Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
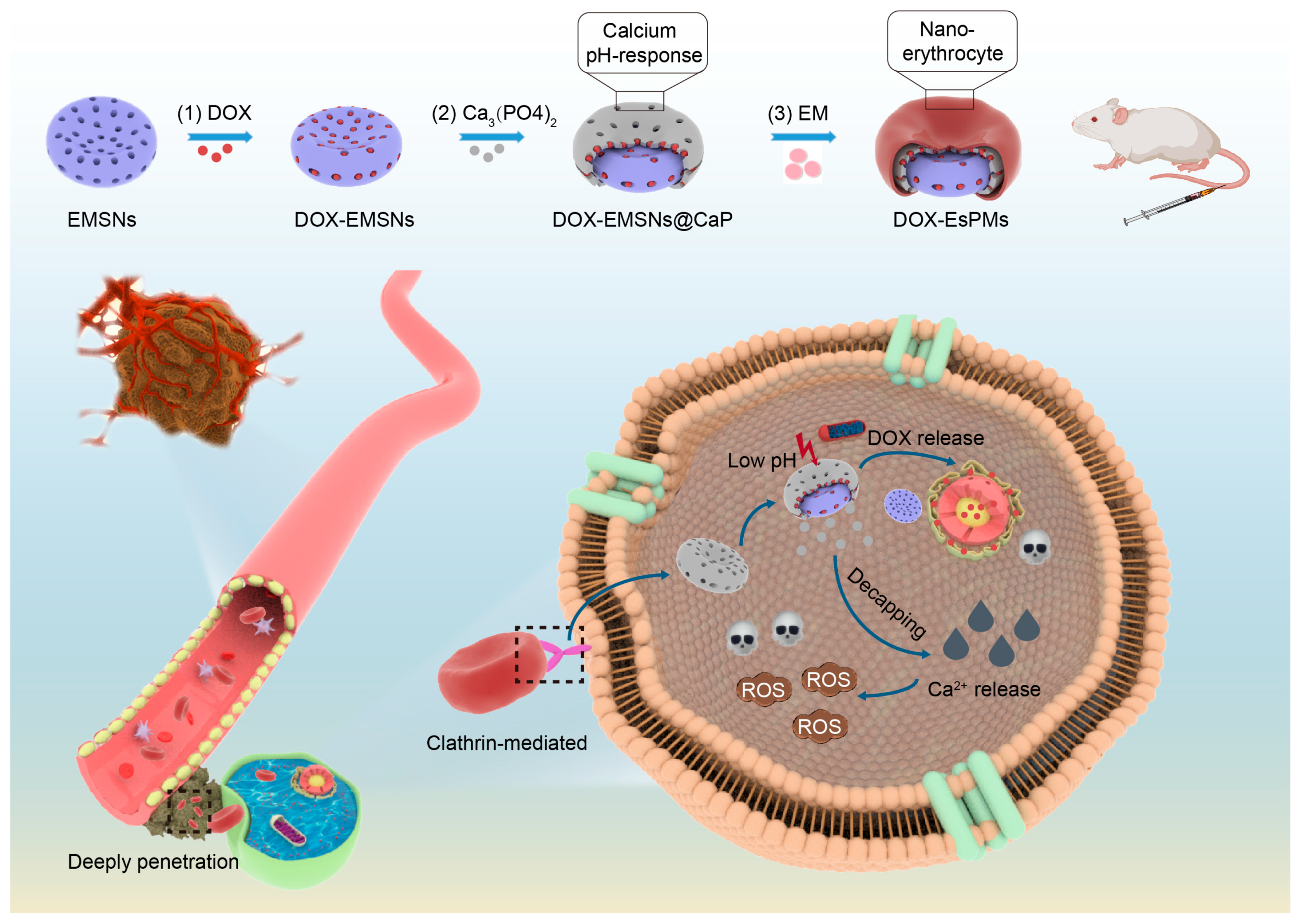
2.2. The Synthesis of Nanoparticles
2.3. The Characterization of Nanoparticles
2.4. An In Vitro Ca2+ Release Study
2.5. Drug Loading Capacity
2.6. An In Vitro Drug-Release Study
2.7. The Hemolysis Test and Non-Specific Protein Adsorption
2.8. The Degradation Study
2.9. Multiple Particle Tracking (MPT)
2.10. In Vitro Tumor Sphere Penetration
2.11. The Cell Cytotoxicity Study
2.12. Cellular Uptake and Mechanism Studies
2.13. Mitochondrial Co-Localization
2.14. Intracellular Ca2+ Release and ROS Detection Studies
2.15. The In Vivo Circulation Study
2.16. In Vivo Pharmacodynamics and Biosafety Studies
2.17. Statistical Analysis
3. Results and Discussion
3.1. The Characterization of Nanoparticles
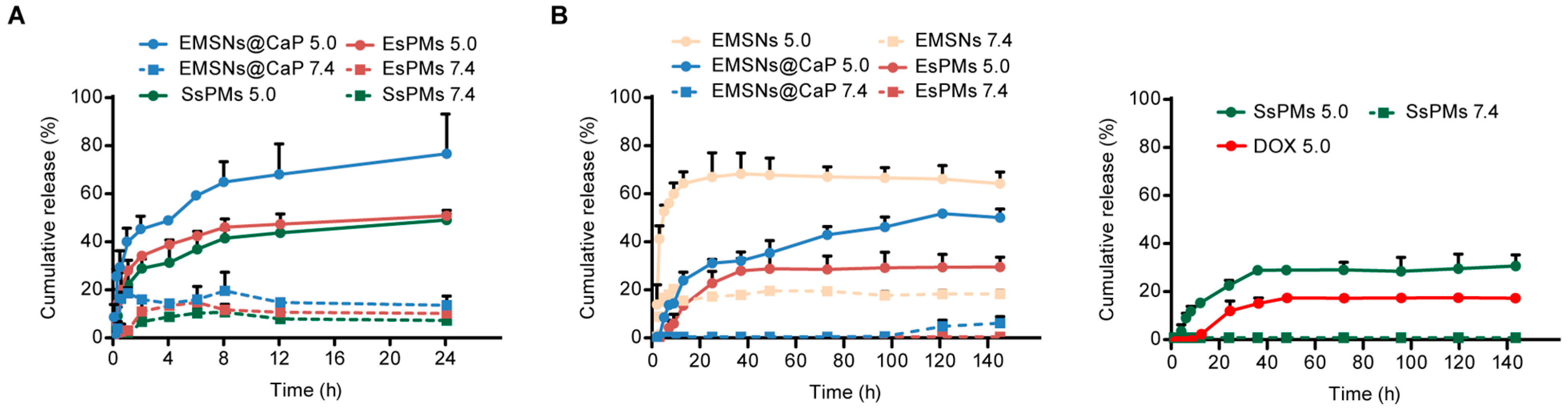
3.2. The In Vitro pH-Responsive Ca2+-Release Study
3.3. Drug Loading and In Vitro pH-Responsive Release Studies
3.4. Biocompatibility and Degradation Evaluations
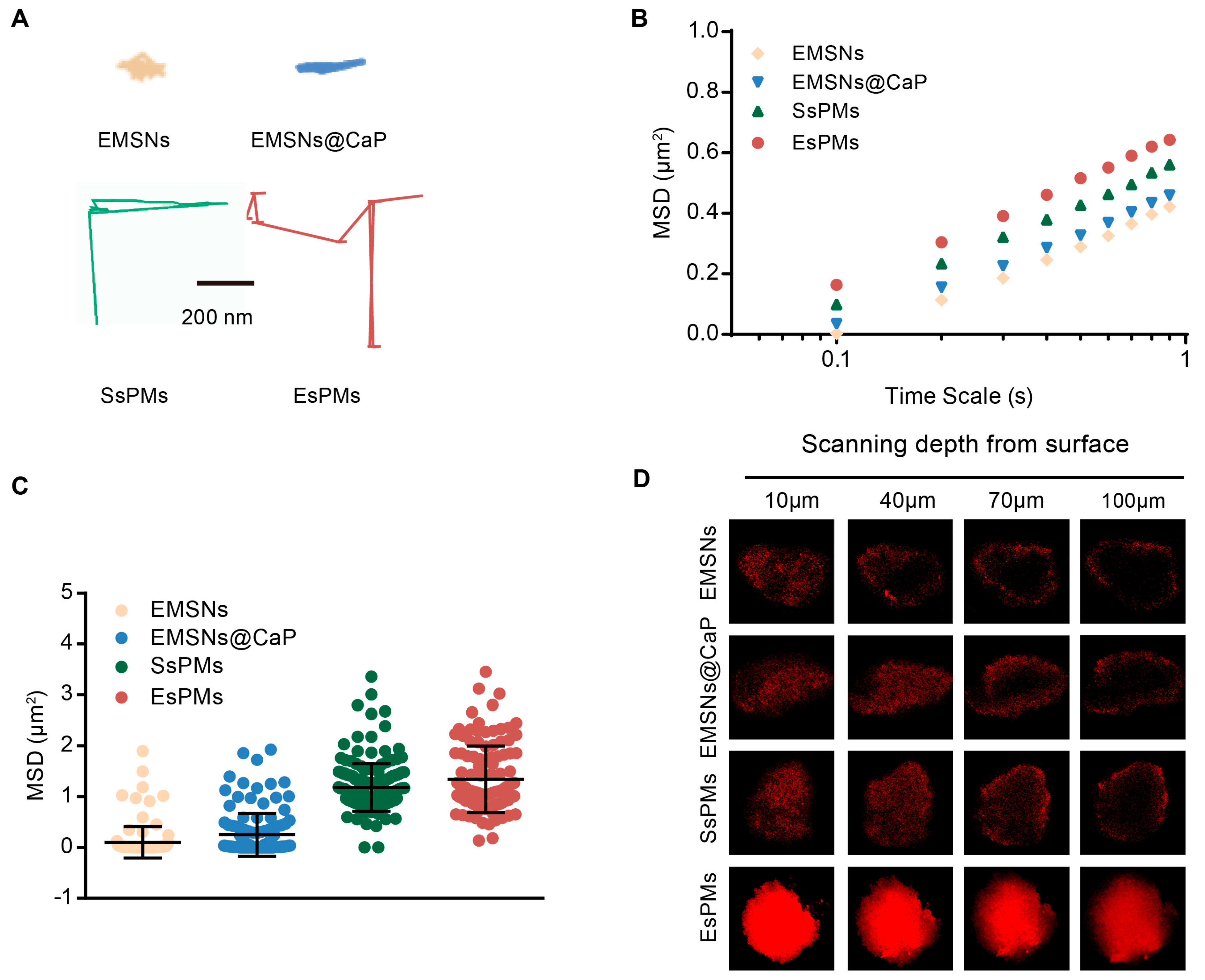
3.5. In Vitro Diffusion and Penetration Ability Studies
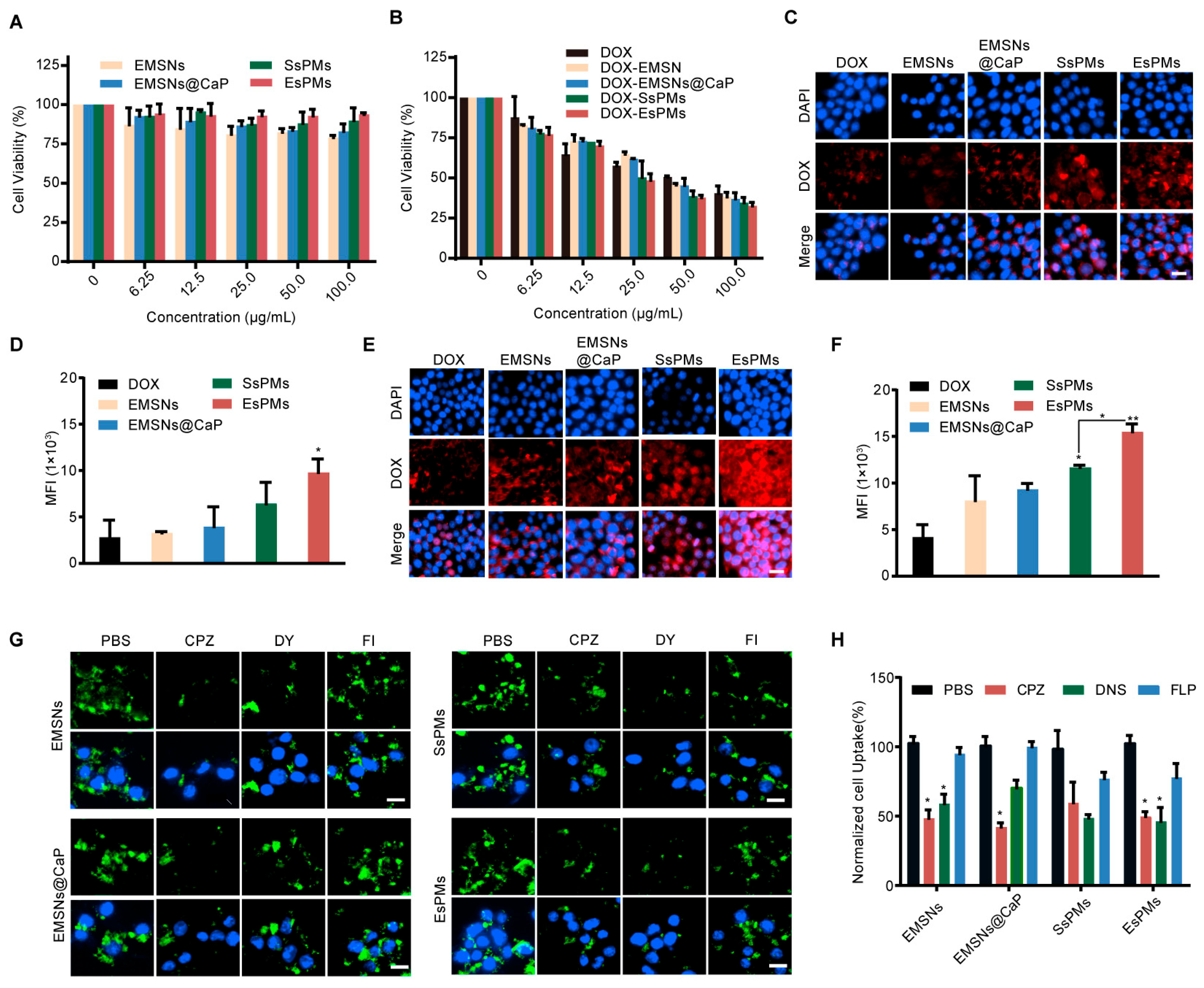
3.6. Cell Viability Study
3.7. Cellular Uptake and Detailed Mechanism Studies
3.8. Mitochondrial Co-Localization
3.9. Intracellular Ca2+ Release and ROS Detection
