Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Silico Analysis
2.3. Cell Viability Assay
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. PASS Online Prediction of the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac
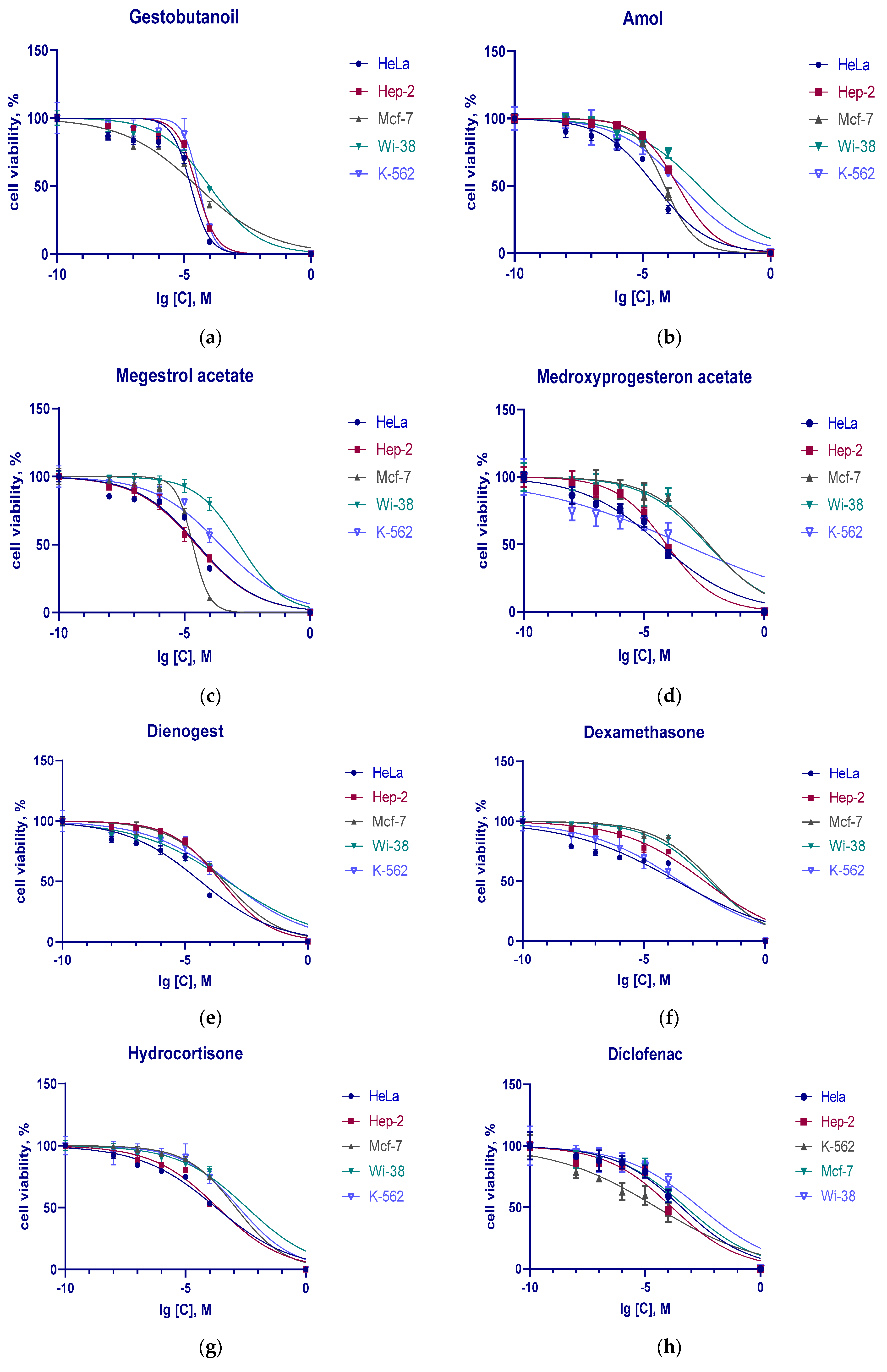
3.2. Cytotoxic Action of Progestins, Glucocorticoids and Diclofenac on Tumor Cells
