Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix[4]arene with Hydroxyl and Amine Groups
Abstract
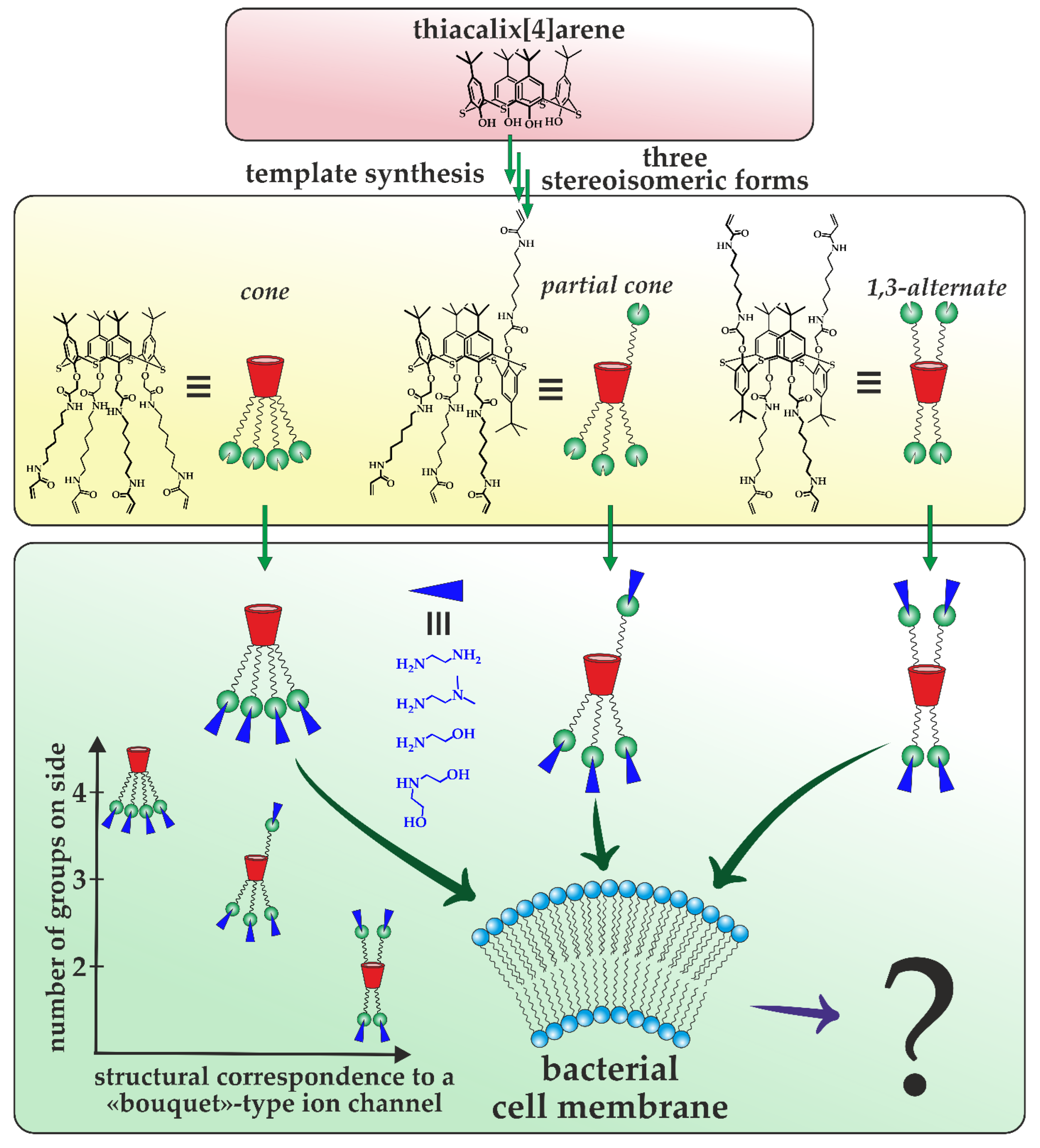
:1. Introduction
2. Materials and Methods
2.1. General Experimental Information
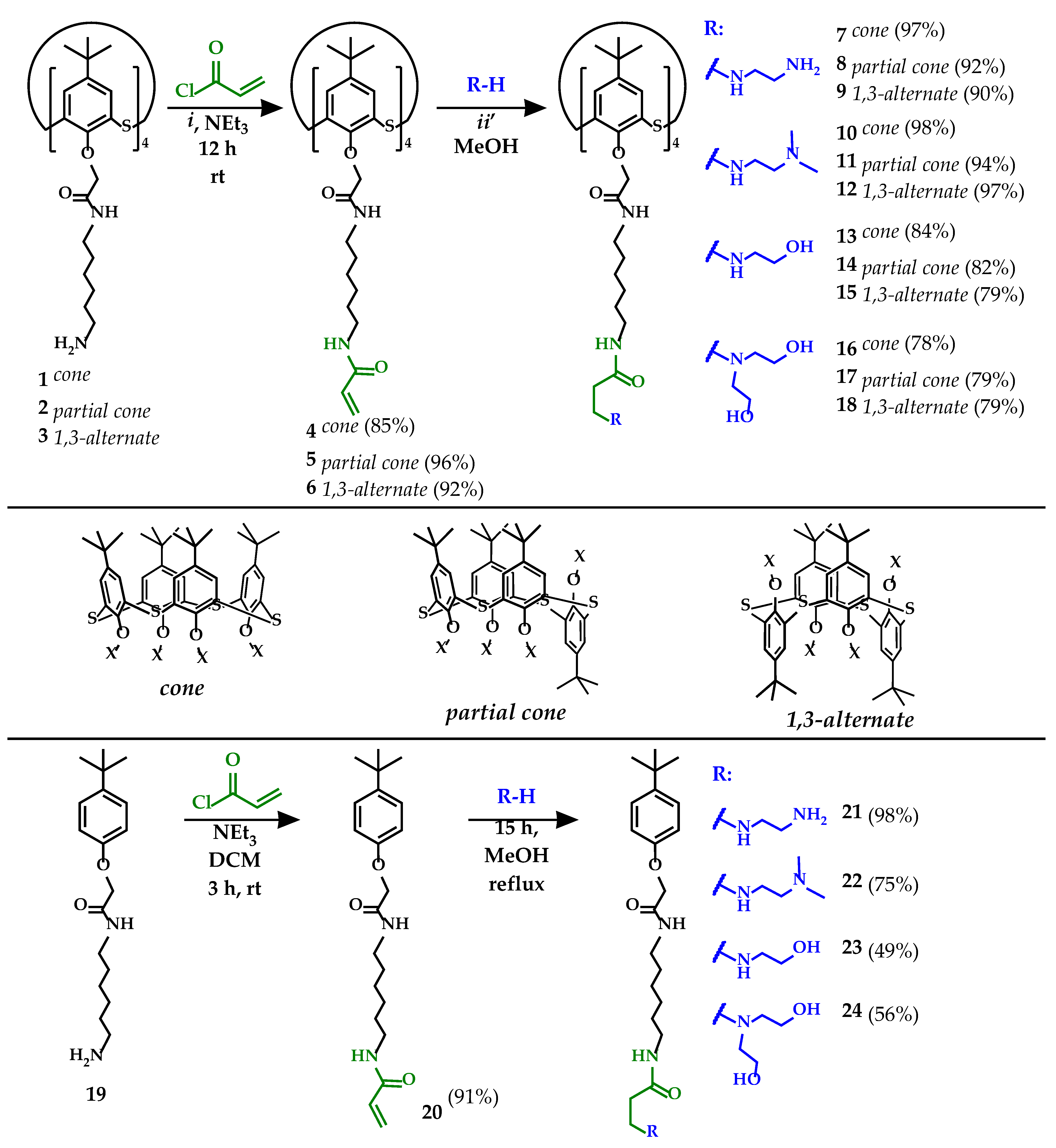
2.2. General Procedure for the Synthesis of Compounds 4–6
2.2.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(acrylamido)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 4 in cone conformation. Yield: 0.98 g (85%). White Powder, mp 88 °C
2.2.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(acrylamido)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 5 in partial cone conformation. Yield: 1.11 g (96%). White Powder, mp 84 °C
2.2.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(acrylamido)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 6 in 1,3-alternate conformation. Yield: 1.07 g (92%). White Powder, mp 91 °C
2.3. General Procedure for the Synthesis of Compounds 7–12
2.3.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-aminoethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 7 in cone conformation. Yield: 0.11 g (97%). White Solid Foam, mp 90 °C
2.3.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-aminoethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 8 in partial cone conformation. Yield: 0.11 g (92%). White Solid Foam, mp 88 °C
2.3.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-aminoethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 9 in 1,3-alternate conformation. Yield: 0.10 g (90%). White Solid Foam, mp 88 °C