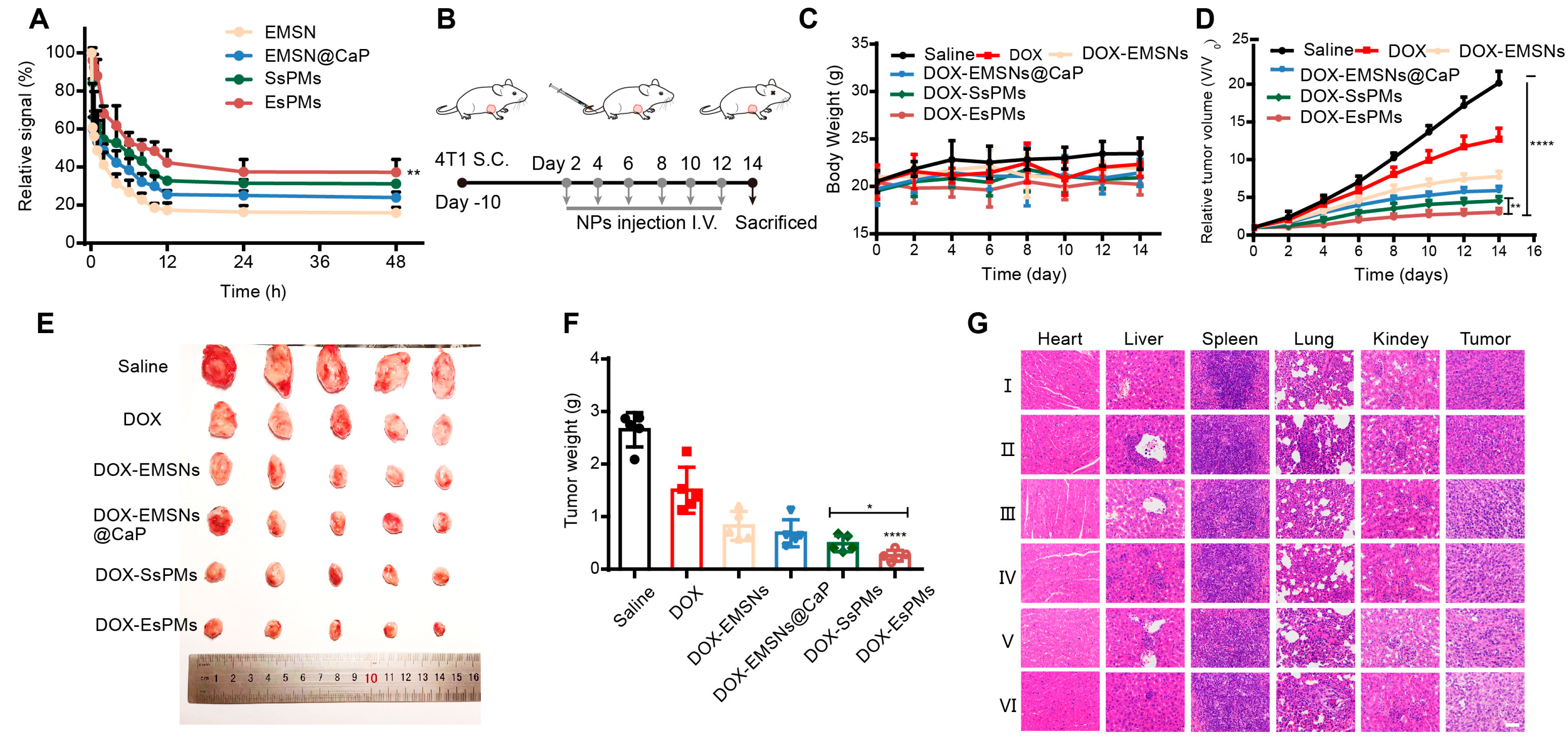
3.10. The In Vivo Circulation Performance
3.11. The In Vivo Antitumor Effect and Biosafety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef]
- Lee, B.J.; Cheema, Y.; Bader, S.; Duncan, G.A. Shaping nanoparticle diffusion through biological barriers to drug delivery. JCIS Open 2021, 4, 100025. [Google Scholar] [CrossRef]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N.; et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, R.; Yu, L.; Wang, W. Facile preparation of hierarchical mesoporous silica microspheres with tunable porous structure and particle sizes. Ceram. Int. 2023, 49, 3030–3040. [Google Scholar] [CrossRef]
- Lai, S.-M.; Lai, H.-Y.; Chou, M.-Y. A facile approach for the tunable wormlike or ordered pore morphology of mesoporous silica: Effect of catalyst types and polyethylene glycol. Microporous Mesoporous Mater. 2014, 196, 31–40. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, H.; Wang, X. Polydopamine-doped virus-like mesoporous silica coated reduced graphene oxide nanosheets for chemo-photothermal synergetic therapy. J. Biomater. Appl. 2020, 35, 28–38. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Biomimetic hierarchical walnut kernel-like and erythrocyte-like mesoporous silica nanomaterials: Controllable synthesis and versatile applications. Microporous Mesoporous Mater. 