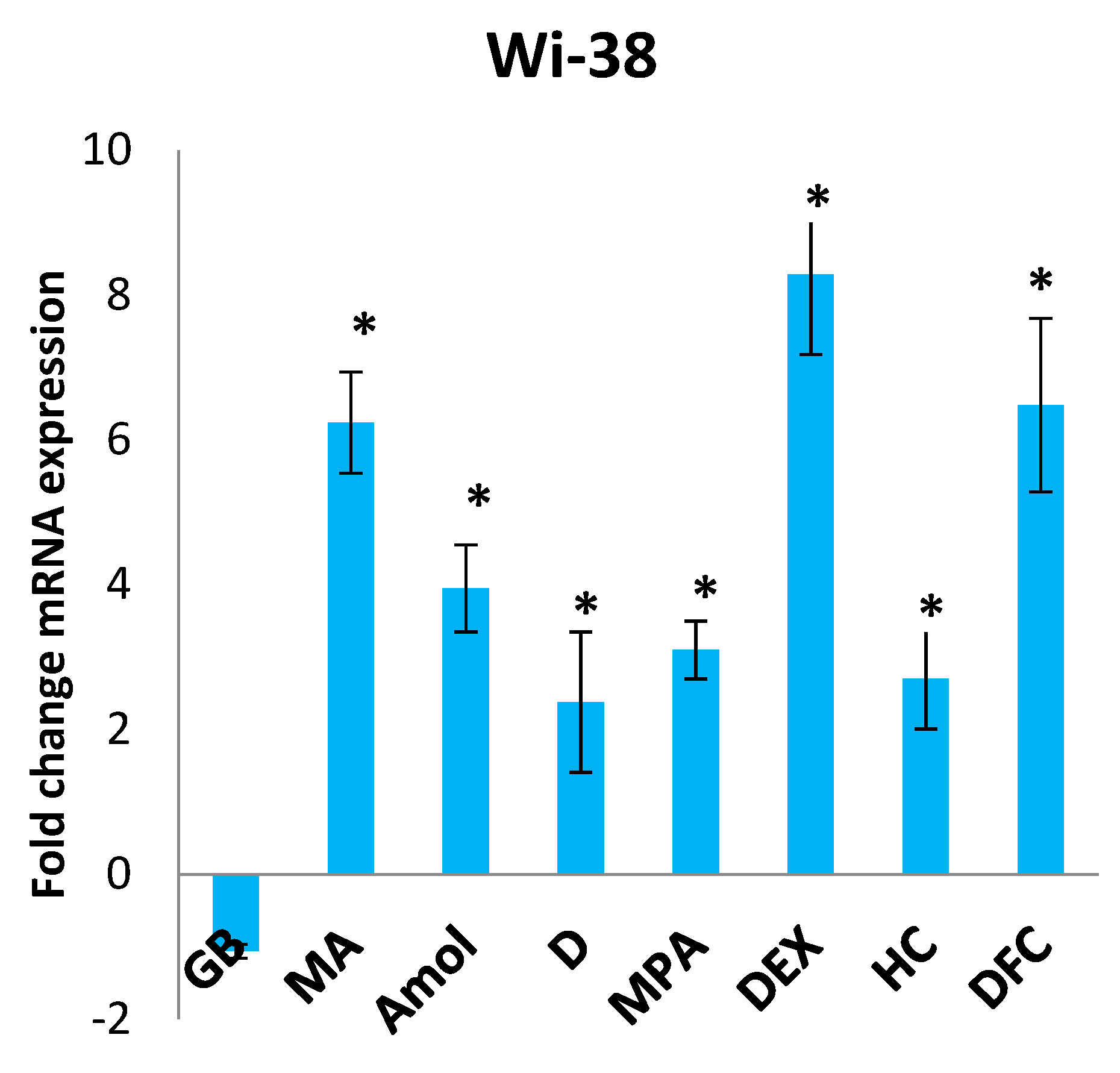
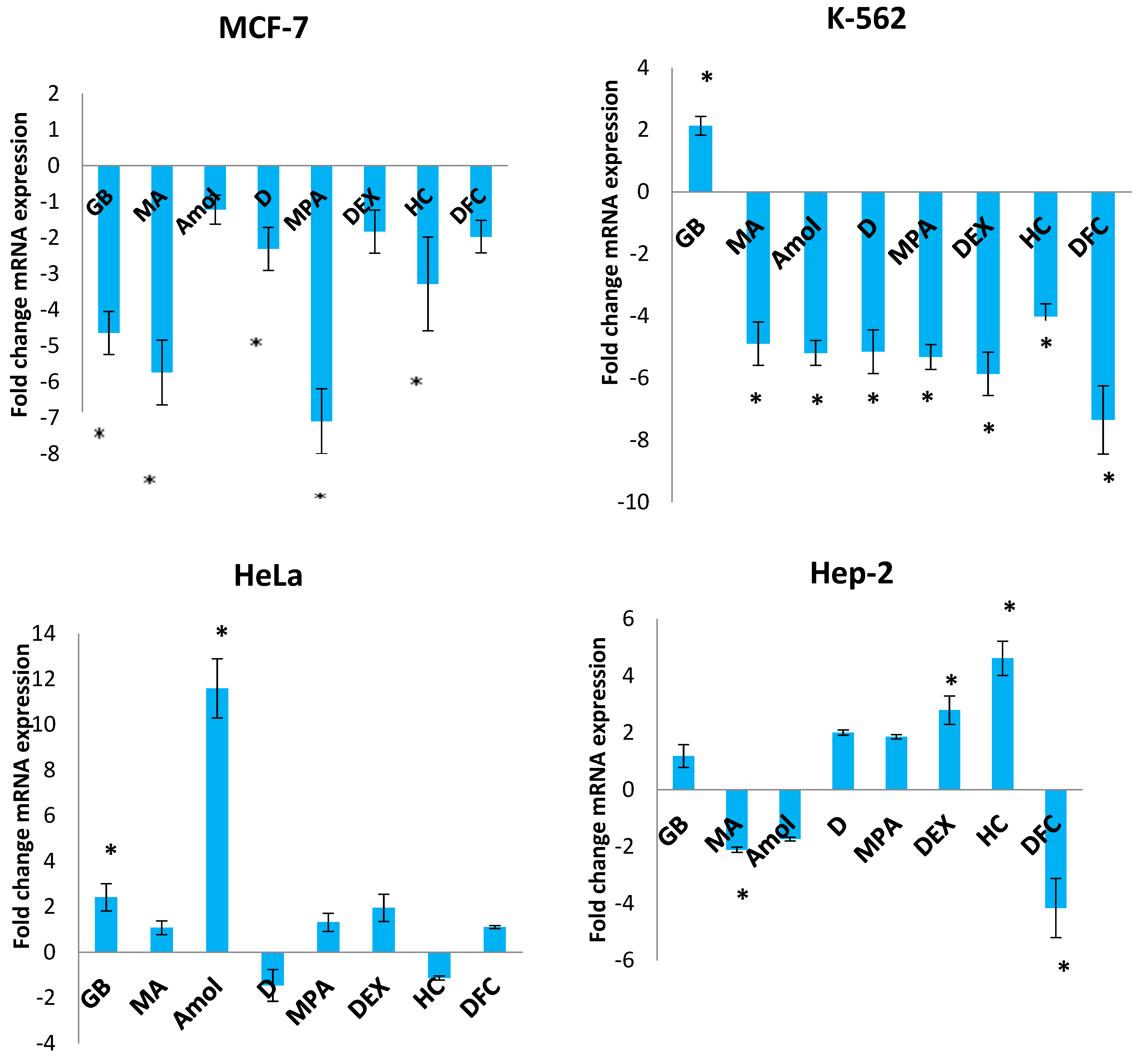
3.3. The Influence of Progestins, GCs, and DCF on the Expression of Adenine Nucleotide Translocase ANT1 in Tumor Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANT1 (SLC25A4 Mitochondrial ADP/ATP carrier 1 (AAC1) | adenine nucleotide translocase 1 |
| COX | cyclooxigenase |
| GB | gestobutanoyl |
| MPTP | mitochondrial permeability transition pore |
| MTT | 3-[4,5-di-methylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| NSAID | non-steroidal anti-inflammatory drugs |
| P4 | progesterone |
| PR | progesterone receptor |
References
- Available online: https://www.cancer.gov/about-cancer/treatment/drugs/megestrolacetate (accessed on 30 September 2023).