2.3.4. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-(N,N-dimethylamino)ethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 10 in cone conformation. Yield: 0.12 g (98%). White Solid Foam, mp 60 °C
2.3.5. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-(N,N-dimethylamino)ethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 11 in partial cone conformation. Yield: 0.11 g (94%). White Solid Foam, mp 66 °C
2.3.6. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-(N,N-dimethylamino)ethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 12 in 1,3-alternate conformation. Yield: 0.12 g (97%). White Solid Foam, mp 62 °C
2.4. General Procedure for the Synthesis of Compounds 13–15
2.4.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-hydroxyethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 13 in cone conformation. Yield: 0.19 g (84%). White Powder, mp 72 °C
2.4.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-hydroxyethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 14 in partial cone conformation. Yield: 0.19 g (82%). White Powder, mp 65 °C
2.4.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(2-hydroxyethyl)aminopropanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 15 in 1,3-alternate conformation. Yield: 0.18 g (79%). White Powder, mp 80 °C
2.5. General Procedure for the Synthesis of Compounds 16–18
2.5.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(N,N-di(2-hydroxyethyl)amino)propanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 16 in cone conformation. Yield: 0.20 g (78%). White Powder, mp 85 °C
2.5.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(N,N-di(2-hydroxyethyl)amino)propanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 17 in partial cone conformation. Yield: 0.20 g (79%). White Powder, mp 81 °C
2.5.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N-(3-(N,N-di(2-hydroxyethyl)amino)propanoyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene 18 in 1,3-alternate conformation. Yield: 0.20 g (79%). White Powder, mp 92 °C
2.6. Procedure for the Synthesis of Compound 20
N-(6-(2-(4-(tert-butyl)phenoxy)acetamido)hexyl)acrylamide 20. Yield: 0.58 g (91%). White Powder, mp 77 °C
2.7. General Procedure for the Synthesis of Compounds 21 and 22
2.7.1. 3-((2-aminoethyl)amino)-N-(6-(2-(4-(tert-butyl)phenoxy)acetamido)hexyl)propanamide 21. Yield: 0.16 g (98%). Viscous Oil
2.7.2. N-(6-(2-(4-(tert-butyl)phenoxy)acetamido)hexyl)-3-((2-(dimethylamino)ethyl)amino)propanamide 22. Yield: 0.13 g (75%). Viscous Oil
2.8. General Procedure for the Synthesis of Compounds 23 and 24
2.8.1. N-(6-(2-(4-(tert-butyl)phenoxy)acetamido)hexyl)-3-((2-hydroxyethyl)amino)propanamide 23. Yield: 0.08 g (49%). Viscous Oil
2.8.2. 3-(Bis(2-hydroxyethyl)amino)-N-(6-(2-(4-(tert-butyl)phenoxy)acetamido)hexyl)propanamide 24. Yield: 0.10 g (56%). Viscous Oil
3. Results and Discussion
3.1. Development of an Approach to the Synthesis of Multivalent Derivatives of Thiacalix[4]arene
3.2. Antibacterial Properties and Cytotoxicity of the Obtained Multivalent Derivatives of Thiacalix[4]arene
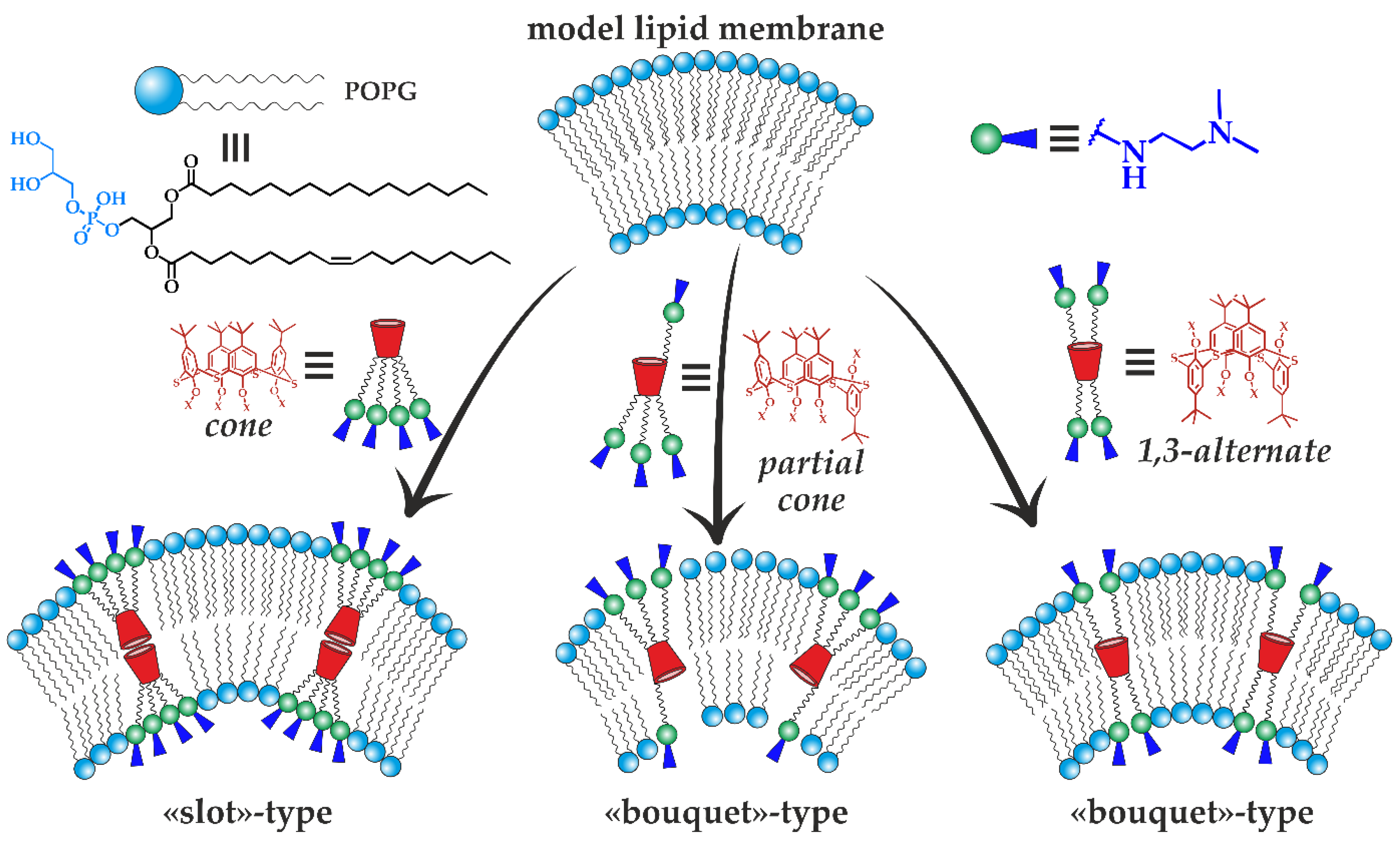
3.3. Study of the Mechanism of Antibacterial Activity of the Obtained Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Fact Sheet—Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 November 2023).