2018, 261, 144–149. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Wang, J.; Nie, D.; Jiang, X.; Zhuo, Y.; Yu, M. Shape-directed drug release and transport of erythrocyte-like nanodisks augment chemotherapy. J. Control. Release 2022, 350, 886–897. [Google Scholar] [CrossRef]
- Roggers, R.A.; Joglekar, M.; Valenstein, J.S.; Trewyn, B.G. Mimicking Red Blood Cell Lipid Membrane to Enhance the Hemocompatibility of Large-Pore Mesoporous Silica. ACS Appl. Mater. Interfaces 2014, 6, 1675–1681. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.; Shao, D.; Chang, Z.; Hu, R.; Li, L.; Luo, S.-Z.; Dong, W.-F. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv. 2018, 8, 40288–40297. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chen, G.-B.; Chen, H.-H.; Zhang, J.-B.; Li, H.-Z.; Sheng, M.-X.; Weng, W.-B.; Guo, S.-M. Cancer cell membrane-cloaked mesoporous silica nanoparticles with a pH-sensitive gatekeeper for cancer treatment. Colloids Surf. B Biointerfaces 2019, 175, 477–486. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, L.; Chen, H.; Zhuo, M.; Yang, X.; Zhao, D.; Zeng, S.; Xiao, X. Natural material-decorated mesoporous silica nanoparticle container for multifunctional membrane-controlled targeted drug delivery. Int. J. Nanomed. 