- Available online: https://go.drugbank.com/drugs/DB00603 (accessed on 7 March 2023).
- Available online: https://www.cancer.org/cancer/endometrial-cancer/treating/hormone-therapy.html (accessed on 30 September 2023).
- Saeteaw, M.; Sanguanboonyaphong, P.; Yoodee, J.; Craft, K.; Sawangjit, R.; Ngamphaiboon, N.; Shantavasinkul, P.C.; Subongkot, S.; Chaiyakunapruk, N. Efficacy and safety of pharmacological cachexia interventions: Systematic review and network meta-analysis. BMJ Support. Palliat. Care 2021, 11, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Kullolli, O.; Maggi, E.; Barkley, M.S. Medroxyprogesterone Acetate Decreases Th1, Th17, and Increases Th22 Responses via AHR Signaling Which Could Affect Susceptibility to Infections and Inflammatory Disease. Front. Immunol. 2019, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.; Dienstmann, R.; Obadia, R.M.; Branco, L.G.P.; Quintella, D.C.; Castro, T.M.; Camacho, P.G.; Soares, F.A.; Costa, M.E.F. Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: A phase II trial. Ann. Oncol. 2014, 25, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Moe, B.T.; Vereide, A.B.; Orbo, A.; Jaeger, R.; Sager, G. Levonorgestrel, medroxyprogesterone and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009, 29, 1047–1052. [Google Scholar]
- Cao, C.; Zhou, J.Y.; Xie, S.W.; Guo, X.J.; Li, G.T.; Gong, Y.J.; Yang, W.J.; Li, Z.; Zhong, R.H.; Shao, H.H.; et al. Metformin Enhances Nomegestrol Acetate Suppressing Growth of Endometrial Cancer Cells and May Correlate to Downregulating mTOR Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 3308. [Google Scholar] [CrossRef]
- Sergeev, P.V.; Fedotcheva, T.A.; Rzheznikov, V.M.; Grinenko, G.S.; Semeĭkin, A.V.; Vetchinkina, V.B.; Atroshkin, K.A.; Shimanovskiĭ, N.L. A new Russian gestagen with anticancer activity. Vestn. Ross. Akad. Med. Nauk. 2007, 5, 27–32. [Google Scholar]
- Katsuki, Y.; Shibutan, I.Y.; Aoki, D.; Nozawa, S. Dienogest, a novel synthetic steroid, overcomes hormone-dependent cancer in a different manner than progestins. Cancer 1997, 79, 169–176. [Google Scholar] [CrossRef]
- Shimizu, Y.; Takeuchi, T.; Mita, S.; Mizuguchi, K.; Kiyono, T.; Inoue, M.; Kyo, S. Dienogest, a synthetic progestin, inhibits the proliferation of immortalized human endometrial epithelial cells with suppression of cyclin D1 gene expression. Mol. Hum. Reprod. 2009, 15, 693–701. [Google Scholar] [CrossRef]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; LaRiviere, M.; Macduffie, E.; White, C.A.; Jordan-Luft, M.M.; Anderson, E.; Ziegler, M.; Radcliff, J.A.; Jones, J. Use of Glucocorticoids in Patients With Cancer: Potential Benefits, Harms, and Practical Considerations for Clinical Practice. Pract. Radiat. Oncol. 2023, 13, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Bhadri, V.A.; Beck, D.; Thoms, J.A.; Yakob, N.A.; Wong, J.W.; Knezevic, K.; Pimanda, J.E.; Lock, R.B. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood 2015, 125, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kalfeist, L.; Galland, L.; Ledys, F.; Ghiringhelli, F.; Limagne, E.; Ladoire, S. Impact of Glucocorticoid Use in Oncology in the Immunotherapy Era. Cells 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Miller, A.V.; Hicks, M.A.; Marking, C.B.; Harada, H. Glucocorticoid-mediated BIM induction and apoptosis are regulated by Runx2 and c-Jun in leukemia cells. Cell Death Dis. 2012, 3, e349. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cancer.gov/about-cancer/treatment/drugs/dexamethasone (accessed on 7 March 2023).