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in Bacterial Resistance Mechanisms and the Potential Ways to Combat Them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Nwobodo, D.C.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Chan, L.W.; Hern, K.E.; Ngambenjawong, C.; Lee, K.; Kwon, E.J.; Hung, D.T.; Bhatia, S.N. Selective Permeabilization of Gram-Negative Bacterial Membranes Using Multivalent Peptide Constructs for Antibiotic Sensitization. ACS Infect. Dis. 2021, 7, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Laroque, S.; Reifarth, M.; Sperling, M.; Kersting, S.; Klöpzig, S.; Budach, P.; Storsberg, J.; Hartlieb, M. Impact of Multivalence and Self-Assembly in the Design of Polymeric Antimicrobial Peptide Mimics. ACS Appl. Mater. Interfaces 2020, 12, 30052–30065. [Google Scholar] [CrossRef]
- Yin, F.; Li, J.-J.; Shi, B.; Zhang, K.; Li, X.-L.; Wang, K.-R.; Guo, D.-S. Carbohydrate–Macrocycle Conjugates for Biomedical Applications. Mater. Chem. Front. 2023, 7, 5263–5287. [Google Scholar] [CrossRef]
- Szymura, A.; Ilyas, S.; Horn, M.; Neundorf, I.; Mathur, S. Multivalent Magnetic Nanoaggregates with Unified Antibacterial Activity and Selective Uptake of Heavy Metals and Organic Pollutants. J. Mol. Liq. 2020, 317, 114002. [Google Scholar] [CrossRef]
- Hoyos, P.; Perona, A.; Juanes, O.; Rumbero, Á.; Hernáiz, M.J. Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity. Chem.—Eur. J. 2021, 27, 7593–7624. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 201801381. [Google Scholar] [CrossRef] [PubMed]
- Andriianova, A.N.; Latypova, L.R.; Vasilova, L.Y.; Kiseleva, S.V.; Zorin, V.V.; Abdrakhmanov, I.B.; Mustafin, A.G. Antibacterial Properties of Polyaniline Derivatives. J. Appl. Polym. Sci. 2021, 138, 51397. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2019, 32, 201904106. [Google Scholar] [CrossRef] [PubMed]
- Djouhri-Bouktab, L.; Rolain, J.M.; Brunel, J.M. Mini-Review: Polyamines Metabolism, Toxicity and Potent Therapeutical Use. Anti-Infect. Agents 2014, 12, 95–103. [Google Scholar] [CrossRef]
- Gorbunova, M.; Lemkina, L.; Borisova, I. New Guanidine-Containing Polyelectrolytes as Advanced Antibacterial Materials. Eur. Polym. J. 2018, 105, 426–433. [Google Scholar] [CrossRef]
- Stelmakh, S.A.; Grigor’eva, M.N.; Garkusheva, N.M.; Lebedeva, S.N.; Ochirov, O.S.; Mognonov, D.M.; Zhamsaranova, S.D.; Batoev, V.B. Studies of New Biocidal Polyguanidines: Antibacterial Action and Toxicity. Polym. Bull. 2020, 78, 1997–2008. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Hou, P.; Liu, J.; Fu, S. Design, Synthesis, Antibacterial, and Antitumor Activity of Linear Polyisocyanide Quaternary Ammonium Salts with Different Structures and Chain Lengths. Molecules 2021, 26, 5686. [Google Scholar] [CrossRef]
- Pham, P.; Oliver, S.; Boyer, C. Design of Antimicrobial Polymers. Macromol. Chem. Phys. 2022, 224, 2200226. [Google Scholar] [CrossRef]
- Cuneo, T.; Gao, H. Recent Advances on Synthesis and Biomaterials Applications of Hyperbranched Polymers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1640. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Wong, E.H.H.; Boyer, C. Synthetic Antimicrobial Polymers in Combination Therapy: Tackling Antibiotic Resistance. ACS Infect. Dis. 2021, 7, 215–253. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers Showing Intrinsic Antimicrobial Activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; Pechar, M.; Chytil, P.; Etrych, T. Polymer-Based Drug-Free Therapeutics for Anticancer, Anti-Inflammatory, and Antibacterial Treatment. Macromol. Biosci. 2021, 21, 202100135. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Karanastasis, A.; Casey, K.R.; Necelis, M.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 21270–21282. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E.V. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int. J. Mol. Sci. 2022, 23, 16144. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Shaikhutdinova, Z.M.; Mironov, V.F.; Bogdanov, A.V. Ammonium Amphiphiles Based on Natural Compounds: Design, Synthesis, Properties, and Biomedical Applications. A Review. Dokl. Chem. 2023, 509, 71–88. [Google Scholar] [CrossRef]