2017, 12, 8411–8426. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, Y.; Sun, H.; Li, D.; Zhou, S. Biodegradable magnetic calcium phosphate nanoformulation for cancer therapy. Eur. J. Pharm. Biopharm. 2014, 87, 90–100. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Zhang, H.; Mu, Z.; Li, L.; Wang, C. Rationally Designed Calcium Phosphate/Small Gold Nanorod Assemblies Using Poly(acrylic acid calcium salt) Nanospheres as Templates for Chemo-photothermal Combined Cancer Therapy. ACS Biomater. Sci. Eng. 2017, 3, 3215–3221. [Google Scholar] [CrossRef]
- Duc-Viet, N.; Jiang, S.; He, C.; Lin, Z.; Lin, N.; Anh-Tuan, N.; Kang, L.; Han, M.-Y.; Liu, X.-Y. Elevating Biomedical Performance of ZnO/SiO2@Amorphous Calcium Phosphate—Bioinspiration Making Possible the Impossible. Adv. Funct. Mater. 2016, 26, 6921–6929. [Google Scholar] [CrossRef]
- Nomoto, T.; Fukushima, S.; Kumagai, M.; Miyazaki, K.; Inoue, A.; Mi, P.; Maeda, Y.; Toh, K.; Matsumoto, Y.; Morimoto, Y.; et al. Calcium phosphate-based organic-inorganic hybrid nanocarriers with pH-responsive on/off switch for photodynamic therapy. Biomater. Sci. 2016, 4, 826–838. [Google Scholar] [CrossRef]