- Liu, L.; Han, S.; Xiao, X.; An, X.; Gladkich, J.; Hinz, U.; Hillmer, S.; Hoppe-Tichy, T.; Xu, Y.; Schaefer, M.; et al. Glucocorticoid-induced microRNA-378 signaling mediates the progression of pancreatic cancer by enhancing autophagy. Cell Death Dis. 2022, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Kumar, R. Crosstalk between NFkB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett. 2012, 322, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Marinov, L.; Georgieva, A.; Voynikov, Y.; Toshkova, R.; Nikolova, I.; Malchev, M. Cytotoxic and antiproliferative effects of the nonsteroidal anti-inflammatory drug diclofenac in human tumour cell lines. Biotech. Biotech. Equip. 2021, 35, 1118–1126. [Google Scholar] [CrossRef]
- Okamoto, K.; Saito, Y.; Narumi, K.; Furugen, A.; Iseki, K.; Kobayashi, M. Anticancer effects of non-steroidal anti-inflammatory drugs against cancer cells and cancer stem cells. Toxicol. Vitr. 2021, 74, 105155. [Google Scholar] [CrossRef]
- Kobyakova, M.; Lomovskaya, Y.; Senotov, A.; Lomovsky, A.; Minaychev, V.; Fadeeva, I.; Shtatnova, D.; Krasnov, K.; Zvyagina, A.; Odinokova, I.; et al. The Increase in the Drug Resistance of Acute Myeloid Leukemia THP-1 Cells in High-Density Cell Culture Is Associated with Inflammatory-like Activation and Anti-Apoptotic Bcl-2 Proteins. Int. J. Mol. Sci. 2022, 23, 7881. [Google Scholar] [CrossRef]
- Esteruelas, G.; Souto, E.B.; Espina, M.; García, M.L.; Świtalska, M.; Wietrzyk, J.; Gliszczyńska, A.; Sánchez-López, E. Diclofenac Loaded Biodegradable Nanoparticles as Antitumoral and Antiangiogenic Therapy. Pharmaceutics 2023, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Motafeghi, F.; Mortazavi, P.; Ghassemi-Barghi, N.; Zahedi, M.; Shokrzadeh, M. Dexamethasone as an anti-cancer or hepatotoxic. Toxicol. Mech. Methods 2023, 33, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, H.; Ni, D.; Hu, Y.; Liu, J.; Qin, Y.; Zhou, Q.; Yi, Q.; Xie, Y. High-Dose Dexamethasone Manipulates the Tumor Microenvironment and Internal Metabolic Pathways in Anti-Tumor Progression. Int. J. Mol. Sci. 2020, 21, 1846. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, P.; Bai, M.; Huang, Y.; Li, L.; Feng, Y.; Liao, H.; Zheng, W.; Chen, X.; Zhang, Z. Progestin Resistance and Corresponding Management of Abnormal Endometrial Hyperplasia and Endometrial Carcinoma. Cancers 2022, 14, 6210. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef]
- De Nicola, A.F.; Garay, L.I.; Meyer, M.; Guennoun, R.; Sitruk-Ware, R.; Schumacher, M.; Gonzalez Deniselle, M.C. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J. Neuroendocrinol. 2018, 30, 12502. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progestins as Anticancer Drugs and Chemosensitizers, New Targets and Applications. Pharmaceutics 2021, 13, 1616. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Fedotcheva, T.A.; Rzheznikov, V.M.; Shimanovskii, N.L. Effect of progesterone and its synthetic analogues on the activity of mitochondrial permeability transition pore in isolated rat liver mitochondria. Biochem. Pharmacol. 2009, 78, 1060–1068. [Google Scholar] [CrossRef]
