Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (iSRB)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Immunogenic Smart Radiotherapy Biomaterial (iSRB)
2.2. Toxicity Studies in Healthy Mice
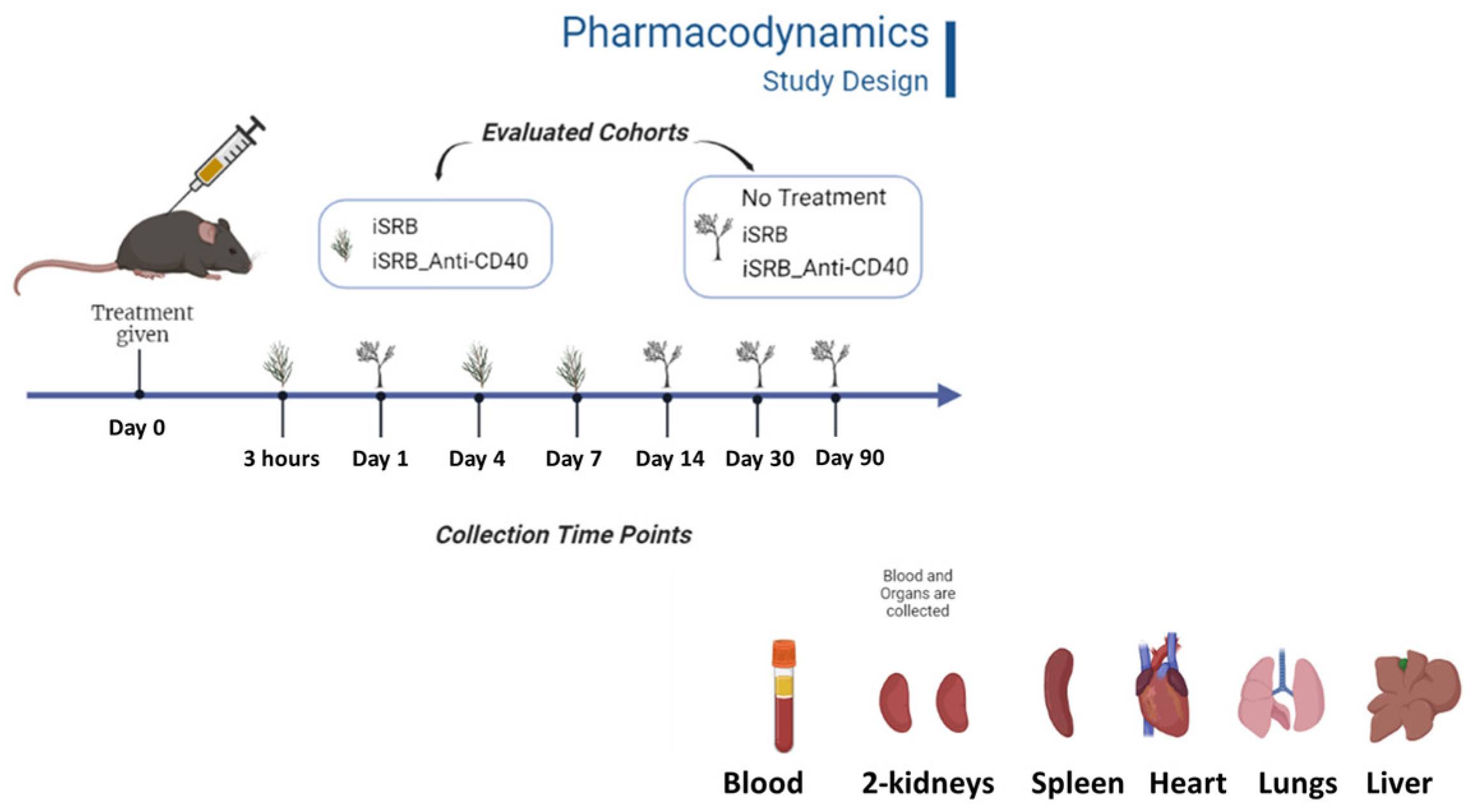
2.2.1. Study Design
2.2.2. Body Score and Body Weight Analyses
2.2.3. Liver and Kidney Function and Serum Total Cholesterol Analyses
2.2.4. Complete Blood Count (CBC) Analysis
2.2.5. Histopathology Analysis
2.2.6. Colitis Assessment
2.2.7. Statistics
3. Results
3.1. Body Weight and Body Score
3.2. Renal and Hepatic Function
3.2.1. Hepatic Function
3.2.2. Renal (Kidney) Function and Serum Cholesterol Levels
3.3. Hematological Analyses
3.4. Histopathology Report
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahn, A.W.; Gill, D.M.; Pal, S.K.; Agarwal, N. The Future of Immune Checkpoint Cancer Therapy after PD-1 and CTLA-4. Immunotherapy 2017, 9, 681–692. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- Van Mierlo, G.J.D.; den Boer, A.T.; Medema, J.P.; van der Voort, E.I.H.; Fransen, M.F.; Offringa, R.; Melief, C.J.M.; Toes, R.E.M. CD40 Stimulation Leads to Effective Therapy of CD40 − Tumors through Induction of Strong Systemic Cytotoxic T Lymphocyte Immunity. Proc. Natl. Acad. Sci. USA 2002, 99, 5561–5566. [Google Scholar] [CrossRef]
- Byrne, K.T.; Vonderheide, R.H. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep. 2016, 15, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Lanzavecchia, A. Licence to Kill. Nature 1998, 393, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 Agonists Alter Tumor Stroma and Show Efficacy Against Pancreatic Carcinoma in Mice and Humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef]
- Buhtoiarov, I.N.; Lum, H.; Berke, G.; Paulnock, D.M.; Sondel, P.M.; Rakhmilevich, A.L. CD40 Ligation Activates Murine Macrophages via an IFN-γ-Dependent Mechanism Resulting in Tumor Cell Destruction In Vitro. J. Immunol. 2005, 174, 6013–6022. [Google Scholar] [CrossRef] [PubMed]
- Long, K.B.; Gladney, W.L.; Tooker, G.M.; Graham, K.; Fraietta, J.A.; Beatty, G.L. IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016, 6, 400–413. [Google Scholar] [CrossRef]
- Ingoglia, G.; Yalamanoglu, A.; Pfefferlé, M.; Dubach, I.L.; Schaer, C.A.; Valkova, K.; Hansen, K.; Schulthess, N.; Humar, R.; Schaer, D.J.; et al. Line-Selective Macrophage Activation with an Anti-CD40 Antibody Drives a Hemophagocytic Syndrome in Mice. Blood Adv. 2020, 4, 2751–2761. [Google Scholar] [CrossRef]