- Xu, L.; Tong, G.; Song, Q.; Zhu, C.; Zhang, H.; Shi, J.; Zhang, Z. Enhanced Intracellular Ca2+ Nanogenerator for Tumor-Specific Synergistic Therapy via Disruption of Mitochondrial Ca2+ Homeostasis and Photothermal Therapy. ACS Nano 2018, 12, 6806–6818. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Jin, S.; Liang, X.-J.; Ma, X. Enhanced anti-tumor activity of a drug through pH-triggered release and dual targeting by calcium phosphate-covered mesoporous silica vehicles. J. Mater. Chem. B 2022, 10, 384–395. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.-L.; Xu, J.-H.; Cai, B.; Yu, G.-T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.-S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Schuerch, C.M.; Forster, S.; Bruehl, F.; Yang, S.H.; Felley-Bosco, E.; Hewer, E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology 2018, 7, e1373235. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Li, M.; Jiang, S.; Simon, J.; Passlick, D.; Frey, M.-L.; Wagner, M.; Mailaender, V.; Crespy, D.; Landfester, K. Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Shen, Y.; Yu, Q.; Gan, Z. Shell-Sheddable Poly(N-2-hydroxypropyl methacrylamide) Polymeric Micelles for Dual-Sensitive Release of Doxorubicin. Macromol. Rapid Commun. 2018, 39, 1800139. [Google Scholar] [CrossRef]