- Filippova, S.S.; Deriabin, K.V.; Perevyazko, I.; Shamova, O.V.; Orlov, D.S.; Islamova, R.M. Metal- and Peroxide-Free Silicone Rubbers with Antibacterial Properties Obtained at Room Temperature. ACS Appl. Polym. Mater. 2023, 5, 5286–5296. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, W.; Wang, C.; Wang, Y.; Zhang, Y.; Ye, Z.; Zhang, J.; Deng, L.; Dong, A. Screening and Matching Amphiphilic Cationic Polymers for Efficient Antibiosis. Biomacromolecules 2020, 21, 5269–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Hou, P.; Pan, H.; Fu, S. Polyisocyanide Quaternary Ammonium Salts with Exceptionally Star-Shaped Structure for Enhanced Antibacterial Properties. Polymers 2022, 14, 1737. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and Hyperbranched Structures for Biomedical Applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; de la Mata, F.; Galdiero, M. Function Oriented Molecular Design: Dendrimers as Novel Antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Sanchez, S.; Gómez-Casanova, N.; Sánchez-Nieves, J.; Gómez, R.; Rachuna, J.; Wąsik, S.; Semaniak, J.; Maciejewska, B.; Drulis-Kawa, Z.; Ciepluch, K.; et al. The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin. Int. J. Mol. Sci. 2022, 23, 1873. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Alfei, S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Apartsin, E.; Akhir, A.; Kaul, G.; Saxena, D.; Laurent, R.; Srivastava, K.K.; Mignani, S.; Majoral, J.-P.; Chopra, S. Low-Generation Cationic Phosphorus Dendrimers: Novel Approach to Tackle Drug-Resistant S. Aureus In Vitro and In Vivo. Biomacromolecules 2023, 24, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Ramchuran, E.J.; Pérez-Guillén, I.; Bester, L.A.; Khan, R.; Albericio, F.; Viñas, M.; de la Torre, B.G. Super-Cationic Peptide Dendrimers—Synthesis and Evaluation as Antimicrobial Agents. Antibiotics 2021, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Manov, H.; Yordanova, S.; Vasileva-Tonkova, E.; Stoyanov, S.; Grabchev, I. Synthesis, Spectral Properties and Antimicrobial Activity of a New Cationic Water-soluble pH-dependent Poly(Propylene Imine) Dendrimer Modified with 1,8-naphthalimides. Luminescence 2020, 35, 947–954. [Google Scholar] [CrossRef]
- Hernando-Gozalo, M.; Aguilera-Correa, J.J.; Rescalvo-Casas, C.; Seijas-Pereda, L.; García-Bertolín, C.; de la Mata, F.J.; Sánchez-Nieves, J.; Cuadros, J.; Pérez-Tanoira, R. Study of the Antimicrobial Activity of Cationic Carbosilane Dendrimers against Clinical Strains of Multidrug-Resistant Bacteria and Their Biofilms. Front. Cell. Infect. Microbiol. 2023, 13, 1203991. [Google Scholar] [CrossRef]
- Dhumal, D.; Maron, B.; Malach, E.; Lyu, Z.; Ding, L.; Marson, D.; Laurini, E.; Tintaru, A.; Ralahy, B.; Giorgio, S.; et al. Dynamic Self-Assembling Supramolecular Dendrimer Nanosystems as Potent Antibacterial Candidates against Drug-Resistant Bacteria and Biofilms. Nanoscale 2022, 14, 9286–9296. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Song, C.; Ouyang, Z.; Shen, M.; Janaszewska, A.; Klajnert-Maculewicz, B.; Shi, X. Synthesis and Shaping of Core–Shell Tecto Dendrimers for Biomedical Applications. Bioconjugate Chem. 2021, 32, 225–233. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional Supramolecular Systems: Design and Applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Dang, Y.-Y.; Li, H.; Li, H.; Liu, J.; Zhong, R.; Chen, Y.; Liu, S.; Lin, S. Membrane-Active Antibacterial Agents Based on Calix[4]Arene Derivatives: Synthesis and Biological Evaluation. Front. Chem. 2022, 10, 816741. [Google Scholar] [CrossRef] [PubMed]
- Razuvayeva, Y.; Kashapov, R.; Zakharova, L. Calixarene-Based Pure and Mixed Assemblies for Biomedical Applications. Supramol. Chem. 2020, 32, 178–206. [Google Scholar] [CrossRef]
- Surur, A.S.; Sun, D. Macrocycle-Antibiotic Hybrids: A Path to Clinical Candidates. Front. Chem. 2021, 9, 659845. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, L.; Consoli, G.M.L.; Clarizia, G.; Zampino, D.C.; Nostro, A.; Granata, G.; Ginestra, G.; Giuffrida, M.L.; Zimbone, S.; Bernardo, P. A Novel Material Based on an Antibacterial Choline-Calixarene Nanoassembly Embedded in Thin Films. J. Mater. Sci. 2022, 57, 20685–20701. [Google Scholar] [CrossRef]