- Sargent, L.M.; Hubbs, A.F.; Young, S.-H.; Kashon, M.L.; Dinu, C.Z.; Salisbury, J.L.; Benkovic, S.A.; Lowry, D.T.; Murray, A.R.; Kisin, E.R.; et al. Single-Walled Carbon Nanotube-Induced Mitotic Disruption. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 28–37. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Kaida, T.; Kobayashi, K.; Adachi, M.; Suzuki, F. Optical Characteristics of Titanium Oxide Interference Film and the Film Laminated with Oxides and Their Applications for Cosmetics. J. Cosmet. Sci. 2004, 55, 219–220. [Google Scholar]
- Wang, J.J.; Sanderson, B.J.S.; Wang, H. Cyto- and Genotoxicity of Ultrafine TiO2 Particles in Cultured Human Lymphoblastoid Cells. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 628, 99–106. [Google Scholar] [CrossRef]
- Wolf, R.; Matz, H.; Orion, E.; Lipozencić, J. Sunscreens—The Ultimate Cosmetic. Acta Dermatovenerol. Croat. 2003, 11, 158–162. [Google Scholar]
- Riu, J.; Maroto, A.; Rius, F. Nanosensors in Environmental Analysis. Talanta 2006, 69, 288–301. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, J.; Xu, J.; Deng, J.; Guo, J. TiO2 Nanoparticles Co-Doped with Silver and Nitrogen for Antibacterial Application. J. Nanosci. Nanotechnol. 2010, 10, 4868–4874. [Google Scholar] [CrossRef]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of Metallic Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef]
- Carlander, U.; Li, D.; Jolliet, O.; Emond, C.; Johanson, G. Toward a General Physiologically-Based Pharmacokinetic Model for Intravenously Injected Nanoparticles. Int. J. Nanomed. 2016, 625. [Google Scholar] [CrossRef]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H. Abnormal Trace Metals in Man: Titanium. J. Chronic Dis. 1963, 16, 55–69. [Google Scholar] [CrossRef]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The Application of Titanium Dioxide, Zinc Oxide, Fullerene, and Graphene Nanoparticles in Photodynamic Therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Zhang, J.; Huang, Y.; Deng, L.; Li, C.; Tai, G.; Ruan, B. Local Penetration of Doxorubicin via Intrahepatic Implantation of PLGA Based Doxorubicin-Loaded Implants. Drug Deliv. 2019, 26, 1049–1057. [Google Scholar] [CrossRef]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef]
- Moreau, M.; Yasmin-Karim, S.; Kunjachan, S.; Sinha, N.; Gremse, F.; Kumar, R.; Chow, K.F.; Ngwa, W. Priming the Abscopal Effect Using Multifunctional Smart Radiotherapy Biomaterials Loaded with Immunoadjuvants. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Yasmin-Karim, S.; Ziberi, B.; Wirtz, J.; Bih, N.; Moreau, M.; Guthier, R.; Ainsworth, V.; Hesser, J.; Makrigiorgos, G.M.; Chuong, M.D.; et al. Boosting the Abscopal Effect Using Immunogenic Biomaterials with Varying Radiation Therapy Field Sizes. Int. J. Radiat. Oncol. 2022, 112, 475–486. [Google Scholar] [CrossRef]