- Maso, K.; Grigoletto, A.; Raccagni, L.; Bellini, M.; Marigo, I.; Ingangi, V.; Suzuku, A.; Hirai, M.; Kamiya, M.; Yashioka, H.; et al. Poly(L-glutamic acid)-co-poly(ethylene glycol) block copolymers for protein conjugation. J. Control. Release 2020, 324, 228–237. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Wang, J.; Pan, H.; Li, J.; Nie, D.; Zhuo, Y.; Lv, Y.; Wang, N.; Chen, H.; Guo, S.; Gan, Y.; et al. Cell membrane-coated mesoporous silica nanorods overcome sequential drug delivery barriers against colorectal cancer. Chin. Chem. Lett. 2023, 34, 107828. [Google Scholar] [CrossRef]
- Dong, N.; Liu, Z.; He, H.; Lu, Y.; Qi, J.; Wu, W. “Hook&Loop” multivalent interactions based on disk-shaped nanoparticles strengthen active targeting. J. Control. Release 2023, 354, 279–293. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef]
- Varga, G.; Somosi, Z.; Kónya, Z.; Kukovecz, A.; Pálinkó, I.; Szilagyi, I. A colloid chemistry route for the preparation of hierarchically ordered mesoporous layered double hydroxides using surfactants as sacrificial templates. J. Colloid Interface Sci. 2021, 581, 928–938. [Google Scholar] [CrossRef]
- Muráth, S.; Varga, T.; Kukovecz, A.; Kónya, Z.; Sipos, P.; Palinko, I.; Varga, G. Morphological aspects determine the catalytic activity of porous hydrocalumites: The role of the sacrificial templates. Mater. Today Chem. 2022, 23, 100682. [Google Scholar] [CrossRef]