- Smirnov, A.S.; Suponin, D.A.; Shilov, B.V.; Fedotcheva, T.A. Modeling of the Interaction of a Series of Substances from the Class of Pregnanic Steroids with Human ADP/ATP Translocase. In VIII International Scientific and Practical Conference of Young Students: Biophysicists, Biotechnologists, Molecular Biolves and Virusocolv; Collection of Abstracts of the Conference Held as Part of the Open Communications Platform OpenBio-2021; OpenBio: Novosibirsk, Russia, 2021; pp. 338–339. [Google Scholar]
- Clémençon, B.; Babot, M.; Trézéguet, V. The mitochondrial ADP/ATP carrier (SLC25 family): Pathological implications of its dysfunction. Mol. Asp. Med. 2013, 34, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.A.; Sveshnikova, E.D.; Sheina, N.I.; Shimanovskii, N.L.; Sokolov, M.N.; Kudryavtsev, K.V.; Fedotcheva, N.I. Synthesis and cytostatic activity of new mepregenol 17-acetate derivatives with respect to Hela cancer cell culture. Pharm. Chem. J. 2020, 54, 119–125. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, S.; Park, J.; Lee, S.E.; Kim, C.; Kang, D. Diclofenac: A Nonsteroidal Anti-Inflammatory Drug Inducing Cancer Cell Death by Inhibiting Microtubule Polymerization and Autophagy Flux. Antioxidants 2022, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Fjelldal, R.; Moe, B.T.; Ørbo, A.; Sager, G. MCF-7 cell apoptosis and cell cycle arrest: Non-genomic effects of progesterone and mifepristone (RU-486). Anticancer Res. 2010, 30, 4835–4840. [Google Scholar] [PubMed]
- Bajalovic, N.; Or, Y.Z.; Woo, A.R.E.; Lee, S.H.; Lin, V.C.L. High Levels of Progesterone Receptor B in MCF-7 Cells Enable Radical Anti-Tumoral and Anti-Estrogenic Effect of Progestin. Biomedicines 2022, 10, 1860. [Google Scholar] [CrossRef]
- Winter, J.; Hammer, E.; Heger, J.; Schultheiss, H.-P.; Rauch, U.; Landmesser, U.; Dörner, A. Adenine Nucleotide Translocase 1 Expression Is Coupled to the HSP27-Mediated TLR4 Signaling in Cardiomyocytes. Cells 2019, 8, 1588. [Google Scholar] [CrossRef]
- Garcia-Guasch, M.; Escrich, E.; Moral, R.; Duarte, I.F. Metabolomics Insights into the Differential Response of Breast Cancer Cells to the Phenolic Compounds Hydroxytyrosol and Luteolin. Molecules 2023, 28, 3886. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, L.B.; Yang, H.Y.; Zhang, H.P. Effects of estradiol and progesterone on the growth of HeLa cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3959–3965. [Google Scholar] [PubMed]