- Mueller, R.; Moreau, M.; Yasmin-Karim, S.; Protti, A.; Tillement, O.; Berbeco, R.; Hesser, J.; Ngwa, W. Imaging and Characterization of Sustained Gadolinium Nanoparticle Release from Next Generation Radiotherapy Biomaterial. Nanomaterials 2020, 10, 2249. [Google Scholar] [CrossRef]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6Chi Monocytes in the Inflamed Colon Give Rise to Proinflammatory Effector Cells and Migratory Antigen-Presenting Cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Rüter, J.; Antonia, S.J.; Burris, H.A.; Huhn, R.D.; Vonderheide, R.H. Immune Modulation with Weekly Dosing of an Agonist CD40 Antibody in a Phase I Study of Patients with Advanced Solid Tumors. Cancer Biol. Ther. 2010, 10, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. Prospect of Targeting the CD40 Pathway for Cancer Therapy. Clin. Cancer Res. 2007, 13, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Scotland, R.S.; Stables, M.J.; Madalli, S.; Watson, P.; Gilroy, D.W. Sex Differences in Resident Immune Cell Phenotype Underlie More Efficient Acute Inflammatory Responses in Female Mice. Blood 2011, 118, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Bous, M.; Schmitt, C.; Hans, M.C.; Weber, R.; Nourkami-Tutdibi, N.; Tenbruck, S.; Haj Hamoud, B.; Wagenpfeil, G.; Kaiser, E.; Solomayer, E.-F.; et al. Sex Differences in the Frequencies of B and T Cell Subpopulations of Human Cord Blood. Int. J. Mol. Sci. 2023, 24, 11511. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Arnold, A.P.; Reue, K. A Guide for the Design of Pre-Clinical Studies on Sex Differences in Metabolism. Cell Metab. 2017, 25, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Lian, K.; Yan, X.; Wang, R.; Lin, J.; Feng, X.; Tu, X.; Wang, C.; Zheng, L.; Xu, X.; et al. 447 Evaluation of Efficacy and Toxicity of Human CD40 Agonistic Antibodies in C57BL/6-HCD40/HFcγRIIB HuGEMM TM. In Proceedings of the Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2023; p. A497. [Google Scholar]
- Moreno, V.; Perets, R.; Peretz-Yablonski, T.; Fourneau, N.; Girgis, S.; Guo, Y.; Hellemans, P.; Verona, R.; Pendás, N.; Xia, Q.; et al. A Phase 1 Study of Intravenous Mitazalimab, a CD40 Agonistic Monoclonal Antibody, in Patients with Advanced Solid Tumors. Investig. New Drugs 2023, 41, 93–104. [Google Scholar] [CrossRef]