- Kuai, R.; Singh, P.B.; Sun, X.; Xu, C.; Najafabadi, A.H.; Scheetz, L.; Yuan, W.; Xu, Y.; Hong, H.; Keskin, D.B.; et al. Robust Anti-Tumor T Cell Response with Efficient Intratumoral Infiltration by Nanodisc Cancer Immunotherapy. Adv. Ther. 2020, 3, 2000094. [Google Scholar] [CrossRef]
- Plaze, M.; Attali, D.; Prot, M.; Petit, A.-C.; Blatzer, M.; Vinckier, F.; Levillayer, L.; Chiaravalli, J.; Perin-Dureau, F.; Cachia, A.; et al. Inhibition of the replication of SARS-CoV-2 in human cells by the FDA-approved drug chlorpromazine. Int. J. Antimicrob. Agents 2021, 57, 106274. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Fishman, P.H. Filipin-dependent inhibition of cholera toxin: Evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998, 141, 905–915. [Google Scholar] [CrossRef]
- Abban, C.Y.; Bradbury, N.A.; Meneses, P.I. HPV16 and BPV1 infection can be blocked by the dynamin inhibitor dynasore. Am. J. Ther. 2008, 15, 304–311. [Google Scholar] [CrossRef]
- Yao, L.-H.; Rao, Y.; Varga, K.; Wang, C.-Y.; Xiao, P.; Lindau, M.; Gong, L.-W. Synaptotagmin 1 Is Necessary for the Ca2+ Dependence of Clathrin-Mediated Endocytosis. J. Neurosci. 2012, 32, 3778–3785. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Hajra, S.; Patra, A.R.; Basu, A.; Bhattacharya, S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018, 101, 228–243. [Google Scholar] [CrossRef]