- Brunings, J.W.; Schepens, J.J.; Peutz-Kootstra, C.J.; Kross, K.W. The expression of estrogen and progesterone receptors in the human larynx. J. Voice 2013, 27, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.D.; Anderson, D.J. Progesterone receptor expression by human leukocyte cell lines: Molecular mechanisms of cytokine suppression. Clin. Exp. Obstet. Gynecol. 2007, 34, 14–24. [Google Scholar] [PubMed]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell. Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.S.; Makarenkova, L.M.; Chistyakov, V.V.; Fedotcheva, T.A.; Parshin, V.A.; Shimanovsky, N.L. Metabolism of Gestobutanoil, a Novel Drug of Progestin Group. Sovr. Tehn. Med. 2019, 11, 48. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-diclofenac as an anti-cancer agent. Ecancermedicalscience 2016, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Lae Lae Phoo, N.; Sukhamwang, A.; Dejkriengkraikul, P.; Yodkeeree, S. Diclofenac Sensitizes Signet Ring Cell Gastric Carcinoma Cells to Cisplatin by Activating Autophagy and Inhibition of Survival Signal Pathways. Int. J. Mol. Sci. 2022, 23, 12066. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.B.; Fang, H.Y.; Tao, D.Y.; Chen, X.P.; Cao, F.L. COX-2 potentiates cisplatin resistance of non-small cell lung cancer cells by promoting EMT in an AKT signaling pathway-dependent manner. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3838–3846. [Google Scholar] [CrossRef]
- Bell, C.R.; Pelly, V.S.; Moeini, A.; Chiang, S.C.; Flanagan, E.; Bromley, C.P.; Clark, C.; Earnshaw, C.H.; Koufaki, M.A.; Bonavita, E.; et al. Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations. Nat. Commun. 2022, 13, 2063. [Google Scholar] [CrossRef]
- Gottfried, E.; Lang, S.A.; Renner, K.; Bosserhoff, A.; Gronwald, W.; Rehli, M.; Einhell, S.; Gedig, I.; Singer, K.; Seilbeck, A.; et al. New aspects of an old drug--diclofenac targets MYC and glucose metabolism in tumor cells. PLoS ONE 2013, 8, e66987. [Google Scholar] [CrossRef]
- Renner, K.; Bruss, C.; Schnell, A.; Koehl, G.; Becker, H.M.; Fante, M.; Menevse, A.N.; Kauer, N.; Blazquez, R.; Hacker, L.; et al. Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. Cell Rep. 2019, 29, 135–150.e9. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.T.; Smaglyukova, N.; Ørbo, A.; Sager, G. Expression of nuclear progesterone receptors (nPRs), membrane progesterone receptors (mPRs) and progesterone receptor membrane components (PGRMCs) in the human endometrium after 6 months levonorgestrel low dose intrauterine therapy. J. Steroid Biochem. Mol. Biol. 2020, 202, 105701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.; Liu, J.; Ma, L.; Zhang, W. Adenine nucleotide translocase: Current knowledge in post-translational modifications, regulations and pathological implications for human diseases. FASEB J. 2023, 37, e22953. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Choi, Y.; Jeon, Y.-K.; Aung, K.C.A.; Kim, C.-W. Over-expression of Adenine Nucleotide Translocase 1 (ANT1) Induces Apoptosis and Tumor Regression in vivo. BMC Cancer 2008, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, O.; Kunji, E.R.S. Expression and putative role of mitochondrial transport proteins in cancer. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Rechichi, M.; Salvetti, A.; Vecchio, D.; Evangelista, M.; Rainaldi, G.; Gremigni, V.; Rossi, L. The silencing of adenine nucleotide translocase isoform 1 induces oxidative stress and programmed cell death in ADF human glioblastoma cells. FEBS J. 2010, 277, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Chevrollier, A.; Loiseau, D.; Reynier, P.; Stepien, G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta 2011, 1807, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.A.; Fedotcheva, N.I. Protectors of the Mitochondrial Permeability Transition Pore Activated by Iron and Doxorubicin. Curr. Cancer Drug Targets 2021, 21, 514–525. [Google Scholar] [CrossRef]
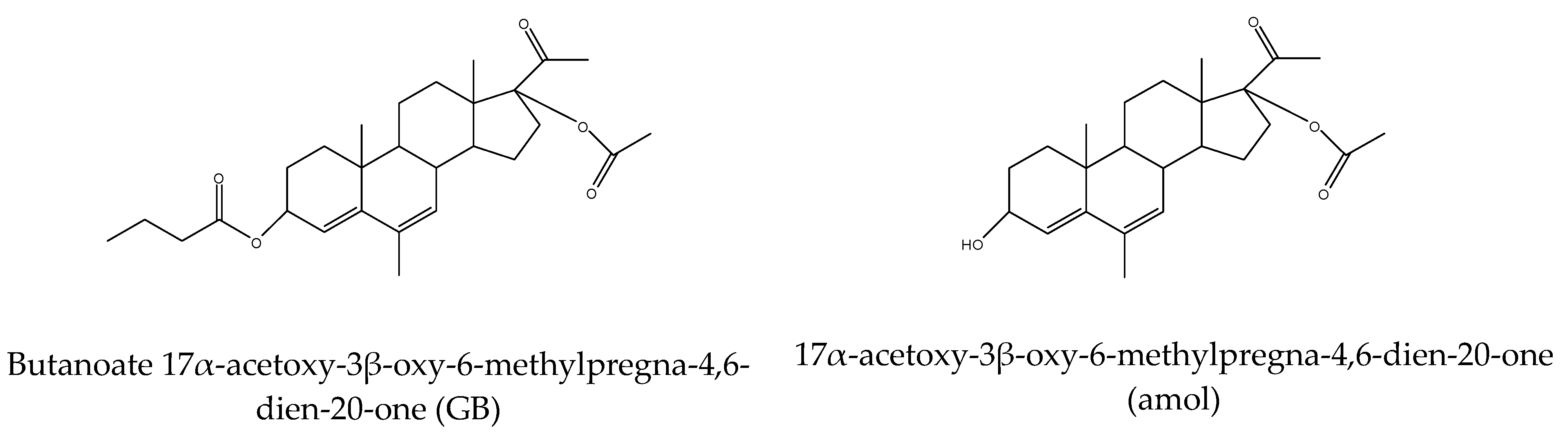
| Predicted Activity | GB | MA | Amol | D | MPA | DEX | HC | DFC |
|---|---|---|---|---|---|---|---|---|
| Anti-inflammatory | 0.785 | 0.84 | 0.811 | 0.597 | 0.883 | 0.985 | 0.94 | 0.791 |
| Cytotoxic (antitumor) | 0.814 | 0.767 | 0.774 | 0.532 | 0.727 | - | 0.679 | - |
| GB | MA | Amol | D | MPA | DEX | HC | DCF | |
|---|---|---|---|---|---|---|---|---|
| HeLa | 18.3 ± 2.18 | 32.9 ± 3.29 | 31.3 ± 4.7 | 44 ± 4.8 | 49.9 ± 8.73 | 378 ± 109.24 | 215 ± 32.3 | 384 ± 99.1 |
| Hep-2 | 31 ± 4.96 | 29.2 ± 5.0 | 238 ± 39.7 | 296 ± 44 | 85.1 ± 14.21 | 3160 ± 543 | 217 ± 30.16 | 146 ± 22.5 |
| MCF-7 | 31.9 ± 5.17 | 21.3 ± 2.85 | 73.7 ± 12.7 | 387 ± 61.53 | 4910 ± 85.3 | 6540 ± 1294.9 | 1230 ± 212.79 | 595 ± 119.0 |
| K-562 | 38.2 ± 8.4 | 266 ± 62 | 326 ± 60 | 611 ± 123 | 441 ± 121.72 | 304 ± 63.8 | 1660 ± 417 | 31.5 ± 8.45 |
| Wi-38 | 98.9 ± 21.1 | 1540 ± 297 | 1620 ± 460.1 | 546 ± 115 | 4200 ± 721.4 | 5080 ± 1132.8 | 3530 ± 687.2 | 2330 ± 459.4 |
| Drug Name | SI | |||
|---|---|---|---|---|
| HeLa | Hep-2 | MCF-7 | K-562 | |
| GB | 5.40 | 3.19 | 3.10 | 2.59 |
| MA | 46.81 | 52.74 | 72.30 | 5.79 |
| A | 51.76 | 6.81 | 21.98 | 4.97 |
| D | 12.41 | 1.84 | 1.41 | 0.89 |
| MPA | 84.17 | 49.35 | 0.86 | 9.52 |
| DEX | 13.44 | 1.61 | 0.78 | 16.71 |
| HC | 16.42 | 16.27 | 2.87 | 2.13 |
| DCF | 6.06 | 15.96 | 3.92 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulchenko, D.; Miloykovich, L.; Zemlyanaya, O.; Shimanovsky, N.; Fedotcheva, T. Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells. Pharmaceutics 2023, 15, 2787. https://doi.org/10.3390/pharmaceutics15122787
Ulchenko D, Miloykovich L, Zemlyanaya O, Shimanovsky N, Fedotcheva T. Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells. Pharmaceutics. 2023; 15(12):2787. https://doi.org/10.3390/pharmaceutics15122787
Chicago/Turabian StyleUlchenko, Darya, Lilia Miloykovich, Olga Zemlyanaya, Nikolay Shimanovsky, and Tatiana Fedotcheva. 2023. "Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells" Pharmaceutics 15, no. 12: 2787. https://doi.org/10.3390/pharmaceutics15122787
APA StyleUlchenko, D., Miloykovich, L., Zemlyanaya, O., Shimanovsky, N., & Fedotcheva, T. (2023). Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells. Pharmaceutics, 15(12), 2787. https://doi.org/10.3390/pharmaceutics15122787