- Otto, G.P.; Rathkolb, B.; Oestereicher, M.A.; Lengger, C.J.; Moerth, C.; Micklich, K.; Fuchs, H.; Gailus-Durner, V.; Wolf, E.; Hrabě de Angelis, M. Clinical Chemistry Reference Intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus Musculus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 375–386. [Google Scholar]
- Moorhead, K.A.; Discipulo, M.L.; Hu, J.; Moorhead, R.C.; Johns, J.L. Alterations Due to Dilution and Anticoagulant Effects in Hematologic Analysis of Rodent Blood Samples on the Sysmex XT-2000iV. Vet. Clin. Pathol. 2016, 45, 215–224. [Google Scholar] [CrossRef]
- McClure, D.E. Clinical Pathology and Sample Collection in the Laboratory Rodent. Vet. Clin. N. Am. Exot. Anim. Pract. 1999, 2, 565–590. [Google Scholar] [CrossRef]
- Schnell, M.A.; Hardy, C.; Hawley, M.; Propert, K.J.; Wilson, J.M. Effect of Blood Collection Technique in Mice on Clinical Pathology Parameters. Hum. Gene Ther. 2002, 13, 155–161. [Google Scholar] [CrossRef]
- Friedel, R.; Trautschold, I.; Gärtner, K.; Helle-Feldmann, M.; Gaudssuhn, D. Effects of Blood Sampling on Enzyme Activities in the Serum of Small Laboratory Animals. Z. Klin. Chem. Klin. Biochem. 1975, 13, 499–505. [Google Scholar]
- Weingand, K.; Brown, G.; Hall, R.; Davies, D.; Gossett, K.; Neptun, D.; Waner, T.; Matsuzawa, T.; Salemink, P.; Froelke, W.; et al. Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies. The Joint Scientific Committee for International Harmonization of Clinical Pathology Testing. Fundam. Appl. Toxicol. 1996, 29, 198–201. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, M.; Acter, S.; Ngema, L.M.; Bih, N.; Sy, G.; Keno, L.S.; Chow, K.F.; Sajo, E.; Nebangwa, O.; Walker, J.; et al. Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (iSRB). Pharmaceutics 2023, 15, 2778. https://doi.org/10.3390/pharmaceutics15122778
Moreau M, Acter S, Ngema LM, Bih N, Sy G, Keno LS, Chow KF, Sajo E, Nebangwa O, Walker J, et al. Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (iSRB). Pharmaceutics. 2023; 15(12):2778. https://doi.org/10.3390/pharmaceutics15122778
Chicago/Turabian StyleMoreau, Michele, Shahinur Acter, Lindokuhle M. Ngema, Noella Bih, Gnagna Sy, Lensa S. Keno, Kwok Fan Chow, Erno Sajo, Oscar Nebangwa, Jacques Walker, and et al. 2023. "Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (iSRB)" Pharmaceutics 15, no. 12: 2778. https://doi.org/10.3390/pharmaceutics15122778
APA StyleMoreau, M., Acter, S., Ngema, L. M., Bih, N., Sy, G., Keno, L. S., Chow, K. F., Sajo, E., Nebangwa, O., Walker, J., Oh, P., Broyles, E., Ngwa, W., & Yasmin-Karim, S. (2023). Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (iSRB). Pharmaceutics, 15(12), 2778. https://doi.org/10.3390/pharmaceutics15122778