- Baldini, L.; Casnati, A.; Sansone, F. Multivalent and Multifunctional Calixarenes in Bionanotechnology. Eur. J. Org. Chem. 2020, 2020, 5056–5069. [Google Scholar] [CrossRef]
- Burilov, V.; Makarov, E.; Mironova, D.; Sultanova, E.; Bilyukova, I.; Akyol, K.; Evtugyn, V.; Islamov, D.; Usachev, K.; Mukhametzyanov, T.; et al. Calix[4]Arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding. Int. J. Mol. Sci. 2022, 23, 14889. [Google Scholar] [CrossRef]
- Lee, J.-S.; Song, I.; Shinde, P.B.; Nimse, S.B. Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents. Antioxidants 2020, 9, 859. [Google Scholar] [CrossRef]
- Crowley, P.B. Protein–Calixarene Complexation: From Recognition to Assembly. Acc. Chem. Res. 2022, 55, 2019–2032. [Google Scholar] [CrossRef]
- Mourer, M.; Duval, R.E.; Constant, P.; Daffé, M.; Regnouf-de-Vains, J. Impact of Tetracationic Calix[4]Arene Conformation—From Conic Structure to Expanded Bolaform—On Their Antibacterial and Antimycobacterial Activities. ChemBioChem 2019, 20, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hu, X.; Guo, D.-S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2020, 60, 2768–2794. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Syakaev, V.V.; Latypov, S.K.; Nizameev, I.R.; Kadirov, M.K.; et al. N-Methyl-d-Glucamine–Calix[4]Resorcinarene Conjugates: Self-Assembly and Biological Properties. Molecules 2019, 24, 1939. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, H.; Zheng, B.; Huang, F. Combating Antibiotic Resistance: Current Strategies for the Discovery of Novel Antibacterial Materials Based on Macrocycle Supramolecular Chemistry. Giant 2021, 7, 100066. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Granata, G.; Ginestra, G.; Marino, A.; Toscano, G.; Nostro, A. Antibacterial Nanoassembled Calix[4]Arene Exposing Choline Units Inhibits Biofilm and Motility of Gram Negative Bacteria. ACS Med. Chem. Lett. 2022, 13, 916–922. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Di Bari, I.; Blanco, A.R.; Nostro, A.; D’Arrigo, M.; Pistarà, V.; Sortino, S. Design, Synthesis, and Antibacterial Activity of a Multivalent Polycationic Calix[4]Arene–NO Photodonor Conjugate. ACS Med. Chem. Lett. 2017, 8, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Granata, G.; Picciotto, R.; Blanco, A.R.; Geraci, C.; Marino, A.; Nostro, A. Design, Synthesis and Antibacterial Evaluation of a Polycationic Calix[4]Arene Derivative Alone and in Combination with Antibiotics. Med. Chem. Commun. 2018, 9, 160–164. [Google Scholar] [CrossRef]
- Mourer, M.; Regnouf-de-Vains, J.-B.; Duval, R.E. Functionalized Calixarenes as Promising Antibacterial Drugs to Face Antimicrobial Resistance. Molecules 2023, 28, 6954. [Google Scholar] [CrossRef]
- Padnya, P.; Mostovaya, O.; Ovchinnikov, D.; Shiabiev, I.; Pysin, D.; Akhmedov, A.; Mukhametzyanov, T.; Lyubina, A.; Voloshina, A.; Petrov, K.; et al. Combined Antimicrobial Agents Based on Self-Assembled PAMAM-Calix-Dendrimers/Lysozyme Nanoparticles: Design, Antibacterial Properties and Cytotoxicity. J. Mol. Liq. 2023, 389, 122838. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Shiabiev, I.E.; Stoikov, I.I. Novel Lactide Derivatives of P-Tert-Butylthiacalix[4]Arene: Directed Synthesis and Molecular Recognition of Catecholamines. J. Mol. Liq. 2021, 327, 114806. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael Reaction: Achievements and Prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael Reaction: A Decade Later—Is the Research Over? Eur. J. Org. Chem. 2023, 26, e202300451. [Google Scholar] [CrossRef]