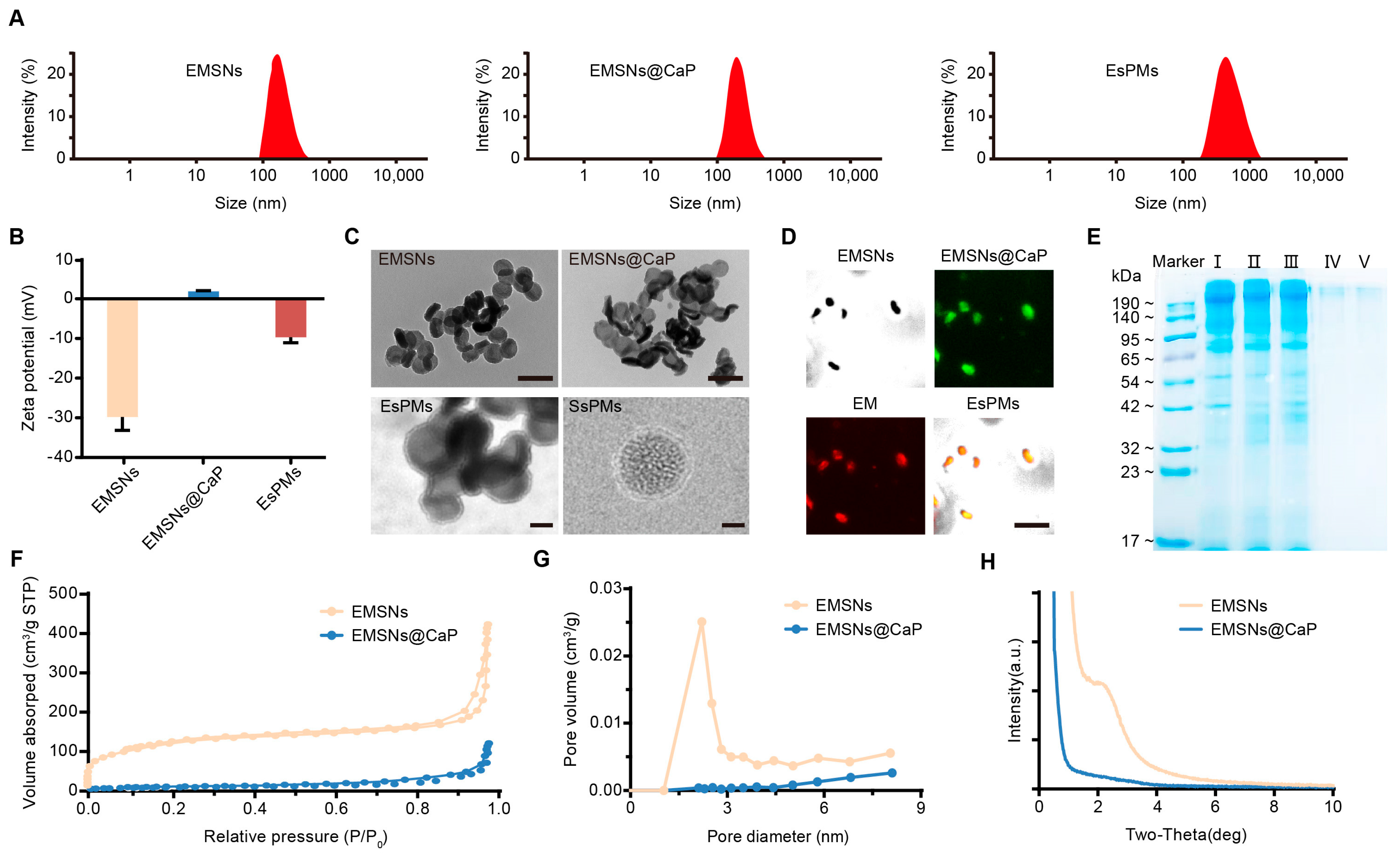


| Samples | SBET (m2/g) | WBJH (nm) | Vt (cm3/g) |
|---|---|---|---|
| EMSNs | 415.43 | 2.21 | 0.64 |
| EMSNs@CaP | 31.95 | 2.07 | 0.17 |
| Drug-Loaded Samples | Drug-Loading Efficiency (%) |
|---|---|
| DOX/EMSNs | 30.31 |
| DOX/EMSNs@CaP | 28.08 |
| DOX/EsPMs | 27.89 |
| DOX/SsPMs | 27.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Jiang, Y.; Ma, Z.; Li, Q.; Xi, X.; Fan, C.; Zhu, S.; Zhang, J.; Xu, L. Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect. Pharmaceutics 2023, 15, 2785. https://doi.org/10.3390/pharmaceutics15122785
Xi Z, Jiang Y, Ma Z, Li Q, Xi X, Fan C, Zhu S, Zhang J, Xu L. Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect. Pharmaceutics. 2023; 15(12):2785. https://doi.org/10.3390/pharmaceutics15122785
Chicago/Turabian StyleXi, Ziyue, Yingying Jiang, Zitong Ma, Qun Li, Xinran Xi, Chuanyong Fan, Shuang Zhu, Junjie Zhang, and Lu Xu. 2023. "Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect" Pharmaceutics 15, no. 12: 2785. https://doi.org/10.3390/pharmaceutics15122785
APA StyleXi, Z., Jiang, Y., Ma, Z., Li, Q., Xi, X., Fan, C., Zhu, S., Zhang, J., & Xu, L. (2023). Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect. Pharmaceutics, 15(12), 2785. https://doi.org/10.3390/pharmaceutics15122785






