug resistance | [92] |
| 10 | CBZ | Poloxamer 188:PLGA:PVA (130–150, ~0.2) | PD: Wistar rats, INH (acute model); i.v., 0.7–5 mg/kg, single dose (free CBZ) | Minimum effective dose of 1 mg/kg vs. 30 mg/kg of free compound Delayed seizure onset and reduced their duration and intensity | Reduced drug resistance | [93] |
| 11 | Lamotrigine | Poloxamer 403 and 407 *-CDI:tryptophan derivative (20) | PK: Sprague-Dawley rats, pilocarpine; i.v., 10 mg/kg, single dose (free lamotrigine) | _ | Reduced drug resistance (in vitro and ex vivo) Increased brain penetration | [94] |
| Entry | Bioactive Compound | Main NP Composition (Size [nm], PdI) | Type of Study: Animal Model; Protocol (Positive Control) | PD Effect | Advantages of NPs | Reference |
|---|---|---|---|---|---|---|
| 1 | EGCG | PLGA:PEG, Tween 80 (168.5 ± 9.9, <0.1) | PD: C57BL/6J mice, KA (acute model); i.p., 30 mg/kg, single dose (free EGCG) | Significant reduction of the temporal lobe epilepsy patterns (56.1% versus 36.6% for free compound) | Prolonged duration of action Protection from degradation | [98] |
| 2 | Piperine | Eudragit S100: poloxamer 188 (130.2 ± 1.6, 0.195 ± 0.002) | PD: Kunming mice, PTZ (acute model); p.o., 7.5 and 15 mg/kg, single dose (free piperine) | No seizure (15 mg/kg) or reduced seizure frequency and delayed onset of seizure (7.5 mg/kg) was found for piperine nanosuspensions. Free drug failed to prevent the PTZ-induced seizure | Increased oral bioavailability Increased brain penetration | [99] |
| 3 | Curcumin | SA:lecithin (117.9) | PD: C57BL/6 mice, KA (chronic model); single dose (free curcumin) | Mice showed greater exploring ability than free curcumin in the open field test | Increased brain penetration | [100] |
| 4 | GABA | NMBAc-DMAc-PEG-2000 (124.4 ± 0.8, 0.238 ± 0.016) | PD: Wistar rats, PTZ (acute model); i.p., 100 mg/kg, single dose (free GABA) | Retarded latency time; decreased ending time and duration of seizure, compared to free GABA | Increased brain penetration | [101] |
| 5 | Curcumin | CH-ALG-STPP (50) | PD: NMRI mice, PTZ-induced kindling (chronic model); i.p., 12.5 and 25 mg/kg, daily, 10 days (free curcumin) | Decreased seizures stage and reduced duration of generalized tonic-clonic seizures, compared to vehicle and free curcumin groups | Increased aqueous solubility | [102] |
| 6 | Muscimol | DPPC-albumin-lactose (400–500) | PD: Sprague–Dawley rats, pilocarpine (chronic model); intrahippocampal injections, 5 µg (free muscimol) | The rise of the trajectory in behaviour scores slower than the positive control | Reduced side effects | [103] |
| Entry | Bioactive Compound | Main NP Composition (Size [nm], PdI) | Type of Study: Animal Model; Protocol (Positive Control) | PD Effect | Advantages of NPs | Reference |
|---|---|---|---|---|---|---|
| 1 | Oxcarbazepine | PLGA (256.2 ± 2.9, 0.144 ± 0.02) | PD: Wistar rats, PTZ (acute model); i.n.; 0.5 mg/kg, 3, 11 and 16 administrations (free oxcarbazepine) | Reduced symptoms and their duration | Prolonged duration of action | [111] |
| 2 | CBZ | Carboxymethyl CH (218.8 ± 2.4 nm) | PK: C57BL mice; i.n., single dose (free CBZ) | _ | Increased brain penetration | [112] |
| 3 | Clonazepam | GMS:SA:compritol:OA:GO (210.2 ± 12.7, 0.197 ± 0.08) | PD: Swiss Albino mice, PTZ (acute model); i.n., 0.2 mg/kg, single dose | Prolonged convulsion onset time (64.9 s vs. 41.7 s for control) and the onset time of death (552 s versus 113.5 s for control) | Long retention at application site | [113] |
| 4 | Combination of phenytoin and fosphenytoin | Capryol 90:Im-witor 988:Kolliphor EL:Albumin | PK: CD-1 mice; i.n., 5.8 mg/kg of phenytoin equivalents, single dose (free fosphenytoin i.v. i.n. fosphenytoin solution and i.n. nanoformulation fosphenytoin only) | _ | Increased brain bioavailability | [114] |
| 5 | Amiloride | OA:Tween-80:Carbitol (89.36 ± 6.19, 0.231 ± 0.018) | PD: Swiss Albino mice, ICES, PTZ (acute models); i.n., 0.5 mg/kg, single dose (free amiloride) | Higher protection than free drug; reduced onset of myoclonic jerks with clonic generalized seizures than free drug | Increased brain penetration | [115] |
| 6 | Catechin hydrate | CH:PLGA:PVA (93.46 ± 3.94, 0.106 ± 0.01) | PD: Albino rats, PTZ, ICES (acute models); i.n. 10 mg/kg, single dose (free catechin hydrate) | Higher protection in both models, compared to free compound | Increased brain penetration | [116] |
| 7 | Thymoquinone | PLGA:PVA (97.36 ± 2.01, 0.263 ± 0.004) | PD: Albino rats, ICES (acute model); i.n., 10 mg/kg, single dose (free thymoquinone) | Increased ICES threshold and decreased the recovery period, when compared to free compound | Increased brain bioavailability | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, M.; Santos, A.O.; Silvestre, S.; Alves, G. Fighting Epilepsy with Nanomedicines—Is This the Right Weapon? Pharmaceutics 2023, 15, 306. https://doi.org/10.3390/pharmaceutics15020306
Matias M, Santos AO, Silvestre S, Alves G. Fighting Epilepsy with Nanomedicines—Is This the Right Weapon? Pharmaceutics. 2023; 15(2):306. https://doi.org/10.3390/pharmaceutics15020306
Chicago/Turabian StyleMatias, Mariana, Adriana O. Santos, Samuel Silvestre, and Gilberto Alves. 2023. "Fighting Epilepsy with Nanomedicines—Is This the Right Weapon?" Pharmaceutics 15, no. 2: 306. https://doi.org/10.3390/pharmaceutics15020306
APA StyleMatias, M., Santos, A. O., Silvestre, S., & Alves, G. (2023). Fighting Epilepsy with Nanomedicines—Is This the Right Weapon? Pharmaceutics, 15(2), 306. https://doi.org/10.3390/pharmaceutics15020306