- Mostovaya, O.; Shiabiev, I.; Pysin, D.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Padnya, P.; Stoikov, I. PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding. Pharmaceutics 2022, 14, 2748. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, O.; Padnya, P.; Shiabiev, I.; Mukhametzyanov, T.; Stoikov, I. PAMAM-Calix-Dendrimers: Synthesis and Thiacalixarene Conformation Effect on DNA Binding. Int. J. Mol. Sci. 2021, 22, 11901. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, S.; Zhang, X.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary Ammonium Salts: Insights into Synthesis and New Directions in Antibacterial Applications. Bioconjugate Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Saverina, E.A.; Frolov, N.A.; Kamanina, O.A.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. From Antibacterial to Antibiofilm Targeting: An Emerging Paradigm Shift in the Development of Quaternary Ammonium Compounds (QACs). ACS Infect. Dis. 2023, 9, 394–422. [Google Scholar] [CrossRef] [PubMed]
- Seferyan, M.A.; Saverina, E.A.; Frolov, N.A.; Detusheva, E.V.; Kamanina, O.A.; Arlyapov, V.A.; Ostashevskaya, I.I.; Ananikov, V.P.; Vereshchagin, A.N. Multicationic Quaternary Ammonium Compounds: A Framework for Combating Bacterial Resistance. ACS Infect. Dis. 2023, 9, 1206–1220. [Google Scholar] [CrossRef]
- Padnya, P.L.; Terenteva, O.S.; Akhmedov, A.A.; Iksanova, A.G.; Shtyrlin, N.V.; Nikitina, E.V.; Krylova, E.S.; Shtyrlin, Y.G.; Stoikov, I.I. Thiacalixarene Based Quaternary Ammonium Salts as Promising Antibacterial Agents. Bioorg. Med. Chem. 2021, 29, 115905. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Antipin, I.S.; Konovalov, A.I. Artificial Ion Channels. Russ. Chem. Rev. 2003, 72, 1055–1077. [Google Scholar] [CrossRef]
- Pregel, M.J.; Jullien, L.; Canceill, J.; Lacombe, L.; Lehn, J.-M. Channel-Type Molecular Structures. Part 4. Transmembrane Transport of Alkali-Metal Ions by ‘Bouquet’ Molecules. J. Chem. Soc. Perkin Trans. 2 1995, 417–426. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez, M.A.; Castellano, V.; Martínez-Larrañaga, M.R. The Role of in Vitro Methods as Alternatives to Animals in Toxicity Testing. Expert Opin. Drug Metab. Toxicol. 2013, 10, 67–79. [Google Scholar] [CrossRef]
- Krok, E.; Stephan, M.; Dimova, R.; Piatkowski, L. Tunable Biomimetic Bacterial Membranes from Binary and Ternary Lipid Mixtures and Their Application in Antimicrobial Testing. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184194. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, H.; Onishi, M.; Kamimori, H. Lipid Membrane-Binding Properties of Daptomycin Using Surface Plasmon Resonance. Anal. Sci. 2013, 29, 297–301. [Google Scholar] [CrossRef]
- Li, S.; Ren, R.; Lyu, L.; Song, J.; Wang, Y.; Lin, T.-W.; Brun, A.L.; Hsu, H.-Y.; Shen, H.-H. Solid and Liquid Surface-Supported Bacterial Membrane Mimetics as a Platform for the Functional and Structural Studies of Antimicrobials. Membranes 2022, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.D.; Gumerova, S.K.; Sapunova, A.S.; Kulik, N.V.; Mirgorodskaya, A.B.; Kotenko, A.A.; Prokopyeva, T.M.; Mikhailov, V.A.; Zakharova, L.Y.; Sinyashin, O.G. The Structure—Activity Correlation in the Family of Dicationic Imidazolium Surfactants: Antimicrobial Properties and Cytotoxic Effect. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129728. [Google Scholar] [CrossRef]
- Agarkov, A.S.; Nefedova, A.A.; Gabitova, E.R.; Mingazhetdinova, D.O.; Ovsyannikov, A.S.; Islamov, D.R.; Amerhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Litvinov, I.A.; et al. (2-Hydroxy-3-Methoxybenzylidene)Thiazolo[3,2-a]Pyrimidines: Synthesis, Self-Assembly in the Crystalline Phase and Cytotoxic Activity. Int. J. Mol. Sci. 2023, 24, 2084. [Google Scholar] [CrossRef] [PubMed]
| Compounds | miLogP | Terminal Fragment | S. aureus | B. cereus | E. faecalis | E. coli | P. aeruginosa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |||
| 7 (cone) | 8.74 | 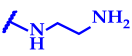 | 62.5 ± 5.3 | 62.5 ± 4.8 | 125 ± 11 | 250 ± 19 | 62.5 ± 4.9 | 62.5 ± 5.2 | 62.5 ± 5.3 | 62.5 ± 4.9 | 250 ± 19 | 250 ± 20 |
| 8 (partial cone) | 31.3 ± 2.5 | 31.3 ± 2.5 | 31.3 ± 2.1 | 31.3 ± 2.3 | 15.6 ± 1.3 | 250 ± 21 | 15.6 ± 1.4 | 15.6 ± 1.3 | 15.6 ± 1.2 | 15.6 ± 1.2 | ||
| 9 (1,3-alternate) | 31.3 ± 2.6 | 31.3 ± 2.4 | 62.5 ± 5.4 | >500 | 62.5 ± 4.8 | 62.5 ± 5.5 | 15.6 ± 1.2 | 15.6 ± 1.2 | 15.6 ± 1.3 | 15.6 ± 1.3 | ||
| 21 (monomer) | 1.97 | 125 ± 11 | 125 ± 9 | 250 ± 19 | >500 | 125 ± 9 | 125 ± 10 | 250 ± 20 | 250 ± 21 | 250 ± 21 | 250 ± 19 | |
| 10 (cone) | 9.95 | 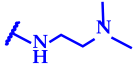 | 15.6 ± 1.3 | 15.6 ± 1.3 | 62.5 ± 4.8 | 125 ± 10 | 3.9 ± 0.4 | 3.9 ± 0.3 | 31.3 ± 2.6 | 31.3 ± 2.4 | 125 ± 11 | 125 ± 9 |
| 11 (partial cone) | 0.90 ± 0.01 | 1.9 ± 0.1 | 15.6 ± 1.2 | 15.6 ± 1.2 | 1.9 ± 0.2 | 3.9 ± 0.3 | 7.8 ± 0.6 | 7.8 ± 0.6 | 15.6 ± 1.2 | 15.6 ± 1.3 | ||
| 12 (1,3-alternate) | 7.8 ± 0.7 | 7.8 ± 0.7 | 62.5 ± 5.3 | 62.5 ± 4.9 | 7.8 ± 0.6 | 7.8 ± 0.8 | 7.8 ± 0.7 | 7.8 ± 0.6 | 62.5 ± 5.2 | 62.5 ± 5.3 | ||
| 22 (monomer) | 3.20 | 250 ± 19 | 500 ± 45 | 250 ± 21 | >500 | 125 ± 11 | 125 ± 11 | 250 ± 19 | 250 ± 20 | 500 ± 44 | 500 ± 45 | |
| 13 (cone) | 9.47 | 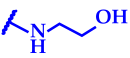 | 7.8 ± 0.6 | 7.8 ± 0.7 | 125 ± 11 | 125 ± 10 | 7.8 ± 0.6 | 125 ± 11 | 125 ± 10 | 125 ± 10 | 62.5 ± 4.8 | 62.5 ± 5.2 |
| 14 (partial cone) | 1.9 ± 0.1 | 3.9 ± 0.3 | 31.3 ± 2.3 | 31.3 ± 2.5 | 3.9 ± 0.3 | 62.5 ± 5.3 | 31.3 ± 2.4 | 31.3 ± 2.5 | 125 ± 9 | 125 ± 11 | ||
| 15 (1,3-alternate) | 3.9 ± 0.3 | 3.9 ± 0.4 | 31.3 ± 2.5 | >500 | 7.8 ± 0.6 | 31.3 ± 2.8 | 31.3 ± 2.5 | 31.3 ± 2.3 | 250 ± 20 | 250 ± 19 | ||
| 23 (monomer) | 2.54 | 250 ± 19 | 250 ± 19 | 250 ± 21 | >500 | 250 ± 21 | 250 ± 19 | 500 ± 45 | 500 ± 46 | 500 ± 47 | >500 | |
| 16 (cone) | 9.44 |  | 7.8 ± 0.7 | 7.8 ± 0.6 | 250 ± 21 | 500 ± 44 | 15.6 ± 1.2 | 125 ± 9 | 500 ± 42 | 500 ± 45 | >500 | >500 |
| 17 (partial cone) | 7.8 ± 0.6 | 7.8 ± 0.7 | 125 ± 10 | >500 | 62.5 ± 5.4 | 62.5 ± 4.9 | >500 | >500 | >500 | >500 | ||
| 18 (1,3-alternate) | 62.5 ± 5.6 | 250 ± 18 | 500 ± 45 | >500 | 31.3 ± 2.4 | >500 | 500 ± 44 | 500 ± 44 | >500 | >500 | ||
| 24 (monomer) | 2.50 | 125 ± 10 | 250 ± 21 | 250 ± 19 | >500 | 250 ± 20 | >500 | >500 | >500 | >500 | >500 | |
| Ciprofloxacin | –0.70 | – | 0.50 ± 0.04 | 0.50 ± 0.04 | 0.50 ± 0.03 | 0.50 ± 0.03 | 3.9 ± 0.4 | 3.9 ± 0.4 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.5 ± 0.04 | 0.5 ± 0.04 |
| Norfloxacin | –0.69 | – | 3.9 ± 0.4 | 3.9 ± 0.4 | 7.8 ± 0.6 | 7.8 ± 0.6 | 7.8 ± 0.6 | 15.6 ± 1.2 | 1.5 ± 0.1 | 7.8 ± 0.6 | 3.9 ± 0.2 | 15.6 ± 1.3 |
| Compounds | IC50 | Selective Index (SI = IC50/MIC) | ||||
|---|---|---|---|---|---|---|
| S. aureus | B. cereus | E. faecalis | E. coli | P. aeruginosa | ||
| 10 (cone) | 52.0 ± 4.2 | 3.3 | 0.8 | 13.3 | 1.7 | 0.4 |
| 11 (partial cone) | 3.6 ± 0.3 | 4.0 | 0.2 | 1.9 | 0.5 | 0.2 |
| 12 (1,3-alternate) | 25.3 ± 1.8 | 3.2 | 0.4 | 3.2 | 3.2 | 0.4 |
| Supramolecular System | POPG/Macrocycle Ratio | D, nm | PDI | Zeta-Potential, mV |
|---|---|---|---|---|
| POPG | 1:0 | 106 ± 2 | 0.09 | –28.2 ± 4.0 |
| POPG + 10 (cone) | 1:0.1 | 145 ± 3 | 0.17 | –19.6 ± 2.7 |
| 1:1 | 159 ± 4 | 0.21 | +26.2 ± 3.0 | |
| POPG + 11 (partial cone) | 1:0.1 | 127 ± 2 | 0.13 | –5.8 ± 5.8 |
| 1:1 | 571 ± 4 | 0.56 | +19.7 ± 2.9 | |
| POPG + 12 (1,3-altenate) | 1:0.1 | 143 ± 7 | 0.17 | –11.2 ± 2.3 |
| 1:1 | 290 ± 41 | 0.22 | +30.4 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiabiev, I.; Pysin, D.; Akhmedov, A.; Babaeva, O.; Babaev, V.; Lyubina, A.; Voloshina, A.; Petrov, K.; Padnya, P.; Stoikov, I. Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix[4]arene with Hydroxyl and Amine Groups. Pharmaceutics 2023, 15, 2731. https://doi.org/10.3390/pharmaceutics15122731
Shiabiev I, Pysin D, Akhmedov A, Babaeva O, Babaev V, Lyubina A, Voloshina A, Petrov K, Padnya P, Stoikov I. Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix[4]arene with Hydroxyl and Amine Groups. Pharmaceutics. 2023; 15(12):2731. https://doi.org/10.3390/pharmaceutics15122731
Chicago/Turabian StyleShiabiev, Igor, Dmitry Pysin, Alan Akhmedov, Olga Babaeva, Vasily Babaev, Anna Lyubina, Alexandra Voloshina, Konstantin Petrov, Pavel Padnya, and Ivan Stoikov. 2023. "Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix[4]arene with Hydroxyl and Amine Groups" Pharmaceutics 15, no. 12: 2731. https://doi.org/10.3390/pharmaceutics15122731
APA StyleShiabiev, I., Pysin, D., Akhmedov, A., Babaeva, O., Babaev, V., Lyubina, A., Voloshina, A., Petrov, K., Padnya, P., & Stoikov, I. (2023). Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix[4]arene with Hydroxyl and Amine Groups. Pharmaceutics, 15(12), 2731. https://doi.org/10.3390/pharmaceutics15122731








